SUMMARY
Down syndrome (DS) is a genetic disorder that causes cognitive impairment. The staggering effects associated with an extra copy of human chromosome 21 (HSA21) complicates mechanistic understanding of DS pathophysiology. We examined the neuron-astrocyte interplay in a fully recapitulated HSA21 trisomy cellular model differentiated from DS-patient-derived induced pluripotent stem cells (iPSCs). By combining calcium imaging with genetic approaches, we discovered the functional defects of DS astroglia and their effects on neuronal excitability. Compared with control isogenic astroglia, DS astroglia exhibited more-frequent spontaneous calcium fluctuations, which reduced the excitability of co-cultured neurons. Furthermore, suppressed neuronal activity could be rescued by abolishing astrocytic spontaneous calcium activity either chemically by blocking adenosine-mediated signaling or genetically by knockdown of inositol triphosphate (IP3) receptors or S100B, a calcium binding protein coded on HSA21. Our results suggest a mechanism by which DS alters the function of astrocytes, which subsequently disturbs neuronal excitability.
Graphical Abstract
In Brief
To understand how Down syndrome (DS) affects neural networks, Mizuno et al. used a DS-patient-derived stem cell model and calcium imaging to investigate the functional defects of DS astrocytes and their effects on neuronal excitability. Their study reveals that DS astroglia exhibited more frequent spontaneous calcium fluctuations, which impair neuronal excitability.
INTRODUCTION
Down syndrome (DS) is a neurodevelopmental disorder occurring in 1 in 750 live births worldwide. DS is caused by trisomy of chromosome 21 (Ts21) (Dierssen, 2012), leading to triplication of up to 400 genes, resulting in an array of phenotypes, including profoundly impaired cognitive function. The brains of DS patients demonstrate consistent pathophysiological changes, such as reduced volume, altered neuronal densities and structure, and disturbed balance of all cell types. Confronted with this genetic complexity, it is difficult to determine precise molecular and cellular mechanisms of disease establishment and maintenance. Consequently, there are no therapeutic approaches to mitigate the effects of DS.
To date, DS pathophysiology has been primarily studied in rodent models (Ts65Dn, Ts1cje, and Ts1Rhr; Das and Reeves, 2011). Though useful information has been revealed, rodent models do not faithfully reproduce DS pathophysiology, due in part to incomplete synteny between HSA21 and homologous mouse regions. Furthermore, rodent modeling of complex neurodevelopmental disorders, such as DS, is limited by the fact that the human brain is far more complicated than the rodent brain in terms of structure of the neural circuitry, plasticity, and cognitive capacity.
Advances in induced pluripotent stem cell (iPSC) technology have enabled the modeling of complex diseases, such as DS in the context of human cell biology (Chen et al., 2014; Murray et al., 2015; Weick et al., 2013). These models are highly desirable for understanding disease neuropathophysiology and for developing therapeutics. By culturing iPSCs from DS individuals, it is possible to achieve full expression of the human HSA21 region. In addition, the use of isogenic control lines eliminates inter-individual variability, restricting genotype differences solely to HSA21 dosage.
Recent studies using Ts21-iPSC-derived DS models have revealed deficits in human neurons or astroglia associated with DS (Chen et al., 2014; Huo et al., 2018; Murray et al., 2015; Shi et al.,2012). Weick et al. (2013) established Ts21-iPSC lines from two sets of human fibroblasts and differentiated them into neurons. They found that Ts21-cortical neurons displayed reduced synaptic activity compared to control neurons, while maintaining the ratio of differentiated excitatory and inhibitory neurons. Furthermore, a recent follow-up study using similar model reported impaired migration of DS inhibitory neurons (Huo et al., 2018). Chen et al. (2014), on the other hand, engineered Ts21 iPSCs from a different human fibroblast line and reported that conditioned medium from Ts21-iPSC-derived astroglia had a toxic effect on neuronal maturation and survival.
However, these studies only focus on morphological view of differentiated cells and examined neurons and astrocytes in isolation. Growing evidence suggests that astrocytes substantially contribute to neurological and psychiatric disorders by affecting neuronal function (Cao et al., 2013; Di Giorgio et al., 2008; Marchetto et al., 2008; Molofsky et al., 2012; Tong et al., 2014). Indeed, astrocytes have been implicated in multiple rodent studies as playing an important role in DS (Ballestin et al., 2014; Bambrick et al., 2003). A number of genes involved in DS, including THBS1 and APP, have been shown to be expressed in astrocytes and have been implicated in Alzheimer’s disease (Garcia et al., 2010; Torres et al., 2018). A complete mechanistic understanding of DS pathophysiology requires studying the communication between neurons and astrocytes at the network level.
Unlike neurons, whose excitable membranes allow action potentials to be transmitted cell-wide within milliseconds, astrocyte-wide signaling occurs via intracellular calcium (Ca2+) transients lasting for seconds (Khakh and McCarthy, 2015). These intracellular Ca2+ transients can be triggered by neuronal activity (Wang et al., 2006) and are thought to induce release of gliotransmitters (Angulo et al., 2004; Lee et al., 2010; Mothet et al., 2005; Newman, 2001), which in turn modulate neural activity. Although gliotransmitter identity and release mechanisms are controversial (Ota et al., 2013; Sloan and Barres, 2014; Wolosker et al., 2016), intracellular Ca2+ dynamics are generally acknowledged to encode astrocyte activity. More importantly, altered astrocyte calcium dynamics were reported in cultured cells from the rodent DS models (Busciglio et al., 2002; Garcia et al., 2010).
Based on these previous studies, we hypothesized that DS could affect neuronal excitability through altered astrocytic Ca2+ dynamics, leading to alterations in astrocyte-neuron signaling pathways. Therefore, we differentiated the Ts21-iPSC lines reported in Weick et al. (2013) to astrocytes and neurons to establish a Ts21-iPSC-derived neuron-astrocyte co-culturing system to uncover functional deficits of neural networks. We focused on astrocytic Ca2+ dynamics and the specific interactions between astrocytes and neurons.
RESULTS
Generation and Differentiation of Astroglia from Human Ts21 iPSCs
Using established protocols (Krencik et al., 2011), we differentiated astroglia from previously reported iPSC lines by Weick et al. (2013), DS1 and DS4, which are trisomic for chromosome 21, and DS2U, a control isogenic line (Figures S1A and S1B). After 120 days, all three iPSC lines robustly expressed CD44, GFAP, and AQP4 (Figures S1D–S1F; Table S1). Karyotype analysis prior to and after experiments confirmed trisomy of DS1- and DS4-derived astroglia (DS1A and DS4A) and disomy of DS2U-induced astroglia (DS2UA) (Figure S1B). Using qRT-PCR, we further observed global expression of a panel of astrocyte-specific markers, such as EAAT1, ALDOC, CX43, SOX9, and NFIA, in all three lines (Figure S1C; Table S1; Molofsky et al., 2012), indicating successful astroglia differentiation of the iPSCs. Consistent with previous reports, DS astroglia showed increased expression levels of HSA21 genes compared to control astroglia, including S100B (Esposito et al., 2008), APP (Busciglio et al., 2002), and ETS2 (Wolvetang et al., 2003), as well as higher levels of non-HSA21 genes associated with oxidative stress, such as CAT (Busciglio and Yankner, 1995) and CRYZ (Weick et al., 2013; Figure S1C; Table S1). Morphologically, DS astroglia occupied larger territories than DS2UA; the total arborization size of DS astrocytes was significantly greater than that of control isogenic astroglia (Figure S1G).
DS Astroglia Inhibit the Excitability of Co-cultured Neurons
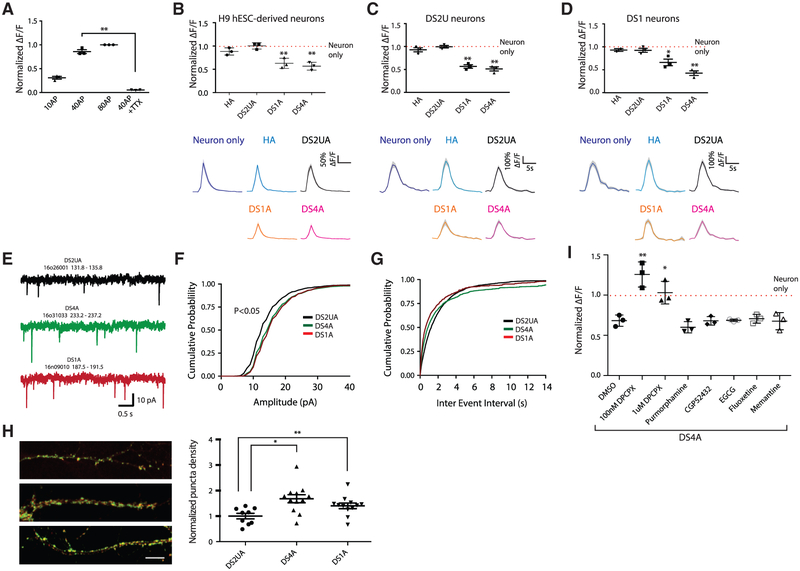
We next studied the potential influence of DS astroglia on co-cultured neurons. Using established protocols (Chambers et al., 2009; Zhang et al., 2001), three lines of cortical TUJ1+ neurons were differentiated from the DS1 and DS2U iPSC lines and a control H9 human embryonic stem cell (hESC) line (Figures S2A and S2B). Differentiated neurons were infected with lentivirus encoding synapsin-1 -GCaMP6m (Figure S2D). To establish a baseline of neuronal excitability, we monitored fluorescence changes in neurons in response to a series of electrically evoked field potentials (FPs) in the absence of astrocytes. The magnitude of evoked Ca2+ transients in neurons increased with the number of applied FPs (Figure 1A). Evoked signals were abolished by addition of 1 μM tetrodotoxin (TTX) (Figure 1A), suggesting that Ca2+ signals in neurons were triggered by action potentials. The expression of multiple voltage-gated sodium-channel isoforms in differentiated neurons was confirmed by qRT-PCR (Figure S2E).
Figure 1. DS Astroglia Inhibit Neuronal Excitability during Co-culture.
(A) The fluorescence changes (ΔF/F) of H9 hESC-derived neurons in response to a variety of FP stimuli; ΔF/F at 10 FPs, 40 FPs, and 40 FPs in the presence of 1 μM TTX were normalized to ΔF/F at 80 FPs.
(B–D) The responses of H9 hESC- (B), isogenic DS2U- (C), and DS1-iPSC- (D) derived neurons to FP stimuli (40 FPs at 30 Hz) when co-cultured with or without astroglia. ΔF/F induced by FP stimuli in the presence of astrocytes was normalized to that of neurons alone (red dotted lines). Representative traces showing Ca2+ transients triggered by FPs in neurons are shown (right panel).
(E) Example recordings of mEPSCs from 1 neuron from each group.
(F) Cumulative probability of the mEPSC amplitude shifted rightward in both DS4A and DS1A groups compared with the DS2UA group.
(G) No change was seen in the cumulative probability of the mEPSC inter-event interval.
(H) Representative images and quantification of puncta density expressing both synapsin and PSD95 (n = 3 images of immunostaining).
(I) The fluorescence changes of H9 hESC-derived neurons in response to 40 FPs stimuli when co-cultured with DS4A in the presence of DMSO or a series of drugs are shown and normalized to changes when co-cultured with DS2UA. For each cell line, n = 3 Ca2+-imaging sessions (each session contains 3 fields of view). Error bars are shown as mean ± SEM, *p < 0.05, **p < 0.01.
After confirming the basis of neuronal excitability, we recorded neuronal activity when co-cultured with DS1-, DS4-, or DS2U-derived astroglia, as well as human primary astrocytes (HA) (Figure S2C). H9 hESC-derived neurons co-cultured with DS astroglia (DS1A or DS4A) showed significantly decreased FP-evoked Ca2+ amplitudes relative to neurons cultured alone (normalized ΔF/F; DS1A: 0.63 ± 0.06, p < 0.01; DS4A: 0.57 ± 0.05, p < 0.001), whereas neurons co-cultured with control isogenic astrocytes (DSU2A) or human primary astrocytes were not significantly affected (DS2UA: 1.00 ± 0.04, p = 0.93; HA: 0.88 ± 0.04, p = 0.059; Figure 1B).
Similar neuronal-activity suppression imposed by DS astroglia was also observed in neurons derived from the two other iPSC lines. DS2U-derived neurons co-cultured with DS astroglia (DS1A or DS4A) showed significantly decreased FP-evoked Ca2+ amplitudes relative to neurons cultured alone (normalized ΔF/F; DS1A: 0.57 ± 0.04, p < 0.001; DS4A: 0.51 ± 0.04, p < 0.001; DS2UA: 0.99 ± 0.03, p = 0.89; HA: 0.93 ± 0.04, p = 0.18; Figure 1C). Likewise, DS1-derived neurons cocultured with DS astroglia (DS1A or DS4A) showed significantly decreased FP-evoked Ca2+ amplitudes relative to neurons cultured alone (normalized ΔF/F; DS1A: 0.66 ± 0.07, p < 0.01; DS4A: 0.43 ± 0.05, p < 0.001; DS2UA: 0.92 ± 0.04, p = 0.15; HA: 0.95 ± 0.03; p = 0.18; Figure 1D). Decreased neuronal activity in the presence of DS astroglia was observed under a variety of stimulation conditions but was most prominent during modest stimulation, such as 10FPs (Figure S2F). In addition, all co-cultured astrocytes significantly accelerated decay to baseline of evoked neuronal Ca2+ transients (T0.5 = 1.62 ± 0.14 for neuron-alone; 1.25 ± 0.12, 1.11 ± 0.13, and 1.18 ± 0.1 for neurons co-cultured with DS2UA, DS1A, and DS4A, respectively; p < 0.01; Figure S2G). Taken together, DS astroglia inhibited neuronal excitability of neurons derived from either trisomy or disomy iPSC lines.
DS Astroglia Promote Synaptic Connectivity
As DS astroglia suppress neuronal activity, we next sought to determine whether DS astroglia influence synaptic function. DS astroglia were co-cultured with dissociated rat hippocampal neurons, and miniature excitatory postsynaptic currents (mEPSCs) were recorded in the presence of TTX, NMDA receptor antagonist D-AP5, and GABAA antagonist bicuculline (Figures 1E–1G, S2H, and S2I). Cumulative distribution plots showed that the mean amplitude of mEPSCs was significantly larger in neurons co-cultured with either DS4A or DS1A groups compared with control DS2UA (DS2UA: 14.21 ± 0.42; DS1A: 16.35 ± 0.78, p < 0.05; DS4A: 16.26 ± 0.73, p < 0.05; Figures 1F and S2H). mEPSC frequency was similar in all three groups, with a trend toward higher mEPSC frequencies in the neurons co-cultured with DS4A and DS1A groups (p = 0.204; DS2UA: 0.56 ± 0.06; DS1A: 1.29 ± 0.45; DS4A: 1.10 ± 0.36; Figures 1G and S2I). Next, using quantitative image analysis (Thomazeau et al., 2014), we found that synapse density significantly increased by 1.5- and 1.3-fold in neurons co-cultured with DS astrocytes (DS1A, p < 0.01 and DS4A, p < 0.05, respectively) compared with those co-cultured with isogenic control astrocytes (Figure 1H). Taken together, these results suggest that DS astroglia are capable of modulating neuronal excitability as well as synaptic activity and density.
Pharmacological Rescue of Suppressed Neuronal Excitability
We next examined whether pharmacological drugs that block astrocyte-neuron communication could rescue the suppressed neuronal excitability. We first examined a panel of small-molecule drugs that have been shown to rescue synaptic abnormalities in DS mouse models (Busciglio et al., 2013). These compounds, including purmorphamine (sonic hedgehog agonist), CGP52432 (GABABR antagonist), epigallocatechin-3-gallate (EGCG) (DYRK1A inhibitor), fluoxetine (serotonin reuptake inhibitor), and memantine (NMDA receptor antagonist), however, failed to rescue decreased neuronal activity associated with DS astroglia (normalized ΔF/F = 0.60 ± 0.04, 0.68 ± 0.03, 0.70 ± 0.02, 0.71 ± 0.03, and 0.68 ± 0.06 from purmorphamine to memantine; p = 0.22, 0.95, 0.73, 0.67, and 0.93; n = 3; Figure 1I).
We next tested whether suppressed neuronal excitability is caused by adenosine-mediated signaling that has been shown to inhibit synaptic activity (Adair, 2005; Delekate et al., 2014; Kawamura and Kawamura, 2011; Koizumi, 2010; Nam et al., 2012). We treated H9 neurons co-cultured with DS astroglia (DS4A) with a Gi-coupled A1 adenosine receptor antagonist, followed by imaging FP-evoked neuronal activity. In particular, the A1 receptor antagonist DPCPX fully rescued suppressed neuronal activity, especially at lower concentrations (100 nM: normalized ΔF/F = 1.20 ± 0.09, p < 0.01; 1 μM: 0.98 ± 0.08, p < 0.05; n = 3; Figure 1I). We also treated H9 hESC derived with 100 μM adenosine, which resulted in suppressed neuronal activity after one hour incubation (normalized ΔF/F of 100 μM adenosine to without adenosine = 0.20 ± 0.06; p < 0.01; Figure S2J). Furthermore, astroglia-conditioned medium (ACM) from DS4A also showed a trend of suppressed neuronal excitability compared to no ACM (Figure S2K).
DS Astroglia Exhibit Abnormally Frequent Spontaneous Ca2+ Fluctuations
Astrocytic Ca2+ signaling has been proposed to modulate neural-circuit activity and structure (Anderson et al., 2016; Bazargani and Attwell, 2016); the suppressed excitability of neurons was specific to DS astroglia and could be rescued when astrocyteneuron communication was blocked by an adenosine receptor antagonist. This evidence led us to further investigate Ca2+ dynamics in astroglia. We focused on optical recordings of calcium dynamics in astroglia using GCaMP6m (Chen et al., 2013). We used the machine-learning software Functional Astrocyte Phenotyping (FASP) (Wang et al., 2016) to facilitate automated detection and analysis of complex Ca2+ dynamics in astroglia.
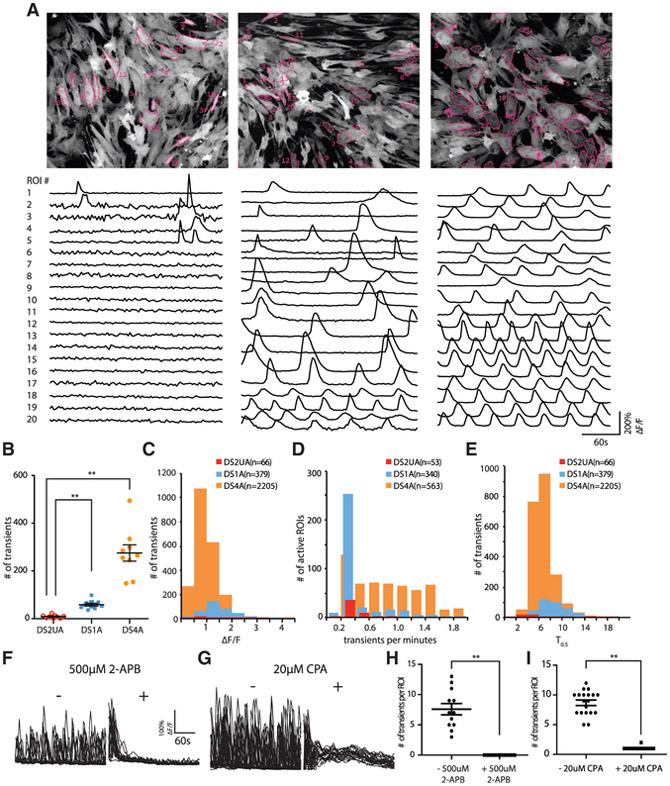
The differentiated astroglia indeed displayed prominent spontaneous Ca2+ transients, which were frequently periodic and especially apparent in DS astroglia (Figure 2A; Videos S1 and S2). DS astroglia exhibited significantly more (7- to 34-fold) Ca2+ transients than control isogenic astroglia (averaged number of calcium transients in a 5-min imaging session: DS1A: 58 ± 6, DS4A: 275 ± 34, DS2UA: 8 ± 2; mean ± SEM; p < 0.0001; unpaired t test; n = 9 imaging sessions; Figure 2B). The average amplitude (ΔF/F; DS1A: 1.45 ± 0.2, DS4A: 0.98 ± 0.15; p < 0.01; Figure 2C) and frequency (transients/min; DS1A: 0.41 ± 0.10, DS4A: 0.88 ± 0.16; p < 0.01; Figure 2D) of Ca2+ transients were significantly different between DS1A and DS4A, whereas the kinetics were similar (T1/2, s; DS1A: 8.59 ± 1.01; DS4A: 6.98 ± 0.90; p = 0.18; Figure 2E). These disparities are potentially due to epigenetic changes between the cell lines.
Figure 2. Imaging Ca2+ Events in Human-iPSC-Derived Isogenic and DS Astroglia.
(A) Spontaneous Ca2+ responses in isogenic DS2UA and two DS astroglia (DS1A and DS4A). Representative ROIs (n = 20) in the field of view showing Ca2+ fluctuations in DS2UA, DS1A, and DS4A. All ROIs were detected using FASP and marked with magenta outlines. The scale bar represents 100 μm.
(B) DS1A and DS4A displayed a significantly increased number of Ca2+ fluctuations in 5 min of imaging sessions compared with DS2UA (9 independent imaging sessions).
(C–E) Features of Ca2+ fluctuations in DS astroglia: averaged kinetics (C), frequency (D), and propagation speed (E) of DS astroglia. Data were collected from 81 cells of DS1A and 188 cells of DS4A.
(F–I) The Ca2+ fluctuations in DS4A could be abolished by incubation with IP3R antagonist (500 μM 2-APB; 17 ROIs; F and H) ordepleting ER Ca2+ store (20 μM CPA; 23 ROIs; G and I).
Error bars are shown as mean ± SEM, *p < 0.05, **p < 0.01.
Inositol triphosphate (IP3)-triggered Ca2+ release from the endoplasmic reticulum (ER) is considered a primary mechanism responsible for intracellular global Ca2+ waves (Tong et al., 2013). Application of the IP3 receptor (IP3R) antagonist 2-aminoethoxydiphenyl borate (2-APB) abolished spontaneous Ca2+ fluctuations (Figures 2F–2I), as did depletion of intracellular stores by cyclopiazonic acid (CPA), suggesting that IP3-ER Ca2+ underlies both spontaneous and evoked events in DS astroglia.
Wavefront analysis of the spontaneous events revealed 33 clusters of cells (Figure S3A, left) with temporally correlated Ca2+ fluctuations (Figure S3A, right) that were spatially intermingled (Figure S3B), suggesting that Ca2+ fluctuations do not propagate to adjacent cells. To further examine whether spontaneous fluctuations travel between cells, we performed Ca2+ imaging in a mixed culture of GCaMP6m-expressing control isogenic astroglia with unlabeled DS4A in a variety of ratios. Culturing with DS astroglia did not significantly increase the number of Ca2+ transients in control isogenic astroglia, even with a 10-fold excess of DS4A (Figure S3C), suggesting that spontaneous Ca2+ fluctuations were not induced in previously silent control isogenic cells. In addition, application of 10 μM n-octanol, a gap junction blocker, showed no effect on Ca2+fluctuations (Figure S3D).
Acutely purified human astrocytes acquire sensitivity to extracellular cues, such as neurotransmitter ATP and glutamate (Zhang et al., 2016). To exclude the possibility that differences in functional maturation of differentiated astroglia contribute to suppressed neuronal excitability, we examined transmitter-evoked Ca2+ responses of DS astroglia and compared with isogenic controls. Only previously, silent astrocytes were selected for quantification of evoked responses in order to differentiate evoked from spontaneous activity. Both DS and control isogenic astroglia responded robustly to ATP (representative traces of evoked Ca2+ transients shown in Figures S3E and S3F) in terms of the number and amplitude of evoked intracellular Ca2+ transients. Similarly, both DS astroglia and control isogenic astroglia responded to glutamate at micromolar concentrations (Figures S3G andS3H). Thus, DS and control astroglia respond similarly to neurotransmitters, further suggesting that Ts21 does not influence functional maturation of differentiated astrocytes.
Blocking Spontaneous Ca2+ Fluctuations in DS Astroglia Rescues Suppressed Neuronal Excitability
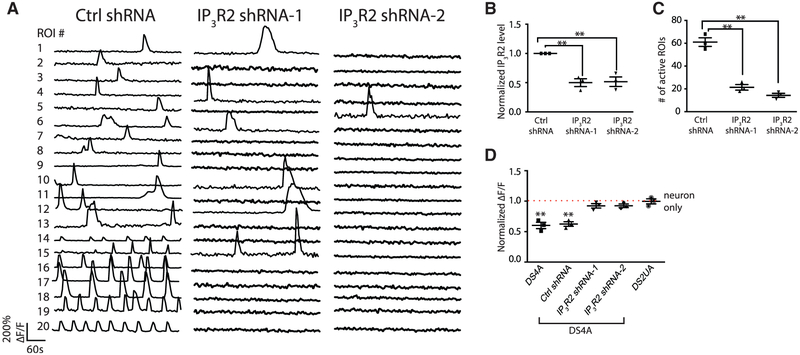
We tested whether the suppression of neuronal activity might be caused by the abnormally frequent spontaneous Ca2+ fluctuations observed in DS astroglia. Because pharmacological block of IP3 receptors abrogated spontaneous Ca2+ waves (Figures 2F–2I), we knocked down (KD) the expression of IP3R2 in DS astroglia DS4A alone, the main IP3R isoform in astrocytes, with short hairpin RNAs (shRNAs). IP3R2 KD in DS astroglia DS4A (Figure 3B) significantly reduced the number of active regions of interest (ROIs) showing spontaneous Ca2+ transients (scrambled shRNA: 61.0 ± 3.8; IP3R2 shRNA-1: 21.3 ± 2.4 [35%]; IP3R2 shRNA-2: 14.3 ± 1.8 [24%]; p < 0.001; Figures 3A and 3C).
Figure 3. DS Astroglial Ca2+ Fluctuations Are Regulated by IP3R-ER Pathway.
(A–C) The Ca2+ events in DS4A were significantly decreased by knocking down the expression of IP3R. Representative ROIs (n = 20) showing Ca2+ fluctuations in DS4A expressing scrambled shRNA (ctrl shRNA) and two shRNAs for IP3R (IP3R shRNA-1/2; A). Real-time PCR confirmed the decreased expression of IP3R in the presence of IP3R shRNAs (3 RNA samples; B), corresponding to a decreased number of Ca2+ events in 5 min (3 imaging sessions; C).
(D) Normalized fluorescence changes of H9 hESC-derived neurons in responseto 40 FPs co-cultured with DS4A or DS4A expressing scrambled or IP3R shRNAs to those of neurons alone (dotted red line).
Error bars are shown as mean ± SEM, *p < 0.05, **p < 0.01.
We then imaged the activity of neurons co-cultured with DS4A astroglia with IP3R2 knocked down, which rescued the reduced amplitude of evoked neuronal Ca2+ transients (measured as normalized ΔF/F; IP3R2 shRNA-1: 0.91 ± 0.0.4; shRNA-2: 0.93 ± 0.03) to the level of isogenic control astroglia (1.01 ± 0.04; p = 0.28). In contrast, DS4A with no shRNA (0.60 ± 0.05; p < 0.01) or control-scrambled shRNA (0.62 ± 0.04; p < 0.01) showed significantly decreased neural activity (Figure 3D). Therefore, elevated intracellular Ca2+ fluctuation mediated by IP3R2 is necessary to suppress neuronal excitability.
Spontaneous Ca2+ Fluctuations in DS Astroglia Are Not Driven by Extracellular Cues
As elevated spontaneous astroglia Ca2+ activity directly contributed to suppressed neuronal activity, we next sought to determine the factors driving elevated Ca2+ activity in DS astroglia. We first performed single-cell analysis of gene expression related to Ca2+ signaling pathways (Figure S4A) in DS astroglia. We also monitored the expression of a panel of astrocytic markers to account for the differentiation state of individual cells (Figures S4A and S4B). We then performed unsupervised clustering analysis of the cells by their gene expression patterns. We found that DS astroglia (DS4A) clustered into two groups (Figure S4C), distinguished by elevated expression of Ca2+ handling genes, such as ATP2B1, NCX1, RYR1/3, STIM1, NCLX, IP3R3, ORAI1, and chromosome 21 gene S100B (Table S1). This suggests that a subset of DS astroglia may display elevated spontaneous Ca2+ fluctuations. In DS astroglia, astrocytic markers, such as CD44, CX43, AQP4, NF1A, and ALDOC, were not differentially expressed between the two clusters. In contrast, we failed to identify significant clustering (Figure S4D) of genes related to the Ca2+-handling toolkit in control isogenic astroglia (DS2UA).
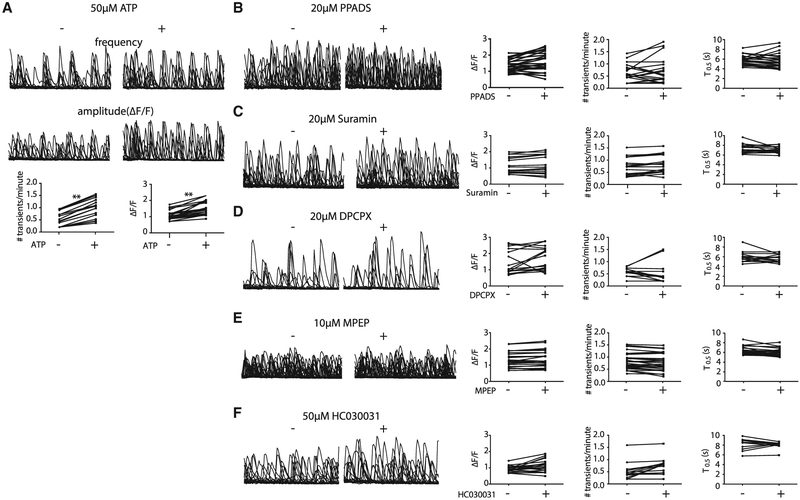
Moreover, from the single-cell gene analysis, we found that metabotropic glutamate receptors (GRM1/2/3/4/5/6/7/8) and purinergic receptors were elevated in a subset of DS4UA. We next investigated whether spontaneous fluctuations in DS astroglia could be modulated by pharmacological manipulation of these receptors. ATP treatment led to a 2-fold increase in the frequency and a 1.4-fold increase in the amplitude of spontaneous Ca2+ fluctuations in ~40% of ROIs (Figure 4A). However, treatment with P2-isotype-specific ATP receptor antagonists (PPADS for P2X; MRS2179for P2Y; Figures 4B and S5A), non-specific P2 antagonists (suramin; Figure 4C), or an adenosine A1-receptor antagonist (DPCPX; Figure 4D) had no significant effect on spontaneous Ca2+ fluctuations, suggesting that, although ATP can modulate spontaneous Ca2+ events in DS astroglia, it is not necessary to evoke them. CHPG (a selective mGluR5 agonist) showed no significant effects on amplitude, frequency, or kinetics of spontaneous Ca2+ fluctuations (Figure S5B). Similarly, mGluR5-selective (MPEP), non-selective mGluR (MCPG), and mGluR2/3-selective (LY341495) antagonists, as well as a glutamate transporter inhibitor (TFB-TBOA), also had no effect (Figures 4E and S5C–S5E). The TRPA1 channel antagonist HC030031 also had no significant effect on spontaneous Ca2+ fluctuations (Figure 4F), consistent with the lack of microdomain Ca2+ activity observed (Shigetomi et al., 2011). In summary, whereas both intrinsically and extrinsically driven calcium transients depend on IP3-mediated release from ER stores, our results suggest that spontaneous fluctuations are unlikely to be driven, though can be modified, by extracellular cues.
Figure 4. Spontaneous Fluctuations in DS Astroglia Could Not Be Modulated by Pharmacological Manipulation.
(A) ATP increased the frequency and amplitude of Ca2+ events in previously active cells (20 ROIs; p < 0.01).
(B and C) Purinergic receptor antagonist (20 μM PPADS; 25 ROIs, B; and 20 μM suramin; 18 ROIs, C) failed to modulate the Ca2+ fluctuations in DS4A.
(D–F) A1 adenosine receptor antagonist (DPCPX 20 μM; 18 ROIs; D), mGluR5 antagonist (10 μM MPEP; 42 ROIs; E), and TRPA inhibitor (50 μM HC030031; 20 ROIs; F) failed to modulate the Ca2+ fluctuations in DS4A. (Left) Representative traces are shown. (Right) Quantification of amplitude, frequency, and kinetics is shown.
*p < 0.01, **p < 0.01.
S100B Regulates Spontaneous Ca2+ Fluctuations in DS Astroglia
From gene analysis, we also noticed that S100B, a Ca2+-binding protein located on HSA21 and primarily expressed in astrocytes, was one of the top genes differentially expressed across DS4A cells. S100B elicits neurotrophic effects and regulates synaptic plasticity and rhythmic neuronal activity by chelating extracellular calcium (Morquette et al., 2015; Nishiyama et al., 2002). Given the particular interest in the context of Ts21 DS, we thus asked whether elevated S100B might contribute to the spontaneous intracellular Ca2+ fluctuations in DS astroglia.
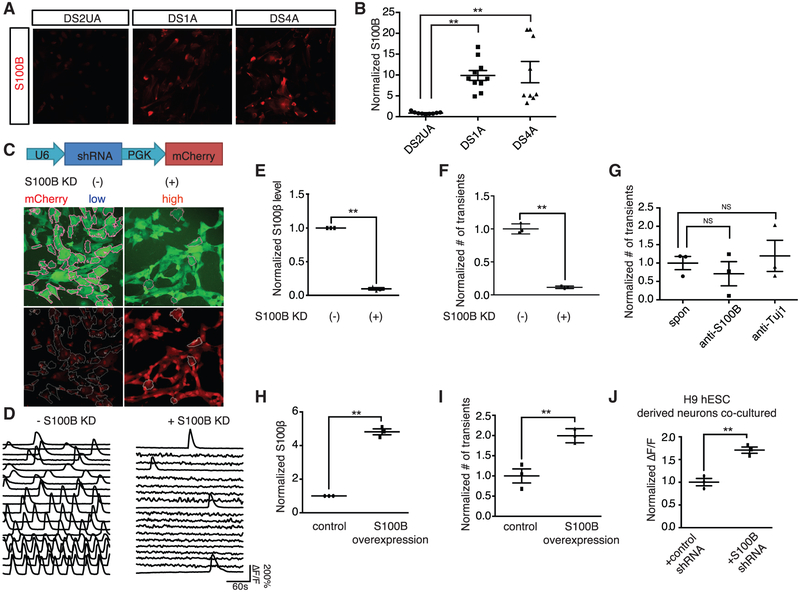
We first quantified the expression level of S100B in Ts21-derived astroglia. qRT-PCR analysis showed an averaged 11-fold greater expression of S100B in DS astroglia (DS1A and DS4A) compared with control isogenic DS2UA cells (Figure S1C). Expression of S100B protein was enriched in DS astroglia compared to DS2UA (Figures 5A and 5B; 9.9- and 10.7-fold increased expression S100B for DS1A and DS4A, respectively, compared to DS2UA).
Figure 5. S100B Regulates Spontaneous Ca2+ Fluctuations in DS Astroglia.
(A and B) Immunostaining of S100B in iPSC-derived astroglia (A) revealed increased expression in DS astroglia normalized to isogenic DS2UA (three images of immunostaining; 12.5% ± 1.0% for DS2UA; 80.4% ± 1.8% for DS1A; 75.3% ± 2.9% for DS4A; p < 0.01; B). Scale bar: 10 μm.
(C) Schematic representation of lentiviral construct encoding S100B shRNA-nls-mCherry. Scale bar: 50 μm.
(D) Representative ROIs (n = 20) highlighted in (C) showing spontaneous Ca2+ fluctuations in populations of DS4A without (left) and with S100B KD (right). The scale bars represent 50 μm.
(E and F) The S100B expression levels normalized to without S100B KD (3 RNA samples; E) and the number of Ca2+ events (3 imaging sessions of 5 min; F) with and without S100B KD are shown.
(G) Extracellular application of anti-S100B antibody did not influence the number of spontaneous Ca 2+ fluctuations in DS4A (3 imaging sessions).
(H and I) Overexpression of S100B increased the number of Ca2+ events in DS1A. qPCR analysis confirmed elevated expression of S100B in DS1A when S100B was overexpressed (3 RNA samples; 4.8- ± 0.2-fold normalized to empty vector group; p < 0.01; H). 2-fold more Ca2+ events in 5 min were detected in DS1A when S100B was overexpressed normalized to control (3 imaging sessions; I).
(J) Blocking intracellular Ca2+ events by S100B KD increased activity of H9 hESC-derived neurons co-cultured with DS4A. The fluorescence changes of H9 hESC-derived neurons in response to 40 FPs stimuli co-cultured with DS4A with S100B shRNA normalized to DS4A with control shRNA are shown. Error bars are shown as mean ± σEM, *p < 0.05, **p < 0.01.
We next selectively knocked down S100B in DS4A and performed Ca2+ imaging. Using mCherry as a proxy for the extent of S100B KD, we used fluorescence-activated cell sorting (FACS) to select the top 15% of cells showing potent S100B KD and use the bottom 15% of cells as a control group showing normal S100B levels (Figures 5C–5F). The S100B KD population showed a 3.5-fold decrease in spontaneous Ca2+ transients during a 5-min window (p < 0.001; Figures 5D and 5F), which corresponds to ~10-fold lower S100B levels compared to the control group (p < 0.001; Figure 5E). These data suggest that S100B modulates spontaneous Ca2+ fluctuations in DS astroglia.
Given the reported role of secreted S100B protein in modulating neural activity, we incubated the cultures with antibodies against S100B or Tuj1 (without permeabilization). After 10 min incubation, there was no effect on spontaneous Ca2+ events of either antibody (Figure 5G), suggesting that the spontaneous Ca2+ events are mediated by intracellular S100B.
We then asked whether overexpression of S100B protein would also modulate spontaneous Ca2+ fluctuations. We observed 2-fold increase in the number of Ca2+ transients when S100B was overexpressed in DS1A (Figures 5H and 5I; p < 0.01), in which the number of spontaneous Ca2+ transients is less abundant than DS4A.
Finally, we examined whether DS astroglia with spontaneous Ca2+ fluctuations alleviated by S100B KD still suppressed neuronal excitability. We recorded evoked Ca2+ events in response to FP stimuli in H9 neurons co-cultured with DS4A with or without S100B KD. H9 neurons co-cultured with DS4A with potent S100B KD displayed significantly larger (1.7-fold; p < 0.01) neural activity than those without S100B KD (Figure 5J) suggesting that S100B KD successfully rescued neuronal activity suppressed by DS4A. Thus, we conclude that blocking Ca2+ fluctuations in DS astroglia by genetic ablation of either IP3R2 oi S100B is sufficient to rescue the excitability decreases o co-cultured neurons.
DISCUSSION
Combining human stem cell technology with Ca2+ imaging and quantitative analysis tools provides a powerful platform to study neuron-astrocyte interaction in both physiological and pathological conditions, especially at early developmental stages. Using this platform, we imaged and characterized the effect of Ts21-iPSC-derived astroglia on neuronal networks. DS astroglia produced structural and functional deficits in co-cultured neurons Specifically, neurons co-cultured with DS astroglia displayed decreased global excitability. Such decreased global excitability of neurons corresponded with increased amplitudes of post-synaptic activity and synaptic density, consistent with accepted mechanisms of homeostatic synaptic plasticity and synaptic scaling (Turrigiano, 2012). Our data are in line with a rodent DS model study, in which the dendritic spine density and mEPSC amplitude increased although frequency of mEPSCs remained unchanged in prefrontal cortical pyramidal neurons (Thomazeau et al., 2014). Though abnormal synaptic morphology, such as reduced synaptic density, has been reported in other iPSC-derived DS models and in Ts65Dn adult mice, none of these studies have examined the function of astrocytes and potential effect imposed by astrocytes at early stages of brain development. Therefore, the effect of DS astrocytes on synaptic properties is worth further investigating across the whole spectrum of neuronal differentiation and development in iPSC-based DS models. In addition, transplantation of DS astrocytes into rodent models can be performed to directly examine the effect imposed by astrocytes on synaptic properties at early stages of brain development.
We further showed functional differences between DS astroglia and control isogenic astroglia in terms of intracellular Ca2+ dynamics. We observed elevated spontaneous Ca2+ fluctuations that are frequent and periodic only in DS-derived astroglia, but not in an isogenic control cell. These aberrant Ca2+ fluctuations in DS astroglia are necessary to drive suppression of global excitability in co-cultured neurons, as evidenced by rescue by genetic or pharmacological block.
What causes aberrant Ca2+ fluctuations in DS astroglia? Here, we demonstrate that overexpression of cellular S100B in DS astroglia mediates elevated spontaneous Ca2+ fluctuations (Figures 5H and 5I), which subsequently regulate neuronal excitability (Figure 5J). This finding is of particular interest, as S100B is a Ca2+-binding protein. Previous research (Barger et al., 1992) has shown that secreted S100B stimulates a rise in intracellular Ca2+ concentration in both neurons and glia. Furthermore, extracellular S100B regulates the firing patterns of neurons by reducing extracellular Ca2+ concentrations (Morquette et al., 2015). In our studies, extracellular S100B did not influence spontaneous Ca2+ fluctuations in DS astroglia, whereas cytosolic of S100B did. Further investigation is necessary to parse the various functions of secreted and cytosolic S100B in healthy and disease model astrocytes and neurons.
A major open question in DS research is the mechanism by which the overdose of hundreds of genes on HSA21 disrupts brain function. To date, several candidate genes have been identified, including DYRK1A, SIM2, DSCAM, KCNJ6, NKCC1, and miR-155 (Deidda et al., 2015; Dierssen, 2012; Wang et al., 2013; Table S1). Overexpression of S100B, at the distal end of the HSA21 long arm, has been shown to generate reactive oxygen species (ROS) (Esposito et al., 2008) in human induced pluripotent stem cells (hiPSC)-derived DS astroglia, leading to neuronal apoptosis (Chen et al., 2014). Previous research reported that ROS induce lipid peroxidation, activate the PLC-IP3R pathway, and cause Ca2+ increases in astrocytes (Vaarmann et al., 2010). Indeed, we found that spontaneous Ca2+ activity was mediated by IP3R2-regulated ER stores. Though we do not have direct evidence to link S100B, ROS, and PLC-IP3R, S100B might mediate perturbed Ca2+ dynamics via ROS in DS astroglia.
Our study provides additional evidence to support the hypothesis that astrocytic Ca2+ signaling modulates neural activity, critical for brain function during development. A grand challenge is to elucidate the pathways regulating astrocyte-neuron interplay during development. In the present study, our results indicate that astrocyte-neuron interaction via purinergic signaling might be a significant contributor linking aberrant astrocytic Ca2+ to neuronal functional deficits in DS. We showed that treatment with 100 μM adenosine in H9 hESC-derived neurons without the presence of astrocytes suppressed neuronal activity. Furthermore, treatment with DPCPX, an adenosine A1 receptor antagonist, rescued the suppressed Ca2+ activity of H9 hESC-derived neurons co-cultured with DS astroglia (Figure 1I). Previous research has shown that adenosine predominantly inhibits synaptic activity via A1 receptors (Delekate et al., 2014; Koizumi, 2010; Nam et al., 2012). However, future efforts should focus on further elucidating the source of release and other co-factors involved in the neuronal inhibition.
In conclusion, the combination of a human iPSC DS model with functional imaging and pharmacological and genetic manipulation provides a platform for quantitative measurement of human cellular physiology and for mechanistic studies of disease pathophysiology. Though animal models of neurological disorders play an important role in studying the effects of specific genetic and experimental perturbations and in testing potential treatments, they often fail to faithfully recapitulate the full spectrum of human phenotypes, which can lead to false conclusions owing to molecular and cellular differences between the systems. Future improvements to iPSC models will include 3-dimensional culture (Paşca et al., 2015), multi-color imaging, and incorporating genetically encoded indicators for other molecules and cellular states (e.g., glutamate; Marvin et al., 2013). Our imaging platform can be applied to the study of other neurological diseases as well, even to the level of testing specific drug combinations on neuron-astrocyte co-cultures developed from single healthy or diseased individuals.
EXPERIMENTAL PROCEDURES
Neural Differentiation of Human ESCs and iPSCs
H9 human ESCs were obtained from WiCell (Madison, WI). Control isogenic trisomy 21 and euploid iPSCs, DS1, DS2U, and DS4, were engineered in Dr. Anita Bhattacharyya’s lab, as previously described (Weick et al., 2013). H9 ESCs and iPSCs were maintained on Matrigel (Becton Dickinson; 356234) in mTeSR1 medium (StemCell Technologies; 05850). Mycoplasma contamination was routinely tested. We used previously described protocols for neural differentiation (Zhang et al., 2001), with minor modifications. Briefly, following detachment of neuroepithelia from adherent conditions at day 14, neurospheres were expanded in fibroblast growth factor (FGF)/epidermal growth factor (EGF) (10 ng/mL). Large spheres were disaggregated into smaller clusters approximately every two weeks until day 90. 20–25 neurospheres or 2.5 × 104 cells/cm2 were seeded on 35-mm Matrigel-coated glass-bottom dishes (MatTek; P35G-1.0-14-C), and once confluent, neurospheres were cultured in neuronal medium (neurobasal medium, 21103-049; 1% N-2 supplement, 17502-048, 2% B-27 supplement, 17504-044; 10 ng/mL BDNF [450-02]; and GDNF [450-10]) for 40 days. Medium components were purchased from Thermo Fisher Scientific and cytokines from Peprotech. Inhibitors of SMAD signaling (10 μM SB431542 and 100 nM LDN193189; Tocris Bioscience) were added for the first 6 days to promote neural induction (Chambers et al., 2009).
Derivation and Culture of Astrocytes
Control isogenic and DS iPSCs were differentiated into neural progenitors and cultured similar to neural differentiation with the following modifications. Astrospheres were then plated at a concentration of 500,000 cells/mL in 12 mL of media in T75 flasks and allowed to adhere to fibronectin-coated dishes (Sigma; F0895). After confluent, astrospheres were dissociated into single cells and cultured in an optimized commercial medium for human primary astrocytes (ScienCell Research Laboratories; 1801). HAs were from ScienCell Research Laboratories (1800). We performed karyotype analysis on DS1-, DS4-, and DS2U-derived astroglia, prior to and after the Ca2+ experiments (Cell Line Genetics). The cell size was analyzed by randomly selecting 5 cells from 3 bright field images (ImageJ).
Ca2+ Imaging and Analysis in Astrocytes
Primary astrocytes or iPSC-derived astrocytes were seeded onto 8-well slides (Ibidi; 80826), coated with fibronectin, and infected with lentiviruses encoding EF1α-GCaMP6m and then subjected to Ca2+ imaging. For IP3R2 KD, DS4A cells were infected with lentiviruses encoding shRNA and GCaMP6m; Ca2+ imaging followed. For S100B KD, DS4A cells were infected with lentiviruses encoding shRNA, sorted into 2 populations by FACS according to mCherry intensity, and infected with GCaMP6m for each population; Ca2+ imaging followed. For each cell line, 3 Ca2+-imaging sessions (each session contains 3 fields of view) were collected from independent samples. For mixed cultures of control isogenic and DS astrocytes, control isogenic DS2UA were first infected with lentiviruses expressing EF1α-GCaMP6m and then seeded with DS4A, followed by Ca2+ imaging. Three days post-infection, frame scans were acquired at 2 Hz (512 × 512 pixels) for a period of 300 s. All imaging was done using a Zeiss LSM 710 confocal microscope (20x magnification; numerical aperture [N.A.] = 0.8 objective). Agonists or antagonists (Tocris) were added at frame 10 during continuous imaging. For quantification of ATP and glutamate-evoked activity, to eliminate the confound of spontaneous activity, only ROIs that were silent during the initial imaging period were analyzed for a response to added ATP or glutamate. Furthermore, we ensured that these evoked responses were time locked to agonist application.
Because of these complex spatiotemporal patterns of Ca2+ dynamics in astrocytes, we developed a computational tool (FASP; Wang et al., 2016) to quantitatively and automatically analyze the large-scale imaging datasets to ensure that the analysis is identical and objective for all cells and across experiments. Additional details are in the Supplemental Information.
Neuron-Astrocyte Co-culture and Astrocyte-Conditioned Media Incubation
Differentiated neurons were infected with lentiviruses expressing Synapsin-1-GCaMP6m. Two days post-infection, astrocytes were seeded on top of neurons to establish co-culture. Neurons were seeded with astrocytes at 1:1 at 5 × 104 in 35 mm on glass bottom dishes in 2 mL of medium or with 100 μg/mL of astrocyte-conditioned media in 8-well μ-slide dishes in 250 μL of media. After 3–7 days, infected neurons were stimulated using a custombuilt field stimulator with platinum wires or 50 μM glutamate. Field stimuli were delivered as 40 V, 30 Hz, 1 ms pulses for the following trains: 10, 20, 40, and 80 field stimuli in Hank’s balanced salt solution (HBSS) with 2 mmol CaCl2 and MgCl2. When chemicals were used, they were applied 3 days prior to imaging, except DPCPX and adenosine, which was acutely applied 1 hr prior to imaging. All chemicals were purchased from Tocris or Sigma.
Single-Cell Expression
DS astrocytes were digested and sorted by FACS to get rid of cell debris and dead cells. The cell suspension was loaded onto a C1 Single-Cell Auto Prep Array for mRNA Seq (10–17 μm; Fluidigm; 100-5760), and single cells were captured and lysed to get cDNA on Fluidigm’s C1 platform. Gene expression patterns of single cells (n = 46) were studied using the 48.48 Dynamic Array Chip for Gene Expression following the manufacturer’s instructions (Fluidigm; BMK-M48.48).
Animals
Animal studies were conducted in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Davis or the relevant institutional regulatory body.
Statistical Analysis
All values are shown as mean ± SEM. To determine significant differences between groups, comparisons were made using a two-tailed unpaired t test. For mEPSC analysis, a one-way ANOVA was used to compare mEPSC amplitude and frequency among groups, followed by Fisher’s least significant difference (LSD) pairwise comparison when appropriate. For single-cell expression analysis, a permutation test was applied for unsupervised clustering, and the differences of each gene between the two clusters were determined using two-sample unpaired Wilcoxon rank-sum test. A p value smaller than 0.05 was accepted for statistical significance p < 0.01 (**) or 0.05 (*). The sample size for each experiment was determined either by power analysis (2-sample, 2-sided equality) or by referring to the sample size in similar studies (Chen et al., 2014; Zhang et al., 2016). For Ca2+-imaging experiments, imaging sessions were collected from at least 3 batches of cells, and ROIs were selected either automatically by FASP for astrocyte Ca2+ imaging or manually for neuronal Ca2+ imaging. For gene expression, RNA samples from three batches of cells were used. For immunostaining analysis, three batches of cells were fixed and five fields of view from each sample were selected for imaging and analyzed blinded. No randomization was used. No data were excluded.
Supplementary Material
Highlights.
Neuron-astrocyte interactions in a human DS stem cell model
DS astroglia exhibited more frequent spontaneous Ca2+ fluctuations
Spontaneous DS astroglia Ca2+ reduced excitability of co-cultured neurons
Abolishing astrocytic spontaneous Ca2+ rescued suppressed neuronal activity
ACKNOWLEDGMENTS
This work was supported by the Hartwell Foundation Individual Biomedical Award (L.T.), NIH DP2MH107056 (L.T.), NIH R21NS095325 (L.T.), NIH R01MH110504 (L.T. and G.Y.), NSF1750931 (G.Y.), NIH R03 HD064880 (A.B.), National Institute of General Medical Sciences (NIGMS) 1P20GM109089-01A1 (J.W.), National Institute of Neurological Disorders and Stroke (NINDS) R21NS093442-01 and NSF7566685 (J.W.), and National Institute on Deafness and Other Communication Disorders (INCD) R01HD09325 (W.D.). This project was supported by the University of California, Davis, Flow Cytometry Shared Resource Laboratory with technical assistance from Ms. Bridget McLaughlin and Mr. Jonathan Van Dyke. We would like to give special thanks to Dr. Bart Borghuis for generously sharing the FluoAnalyzer codes, Dr. Karen Zito for critical input, Dr. Brett Mensh for critical discussions, and Lisa Makhoul for editorial assistance.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures, one table, and two videos and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.06.033.
DECLARATION OF INTERESTS
The authors declare no competing interest.
REFERENCES
- Adair TH (2005). Growth regulation of the vascular system: an emerging role foradenosine. Am. J. Physiol. Regul. Integr. Comp. Physiol 289, R283–R296. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, and Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 582, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, and Audinat E (2004). Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci 24, 6920–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestín R, Blasco-Ibáñez JM, Crespo C, Nacher J, López-Hidalgo R, Gilabert-Juan J, Moltó D, and Varea E (2014). Astrocytes of the murine model for Down syndrome Ts65Dn display reduced intracellular ionic zinc. Neurochem. Int 75, 48–53. [DOI] [PubMed] [Google Scholar]
- Bambrick LL, Yarowsky PJ, and Krueger BK (2003). Altered astrocyte calcium homeostasis and proliferation in theTs65Dn mouse, a model of Down syndrome. J. Neurosci. Res 73, 89–94. [DOI] [PubMed] [Google Scholar]
- Barger SW, Wolchok SR, and Van Eldik LJ (1992). Disulfide-linked S100 beta dimers and signal transduction. Biochim. Biophys. Acta 7760, 105–112. [DOI] [PubMed] [Google Scholar]
- Bazargani N, and Attwell D (2016). Astrocyte calcium signaling: the third wave. Nat. Neurosci 19, 182–189. [DOI] [PubMed] [Google Scholar]
- Busciglio J, and Yankner BA (1995). Apoptosisand increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature 378, 776–779. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, and Yankner BA (2002). Altered metabolism ofthe amyloid b precursorprotein isassociated with mitochondrial dysfunction in Down’s syndrome. Neuron 33, 677–688. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Capone G, O’Bryan J, and Gardiner KJ (2013). Down syndrome: genes, model systems, and progress towards pharmacotherapies and clinical trialsforcognitivedeficits. Cytogenet. Genome Res 141,260–271. [DOI] [PubMed] [Google Scholar]
- Cao X, Li L-P, Wang Q, Wu Q, Hu H-H, Zhang M, Fang Y-Y, Zhang J, Li S-J, Xiong W-C, et al. (2013). Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med 19, 773–777. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, and Studer L (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol 27, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, Parast MM, Li S, Pleasure DE, Laurent LC, et al. (2014). Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat. Commun 5, 4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, and Reeves RH (2011). The use of mouse modelsto understand and improve cognitive deficits in Down syndrome. Dis. Model. Mech 4, 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deidda G, Parrini M, Naskar S, Bozarth IF, Contestabile A, and Can- cedda L (2015). Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med 21, 318–326. [DOI] [PubMed] [Google Scholar]
- Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, and Petzold GC (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Com- mun 5, 5422. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, and Eggan KC (2008). Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3, 637–648. [DOI] [PubMed] [Google Scholar]
- Dierssen M (2012). Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci 13, 844–858. [DOI] [PubMed] [Google Scholar]
- Esposito G, Imitola J, Lu J, De Filippis D, Scuderi C, Ganesh VS, Folkerth R, Hecht J, Shin S, Iuvone T, et al. (2008). Genomic and functional profiling of human Down syndrome neural progenitors implicates S100B and aquaporin 4 in cell injury. Hum. Mol. Genet 17, 440–457. [DOI] [PubMed] [Google Scholar]
- Garcia O, Torres M, Helguera P, Coskun P, and Busciglio J (2010). A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down’s syndrome. PLoS ONE 5, e14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H-Q, Qu Z-Y, Yuan F, Ma L, Yao L, Xu M, Hu Y, Ji J, Bhatta- charyya A, Zhang S-C, and Liu Y (2018). Modeling Down syndrome with patient iPSCs reveals cellular and migration deficits of GABAergic neurons. Stem Cell Reports 10, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M Jr., and Kawamura M (2011). Long-term facilitation of spontaneous calcium oscillations in astrocytes with endogenous adenosine in hippocampal slice cultures. Cell Calcium 49, 249–258. [DOI] [PubMed] [Google Scholar]
- Khakh BS, and McCarthy KD (2015). Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol 7, a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S (2010). Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J. 277, 286–292. [DOI] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang Z-J, and Zhang S-C (2011). Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol 29, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon B-E, Berglund K, Oh S-J, Park H, Shin H-S, Augustine GJ, and Lee CJ (2010). Channel-mediated tonic GABA release from glia. Science 330, 790–796. [DOI] [PubMed] [Google Scholar]
- Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, and Gage FH (2008). Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649–657. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T-W, Bargmann CI, et al. (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, and Rowitch DH (2012). Astrocytes and disease: a neuro-developmental perspective. Genes Dev 26, 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette P, Verdier D, Kadala A, Fethifbre J, Philippe AG, Robitaille R, and Kolta A (2015). An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat. Neurosci 18, 844–854. [DOI] [PubMed] [Google Scholar]
- Mothet J-P, Pollegioni L, Ouanounou G, Martineau M, Fossier P, and Baux G (2005). Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. USA 102, 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Letourneau A, Canzonetta C, Stathaki E, Gimelli S, Sloan- Bena F, Abrehart R, Goh P, Lim S, Baldo C, et al. (2015). Brief report: isogenic induced pluripotent stem cell lines from an adult with mosaic down syndrome model accelerated neuronal ageing and neurodegeneration. Stem Cells 33,2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Mclver SR, Hinton DJ, Thakkar MM, Sari Y, Parkinson FE, Haydon PG, and Choi D-S (2012). Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol. Clin. Exp. Res 36, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA (2001). Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J. Neurosci 21, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama H, Knopfel T, Endo S, and Itohara S (2002). Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc. Natl. Acad. Sci. USA 99, 4037–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, and Hallock RM (2013). The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013, 185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park J-Y, O’Rourke NA, Nguyen KD, et al. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, MacLean G, Orkin SH, and Livesey FJ (2012). A human stem cell model of early Alzheimer’s disease pathology in Down syndrome. Sci. Transl. Med 4, 124ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, and Khakh BS (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci 15, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, and Barres BA (2014). Looks can be deceiving: reconsidering the evidence for gliotransmission. Neuron 84, 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazeau A, Lassalle O, lafrati J, Souchet B, Guedj F, Janel N, Chavis P, Delabar J, and Manzoni OJ (2014). Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a. J. Neurosci 34, 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, and Khakh BS (2013). Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19, 274–291. [DOI] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, and Khakh BS (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci 17, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MD, Garcia O, Tang C, and Busciglio J (2018). Dendritic spine pathology and thrombospondin-1 deficits in Down syndrome. Free Radic. Biol. Med 114, 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G (2012). Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol 4, a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarmann A, Gandhi S, and Abramov AY (2010). Dopamine inducesCa2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. J. Biol. Chem 285, 25018–25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian G-F, Peng WG, Han X, Kang J, Takano T, and Nedergaard M (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci 9, 816–823. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao Y, Zhang X, Badie H, Zhou Y, Mu Y, Loo LS, Cai L, Thompson RC, Yang B, et al. (2013). Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat. Med 19, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi G, Miller DJ, Wang Y, Broussard G, Wang Y, Tian L, and Yu G (2016). FASP: a machine learning approach to functional astrocyte phenotyping from time-lapse calcium imaging data. In 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, 2016 (IEEE; ), pp. 351–354. [Google Scholar]
- Weick JP, Held DL, Bonadurer GF 3rd, Doers ME, Liu Y, Maguire C, Clark A, Knackert JA, Molinarolo K, Musser M, et al. (2013). Deficits in human trisomy 21 iPSCs and neurons. Proc. Natl. Acad. Sci. USA 110, 9962–9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Balu DT, and Coyle JT (2016). The riseandfall of the d-serine-mediated gliotransmission hypothesis. Trends Neurosci 39, 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvetang EJ, Wilson TJ, Sanij E, Busciglio J, Hatzistavrou T, Seth A, Hertzog PJ, and Kola I (2003). ETS2 overexpression in transgenic models and in Down syndrome predisposes to apoptosis via the p53 pathway. Hum. Mol. Genet 12, 247–255. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, and Thomson JA (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol 19, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MSB, Li G, et al. (2016). Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.