Abstract
Background.
After stroke, recovery of movement in proximal and distal upper extremity (UE) muscles appears to follow different time courses, suggesting differences in their neural substrates.
Objective.
We sought to determine if presence or absence of motor evoked potentials (MEPs) differentially influences recovery of volitional contraction and strength in an arm muscle versus an intrinsic hand muscle. We also related MEP status to recovery of proximal and distal interjoint coordination and movement fractionation, as measured by the Fugl-Meyer Assessment (FMA).
Methods.
In 45 subjects in the year following ischemic stroke, we tracked the relationship between corticospinal tract (CST) integrity and behavioral recovery in the biceps (BIC) and first dorsal interosseous (FDI) muscle. We used transcranial magnetic stimulation to probe CST integrity, indicated by MEPs, in BIC and FDI. We used electromyography, dynamometry, and UE FMA subscores to assess muscle-specific contraction, strength, and inter-joint coordination, respectively.
Results.
Presence of MEPs resulted in higher likelihood of muscle contraction, greater strength, and higher FMA scores. Without MEPs, BICs could more often volitionally contract, were less weak, and had steeper strength recovery curves than FDIs; in contrast, FMA recovery curves plateaued below normal levels for both the arm and hand.
Conclusions.
There are shared and separate substrates for paretic UE recovery. CST integrity is necessary for interjoint coordination in both segments and for overall recovery. In its absence, alternative pathways may assist recovery of volitional contraction and strength, particularly in BIC. These findings suggest that more targeted approaches might be needed to optimize UE recovery.
Keywords: stroke recovery, transcranial magnetic stimulation, motor evoked potential, motor cortex, neurorehabilitation
Introduction
Persistent upper extremity (UE) hemiparesis is a common problem following stroke affecting the corticospinal tract (CST). In humans with stroke, volitional movement tends to return first at the shoulder, then elbow, then wrist and fingers.1 These findings have led to the classical view that UE movement recovery follows a proximal-to-distal course, although cross-sectional studies have not consistently observed this gradient.2,3 In macaques with pyramidal CST lesions, strength recovers well for proximal muscles but less well for intrinsic hand muscles; fine motor control, conversely, remains persistently poor for both the arm and hand.4–6 These dissociated recovery patterns suggest that the neural pathways mediating recovery for different muscles and motor outcomes may also be different. From behavioral assessments alone, however, one cannot determine if corticospinal inputs are continually absent or if their reemergence relates to these recovery patterns.
Transcranial magnetic stimulation (TMS) over motor cortex (M1) elicits a motor evoked potential (MEP), which indicates the presence of functional CST integrity.7 Despite a large body of TMS research in stroke, the relationship of CST integrity to recovery in different UE muscles has been surprisingly underinvestigated. Most studies have focused on the concurrent or prognostic relationship between a hand or forearm MEP and whole-UE motor behavior.8–11 Only one investigation examined the muscle-specific relevance of MEPs, finding that paretic muscles could be volitionally contracted if their MEPs were present, but only proximal muscles could if their MEPs were absent.12 Because a modest stimulation intensity and single stimulation site were used, it is possible that the cortical representations for proximal muscles were suboptimally probed. Importantly, the longitudinal relationship between MEPs and the recovery of volitional contraction was not addressed. In addition, the relevance of MEPs to strength recovery in different UE muscles or to segmental recovery on the Fugl-Meyer Assessment (FMA), a commonly used clinical impairment measure, were not evaluated in that study or elsewhere.
Here we sought to determine the influence of CST integrity on recovery of volitional muscle contraction and strength in an arm and hand muscle, and on recovery of proximal and distal subscores of the FMA, in the year following a first-ever motor stroke. We used TMS to track MEPs in the first dorsal interosseous (FDI) and biceps (BIC) muscles of the paretic UE. We predicted, based on observations in macaques,4–6 that the BIC would depend less on MEP presence for recovery of contraction and strength compared with FDI. Conversely, we predicted both the arm and the hand would depend on MEP presence for recovery of the FMA, since this scale captures interjoint coordination and movement fractionation during movements in and out of synergy.
Materials and Methods
Experimental Design
We describe a clinical and neurophysiological portion of the multicenter Study of Motor Learning and Acute Recovery Time Course in Stroke (SMARTS); kinematic outcomes are reported elsewhere.13,14 Institutional review board–approved testing occurred at Columbia University, Johns Hopkins University, University Hospital of Zurich, and the cereneo Center for Neurology and Rehabilitation. All sites used identical equipment and data collection procedures unless specified. Subjects gave written informed consent to participate in this study, in accordance with the Declaration of Helsinki.
Our approach was to longitudinally track recovery, the reduction in deficits toward premorbid levels of behavior,15 at the level of single muscles (FDI and BIC) and UE segments (arm and hand). Subjects were evaluated 5 times in the year following ischemic stroke (mean days ± SD): at week 1 (W1; 10.5 ± 3.6), W4 (34.9 ± 5.4), W12 (95.3 ± 10.7), W24 (187.8 ± 12.1), and W52 (369.7 ± 9.7). We assessed 3 motor outcomes—volitional contraction, strength, and FMA—using measures minimally contaminated by compensation. We characterized an outcome’s recovery by its (1) degree of deficit, (2) recovery curve, and (3) extent of recovery.
Subjects
We evaluated 45 adults (≥21 years old) with a first-time, diffusion-weighted imaging-positive ischemic stroke resulting in UE paresis. Exclusion criteria, as well as extended neurophysiology and clinical testing methods, are detailed in Supplemental Materials.
TMS Neurophysiology
Briefly, we taped surface EMG electrodes in a belly-tendon orientation over bilateral FDI and BIC muscles. We placed electrodes at recorded distances from anatomic landmarks and used frameless stereotaxic neuronavigation (Brainsight, Rogue Research) to ensure consistent stimulation and recording locations. TMS pulses were delivered to the motor cortex (M1) with a 70-mm figure-of-eight coil (Magstim Company Ltd). Separate cortical hotspots were identified for the bilateral FDI and BIC at each session. At the hotspot, 10 stimuli at 100% maximal stimulator output (MSO) were delivered with the muscle at rest, with an interstimulus interval of 5 to 7 seconds. Trials containing peakto-peak EMG activity >50 μV in the 150 ms prior to stimulus were discarded offline. Resting MEP presence/absence (MEP+/−) was delineated by ≥2 deflections with peak-to-peak amplitude >50 μV occurring within 40 ms and at the same time poststimulus.
Clinical Testing
Volitional muscle contraction, strength, and FMA in bilateral UEs were assessed following TMS testing. We classified muscle contraction presence or absence (contraction+/−) in 2 ways. Subjects were asked to abduct their index finger (using FDI) or flex their elbow (using BIC) with EMG electrodes in place; volitional contraction was determined by production of EMG activity >50 μV. If EMG data were missing, volitional contraction was inferred from force generation on dynamometry. Congruence between the 2 approaches occurred in 95% (279/295) of observations; with disagreement, contraction was classified as present.
Muscle strength was measured with standardized testing positions and a handheld dynamometer (microFET2, Hogan Health Industries). Maximal voluntary force (MVF) of finger abduction and elbow flexion was averaged from three trials (3-second duration, 60–90 seconds rest). The UE motor portion of the FMA (maximum value 66) assesses interjoint coordination and movement fractionation during movements made in and out of synergy.16 The FMA arm subscore largely reflects abnormal movement in the shoulder, arm, and forearm, excluding wrist (section A; maximum 36). Similarly, the FMA hand subscore reflects abnormal movement at the fingers (section C; maximum 14). The inability to produce a muscle contraction or perform the FMA was scored as zero.
Statistical Analysis
General Approach.
We used generalized estimating equations (GEE) and generalized linear mixed models (GLMM) to analyze categorical and continuous data, respectively. Random subject effects accounted for within-subject correlations occurring with repeated measures. In longitudinal settings, missing data is a common problem. As long as the value of the missing variable is not related to the reason it is missing, as we believe here, standard longitudinal models allowing for unbalanced data (ie, GEE and GLMM) can handle the missingness.17 Muscles and sides were considered independent. Unless noted, results reflect analyses of the paretic side, and nonparetic data are shown for reference.
Influence of MEP Status on Volitional Contraction and Its Recovery Curve.
We first determined if MEP status influenced the ability to volitionally contract the muscle after stroke. At each time point, we categorized the concordance/discordance of MEP and contraction (eg, MEP+/contraction+, MEP+/contraction−, etc) within subject. We calculated group proportions for each category at each time point and across the year. Subjects could cross through categories as they recovered, reflected as changing recovery curves (time effect). We examined if the likelihood of given category differed across muscles (muscle effect) or if their recovery curves differed (time * muscle effect).
Influence of MEP Status on Strength and FMA and Their Recovery Curves.
For strength, we normalized paretic MVF to maximum nonparetic values (Figure 3A) for quantitative comparisons across muscles. We used absolute FMA subscores for qualitative comparisons across segments; see Supplementary Materials for rationale. To characterize deficits, we calculated adjusted means of strength/impairment for the year. We examined MEP influence on this deficit (MEP effect), and whether MEP influence differed across muscles for strength (MEP * muscle) or across segments for impairment. For analysis of recovery curves, we characterized change over the year (time effect). We examined MEP influence on these curves (MEP * time), and whether MEP influence differed across muscles for strength (MEP * time * muscle) or across segments for FMA.
Figure 3.
Effect of MEP status on (A) absolute and (B) normalized strength over time in the FDI and BIC. Single-subject data and average values with SEM are shown for each time point. (A) Absolute MVF is shown for the paretic and nonparetic sides for clinical reference. (B) Normalized MVF is shown for the paretic side. Normalization was within-subject, such that subjects’ paretic MVFs were normalized to their own highest nonparetic MVF value (nonparetic best), irrespective of MEP status. Both paretic hand and arm were weak following stroke, but having an MEP was associated with significantly greater strength than having no MEP, for both paretic FDI (P < .0001) and BIC (P < .001). Paretic BIC strength was less affected by MEP absence than paretic FDI strength (P = .002). Strength recovery curves ran in parallel for FDIs with and without MEPs, but began to converge for the paretic BIC groups (P = .008). This pattern of recovery in the presence/absence of MEPs was significantly different across the hand and arm (P = .047). MEP, motor evoked potential; FDI, first dorsal interosseous; BIC, biceps; SEM, standard error of the mean; MVF, maximum voluntary force.
Influence of Early MEP Status on the Extent of Strength and FMA Recovery.
We finally determined if MEP status within the first 2 weeks after stroke influenced the extent of strength and FMA recovery achieved by 6 months. We undertook this analysis because time course assessments provide an incomplete picture of recovery. For example, patients with less initial deficit may have flatter recovery curves because they are already closer to their performance ceiling (as imposed by the testing instrument or their individual physiology). Extent of recovery takes into account the available “room to move” between a subject’s unique starting baseline and his or her best potential performance. Recovery extent is thus the ratio between observed recovery (delta between paretic measurements at W1 and W24; that is, the change that actually occurs) and maximum potential recovery (delta between the highest nonparetic measurement during the year and the paretic measurement at W1; that is, the best possible change that could potentially occur).10,18,19 A 6-month endpoint was chosen to facilitate comparisons with previous studies10,18,19 and comparisons across measures and muscles/segments, which had all plateaued by this time.13,14
We used linear regression to determine recovery extent, which is mathematically defined as the β coefficient (slope or proportion) of max potential recovery predicting observed recovery.18 For example, a β value of 0.7 means that a group achieved, on average, 70% of its maximum potential recovery. We characterized strength and FMA recovery extents (max potential recovery effect) and examined influence of early MEP status (MEP * max potential recovery). We also examined if early MEP influence differed across muscles or segments (MEP * max potential recovery * muscle). To allow for clinical worsening, regressions included an intercept, reported for clinical interpretation.
Additional Analyses.
The groups in the aforementioned analyses include subjects who converted from MEP− to MEP+ over the recovery course. We additionally explored recovery curves and extents in subjects with and without MEP conversion (Supplemental Results).
Significance for all analyses was set at α = .05. Fisher’s exact tests and t tests were 2-tailed. P values were not corrected for comparisons across sides, as these analyses were secondary. Analyses were performed in R and JMP Pro 13 (SAS Institute Inc).
Results
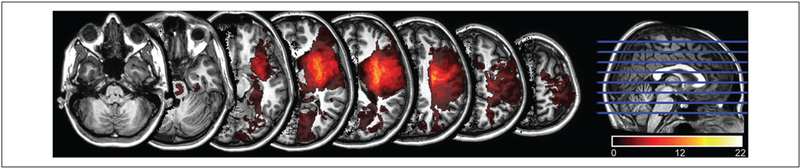
Subject characteristics are shown in Table 1. Fewer subjects had left hemispheric stroke because of exclusion for aphasia. Five subjects had W1 FMA scores of 63 to 65 because of improvement between enrollment in the study and the first formal assessment. All subjects sustained injury to the CST (Figure 1) as confirmed by diffusion tensor imaging of the CST.14 We obtained measurements in 67% to 87% of subjects per time point (Supplementary Materials Table 1).
Table 1.
Participant Characteristics.a
| Participant Characteristics (n = 45) | |
|---|---|
| Demographics | |
| Gender | |
| Male | 31 (69) |
| Female | 14 (31) |
| Age (years) | 60.0 (21.7–68.6) |
| Race | |
| White | 36 (80) |
| Black | 8 (18) |
| Other | 1 (2) |
| Hand dominance | |
| Right | 34 (76) |
| Left | 11 (24) |
| Stroke characteristics | |
| Hemisphere | |
| Right | 29 (64) |
| Left | 16 (36) |
| Locationb | |
| Mixed | 27 (60) |
| Subcortical | 18 (40) |
| Paretic side dominance | |
| Dominant | 13 (29) |
| Nondominant | 32 (71) |
| W1 paretic FMA scorec | 37 (0–65) |
| Psychotropic medications | |
| SSRIs | 15 (33) |
| GABA agonists or antidopaminergics | 5 (11) |
Abbreviations: SSRI, selective serotonin reuptake inhibitors; GABA, γ-aminobutyric acid
Gender, race, hand dominance, and stroke hemisphere, location, and paretic dominance are presented as count with percentage of sample in parentheses. Age and paretic Fugl-Meyer Assessment (FMA) score at first assessment are presented as mean with range in parentheses. Hand Dominance was assessed with the Edinburgh Handedness Inventory. Also shown are the count with percentage of sample in parentheses of patients who took psychotrophic medications at some point within the year.
Stroke location was classified as “mixed” if there was cortical and underlying white matter involvement and “subcortical” if there was white matter ± deep nuclei involvement without cortical involvement.
W1 FMA scores may have exceeded exclusion of ≥ 63 because of interval recovery between screening for enrollment and the first formal assessment.
Figure 1.
Lesion distribution on MRI. The lesion distribution from 43 subjects was mapped to JHU-MNI space and superimposed on one hemisphere, as described in Xu et al.14 The color bar denotes the number of subjects who shared the lesion location. Two missing subjects had striatocapsular lesions. All subjects had lesion involvement of the M1 and/or CST estimated by diffusion tensor imaging (results not shown.) Subjects with pontine lesions (n = 6) had FDI and BIC MEPs, indicating they did not specifically contribute to poorer recovery in the MEP− groups. MRI, magnetic resonance imaging; JHU-MNI, Johns Hopkins University–Montreal Neurological Institute; CST, corticospinal tract; FDI, first dorsal interosseous; BIC, biceps; MEP, motor evoked potential.
Influence of MEP Status on Volitional Muscle Contraction
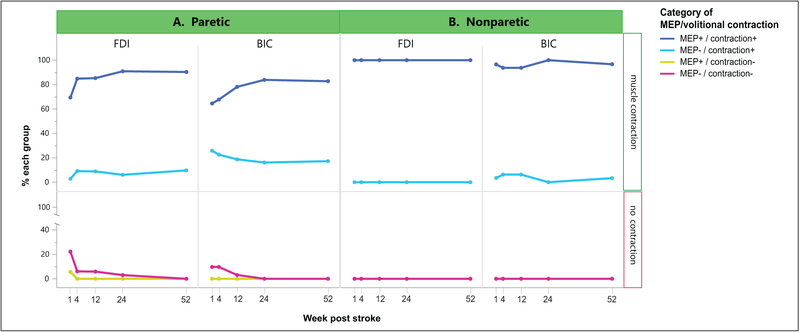
We first characterized the association between MEP status and volitional muscle contraction over the year (Figure 2). The FDI and BIC had similar proportions of MEP+/contraction+ (84.2% vs 74.4%) or MEP−/contraction− (7.4% vs 4.5%) over the year, with a comparable likelihood (muscle: odds ratio [OR], nonsignificant). However, the overall odds of MEP−/contraction+ were 4.6 times higher in BIC than FDI (20.1% vs 7.3%; muscle: OR 4.6, 95% CI 1.7–13.0, P = .004). Of note, the odds of MEP−/contraction+ were also 5.7 times higher in the paretic than nonparetic BIC (20.1% vs 3.9%; OR 5.7, 95% CI 1.7–19.4, P = .005). This indicates that absent paretic BIC MEPs were not simply due to the well-known methodological challenge of eliciting proximal MEPs, and they were significantly more absent than in the setting of normal physiology. MEP+/contraction− was rarely observed, occurring in 1% of observations in the paretic FDI only.
Figure 2.
Relationship of MEPs and volitional muscle contraction over time in the (A) paretic and (B) nonparetic FDI and BIC. At each time point, the presence/absence of an MEP and volitional muscle contraction in the same muscle were categorized for each subject, and the percentage of subjects with each category was calculated. Time courses of these proportions are shown for those with volitional contraction (upper panel) and no contraction (lower panel). The overall odds of MEP−/contraction+ in the paretic BIC were 4.6 times higher than the paretic FDI (P = .004) and 5.7 times higher than nonparetic BIC (P = .005). MEP, motor evoked potential; FDI, first dorsal interosseous; BIC, biceps.
Longitudinally, the odds of MEP+/contraction+ showed a trend for increasing in FDI (time: OR 1.03/week [wk], 95% CI 0.99–1.06, P = .056) and BIC (time: OR 1.02/wk, 95% CI 0.99–1.04, P = .061). The odds of MEP−/contraction− decreased in FDI (time: OR 0.90/wk, 95% CI 0.82–0.99, P = .046) and BIC (time: OR 0.87/wk, 95% CI 0.79–0.95, P = .002). MEP−/contraction+ was stable over time in both muscles (time: nonsignificant). Inspection of individual subject data revealed that this category often served as an intermediate step between early MEP−/contraction− and eventual recovery to MEP+/contraction+, with subjects entering or exiting over time. Importantly, its occurrence indicates that for both muscles, the return of volitional muscle contraction can precede the return of the MEP. The odds of developing any category over the course of a year did not significantly differ between the 2 muscles, indicating similar recovery curves (time * muscle: nonsignificant).
Influence of MEP Status on Deficits of Strength and FMA
We then assessed whether MEP status differentially influenced strength in paretic FDIs and BICs (Figure 3B). As expected, both paretic muscles were weak in the year following stroke, regardless of MEP status. MEP+ FDIs were significantly stronger than MEP− FDIs (60.4% vs 17.9% of maximum nonparetic strength; MEP: t(83) = 5.7, P < .0001). Similarly, MEP+ BICs were significantly stronger than MEP− BICs (71.7% vs 46.1% of maximum nonparetic strength; MEP: t(79) = 5.25, P < .0001). MEP status differentially influenced strength in the muscles: when MEPs were absent, strength was relatively less affected in the BIC than FDI (MEP * muscle: t(263) = 2.7, P = .008). This differential influence was most salient at ≥24 weeks after stroke, when MEPFDIs were substantially weaker than MEP+ FDIs, whereas MEPand MEP+ BICs had similar strength (Figure 3B).
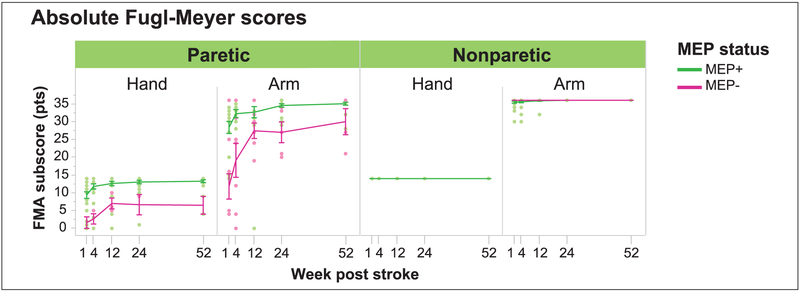
We also investigated whether MEP status differentially influenced FMA subscores in the hand and arm (Figure 4). The paretic hand and arm were impaired in the year following stroke, regardless of MEP status. Hands with MEP+ FDIs had higher FMA subscores than hands with MEP− FDIs (11.4 vs 4.4 FMA pts; MEP: t(149) = 6.5, P < .0001). Arms with MEP+ BICs also had higher FMA subscores than arms with MEP− BICs (31.5 vs 21.1 FMA pts; MEP: t(138) = 5.9, P < .0001). Figure 4 reveals that MEP absence had a similar impact on the FMA in the proximal and distal segments, reducing FMA scores from the maximum by about 5 to 10 points.
Figure 4.
Effect of MEP status on absolute FMA scores over time in the paretic hand and arm. MEPs were assessed in the FDI and BIC. Absolute FMA subscores are shown for the nonparetic sides for clinical reference. Single-subject data and average values with SEM are shown for each time point. Both paretic hand and arm had reduced FMA scores following stroke, but having an MEP was associated with significantly higher FMA subscores than having no MEP, for both the hand (P < .0001) and arm (P < .0001). FMA recovery curves ran in parallel for paretic hands with and without MEPs. FMA recovery was steeper in arms without MEPs than with MEPs (P = .007). However, hand and arm recovery curves behaved in a largely similar fashion in the absence of MEPs, arriving at a recovery plateau at 12 weeks and not converging on their counterparts with MEPs. MEP, motor evoked potential; FMA, Fugl-Meyer Assessment; FDI, first dorsal interosseous; BIC, biceps; SEM, standard error of the mean.
Influence of MEP Status on Recovery Curves of Strength and FMA
We next assessed whether MEP status differentially influenced recovery curves for normalized strength in paretic FDIs and BICs (Figure 3B). Strength recovery occurred in MEP+ FDIs (time: 0.49%/wk, t(97) = 4.2, p < .0001) and MEP− FDIs (time: 0.37%/wk, t(12) = 2.3, P = .042), with recovery curves that were similar (MEP * time: nonsignificant). Strength recovery also occurred in MEP+ BICs (time: 0.19%/wk, t(84) = 2.1, P = .037) and MEP− BICs (time: 0.77%/wk, t(21) = 3.7, P = .001), but interestingly, were significantly steeper for MEP− BICs (MEP * time: t(116) = 2.7, P = .008). The influence of MEP status on strength recovery curves significantly differed across muscles (MEP * time * muscle: t(237) = 2.0, P = .047). Inspection of Figure 3B shows that strength recovery curves for MEP+ and MEP− FDIs ran mostly in parallel, whereas the MEP− BIC recovery curve converged onto the MEP+ BIC recovery curve.
We also assessed whether MEP status differentially influenced recovery curves for FMA in the paretic hand and arm (Figure 4). Recovery occurred in hands with MEP+ FDIs (time: 0.05 pts/wk, t(103) = 4.1, P < .0001) and with MEP− FDIs (time: 0.11 pts/wk, t(10) = 2.7, P = .023), with recovery curves that were similar (MEP * time: nonsignificant). Recovery also occurred in arms with MEP+ BICs (time: 0.07 pts/wk, t(72) = 3.7, P < .001) and MEP− BICs (time: 0.29 pts/wk, t(23) = 2.9, P = .008), but recovery curves were significantly steeper for arms without MEPs (MEP * time: t(109) = 2.7, P = .007). Inspection of Figure 4 shows that FMA recovery curves behaved similarly for the hand and arm. Recovery in MEPsegments showed a rapid recovery until 12 weeks, with no appreciable convergence onto MEP+ segments thereafter.
Influence of Early MEP Status on the Extent of Strength and FMA Recovery
We finally investigated whether having MEPs within 2 weeks after stroke influenced the extent of strength (Figure 5A) and FMA (Figure 5B) recovery. For strength, early MEP+ FDIs attained 55% of their potential recovery (intercept, −2.9 N; max potential recovery: t(13) = 3.7, P = .003), whereas recovery extent in early MEP− FDIs was not significantly different from zero. Accordingly, early MEP+ FDIs attained a greater extent of strength recovery than early MEP− FDIs (MEP * max potential recovery: t(18) = 2.6, P = .019). Early MEP+ BICs attained 100% of their potential strength recovery (intercept, −34.8 N, max potential recovery: t(12) = 3.9, P = .002), and early MEP− BICs attained 46% of their potential recovery (intercept, −6.0 N; t(6) = 3.5, P = .013; Figure 5B). Early MEP+ BICs trended toward attaining a greater extent of strength recovery than early MEP− BICs (MEP * max potential recovery: t(18) = 1.9, P = .071). There was no significant difference in early MEP influence on strength recovery extent across muscles (MEP * max potential recovery * muscle: nonsignificant).
Figure 5.
Effect of early MEP status on extent of (A) strength and (B) FMA recovery in paretic FDI and BIC. MEPs were assessed at W1. Recovery extent is the proportion of maximum potential change that is observed at 24 weeks after stroke, on average, for the group; graphically, it the slope of the regression fit. Shaded areas are 95% confidence intervals for the regression fits of the same color. The stippled line indicates perfect recovery; that is, if observed change = maximum potential change (slope = 1, intercept = 0). (A) FDIs with an early MEP had a greater extent of strength recovery than those without an MEP (P = .019). BICs with an initial MEP showed a trend for greater attainment of strength recovery than those without an MEP (P = .071). There was no significant difference in the influence of early MEP status on the extent of strength recovery in the FDI and BIC. (B) Hands with an initial FDI MEP showed a weak trend for attaining a greater extent of FMA recovery than those without an MEP (P = .095) (of note, recovery extent was not significantly different from zero in the FDI no-MEP group due to the variability of recovery, evidenced by its wide confidence intervals). Arms with and without initial BIC MEPs showed comparable extents of FMA recovery. There was no significant difference in the influence of early MEP status on the extent of FMA recovery in the hand and arm. MEP, motor evoked potential; FMA, FuglMeyer Assessment; FDI, first dorsal interosseous; BIC, biceps.
For FMA subscores, hands with early MEP+ FDIs attained 98% of their potential recovery (intercept, −0.30 pts; max potential recovery: t(17) = 17.1, P < .0001), whereas recovery extent in hands with MEP− FDIs was not significantly different from zero. Hands with MEP+ FDIs trended weakly toward attaining a greater extent of recovery than hands with MEP− FDIs (MEP * max potential recovery: t(22) = 1.7, P = .095). Arms with early MEP+ BICs attained 86% of their potential recovery (intercept, −0.61 pts; max potential recovery: t(13) = 10.7, P < .0001), and arms with MEP− BICs attained 75% of their potential recovery (intercept, −0.47 pts; max potential recovery: t(6) = 4.0, P = .007; Figure 5B). The extent of arm recovery was comparable regardless of early MEP status. There was no significant segmental difference in the influence of early MEP status on FMA recovery extent (MEP * max potential recovery * segment).
Of note, the aforementioned analyses investigated group differences based on MEP status. Groups included subjects whose MEPs converted from MEP− to MEP+ over the recovery course (FDI: n = 5; BIC: n = 6). When characterizing recovery of only those with MEP+ (FDI: n = 34; BIC: n = 26) or MEP− (FDI: n = 4; BIC: n = 8) throughout their time courses, we found results mirroring those above (Supplementary Table 2). We also observed that FDIs with MEP conversion received a boost in both strength and FMA recovery, approximating that of FDIs with MEP+ throughout. BICs with MEP conversion, on the other hand, received a substantial boost in FMA recovery only (Supplementary Figures 1 and 2).
Discussion
In this longitudinal observational study of individuals recovering from ischemic stroke, we examined if MEP status influences clinical deficits and their recovery in an arm and hand muscle. We found that MEP presence similarly benefitted volitional muscle contraction, strength, and FMA scores in both the BIC and FDI. Interestingly, when MEPs were absent, recovery patterns diverged by muscle and motor outcome. Without MEPs, BICs could more often be contracted, were less weak, and had steeper strength recovery curves than FDIs. FMA recovery curves, conversely, appeared comparably limited in both arm and hand.
We assessed the concordance of MEP status and volitional muscle contraction after stroke. When an MEP in the FDI or BIC was present, subjects could almost always volitionally contract the muscle. When an MEP in the FDI or BIC was absent, a modest proportion of subjects could still volitionally contract the muscle. The odds of this MEP−/contraction+ condition were over 4 times higher in the paretic BIC than FDI. Although MEP elicitation is more difficult in proximal than distal representations even in healthy individuals, we believe absent BIC MEPs here represent true pathophysiology; the odds of MEP−/contraction+ were nearly 6 times higher in paretic than nonparetic BICs. We also found that MEP−/contraction+ was commonly a transition state between fully absent and fully present MEP/contraction conditions. These findings suggest that volitional muscle contraction is not exclusively dependent on and can precede the return of MEPs, particularly in the BIC.
Our observations agree with previous work showing that MEP presence after stroke is required for volitional contraction in FDI but not BIC.12 Given that this study used submaximal stimulation intensities (45%–70% MSO; ~1 T) and a single stimulation site for both muscles, it is conceivable that understimulation of the cortical BIC representation could have resulted in spurious differences between muscles. Here we found that even with maximal hotspotspecific stimulation (100% MSO; 2.2 T), volitional activation is still possible despite absent MEPs, especially for BIC.
We furthermore found that MEP status influenced average deficits in strength and FMA scores. The presence of FDI MEPs was associated with greater FDI strength and less hand impairment than if MEPs were absent, consistent with prior reports.8,11,20 Here we newly document a similar relationship in the BIC and arm, with MEP presence conferring greater strength and higher FMA subscores. Comparing muscles, we found that the absence of MEPs had less of a deleterious effect on strength in BIC than FDI. In contrast, for FMA, the two segments seemed to be similarly affected by the absence of MEPs. These results suggest that CST integrity is generally beneficial for the expression of strength and normal movement throughout the UE. They also suggest that for strength, additional neural substrates available to BIC but not FDI may be in operation.
MEP status similarly influenced strength and FMA recovery curves, which has not been previously reported. Recovery curves were generally flatter in muscles/segments with MEPs than those without, likely because of their closer proximity to the recovery ceiling—there was less room to change over time. In the absence of MEPs, recovery curves differed by outcome. For strength, BIC recovery curves converged in those with and without MEPs, whereas FDI recovery curves ran in parallel. For the FMA, recovery curves of both hands and arms without MEPs plateaued at about 12 weeks, with neither converging onto those with MEPs. These findings suggest that different neural substrates may participate in strength recovery in the BIC versus FDI, but that a similar neural substrate, likely the CST, is required for recovery of interjoint coordination in both the arm and hand.
Finally, we found that MEP presence within two weeks after stroke generally predicted moderate to substantial extents of strength and FMA recovery for both muscles/segments. In the absence of early MEPs, recovery extents were highly variable in the FDI/hand, consistent with previous reports,9,10,21–26 but were modest to good in the BIC/arm. We found no between-muscle/segment differences, underscoring the consistently strong benefit of early MEP presence for predicting adherence to the proportional recovery rule.18,27 The variable or modest recovery extents seen in the early MEP-absent groups could be explained by inclusion of MEP-converters (Supplemental Materials). Those who regained an MEP at a delay recovered higher extents of strength and FMA scores. Interestingly, strength and FMA recovery extents were still modest in BICs that never regained MEPs, although small sample sizes precluded powered conclusions.
To our knowledge, we are the first to comparatively assess the differential relevance of MEP status to recovery of volitional contraction and strength in separate UE muscles, and to recovery of proximal and distal FMA subscores. The observed recovery patterns point to both shared and separate neural recovery substrates for different motor outcomes. At high intensities, cortical stimulation most effectively activates pyramidal cells with fast-conducting, monosynaptic projections to α-motoneurons,7,28 which pri-marily arise from caudal M1.29 MEP size also reflects intracortical, cortico-cortical, and subcortical contributions.30 We used high-intensity stimulation to directly stimulate pyramidal axons (ie, produce D-waves)31 in an effort to bypass these influences and did not take MEP amplitude into account. From MEP presence, we can infer, at the minimum, the integrity of fast monosynaptic M1 projections. As MEP presence was beneficial for all motor outcomes, we conclude that recovery of both force and movement benefits from availability of these projections.
In the absence of MEPs, recovery of contraction and strength was superior in the BIC compared to FDI. One possible explanation is that CST integrity was intact enough to support recovery but was functionally unable to generate MEPs due to perilesional or motoneuronal hypoexcitability.32,33 Although we attempted to circumvent perilesional influences by using high-intensity stimulation,31 we cannot exclude intraspinal reasons for absent MEPs. Even so, it is unclear why spinal hypoexcitability would preferentially affect arm motoneurons, leading to more “false-negative” BIC MEPs.
Alternatively, our findings may indicate that ipsilesional pathways not contributing to the MEP could participate in recovery. MEP status does not reflect the integrity of slow-conducting mono-, di-, and oligosynaptic projections from primary and secondary motor areas, such as the premotor cortex (PMC) and supplementary motor area (SMA). These ipsilesional CST projections may remain uninjured after stroke and could participate in recovery, as occurs in macaques.34–36 However, these projections are more abundant and functionally stronger for distal muscles,29 suggesting that if they were to support recovery, the FDI MEP− group should have recovered better. As we did not observe this, we speculate that slow CST projections cannot actualize recovery for the entire UE, or they do so selectively for the BIC. We know of no mechanism for this, unless they project to other pathways that have predominantly proximal innervation (eg, the reticulospinal tract; see below).
Other potential pathways to consider include uncrossed CST projections from contralesional M1,35,37,38 the C3–4 priopriospinal system,39 and corticorubral pathways.40 In macaques that have recovered from a pyramidal lesion, uncrossed M1-CST projections to hand and forearm motoneurons do not functionally strengthen (proximal muscle motoneurons not assessed).41 In macaques that have recovered from an isolated frontal lobe stroke, however, uncrossed M1-CST projections show increased terminal density at spinal cord laminae supporting axial/proximal function.38 While these results suggest that uncrossed M1-CST projections could play a role in proximal recovery, their anatomic scantiness should be kept in mind.42 Propriospinal interneurons in the C3–4 spinal cord also demonstrate upregulated excitability after stroke,43,44 but the functional relevance of this pathway in humans is debated.45,46 Ipsilesional corticorubral projections and the red nuclei undergo microstructural changes after stroke,40 but the rubrospinal tract is vestigial in humans and does not project beyond the upper cervical spinal cord.47 It is conceivable that these various pathways upregulate to contribute to motor recovery in humans, but their direct role in restoring volitional contraction and strength is less clear.
In our view, the likeliest pathway to explain the robust BIC recovery in the absence of MEPs is the reticulospinal tract (RST). In humans, the CST and RST are the primary drivers of voluntary UE movement.48 The CST has more numerous and functionally stronger projections to motoneurons of distal muscles,49,50 whereas the RST has more numerous and functionally stronger projections to interneurons and motoneurons of axial and proximal muscles.51–54 The RST and corticoreticular projections (arising from M1 and more densely from PMC and SMA55) may also participate in motor recovery. In CST-lesioned animals, corticoreticular and reticulospinal projections demonstrate increased firing rates, functional strengthening, axonal spouting, and synaptic bouton formation.36,41,56–58 CST-lesioned macaques with a spared RST recover proximal strength and hand grip, while a secondary RST lesion causes reemergence of proximal deficits.4 In humans, the RST has an increased influence on proximal force generation after stroke (distal muscles not tested).59
The functional and anatomical connectivity of the RST could explain why, in the absence of MEPs, the BIC showed superior recovery of contraction and strength—the BIC could more heavily draw upon a reorganizing RST. This pathway may contribute less to inter-joint coordination, which likely requires CST input.60,61 This requirement may explain why both MEP− segments plateaued during FMA recovery: both were afforded some basic movement via the RST—enough to score some FMA points—but lacked the CST input necessary for further coordination and generation of additional FMA points.
Limitations
Some limitations of this study should be considered. Our modest sample consisted mostly of mildly and moderately impaired subjects, reducing generalizability. In addition, strength and FMA measures may have not been identically matched across muscles and segments in terms of their performance demands. Performing dynamometry requires strength for both FDI and BIC, but may also require movement fractionation for the finger (ie, isolating movement of the index finger to undergo testing).60 Conversely, performing the FMA requires interjoint coordination during movement in and out of synergies for the arm but not for the hand. Importantly, the BIC and FDI are not representative of all muscles in the proximal and distal segments. Muscles acting at the same segment may be innervated by different pathways (eg, intrinsic/extrinsic hand muscles62) or be differentially influenced by the same pathway (eg, elbow flexors/extensors63). A pathway may also upregulate for select muscles in the same segment.41 Thus, muscle-specific assessments are necessary to generate a more nuanced characterization of UE recovery substrates. Finally, we speculate that the corticoreticulospinal pathway could be a major contributor to BIC recovery, but we did not assess it directly. Although a few studies have longitudinally assessed the corticoreticulospinal pathway after stroke.12,23,64 none have reported its evolution in relation to behavioral recovery.
Conclusions
In this longitudinal study of ischemic stroke patients, we found that in the presence of MEPs, FDIs and BICs recovered comparably well in all motor outcomes. In the absence of MEPs, the BIC recovered volitional contraction and strength more robustly than FDI, whereas inter-joint coordination recovery, measured with the FMA, remained limited in both the arm and hand. Our findings reinforce the notion that CST integrity is beneficial for recovery generally, but is of particular importance for inter-joint coordination proximally and distally. The results also suggest that alternative pathways, such as the RST, may participate in strength recovery (especially for BIC). Understanding the neural substrates that are more—or less—relevant to various aspects of motor recovery will help develop mechanismdriven approaches to restore function.
Supplementary Material
Acknowledgments
We thank Gianpiero Liuzzi for input on the TMS paradigm; Codruta Chiuzan, Yuan Zhang, and Yuting Xu for statistical assistance; and Isis Martinez-Hernandez, Leopold Zizlsperger, Jeremia Held, and Joachim Cerny for data collection assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was primarily supported by the James S. McDonnell Foundation (JWK), with additional support from K23NS078052 (HMS), R01HD053793 (PAC), and R01HD073147 (PAC).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at https://journals.sagepub.com/home/nnr
References
- 1.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. [DOI] [PubMed] [Google Scholar]
- 2.Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol. 2008;119:2074–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112(pt 3):749–763. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91:15–36. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. [DOI] [PubMed] [Google Scholar]
- 6.Tower SS. Pyramidal lesion in the monkey. Brain. 1940;63: 36–90. [Google Scholar]
- 7.de Noordhout AM, Rapisarda G, Bogacz D, et al. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122(pt 7):1327–1340. [DOI] [PubMed] [Google Scholar]
- 8.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(pt 1):170–180. [DOI] [PubMed] [Google Scholar]
- 9.Hendricks HT, Pasman JW, van Limbeek J, Zwarts MJ. Motor evoked potentials in predicting recovery from upper extremity paralysis after acute stroke. Cerebrovasc Dis. 2003;16:265–271. [DOI] [PubMed] [Google Scholar]
- 10.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015;78:848–859. [DOI] [PubMed] [Google Scholar]
- 11.Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor of functional outcome at 12 months. Brain. 1993;116(pt 6):1371–1385. [DOI] [PubMed] [Google Scholar]
- 12.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JC, Goldsmith J, Harran MD, et al. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair. 2017;31:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Ejaz N, Hertler B, et al. Separable systems for recovery of finger strength and control after stroke. J Neurophysiol. 2017:118:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 17.Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315:407–408. [DOI] [PubMed] [Google Scholar]
- 18.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. [DOI] [PubMed] [Google Scholar]
- 19.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2015;29:614–622. [DOI] [PubMed] [Google Scholar]
- 20.Martinez AC, Tejada J, Tejedor ED. Motor hand recovery after stroke. Prognostic yield of early transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1999;39:405–410. [PubMed] [Google Scholar]
- 21.Palliyath S Role of central conduction time and motor evoked response amplitude in predicting stroke outcome. Electromyogr Clin Neurophysiol. 2000;40:315–320. [PubMed] [Google Scholar]
- 22.Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 1998;29:1854–1859. [DOI] [PubMed] [Google Scholar]
- 23.Alagona G, Delvaux V, Gerard P, et al. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 24.Pennisi G, Rapisarda G, Bella R, Calabrese V, De Noordhout AM, Delwaide PJ. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–2670. [DOI] [PubMed] [Google Scholar]
- 25.Delvaux V, Alagona G, Gerard P, De Pasqua V, Pennisi G, de Noordhout AM. Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol. 2003;114:1217–1225. [DOI] [PubMed] [Google Scholar]
- 26.Hendricks HT, Pasman JW, Merx JL, van Limbeek J, Zwarts MJ. Analysis of recovery processes after stroke by means of transcranial magnetic stimulation. J Clin Neurophysiol. 2003;20:188–195. [DOI] [PubMed] [Google Scholar]
- 27.Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. Proportional motor recovery after stroke: implications for trial design. Stroke. 2017;48:795–798. [DOI] [PubMed] [Google Scholar]
- 28.Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120(pt 5):839–853. [DOI] [PubMed] [Google Scholar]
- 29.Witham CL, Fisher KM, Edgley SA, Baker SN. Corticospinal inputs to primate motoneurons innervating the forelimb from two divisions of primary motor cortex and area 3a. J Neurosci. 2016;36:2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Lazzaro V, Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circuits. 2013;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Lazzaro V, Oliviero A, Pilato F, et al. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol Res. 2003;25:143–150. [DOI] [PubMed] [Google Scholar]
- 32.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh AK, Hu X, Powers RK, Heckman CJ, Suresh NL, Rymer WZ. Changes in motoneuron afterhyperpolarization duration in stroke survivors. J Neurophysiol. 2014;112:1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki S, Isa T, Pettersson LG, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. [DOI] [PubMed] [Google Scholar]
- 35.McNeal DW, Darling WG, Ge J, et al. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darling WG, Ge J, Stilwell-Morecraft KS, Rotella DL, Pizzimenti MA, Morecraft RJ. Hand motor recovery following extensive frontoparietal cortical injury is accompanied by upregulated corticoreticular projections in monkey. J Neurosci. 2018;38:6323–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netz J, Lammers T, Hömberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(pt 9):1579–1586. [DOI] [PubMed] [Google Scholar]
- 38.Morecraft RJ, Ge J, Stilwell-Morecraft KS, et al. Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the nonlesioned hemisphere in monkey (Macaca mulatta). J Comp Neurol. 2016;524:380–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rüber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(pt 7):2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521:4205–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126(pt 4):988–1000. [DOI] [PubMed] [Google Scholar]
- 44.Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–434. [DOI] [PubMed] [Google Scholar]
- 45.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol. 2000;84:698–709. [DOI] [PubMed] [Google Scholar]
- 47.Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105(pt 2):223–269. [DOI] [PubMed] [Google Scholar]
- 48.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- 49.Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex. 2010;20:169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford, England/ New York, NY: Clarendon Press/Oxford University Press; 1993. [Google Scholar]
- 51.Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol. 2004;92:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol. 2012;590:4011–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirschauer TJ, Buford JA. Bilateral force transients in the upper limbs evoked by single-pulse microstimulation in the pontomedullary reticular formation. J Neurophysiol. 2015;113:2592–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. [DOI] [PubMed] [Google Scholar]
- 55.Fregosi M, Contestabile A, Hamadjida A, Rouiller EM. Corticobulbar projections from distinct motor cortical areas to the reticular formation in macaque monkeys. Eur J Neurosci. 2017;45:1379–1395. [DOI] [PubMed] [Google Scholar]
- 56.Herbert WJ, Powell K, Buford JA. Evidence for a role of the reticulospinal system in recovery of skilled reaching after cortical stroke: initial results from a model of ischemic cortical injury. Exp Brain Res. 2015;233:3231–3251. [DOI] [PubMed] [Google Scholar]
- 57.Zaaimi B, Soteropoulos DS, Fisher KM, Riddle CN, Baker SN. Classification of neurons in the primate reticular formation and changes after recovery from pyramidal tract lesion. J Neurosci. 2018;38:6190–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachmann LC, Lindau NT, Felder P, Schwab ME. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J Neurosci. 2014;34: 3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellis MD, Drogos J, Carmona C, Keller T, Dewald JP. Neck rotation modulates flexion synergy torques, indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol. 2012;108:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Haith AM, Krakauer JW. Motor control of the hand before and after stroke In: Kansaku, Cohen, Birbaumer, eds. Clinical Systems Neuroscience: Tokyo, Japan: Springer; 2015:271–289. [Google Scholar]
- 61.Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci. 2015;35:4882–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589(pt 23):5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson AG, Schieber MH, Buford JA. Bilateral spiketriggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Misawa S, Kuwabara S, Matsuda S, Honma K, Ono J, Hattori T. The ipsilateral cortico-spinal tract is activated after hemiparetic stroke. Eur J Neurol. 2008;15:706–711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.