Differentiating solar lentigo from early lentigo maligna (LM) can be clinically challenging. However, dermoscopy can aid in identifying LM, with a reported sensitivity of 89% and a specificity of 96%.1 Dermoscopic criteria associated with LM include pigmentation in and around hair follicles (circles and circles within circles), perifollicular gray dots and globules (annular-granular pattern), angulated lines creating rhomboidal structures, and pigmented blotches (homogeneous areas, obliteration of hair follicles).1,2 Whilethehistopathologiccorrelationshavebeendecipheredforsomeofthesestructures,3,4 thehistopathologiccor-relation of circle within a circle has remained elusive. The aim of this report is to describe a potential reflectance confocal microscopic(RCM) and en face histopathologic correlation of circle within a circle, which has a low sensitivity (4.2%–5.0%) but high specificity (98.1%) for the diagnosis of LM.2,5
Report of a Case |
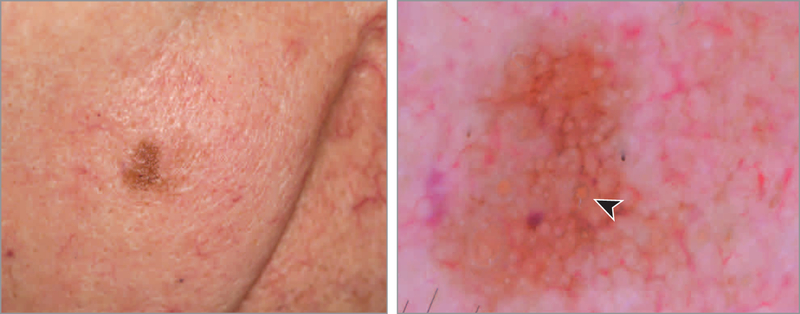
During routine follow-up examination of a man in his 80s with a history of melanoma, an irregular, solitary, pigmented lesion was noted on the right cheek (Figure 1A). Dermoscopy revealed a brown lesion with symmetric and asym metrically pigmented hair follicles presenting as gray circles. Some of these were double-layered circles or circles within a circle (Figure 1B, arrowhead). These findings were highly suggestive of LM.
Figure 1. Lentigo Maligna.
A, Clinically, an ill-defined solitary pigmented macule is seen on the right cheek. B, Dermoscopy shows asymmetric pigmentation of the hair follicles, some with a double layer, a “circle within a circle” (arrowhead) (polarized dermoscopy, original magnification ×10).
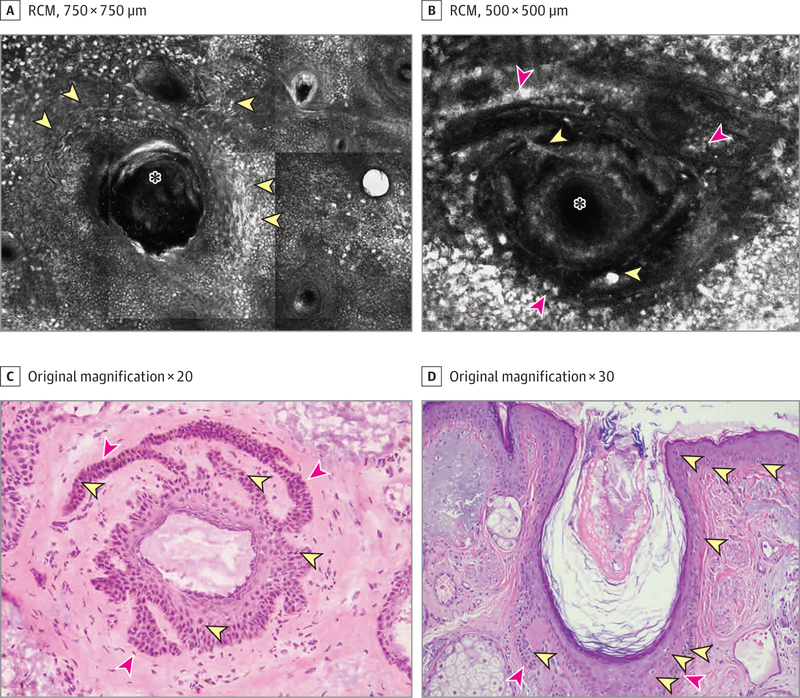
Examination by RCM revealed multiple, large, nucleated, dendritic, hyperreflective cells, surrounding the hair follicles (Figure 2A, yellow arrowheads). Some of the hair follicles had a proliferation of strands of keratinocytes peripherally filled with round and dendritic hyperreflective cells (Figure 2B, red arrowheads showing pigmented keratinocytes and yellow arrowheads showing atypical dendritic melanocytes). After der moscopy-RCM correlation, we noted that these double-layered hair follicles seen with RCM were directly correlated with the circles within a circle seen under dermoscopy (Figures 1B and 2B).
Figure 2. RCM and Histopathologic Images of the Lesion.
A, Reflectance confocal microscopy (RCM) image shows large nucleated, dendritic cells (yellow arrowheads) surrounding the hair follicles (asterisk) at the dermoepidermal junction level. B, An RCM close-upat the superficial dermis level shows that some of the hair follicles (asterisk) had proliferations of strands of pigmented keratinocytes (red arrowheads) and atypical round and dendritic large cells (yellow arrowheads). C, En face histopathologic section showing a hair follicle with an increased number of melanocytes (yellow arrowheads) and radial rete ridge projections with pigmented keratinocytes (red arrowheads); this image correlated with the RCM image shown in panel B (hematoxylin-eosin). D, Vertical histopathologic section showing an increased number of junctional melanocytes surrounding a hair follicle (yellow arrowheads) and a proliferation of strands of keratinocytes arising from the hair follicle (red arrowheads) (hematoxylin-eosin).
To further explore this correlation, we performed a 2-mm punch precision biopsy of the circle within a circle. The tissue was processed in the Mohs laboratory with frozen sections and was oriented en face to permit direct correlation with RCM and dermoscopic images. The biopsy showed epidermis and multiple hair follicles with increased melanin pigment in basilar keratinocytes associated with an increase in the density of junctional melanocytes. The larger follicle showed a circumferential orientation of the rete ridges surrounding the hair apparatus. Both the hair follicle and peripheral rete ridges were highlighted with pigment and an increased number of melanocytes (Figure 2B yellow and red arrowheads and Figure 2C yellow and red arrowheads). The diagnosis of LM was confirmed histologically via traditional formalin-fixed, paraffin-embedded tissue, which also showed an increased number of junctional melanocytes surrounding a hair follicle and rete ridge proliferations (Figure 2D).
Discussion |
Precise correlation of dermoscopic and routine histopathologic findings is difficult because dermoscopy views the lesion en face, and routine histopathologic sections view the lesion vertically. With the advent of RCM, we now have the ability to directly correlate en face dermoscopy with a quasi-histological view of the skin in vivo.6 In addition, once a feature has been identified via dermoscopy and RCM, we can perform small precision biopsies of the feature and process the tissue in a horizontal plane, permitting direct histopathologic correlation. This is exactly the process we followed in the present case and found that the circle within a circle was associated with pigmentation (increased number of melanocytes) in the follicular epithelium as well as pigmentation (increased number of melanocytes) in the rete ridge surrounding the follicle. This pattern of distribution of pigmentation and rete ridge proliferation explains the circle within a circle observed in LM. The knowledge of this dermoscopic feature and corresponding histopathologic pattern is also a helpful diagnostic clue for dermatopathologists, since it is strongly associated with LM.
Additional Contributions:
The authors are grateful for the excellent histological support provided by Steven Wilson, BS, and Reza Afzalneia, MS, Memorial Sloan Kettering Skin Cancer Center, Hauppage, New York. They received no compensation for their contributions.
Footnotes
Conflict of Interest Disclosures: None reported.
References
- 1.Schiffner R, Schiffner-Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42(1 Pt 1): 25–32. doi: 10.1016/S0190-9622(00)90005-7 [DOI] [PubMed] [Google Scholar]
- 2.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167(2):280–287. doi: 10.1111/j.1365-2133.2012.10932.x [DOI] [PubMed] [Google Scholar]
- 3.Yadav S, Vossaert KA, Kopf AW, Silverman M, Grin-Jorgensen C. Histopathologic correlates of structures seen on dermoscopy (epiluminescence microscopy). Am J Dermatopathol. 1993;15(4):297–305. doi: 10.1097/00000372-199308000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Nascimento MM, Shitara D, Enokihara MM, Yamada S, Pellacani G, Rezze GG. Inner gray halo, a novel dermoscopic feature for the diagnosis of pigmented actinic keratosis: clues for the differential diagnosis with lentigo maligna. J Am Acad Dermatol. 2014;71(4):708–715. doi: 10.1016/j.jaad.2014.05.025 [DOI] [PubMed] [Google Scholar]
- 5.Tschandl P, Rosendahl C, Kittler H. Dermatoscopy of flat pigmented facial lesions. J Eur Acad Dermatol Venereol. 2015;29(1):120–127. doi: 10.1111/jdv.12483 [DOI] [PubMed] [Google Scholar]
- 6.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49(1):7–19. doi: 10.1002/lsm.22600 [DOI] [PMC free article] [PubMed] [Google Scholar]