Abstract
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are a major cause of acute respiratory failure in the critically ill patient. ALI and ARDS are characterized by the acute onset of severe hypoxemia and bilateral pulmonary infiltrates in the absence of clinical evidence for left atrial hypertension. These conditions are differentiated from one another by the ratio of the partial pressure of oxygen in the arterial blood to the inspired fraction of oxygen; ARDS requires a more severe oxygenation defect. ALI and ARDS may occur in association with a number of clinical disorders, including sepsis, pneumonia, aspiration, trauma including inhalational injury, and blood transfusions. The mortality rate remains high, in the range of 25% to 40%. The pathophysiology of ALI/ARDS involves resident lung cells, including endothelial and epithelial cells, as well as neutrophils, monocytes/macrophages, and platelets. When ALI/ARDS is complicated by acute kidney injury, mortality increases substantially. Several supportive and pharmacologic therapies have been tested in clinical trials. Of these, a low tidal volume, lung protective ventilation strategy is the only strategy that has been demonstrated in a large, multicenter randomized clinical trial to reduce mortality for patients with ALI/ARDS. Based on a recent randomized trial, a conservative fluid management strategy reduces the duration of mechanical ventilation without increasing the incidence of renal failure. Pharmacologic strategies and other ventilator management strategies have not been successful to date; however, several randomized, placebo controlled treatment trials are ongoing.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) affect approximately 200,000 Americans per year, with an overall mortality rate of approximately 40%. The objective of this article is to provide a review of these disease states, with an emphasis on a working definition, epidemiology, pathogenesis, and latest treatment advances. Where applicable, we will also discuss the implications of therapies for ALI for acid-base, volume status, and azotemia, metabolic parameters that are frequent concerns for the nephrologist.
Definitions
ALI and ARDS are clinical syndromes characterized by the acute onset (less than 7 d) of severe hypoxemia and bilateral pulmonary infiltrates in the absence of clinical evidence for left atrial hypertension (reviewed in (1,2)). The severity of the hypoxemia differentiates ALI from ARDS. The American/European Consensus Conference defined patients with ALI as those who have a ratio of partial pressure of oxygen in arterial blood (Pao2) to the inspired fraction of oxygen (Fio2) of less than 300 and patients with ARDS as those with a Pao2/Fio2 of less than 200 (3). Both ALI and ARDS develop in association with a wide variety of clinical disorders, including sepsis, pneumonia, aspiration, and major trauma, including severe burns. ALI and ARDS can also occur as sequelae of acute pancreatitis, smoke or toxic gas inhalation, massive blood transfusion, or a reaction to a single blood product transfusion (also known as transfusionassociated lung injury).
Importantly, the Consensus Conference definition has been critical for identifying patients who are appropriate for clinical trials and has also provided standardization that allows for comparison of patients across studies. We believe that standardization of definitions for ALI/ARDS has contributed importantly to the advancement of the field and the observed improved survival of subjects with these conditions. For example, in the first ARDS Network clinical trial (4), overall survival of study subjects was 35.4%, compared with 26.8% in the recently completed Fluid and Catheter Treatment Trial (FACTT) trial (5), despite the fact that individuals enrolled in FACTT had higher Acute Physiology and Chronic Health Evaluation III scores. Similarly, a consensus definition has allowed for comparison of study results across the spectrum of sepsis and septic shock. The recently proposed Acute Dialysis Quality Initiative RIFLE (Risk, Injury, Failure, Loss, End Stage Renal Disease) criteria (6) for acute kidney injury or the even more recent Acute Kidney Injury Network modification (7) will hopefully allow for standardization across studies of acute kidney injury and move the field forward. However, we note that all disease definitions are at best, imperfect, and it seems more critical for the community to agree to adopt a single definition, rather than a perfect definition.
The main clinical challenges in differentiating ALI from cardiogenic pulmonary edema have been discussed in detail in a recent review article (8). The combination of history, physical examination, laboratory data, and the chest radiograph is usually sufficient to diagnose cardiogenic versus noncardiogenic pulmonary edema or ALI. In some patients, a transthoracic or transesophageal echocardiogram is required to make the distinction. Finally, there are some patients in whom pulmonary artery catheterization is required to determine both the etiology of the shock and the pathogenesis of the pulmonary edema.
Epidemiology and Incidence
Although considerable progress has been made in understanding the natural history and pathogenesis of ALI and ARDS, its incidence throughout the world remains uncertain. A recent population-based clinical study identified patients with ALI at all 18 hospitals in King County (9). The incidence of ALI in King County was 78.9 per 100,000 person-years and of ARDS was 58.7 cases per 100,000 person-years. Mortality was approximately 40%. By extrapolating the data in this study to the population of the United States, the estimated incidence of ALI is approximately 200,000 patients per year. Of note, the population of King County differs significantly from that of other regions in the United States; in general, the population is younger and more affluent, and it includes more Asians and fewer African Americans. This study also demonstrated that the incidence of and mortality from ALI/ARDS increases with age.
Lower incidence rates have been reported in Europe and Australia (10,11), although the reason for these lower incidence rates remains uncertain (12). These incidence rates have ranged from 18 to 34 cases per 100,000 patient-years for ALI. Nonetheless, the mortality rates reported in these studies were similar to the King County cohort. Differences in these populations may include differences in risk factors for ALI, including race and differing predisposition to conditions underlying ALI/ARDS, as well as differences in case ascertainment and health care delivery. The data on the incidence of ALI/ARDS in children are more limited. A recent prospective study identified 320 children with ALI at two pediatric hospitals in Northern California; the overall mortality rate was 22% (13). Thus, ALI is a major cause of morbidity and mortality in critically ill children and adults.
In addition, the incidence of multiple organ failure, particularly acute kidney injury, is also very common in patients with ALI/ARDS. In a recent analysis of data from the National Heart, Lung, and Blood Institute (NHLBI) ARDS Network factorial clinical trial of ketoconazole, lisofylline, and a low tidal volume ventilation strategy for ALI/ARDS, approximately 35% of subjects developed acute kidney injury over the first week of the study, defined as a rise in creatinine of 50% from baseline (K.D. Liu and M.A. Matthay, unpublished data). These subjects had a 59% mortality rate, compared with an overall mortality rate of 28% within the cohort. Similarly, multiple cohort studies have suggested that refractory hypoxemia is uncommonly the cause of death in patients with ALI/ARDS. Rather, sepsis or multisystem organ dysfunction is often the cause of death (14,15). Indeed, the major clinical predictors of mortality for patients with ALI/ARDS include age, sepsis, the number of associated nonrespiratory organ dysfunctions, other comorbidities, and treatment-associated factors (16,17). Interestingly, the degree of hypoxemia, as measured by the initial PaO2/Fio2, has not been shown to be an independent predictor of mortality in adults (18).
Pathophysiology
The pathophysiology of ALI/ARDS has been reviewed at length (1,2). The early phase of ALI is characterized by diffuse alveolar damage, with disruption of the alveolar epithelium and the presence of fibrin-rich hyaline membranes along the denuded basement membrane. Protein-rich pulmonary edema fluid can be found filling the alveolar spaces, along with neutrophils, macrophages, and erythrocytes. This protein-rich edema fluid accumulates because of increased capillary permeability, resulting in increased movement of fluid from the capillaries to the lung interstitium. Airspace filling is likely exacerbated by epithelial injury and a decrease in the capacity of the injured alveolar epithelium to reabsorb edema fluid (19). Whereas some patients appear to fully recover from ALI, a few patients go on to a later phase of ALI/ARDS characterized by persistent hypoxemia, as well as fibrosis and ongoing inflammation within the airspaces (20).
Multiple compartments of the lung, including the epithelium and endothelium, play critical roles in the pathogenesis of ALI/ARDS (reviewed in (1)). The alveolar epithelium shares many features with the tubular epithelium of the kidney, including the presence of highly polarized epithelial cells characterized by a free apical surface that faces the airspace in the case of the lung and the urinary space in the case of the kidney and a basal surface that is in contact with the basement membrane (Figure 1A). The apical surface is characterized by the presence of channels and transporters that drive ion and fluid reabsorption. In both cases, the epithelial cells are tightly joined to one another by a series of junctional complexes; these tight junctions are fluid-impermeable. The alveolar epithelium consists of type I cells, which comprise approximately 90% of the alveolar surface area, and type II cells, which have several critical functions, including surfactant protein production and vectorial ion transport. Type II cells are also thought to function as progenitor cells for type I cells after cellular injury. Injury to these cells results in the loss of the tight barrier of the epithelium as well as loss of polarity of these cells, which allows for the formation of pulmonary edema fluid and as well as a loss of the ability to efficiently reabsorb this fluid (21) (Figure 1B).
Figure 1.
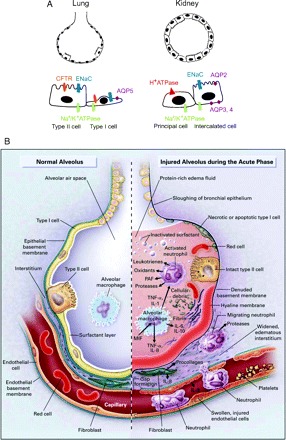
(A) Comparison of the alveolar and renal tubular epithelium. Left, cross section of an alveolus. The alveolar epithelium is composed of a mixture of type I and II cells. Water and ion channels are found on the apical and basolateral surfaces as shown. Right, cross section of the distal convoluted tubule. The renal tubular epithelium at this level is composed of principal cells and intercalated cells. ENaC, epithelial Na+ channel; CFTR, cystic fibrosis transmembrane conductance regulator; AQP, aquaporin. (B) Multiple pathogenic processes contribute to alveolar injury during acute lung injury/acute respiratory distress syndrome.
It is also clear that injury to the endothelial compartment plays a critical role in the pathogenesis of ALI/ARDS. Hemodynamic and ultrastructural studies first demonstrated that large and small vessels within the lung are damaged during ALI/ARDS, manifested as an increase in pulmonary vascular resistance during ALI/ARDS and by the presence of arterial and capillary thrombi on autopsy (22). The pulmonary dead space fraction allows an estimation of the amount of obstruction and destruction within the pulmonary capillary bed. An elevated pulmonary dead space fraction correlates with mortality in mechanically ventilated patients with ALI/ARDS (23). Several markers of dysregulated coagulation, including low levels of protein C and high levels of plasminogen activator inhibitor-1, are associated with increased mortality in patients with ALI/ARDS (24,25).
Increased capillary permeability has been demonstrated by the use of radiolabeled tracer compounds injected into the systemic circulation and comparing concentrations of these compounds in the pulmonary edema fluid and in plasma (26). Additional evidence of endothelial injury comes from studies demonstrating that endothelin-1 and von Willebrand factor levels are elevated in patients with ALI/ARDS (27–29). These proteins are released by endothelial cells following injury. Interestingly, higher levels of von Willebrand factor are associated with increased mortality in patients with ALI/ARDS (30).
Multiple cell types, including neutrophils, activated macrophages, and platelets, play critical roles in the pathogenesis of ALI/ARDS. Neutrophils appear to be retained in the lung following injury and release proteases, reactive oxygen and nitrogen species, inflammatory cytokines and growth factors. Platelets may play a role in enhancing lung endothelial injury in concert with neutrophils (31). Monocytes and macrophages release IL-1β and soluble TNF-α, which in turn stimulate local production of other cytokines, including the neutrophil chemotactic factor IL-8. Endogenous inhibitors of these proinflammatory cytokines, including soluble TNF receptors IL-1 receptor antagonist and anti-inflammatory cytokines such as IL-10 are also up-regulated; the balance of pro-inflammatory and antiinflammatory signals plays a critical role in disease pathogenesis (32). As will be discussed in more detail below, mechanical ventilation with excessive force results in direct lung injury, likely by causing direct capillary stress and through the release of pro-inflammatory cytokines.
It is also clear that there is significant cross-talk between the lung and other organs. Ischemia/reperfusion injury to the kidney results in increased pulmonary vascular permeability, as measured by Evans blue dye permeability (33). Furthermore, attenuation of renal injury with a pharmacologic agent such as α-melanocyte stimulating hormone reduces the degree of ALI (34). Similarly, in animal models, acid and ventilator-associated lung injury can result in acute kidney injury (35,36). The mechanisms by which each organ impacts the other remain unclear. In the case of the effect of ALI/ARDS on the kidney, mechanical ventilation may result in reduced renal perfusion, as well as lead to the expression of a number of inflammatory cytokines, including IL-6 and TNF-α, which may have deleterious consequences for other organs, including the kidney (37).
Treatment
Significant advances in the treatment of ALI have occurred in the past 10 years. To date, the major progress has occurred in identifying better supportive care treatment strategies, rather than identifying novel pharmacotherapies.
Ventilator Treatment
In the 1980s and early 1990s, several experimental and observational studies suggested that the traditional approach to ventilating patients with ALI might exacerbate lung injury (32). Clinical practice had been guided by the concept that a large tidal volume improved oxygenation in the injured lung (reviewed in (38)). Small, randomized clinical trials demonstrated conflicting results for a lower tidal volume strategy, with three studies not supporting a benefit and a single study suggesting that reduction of tidal volumes reduced barotrauma and had a positive impact on patient mortality (39–42). In the late 1990s, the NHLBI supported ARDS Network, a consortium of academic medical centers, carried out a large prospective, randomized clinical trial (n = 861) comparing a traditional ventilation strategy (12 ml/kg predicted body weight) to a lung protective ventilation strategy (6 ml/kg predicted body weight) (4). The lung protective strategy also required that the plateau airway pressure (which measures the pressure applied to small airways and the alveoli) be maintained at less than 30 cmH2O. The lung protective strategy allowed for permissive hypercapnia, that is, tidal volumes were reduced to as low as 4 ml/kg predicted body weight to achieve a plateau pressure of less than 30 cmH2O, provided arterial pH was greater than 7.15. The results showed a significant reduction in mortality from 40% to 31% with the use of the low tidal volume, pressure limited strategy (Table 1). The lung protective strategy was also associated with more ventilator free days and more ICU-free days. A follow-up randomized clinical trial by the ARDS Network demonstrated that the low tidal volume strategy was associated with a further reduction in mortality to 26%, an effect that was not altered by the use of high versus moderate levels of positive end expiratory pressure (43).
Table 1.
NHBLI ARDS Network Low Tidal Volume Treatment Protocola
| Variable | Protocol |
|---|---|
| Ventilator mode | Volume assist-control |
| Tidal volume | ≤6 ml/kg ideal body weight |
| Plateau pressure | ≤30 cmH2O |
| Set respiratory rate (on the ventilator) | 6 to 35 breaths/min, adjusted to achieve arterial pH ≥ 7.30 if possible |
| Arterial pH | No set goal per se, but ventilator rate adjusted as above |
| Oxygenation goal | PaO2 ≥ 55 mmHg or oxyhemoglobin saturation by pulse oximetry of 88% to 95% |
| Fio2/PEEPb (cmH2O) combinations | 0.3/5, 0.4/5, 0.4/8, 0.5/8, 0.5/10, 0.6/10, 0.7/10, 0.7/12, 0.7/14, 0.8/14, 0.9/14, 0.9/16, 0.9/18, 1.0/18, 1.0/20, 1.0/22, 1.0/24; further increases in PEEP allowed to 34 cmH2O allowed but not required |
| Inspiratory flow | Adjusted to achieve inspiratory time/expiratory time of 1:1 to 1:3 |
| Weaning | Attempts to wean to pressure support ventilation when FiO2/PEEP ≤ 0.4/8 |
Adapted from reference 59, with permission.
Fio2, fraction of inspired oxygen; PEEP, positive end expiratory pressure; PaO2, partial pressure of oxygen in arterial blood.
As further evidence of the benefit of this clinical strategy, several mechanistic studies have been published that demonstrated that the low tidal volume strategy is associated with a reduction in the release of inflammatory markers into the plasma of patients with lung injury. For example, patients treated with a low tidal volume strategy have a significant decrease in the pro-inflammatory cytokines IL-6 and IL-8 in the plasma (44). In addition, another study evaluated the release of biologic markers into the airspaces as well as the plasma of patients treated with a lung protective versus conventional ventilation strategy and also found that the severity of the acute inflammation was markedly reduced in the airspaces and plasma of patients treated with a lung protective strategy (45). It should be noted that whether the benefit of the low tidal volume ventilation strategy is attributable to the low tidal volume per se or to the maintenance of a plateau pressure of less than 30 mm H2O or both cannot be determined from the results of the ARDS Network trial; this issue is further discussed in (46).
Many have suggested that in the injured lung, recruitment of atelectatic alveoli (“the open lung concept”) could be important for optimization of respiratory parameters (47). Ventilator strategies designed to open alveoli and to prevent atelectrauma, that is, trauma occurring from repeated opening and closing of alveoli, include the use of higher levels of positive end-expiratory pressure and “recruitment maneuvers.” A recruitment maneuver is performed by increasing the mean airway pressure for a period of 30 to 60 s to open closed alveoli. However, there are many parameters for these maneuvers, and so far none of these has demonstrated a mortality benefit (48). It has been suggested that computed tomographic imaging studies may be helpful in identifying patients with recruitable lung who will respond favorably to these maneuvers (49). However, randomized clinical trials are needed to test this hypothesis. Nonconventional modalities of ventilation, including high frequency ventilation, liquid ventilation, and extracorporeal membrane oxygenation, have not been shown to be of benefit. In general, patients receiving these nonconventional or rescue therapies have highly impaired oxygenation and/or ventilation. Therefore, they may require renal replacement therapy for optimization of volume status and acid-base balance, respectively.
Ventilator Management: Implications for Nephrologists
The landmark clinical trial by the ARDS Network has resulted in a major change in clinical practice, with a general recognition that patients with ALI should be ventilated with lower tidal volumes and a plateau pressure limit, even if there is significant hypercapnia. Thus, patients with ALI/ARDS will often have a mild respiratory acidosis, which is generally well tolerated. Indeed, it has been proposed that hypercapnic acidosis may have an independent protective effect on the lung (50–52). The respiratory acidosis associated with the low tidal volume lung protective ventilation strategy does not require treatment, although clinicians are often reluctant to maintain an arterial pH less than 7.25. Of note, if acute treatment is provided in the form of bicarbonate, the bicarbonate will be converted to CO2 and may exacerbate the respiratory acidosis, especially when bicarbonate is administered rapidly or in bolus form (53). An alternative therapy is THAM (tromethamine; tris-hydroxymethyl aminomethane), a chemically inert base (54). However, THAM is cleared by the kidney and therefore needs to be used with caution in patients with acute or chronic impairments in glomerular filtration rate. More importantly, if the patient has acute or chronic kidney disease and a concomitant metabolic acidosis, early treatment of the metabolic acidosis with renal replacement therapy should be considered to prevent complications of severe, combined respiratory/metabolic acidosis, which may include arrhythmias and hemodynamic instability (55). With regards to modality of renal replacement therapy, hemofiltration may be preferable to dialysis in the patient with severe metabolic acidosis, as hemofiltration allows for delivery of more base equivalents (typically in the form of bicarbonate).
Fluid Management
Another advance in supportive therapy was recently reported by the NHLBI ARDS Network with a prospective, randomized clinical trial that evaluated the use of a liberal versus conservative fluid strategy in patients with ALI (Figure 2) (5). The results demonstrated that a fluid conservative strategy resulted in a significant increase in ventilator-free days and a nonsignificant decrease in mortality by 3%. The conservative fluid management strategy used diuretics to target a central venous pressure less than 4 mmHg or a pulmonary artery occlusion pressure less than 8 mmHg. This treatment strategy resulted in essentially even fluid balance over the first 7 d of the study. Subjects in the fluid liberal arm achieved approximately the same fluid balance as subjects enrolled in previous ARDS Network clinical trials (56). Patients were not treated with diuretic therapy if they had hypotension in the previous 12 h on the study protocol.
Figure 2.
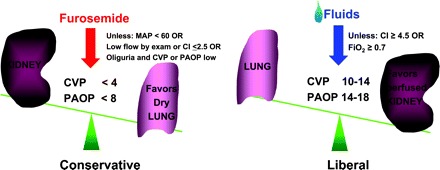
Fluid management strategies in the FACTT trial. Left, fluid conservative strategy, in which diuretics were administered to a target central venous pressure less than 4 mmHg or pulmonary artery occlusion pressure less than 8 mmHg. Right, fluid liberal strategy in which fluids were administered to maintain a central venous pressure between 10 and 14 mmHg or pulmonary artery occlusion pressure between 14 and 18 mmHg.
In the same clinical trial, the investigators also evaluated the value of central venous pressure monitoring versus pulmonary artery pressure monitoring (57). The results showed that there was no benefit of use the pulmonary artery catheter in implementation of the two fluid strategies, thus suggesting that, for most patients with ALI, central venous monitoring is adequate; this result supports the results of several other randomized clinical trials and observational studies. Nevertheless, there are some patients with refractory shock or difficult-to-diagnose pulmonary edema in whom pulmonary artery catheterization still has important value, both for diagnosis and management (8).
Fluid Management: Implications for Nephrologists
Although worsening renal function was a theoretical concern in the fluid management trial, there was no significant difference in the need for dialysis between those treated with the fluid liberal and fluid conservative strategies (14% versus 10%, P = 0.06). Indeed, fewer patients in the fluid conservative arm were treated with dialysis than in the fluid liberal arm. Of note, the primary reason for initiating dialysis was not recorded, so individuals in the two trial arms may have had different indications for dialysis (for example, volume overload/hypoxemia in the fluid liberal group and azotemia in the fluid conservative group). However, the role of dialysis in fluid management in patients with ALI/ ARDS remains uncertain; patients with dialysis-requiring acute kidney injury at the time of study eligibility were excluded from enrollment in the FACTT trial. While the fluid conservative strategy did not result in an additional need for dialysis, the potential impact of the fluid conservative strategy on already established acute kidney injury (especially with regards to renal recovery) remains uncertain. Furthermore, the optimal timing of the initiation of dialysis in the critical care setting remains uncertain.
Pharmacologic Treatments
Numerous clinical trials have evaluated pharmacologic therapies for the treatment of ALI (reviewed in (56,58,59)). Unfortunately, no pharmacologic treatment has yet been shown to reduce mortality. These include several phase II and phase III clinical trials of intravenous glucocorticoids to reduce inflammation in both acute and late phase ARDS. Glucocorticoids have not been shown to be of benefit in either acute phase or late phase ARDS. In a recently completed phase III trial, the ARDS Network demonstrated no mortality benefit to glucocorticoid administration in patients with ARDS for more than 7 d and in fact increased mortality in those treated with glucocorticoids more than 14 d after the onset of ARDS (60). Steroid administration was not associated with an increased risk of infection but with an increase in neuromyopathy.
The use of glucocorticoids for relative adrenal insufficiency in the intensive care unit has recently become more controversial (61,62). It should be noted that steroids in general may increase protein catabolism and azotemia in patients with acute kidney injury, so an understanding of the evidence-based indications for steroid administration in the critical care setting is crucial for nephrologists.
Other pharmacologic therapies for ALI/ARDS have included inhaled nitric oxide, which provides selective pulmonary vasodilation and increases ventilation perfusion matching. However, studies to date have not shown mortality benefit to inhaled nitric oxide; it can be considered as a rescue therapy in patients with severe, refractory hypoxemia. Exogenous surfactant has not been shown to have benefits in adults with ALI/ ARDS, although a recent study has suggested that surfactant may benefit children with ALI/ARDS (63). The Pediatric Acute Lung Injury and Sepsis Interventional group is planning on repeating this study in a larger number of pediatric patients. Antifungal agents (ketoconazole) and phosphodiesterase inhibitors (lisofylline) have been studied in several clinical trials, including negative studies from the ARDS Network. It has been shown in isolated human lung models, animal studies, as well as a small, single-center randomized clinical trial of 40 subjects that β-agonists may accelerate the clearance of extravascular lung water (64,65). It has also been demonstrated in healthy volunteers that β-agonists may reduce inflammation (66). Based on these observations, the ARDS Network is starting a multicenter, randomized clinical trial of inhaled -agonists for the treatment of ALI and ARDS.
Conclusion
Acute lung injury and the ARDS are major causes of acute respiratory failure in the critically ill adult. Similar to acute kidney injury, the pathogenesis of these conditions is complex and involves multiple cell compartments, including the highly differentiated alveolar epithelium, endothelium as well as inflammatory cells, including neutrophils and macrophages. Although multiple pharmacologic therapies have been tried, the only interventions to date that have reduced the mortality of this condition have been supportive care strategies (ventilator management). These advances have been greatly facilitated by a National Institutes of Health Clinical Trials Network whose primary mission is to reduce the morbidity and mortality of patients with ALI and the ARDS as well as by the widespread adoption of a consensus definition for ALI/ARDS that has allowed for more uniform comparison of subjects across studies. While progress has been made toward a consensus definition for acute kidney injury, ultimately, patients with acute kidney injury would benefit from a similar network to carry out clinical trials focused on individuals with early acute kidney injury.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ware LB: Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27: 337–349, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 4.ARDS Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, for the ADQI Workgroup: Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative Group. Crit Care 8: R204–R212. 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA: Clinical practice: acute pulmonary edema. N Engl J Med 353: 2788–2796, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD: Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bersten AD, Edibam C, Hunt T, Moran J: Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 165: 443–448, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hughes M, MacKirdy FN, Ross J, Norrie J, Grant IS: Acute respiratory distress syndrome: an audit of incidence and outcome in Scottish intensive care units. Anaesthesia 58: 838–845, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Avecillas JF, Freire AX, Arroliga AC: Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes [Abstract]. Clin Chest Med 27: 549–557, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Flori HR, Glidden DV, Rutherford GW, Matthay MA: Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 171: 995–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ferring M, Vincent JL: Is outcome from ARDS related to the severity of respiratory failure? Eur Respir J 10: 1297– 1300, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP: Causes and timing of death in patients with ARDS. Chest 128: 525–532, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA: Identification of patients with acute lung injury: predictors of mortality. Am J Respir Crit Care Med 152: 1818–1824, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Ely E, Wheeler A, Thompson B, Ancukiewicz M, Steinberg K, Bernard G: Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med 136: 25–36, 2002 [PubMed] [Google Scholar]
- 18.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, Hammerle AF, Steltzer H: The acute respiratory distress syndrome: definitions, severity and clinical outcome: an analysis of 101 clinical investigations. Intensive Care Med 22: 519–529, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Zimmerman GA: Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson LD, Hough CL: Therapy for late-phase acute respiratory distress syndrome [Abstract]. Clin Chest Med 27: 671–677, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Matthay MA: Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Zapol WM, Jones R: Vascular components of ARDS: clinical pulmonary hemodynamics and morphology. Am Rev Respir Dis 136: 471–474, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA: Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346: 1281–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ware LB, Fang X, Matthay MA: Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 285: L514–L521, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Prabhakaran P, Ware L, White K, Cross M, Matthay M, Olman M: Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 285: L20–L28, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Raijmakers PG, Groeneveld AB, Teule GJ, Thijs LG: Diagnostic value of the gallium-67 pulmonary leak index in pulmonary edema. J Nucl Med 37: 1316–1322, 1996 [PubMed] [Google Scholar]
- 27.Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF: Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis 148: 1169–1173, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Langleben D, DeMarchie M, Laporta D, Spanier AH, Schlesinger RD, Stewart DJ: Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis 148: 1646–1650, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Ware LB, Conner ER, Matthay MA: von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med 29: 2325–2331, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA: Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 170: 766–772, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Zarbock A, Singbartl K, Ley K: Complete reversal of acidinduced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 116: 3211–3219, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F 2nd, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR: Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1896–1903, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H: Renal ischemia/reperfusion leads to macrophagemediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Deng J, Hu X, Yuen PS, Star RA: Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/ reperfusion. Am J Respir Crit Care Med 169: 749–756, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA: Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 167: 1627–1632, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS: Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289: 2104–2112, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Chien CC, King LS, Rabb H: Mechanisms underlying combined acute renal failure and acute lung injury in the intensive care unit. In: Sepsis, Kidney and Multiple Organ Dysfunctioned., edited by Ronco C, Bellomo R, Brendolan A, Basel, Karger, 2004, pp 53–62 [DOI] [PubMed] [Google Scholar]
- 38.Ramnath VR, Hess DR, Thompson BT: Conventional mechanical ventilation in acute lung injury and acute respiratory distress syndrome [Abstract]. Clin Chest Med 27: 601–613, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR, Slutsky AS: Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome: Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 338: 355–361, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F: Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome: the Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 158: 1831–1838, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P Jr, Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S: Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27: 1492–1498, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR: Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Brower R, Lanken P, MacIntyre N, Matthay M, Morris A, Ancukiewicz M, Schoenfeld D, Thompson B, for the National Heart, Lung and Blood ARDS Clinical Trials Network: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327–336, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Parsons P, Eisner M, Thompson B, Matthay M, Ancukiewicz M, Bernard G, Wheeler A, for the NHLBI ARDS Network: Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33: 1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS: Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54–61, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Hager DN, Krishnan JA, Hayden DL, Brower RG: Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med 172: 1241–1245, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papadakos PJ, Lachmann B: The open lung concept of mechanical ventilation: the role of recruitment and stabilization. Crit Care Clin 23: 241–250, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Kacmarek RM, Kallet, RH: Should recruitment maneuvers be used in the management of ALI and ARDS? Respir Care 52: 622–631, discussion 631–625, 2007. [PubMed]
- 49.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G: Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354: 1775–1786, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER: Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med 34: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP: Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med 166: 403–408, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Broccard AF, Hotchkiss JR, Vannay C, Markert M, Sauty A, Feihl F, Schaller MD: Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med 164: 802–806, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Kallet RH, Jasmer RM, Luce JM, Lin LH, Marks JD: The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM). Am J Respir Crit Care Med 161: 1149–1153, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Gehlbach BK, Schmidt GA: Bench-to-bedside review: treating acid-base abnormalities in the intensive care unit: the role of buffers. Crit Care 8: 259–265, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu KD, Matthay MA, Chertow GM: Evolving practices in critical care and potential implications for management of acute kidney injury. Clin J Am Soc Nephrol 1: 869–873, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Calfee CS, Matthay MA: Nonventilatory treatments for acute lung injury and ARDS. Chest 131: 913–920, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 354: 2213–2224, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Cepkova M, Matthay MA: Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 21: 119–143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brower R, Ware L, Berthiaume Y, Matthay M: Treatment of ARDS. Chest 120: 1347–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M: Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671–1684, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E: Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288: 862–871, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Lipiner D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, Briegel J, Keh D, Singer M, Moreno R, Bellissant E, Annane D: Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med 19: 19, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA: Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 293: 470–476, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Perkins GD, McAuley DF, Thickett DR, Gao F: The betaagonist lung injury trial (BALTI): a randomized placebocontrolled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F: Bench-to-bedside review: beta2-Agonists and the acute respiratory distress syndrome. Crit Care 8: 25–32, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maris NA, de Vos AF, Dessing MC, Spek CA, Lutter R, Jansen HM, van der Zee JS, Bresser P, van der Poll T: Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med 172: 878–884, 2005 [DOI] [PubMed] [Google Scholar]


