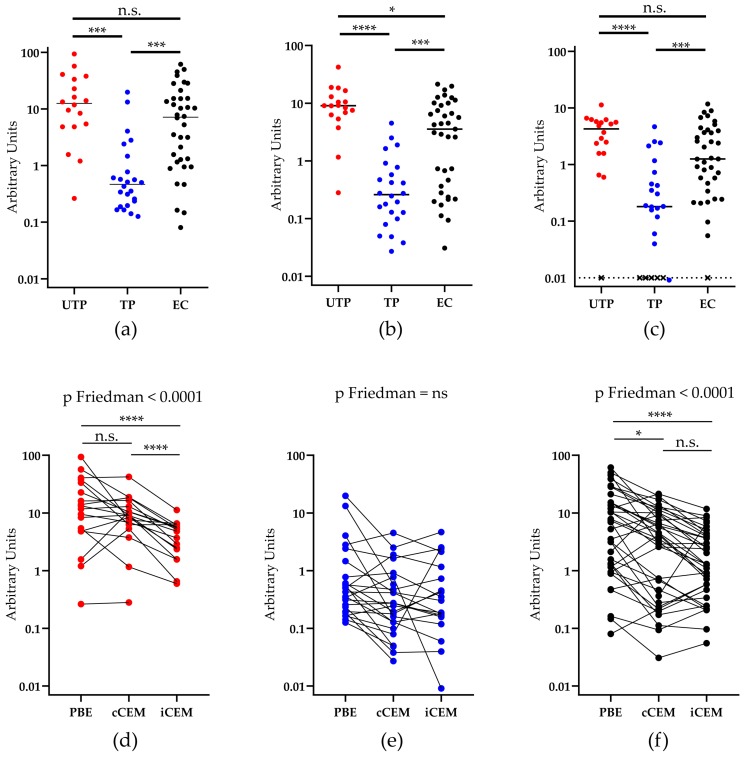
Figure 4.
Quantification of antibodies to rgp120/HIV Envelope-using three methods. The y-axis shows the relative amount of recombinant gp120 or HIV Envelope-specific antibody measured in plasma from three HIV+ subject groups using (a) a plate-based ELISA assay, or by flow cytometry-based assays using (b) cCEM and (c) iCEM cells as target cells. The subject groups being compared are indicated by lines joining two groups and the significance of between-group differences is indicated by “*” symbols over the lines joining the two groups being compared. Anti-rgp120/HIV Envelope-specific antibody levels in 1 untreated progressor, 5 treated progressors and 1 elite controller were below the limit of quantitation when iCEM cells were used as target cells and are represented by an “×” (c). Plasma from (d) untreated progressors, (e) treated ‘progressors, and (f) elite controllers were tested for their capacity to bind rgp120 coated wells in the plate-based ELISA assay to cCEM and to iCEM cells. PBE = plate-based ELISA; UTP = untreated progressors; TP = treated progressors; EC = elite controllers; “*” = p < 0.05; “***” = p < 0.001, “****” = p <0.0001, n.s. = not significant.