Abstract
Background:
A longer duration of untreated psychosis (DUP) has been linked with poor clinical outcomes, as well as variation in resting-state striatal connectivity with central executive regions. However, the link between DUP and task-based activation of executive neurocognition has not previously been examined. The following fMRI study examined the association between DUP and both activation and frontostriatal functional connectivity during a visual working memory (WM) paradigm in patients with first-episode psychosis (FEP).
Methods:
Patients with FEP (N=37) underwent fMRI scanning while performing a visual WM task. At the single-subject level, task conditions were modeled; at the group level, each condition was examined along with DUP. Activation was examined within the dorsolateral prefrontal cortex (DLPFC), a primary region supporting visual WM activation. Frontostriatal functional connectivity during the WM was examined via psychophysical interaction between the dorsal caudate and the DLPFC. Results were compared to a reference range of connectivity values in a matched group of healthy volunteers (N=25). Task performance was also examined in relation to neuroimaging findings.
Results:
No significant association was observed between DUP and WM activation. Longer DUP showed less functional frontostriatal connectivity with the maintenance of increasing WM load. Results were not related to task performance measures, consistent with prior work.
Conclusions:
Our data suggest that DUP may affect frontostriatal circuitry that supports executive functioning. Future work is necessary to examine if these findings contribute to the mechanism underlying the relationship between DUP and worsened clinical outcomes.
Keywords: Schizophrenia, psychosis, frontostriatal connectivity, fMRI, working memory, duration of untreated psychosis
Introduction
Patients with first-episode psychosis (FEP) often interface with mental health treatment following extended periods of untreated psychotic symptoms. A recent calculation of the median duration of untreated psychosis (DUP) from a large community-based sample of patients entering the Recovery After an Initial Schizophrenia Episode study was determined to be 74 weeks in length (1). While causal links remain undetermined, decades of work has established that longer DUP is associated with poorer clinical outcomes, including worse social and occupational functioning, as well as response to antipsychotic treatment (2–5). Therefore, reducing DUP length has become central to preventative interventional strategies across the world aimed at decreasing the morbidity secondary to schizophrenia-spectrum illnesses (6). Coinciding with psychosocial approaches, understanding the neural mechanism associated with DUP will be important for the development of biologically-informed strategies for treatment and prevention.
To date, the precise mechanism underlying DUP remains unknown. The bulk of prior DUP-related studies, focused on neurocognitive assessments and structural brain imaging measures, report differential executive cognition and morphology within the basal ganglia and prefrontal cortex (7–9). However, negative studies have also been published and, overall, efforts in both research domains have not resulted in tangible and replicable findings that inform neurobiological processes (10–14).
More recent work has demonstrated a role for large-scale functional networks in the neural mechanism associated with DUP. Evidence from a large sample of FEP patients reveals DUP-related abnormalities within the hippocampus, suggesting deleterious effects on a subcortical structure with broad cortical functional interactions and a key role in neurocognitive functioning (20). We reported that, in the absence of task-based activation, DUP is associated with overall reduced functional connectivity between the striatum and central executive regions of the cortex, which are important for maintaining and manipulating information and goal-directed behavior. These findings also statistically mediated the negative clinical relationship between DUP and treatment response, shedding light on the mechanism responsible for the DUP-negative outcome association (15). While this work coincided with studies of treatment response that implicate corticostriatal systems in the efficacy of antipsychotic treatment (16–19), it did not address whether DUP-dependent variation exists in neural engagement during executive processing.
Here, we examined the relationship between DUP and working memory (WM) in a cohort of patients with FEP to further explore the neural mechanism associated with untreated psychosis. We focused on maintenance of WM, which has been shown to be a fundamental component of effective executive functioning that reliably activates the executive network (20). Substantial evidence implicates impairments in WM activation, primarily within the dorsolateral prefrontal cortex (DLPFC) in schizophrenia (21–25). However, to date, no study has directly assessed DUP in relation to neuroimaging studies of WM. In this study, we first explored whether DUP relates to WM activation of the DLPFC, and then examined whether frontostriatal connectivity during WM is related to DUP. Consistent with previous findings, we hypothesized that DUP is associated with variation in frontostriatal interactions during WM engagement.
Methods and Materials
Participants
Thirty-seven FEP patients were included in this study, recruited from clinical services at the University of Pittsburgh Medical Center. Patients were between the ages of 12 and 40 and diagnosed with a first episode of a psychotic disorder, including schizophrenia (N=19), schizophreniform disorder (N=6), schizoaffective disorder (N=5), or psychotic disorder, not otherwise specified (N=7). Diagnoses were determined based on consensus discussions of a Structured Clinical Interview for Axis I Diagnostic and Statistical Manual-IV at baseline, as well as follow-up diagnostic interviews six months after baseline evaluations. Clinical interviews were supplemented by information from clinical providers and family members. In the seven individuals with psychotic disorder, not otherwise specified, a schizophrenia spectrum-diagnosis was not given due to either insufficient time criteria (N=3), incomplete ‘criterion A’ psychotic symptoms (N=2), or sub-threshold social/occupational dysfunction (N=2). We did not include individuals with concurrent mood-related diagnoses, to ensure that our patients were more likely to have a schizophrenia-spectrum disorder and not an affective psychotic disorder. Additional assessments were made to rule out a diagnosis of a substance-induced psychotic disorder, as well as concurrent substance abuse or dependence. For all participants, any substance use during the evaluation period, including at time of scanning, was documented by clinical research staff. Exclusion criteria included: medical illness affecting the central nervous system function, intelligence quotient lower than 75 (determined by the Wechsler Abbreviated Scale of Intelligence (26)), or contraindications to magnetic resonance scanning. Clinical ratings were administered at time of study entry via the Brief Psychiatric Rating Scale (BPRS)(27).
Patients underwent treatment as per routine clinical care. Eleven patients were naïve to antipsychotic treatment at time of scanning and the remaining twenty-six patients had been treated for less than two months with antipsychotic drugs, including risperidone (N=16), olanzapine (N=6), aripiprazole (N=1), quetiapine (N=1), and ziprasidone (N=1). Chlorpromazine equivalents of antipsychotic medication dose at time of scanning were calculated to account for possible drug effects on imaging data (28).
Thirty-three of our FEP participants overlap with the cohort examined in previous work (25), which was focused on group differences in WM activation and performance. The present study was not interested in group differences in WM activation. We also did not include participants from this prior work with a mood disorder diagnoses since our focus on DUP in FEP patients likely to have a schizophrenia-spectrum diagnosis. We also included a cohort of twenty-five healthy volunteers (HV) to establish a reference range of normal values for our neuroimaging measures. Healthy participants had no history of a major psychiatric disorder or antipsychotic treatment, no first-degree relatives with history of a psychotic disorder, no neurological disorder, no history of head trauma, and no intellectual impairment as defined by the DSM-IV. All FEP or HV participants or their legal guardians provided written informed consent after study procedures were discussed. Comprehensive demographic information was collected for each participant, including parental socioeconomic status (SES) via the Hollingshead scale (29). All study procedures were approved by the University of Pittsburgh Institutional Review Board.
Length of DUP was defined as the time from the emergence of psychotic symptoms to the initiation of treatment with antipsychotic drugs or the date of scanning for individuals who were treatment naïve. DUP was determined based on clinical records and from structured interviews with the study participants and their families. Measures of DUP were quantified in days and, consistent with prior work, common-log transformed for use as a continuous variable and to account for the skewed distribution of raw DUP values (15). No outlying data points were observed, and Shapiro-Wilks testing confirmed normality of our log-transformed DUP.
WM Task
A description of our task is provided in prior work (25). Briefly, patients underwent fMRI scanning while performing two runs of a six-minute, event-related, spatial WM task diagrammed in Figure 1. Patients were instructed to remember the color of one circle (low load) or the colors of three circles (high load). Each trial consisted of a cue, 700–1400 milliseconds (ms) in length, during which the WM event was presented (encoding phase); a delay period of either one- or three-seconds duration (maintenance phase); and a probe, presented for up to 2 seconds, while the patient indicated via a button press whether a color change occurred (retrieval phase). Subjects completed 64 full trials within the total 12 minutes of data acquisition. The task included 32 “catch” trials of either the cue alone, or cue-and-delay periods, which were used to estimate the task specific hemodynamic response. The number of correct responses and reaction time of correct responses were used to assess WM.
Figure 1. WM Task.
Subjects completed two runs of a 6-minute event-related visuospatial WM task during fMRI acquisition. (Left) Subjects were instructed to remember the color of one (low load) or three (high load) circles on one side of the screen (indicated by an arrow). After a variable delay period, subjects were again presented with colored circles and asked to indicate whether a color change occurred. (Right) An additional 32 partial “catch” trials with either the cue alone (top) or cue and delay (bottom) periods were included.
Image Acquisition
Imaging data were acquired on a 3.0 Tesla Siemens TIM Trio scanner at the University of Pittsburgh Medical Center. Structural images were collected with a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with a voxel size of 1 mm3, and 176 total slices. Parameters for the MPRAGE included the following: 2530 ms TR, 1260 ms TI, multi-echo TE (TE1= 1.74 ms, TE2= 3.6 ms, TE3= 5.46 ms, TE4= 7.32 ms), and a 7° flip angle. Functional images were acquired using a multiband echo-planar sequence sensitive to bold oxygen level-dependent (BOLD) images. Parameters consisted of: TR/TE: 1000/30 ms, flip angle: 55°, voxel size: 2.3 × 2.3 × 2.3 mm in-plane resolution, 60 contiguous axial slices, 360 TRs. Additionally, a high-resolution spin echo sequence was collected with 60 total slices, a TR of 5040 ms, TE of 30 ms, 55° flip angle, and a 220 × 220 × 138 mm FOV.
Image analysis and Preprocessing
Standard preprocessing was performed with tools from AFNI (https://afni.nimh.nih.gov) and FSL (http://www.fmrib.ox.ac.uk). Slice-timing correction and motion correction were performed simultaneously using NIPy (http://nipy.org). Functional images were registered to MNI152 space with affine (FSL FLIRT) and nonlinear (FSL FNIRT) transformations. Field warping on images were applied with FSL FUGUE to correct for spatial distortion. Wavelet despiking was performed with the Brain Wavelet Toolbox (http://www.brainwavelet.org) to remove gross motion confounds (30). Images were spatially smoothed with a 5mm full width at half maximum (FWHM) Gaussian kernel. High-pass filtering at 100 volumes and grand median intensity normalization (10000/global median) were performed to rescale images. Volumes with a framewise displacement (FD) value > 0.9 and/or DVARS > 20 were removed from the analysis to reduce motion related artifacts. Our FEP cohort displayed significantly greater movement than HV based on average FD (p= 0.02). Volumes were removed from 18 FEP individuals (1 to 74, or 0.2% to 20%, volumes removed), and from 12 individuals in our HV group (1 to 21, or 0.2% - 5.8%, volumes removed).
Frontal and Striatal Regions of Interest
In both our activation and functional connectivity analyses, we limited our search space to the DLPFC, bilaterally. A functional region of interest encompassing the DLPFC was defined, a priori, via Neurosynth (06/20/2018, (31)). The search term “dlpfc” was used to generate a reverse inference map that representing boundaries of meta-analytic activation of the DLPFC. The resulting map was used to mask our analyses (Supplemental Figure S2).
In our frontostriatal connectivity analyses described below, we defined striatal seeds, a priori, within a region of the dorsal caudate (DC) that has been shown to connect to lateral portions of the prefrontal cortex. The DC provides distinct contributions to DLPFC for executive functioning, which is supported by its frontostriatal functional connectivity (32). Regions of interest within the left and the right DC were creating based on coordinates used in prior functional connectivity studies that have demonstrated functional interactions between striatum and the DLPFC (15, 33). Spheres were drawn with radius of 2 mm around central voxels (x = ±13, y = 15, z = 9).
WM Activation
To examine WM activation within the DLPFC as a function of task phase at each load, a first-level general linear model (GLM) (2×3) was constructed for each patient. Task phase (encoding, maintenance, retrieval) for each load (low, high) and incorrect task trials at each of the three task phases were modeled as regressors. All regressors were convolved with a double-gamma hemodynamic response function. Individual maps of parameter estimates were created for six contrasts of interest: (1) encoding low load > baseline, (2) encoding high load > baseline, (3) maintenance low load > baseline, (4) maintenance high load > baseline, (5) retrieval low load > baseline, and (6) retrieval high load > baseline. At the group level, we examined activation of all 6 contrasts independently with GLMs that included DUP as a covariate, and also included age and sex as regressors.
Functional connectivity analyses
To examine frontostriatal connectivity during maintenance of WM, psychophysiological interaction (PPI) analyses were conducted (34). The PPI method allows for the measurement of task-specific functional connectivity between activity in separate brain regions. Typically, the time course of a seed ROI is examined along with a task-specific phase to identify regions whose activity depends on an interaction between psychological factors (the task-specific context) and physiological factors (the time course of the seed ROI). The scope of our PPI analyses was limited to maintenance of WM, given the robust characterization of activation deficits within the DLPFC during this phase in schizophrenia (35). First-level PPI analyses consisted of GLMs with the time series from the left or the right DC used as physiological regressor, along with 9 task-based psychological regressors and 2 PPI regressors, one for each WM load. Group analyses were performed to examine DUP in relation to striatal connectivity for both low and high WM loads, along with age and sex, included as explanatory variables.
DLPFC analysis and Statistical Testing
Significance was defined in our main activation and connectivity analyses by a voxel-wise threshold of p <0.005, and familywise error correction at p < 0.05. AFNI’s 3dFWHMx was used to estimate the amount of smoothing present using a spatial autocorrelation function. The resulting values were entered into 3dClustSim to determine, with 10,000 iterations, the number of contiguous voxels needed for small volume correction within our DLFC region on interest at p<0.05. The resulting cluster size was 9 voxels.
Results
Participant Characteristics and WM Performance
Demographic and clinical information for all participants is in Table 1. The median DUP of our cohort of participants was 365 days (Supplemental Figure S2). The mean dose of antipsychotic treatment at time of scanning in chlorpromazine equivalents was 148.62 mg. Average WM accuracy during the low and high loads of the task in the FEP group was 89% and 82%, and reaction times were reaction times were 1,036 ms and 1,046 ms, respectively. Consistent with prior work that includes a sub-set of our study cohort, HV participants showed significantly higher accuracy (load 1: p < 0.009, load 3: p < 0.005) and lower reaction time (load 1: p < 0.01957, load 3: p < 0.004263) than in our FEP group (25). We observed no relationship between accuracy or reaction time in relation to DUP.
Table 1.
Baseline Demographics and Clinical Ratings.
| N = 37 | |
|---|---|
| Mean age in years (±SD) | 22.25 (5.065) |
| # right handed (%) | 29 (83%) |
| # female (%) | 11 (32) |
| Mean WASI IQ (±SD) | 105.4 (13.21) |
| Mean parental SES (±SD) | 40.1 (13.9) |
| # antipsychotic naïve | 12 |
| CPZ equivalents (mg) | 149 mg |
| BPRS Total symptoms (±SD) | 46.29 (7.9) |
| BPRS Positive symptoms (±SD) | 13.48 (3.6) |
| BPRS Negative symptoms (±SD) | 6.89 (2.5) |
| Median DUP (days) | 365 |
WM Activation
Whole-brain, confirmatory analyses were performed to examine WM activation patterns relative to prior studies for validation of our task (25). Activation of canonical WM regions by our task was validated and confirmed by a group-level examination of each condition (Supplemental Figure S3). We then examined whether there was a relationship between DUP and activation within the DLPFC in each phase of the task. No significant association was found at our designated threshold (p <0.05, corrected).
Frontostriatal connectivity
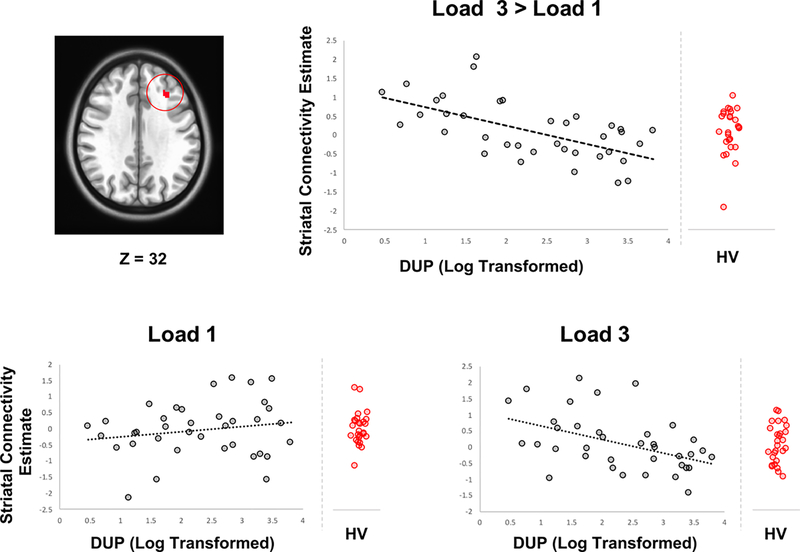
In addition to activation, the relationship between DUP and engagement of frontostriatal circuits during WM maintenance for each task-load was examined via PPI analyses. The time series from seed regions in the left and right DC known to functionally link to the DLPFC were included as physiologic regressors. The interaction between this regressor and the task conditions were assessed along with differences in connectivity between WM loads. During maintenance of the lowest WM load, no significant relationship was observed between striatal connectivity with DLPFC and DUP. In the direct comparison of low versus high load maintenance, longer DUP was associated with less frontostriatal functional connectivity strength between the left DC and a cluster of 25 voxels located in the rostral portion of the DLPFC in Brodmann area 9 (Figure 2). Connectivity estimates of maintenance of higher WM load, by itself, also revealed a negative correlation within the same DFLPC cluster (Figure 2). Estimates from the nonsignificant lower load are displayed in Figure 2 for comparison. Ranges of connectivity in our matched HV group are also displayed for comparison (Figure 2). No significant differences in connectivity were observed between FEP of HV participants with average FD as a covariate to account for differences in motion between these groups.
Figure 2. Frontostriatal Connectivity during WM Maintenance.
Upper panel shows connectivity estimates between the DC in relation to DUP (log transformed) and a cluster of 25 voxels within the DLPFC with a peak at x=−31, y=29, z=32 (Montreal Neurological Institute coordinates). Lower panel shows DUP and connectivity estimates with low and higher WM load. Reference ranges for connectivity from a matched HV group are displayed.
In post-hoc analyses, functional connectivity estimates were extracted from our significant cluster for both low and high loads and examined in relation to clinical symptoms (total symptoms, negative symptoms, and positive symptoms), WM accuracy, WM reaction time, and medication exposure. No performance-related measures (accuracy and reaction time) correlated with frontostriatal connectivity during both WM loads. Consistent with prior studies (15, 36), the positive symptom sub-score of the BPRS was not related to functional connectivity (P < 0.008, Bonferroni corrected). Similarly, connectivity at each WM load was not significantly related to the negative symptoms sub-score, total psychopathology score, and medication exposure.
Additional post-hoc tests on extracted values were performed to confirm that our connectivity results remain significant when accounting the following confounding factors in regression analyses: medication status (naive vs. prior exposure), diagnosis of psychotic disorder, not otherwise specified, and parental SES.
Discussion
We examined whether DUP is related to visuospatial WM activation within the DLPFC, and functional connectivity between the dorsal striatum and the DLPFC during WM maintenance. No significant association was found between DUP and WM performance measures. Similarly, no significant relationships between DUP and WM activation were noted for either low or high WM loads across all task conditions. As hypothesized, DUP was associated with differential engagement of frontostriatal circuitry during maintenance WM that was specific to higher WM load. In addition, the range of connectivity values in our FEP cohort was indistinguishable from a reference range observed in matched HV. These results are the first to demonstrate variation in frontostriatal connectivity during WM in relation to DUP and contribute to our understanding of the mechanisms associated with untreated psychosis.
Numerous studies have described abnormal activation of the DLPFC during WM in schizophrenia, including in patients with FEP (21–25). While some studies have shown a relationship between neurocognitive deficit and longer DUP (7), prior work has also found evidence for preserved cognition in patients with longer DUP (13, 37). In the present report, no significant relationship was observed between DUP and either WM performance or activation within the DLPFC. Our results are consistent with the sum of this literature that demonstrates no overall relationship between DUP and neurocognitive measures (10, 14).
In light of preserved WM activation and neurocognition, the neurobiological mechanism associated with DUP remains elusive. Differences secondary to untreated psychosis may exist in how the DLPFC is engaged at the level of large-scale functional interactions, in particular, between the striatum and cortical regions important for executive functioning. The DC has been shown to have specialized functional relationships with dorsolateral prefrontal regions (32, 33, 38). In a previous study, variation in intrinsic striatal connectivity was found with central executive regions in a large cohort of patients with FEP, a finding that also mediated the relationship between DUP and poor treatment outcome (15). However, this previous work did not directly activate the executive network with a cognitive task. Results of the present study support the finding of DUP-associated variation in frontostriatal functional connectivity with task- based neural engagement. We observed that DUP length is negatively associated with functional connectivity during WM maintenance between the DC and the DLPFC. These results do not deviate from a reference range of connectivity values observed in a matched HV group. Overall, it is unclear whether our functional connectivity findings are a result of a causal relationship with untreated psychosis, or if they represent a trait-related marker present in subsets of patients with longer DUP.
Variation in DUP-related frontostriatal connectivity during WM maintenance may be driven by a mechanism mediated by imbalances in dopamine between the striatum and the prefrontal cortex. Patients with a longer DUP demonstrate treatment resistance to antipsychotic medications (2, 4), which by itself has been associated with normal levels of dopamine in the striatum (39). Our results suggest that a longer DUP may disrupt and alter frontostriatal systems leading to decreased engagement of functional circuits with increasing cognitive demand. The unique specificity of our findings at higher WM load may coincide with evidence suggesting a blunting of normal inverted-U shaped cortical functioning (22, 40). Furthermore, this decreased connectivity with higher WM load may reflect insufficient dopamine release (41), abnormal dopamine D1 signaling (42), or increased glutamatergic tone within the DLPFC secondary to prolonged disruptions in corticostriatal dopamine functioning (43). Future studies are needed to disentangle whether individuals with prolonged psychosis represent a distinct subgroup of patients with unique neurophysiological characteristics, or if prolonged DUP causes alterations in cortico-subcortical cognitive systems.
Our findings also highlight normal functional relationships between the striatal and prefrontal cortex during WM. The striatum has been hypothesized to dynamically gate and update representations maintained in prefrontal regions during WM (44). Recent evidence supports this theory and implicates adequate striatal gating for WM efficiency (45, 46). Our finding of decreased frontostriatal connectivity during higher WM load may be in response to a dopamine-mediated abnormality in selective gating for manipulation and maintenance of information (20, 47). Intact frontostriatal links may also be important for WM functioning given its interplay with reinforcement learning mechanisms associated with the ventral striatum (48). Reward functioning and related recruitment of the striatum and prefrontal cortex have been shown to be blunted in patients with schizophrenia (49, 50). Untreated psychosis may affect reward processing and broadly contribute to impairments in goal-oriented behavior and problem solving. Further investigation is necessary to deconstruct WM processing in the context of frontostriatal links in early-course schizophrenia, as well as how untreated illness affects WM processing in relation to poorer functional recovery.
One important limitation of this study is the lack of longitudinal follow-up assessments. The present study represents a cross-sectional examination of WM in patients with FEP. Longer DUP has been associated with poorer treatment response and functional outcomes (2–5). It is unknown whether our results mediate response to treatment and contribute to long-term social and occupational functioning. Future prospective neuroimaging studies are required to examine the connections between WM processing, DUP, and clinical trajectories. Another limitation of this study is the relatively small cohort of patients examined. While we took steps to minimize the chances of false positive results via the section of a priori regions within the striatum and DLPFC, careful correction for multiple comparisons, and post-hoc analyses, a larger cohort may reveal smaller effects in both activation and connectivity during WM. A larger cohort, followed longitudinally, will also be necessary to evaluate whether DUP uniquely impacts frontostriatal connectivity in individuals with psychotic disorder, not otherwise specified. This group may represent sub-cohort of FEP patients with less severe illness or evolving schizophrenia-spectrum diagnoses. Replication of our findings in future work will also be important considering our limited statistical power. It remains unknown how untreated psychosis impacts normal neural development. Future work in larger adolescent cohorts may allow for the examination of DUP in the context of normal developmental of neurocognitive systems (51).
The findings described here contribute to our understanding of the neural mechanism associated with untreated psychosis. We provide evidence that while DUP length is not significantly associated with WM performance and activation, it does show an important relationship with functional connectivity between the striatum and prefrontal cortex during maintenance of increasing WM load. These results may represent frontostriatal abnormalities in response to detrimental effects of untreated psychosis or a trait-related mechanism that distinguishes patients with significantly longer DUP. Future directions include further deconstruction of WM in the context of untreated illness and treatment-related outcomes in individuals with FEP.
Supplementary Material
Acknowledgements
The project described was supported by a NARSAD Young Investigator Award by the Brain & Behavior Research Foundation (Deepak Sarpal, MD); and the National Institutes of Health through grants: K23MH110661 (Deepak Sarpal, MD, PI), K01 MH112774 (Maria Jalbrzikowski, PhD, PI), P50 MH103204, Conte Center for Translational Mental Health Research (David A. Lewis, MD, Director), and the UL1 TR001857 funded Clinical and Translational Science Institute of the University of Pittsburgh (Steven E. Reis, MD, PI). We thank: the faculty and staff of the WPIC Psychosis Recruitment and Assessment Core for their assistance in diagnostic and psychopathological assessments; Dean Salisbury, Ph.D., and Raymond Cho, M.D., and Carl Olson, Ph.D. for assistance in task development, and finally; and our patients and their families.
Disclosures
The authors have no disclosures to report.
References
- 1.Addington J, Heinssen RK, Robinson DG, Schooler NR, Marcy P, Brunette MF, et al. (2015): Duration of Untreated Psychosis in Community Treatment Settings in the United States. Psychiatr Serv. 66:753–756. [DOI] [PubMed] [Google Scholar]
- 2.Penttila M, Jaaskelainen E, Hirvonen N, Isohanni M, Miettunen J (2014): Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 205:88–94. [DOI] [PubMed] [Google Scholar]
- 3.Loebel AD, Lieberman JA, Alvir JM, Mayerhoff DI, Geisler SH, Szymanski SR (1992): Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry. 149:1183–1188. [DOI] [PubMed] [Google Scholar]
- 4.Perkins D, Lieberman J, Gu H, Tohen M, McEvoy J, Green A, et al. (2004): Predictors of antipsychotic treatment response in patients with first-episode schizophrenia, schizoaffective and schizophreniform disorders. Br J Psychiatry. 185:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM (2004): Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 161:473–479. [DOI] [PubMed] [Google Scholar]
- 6.Srihari VH, Tek C, Pollard J, Zimmet S, Keat J, Cahill JD, et al. (2014): Reducing the duration of untreated psychosis and its impact in the U.S.: the STEP-ED study. BMC Psychiatry. 14:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lappin JM, Morgan K, Morgan C, Hutchison G, Chitnis X, Suckling J, et al. (2006): Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 83:145–153. [DOI] [PubMed] [Google Scholar]
- 8.Malla AK, Mittal C, Lee M, Scholten DJ, Assis L, Norman RM (2002): Computed tomography of the brain morphology of patients with first-episode schizophrenic psychosis. J Psychiatry Neurosci. 27:350–358. [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, Gonzalez-Blanch C, Perez-Iglesias R, Gutierrez A, et al. (2007): Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res. 91:87–96. [DOI] [PubMed] [Google Scholar]
- 10.Rund BR (2014): Does active psychosis cause neurobiological pathology? A critical review of the neurotoxicity hypothesis. Psychol Med. 44:1577–1590. [DOI] [PubMed] [Google Scholar]
- 11.Ho BC, Alicata D, Ward J, Moser DJ, O’Leary DS, Arndt S, et al. (2003): Untreated initial psychosis: relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry. 160:142–148. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KK, Rodrigues M, Mann K, Voineskos A, Mulsant BH, George TP, et al. (2015): Minimal evidence that untreated psychosis damages brain structures: a systematic review. Schizophr Res. 162:222–233. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg TE, Burdick KE, McCormack J, Napolitano B, Patel RC, Sevy SM, et al. (2009): Lack of an inverse relationship between duration of untreated psychosis and cognitive function in first episode schizophrenia. Schizophr Res. 107:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bora E, Yalincetin B, Akdede BB, Alptekin K (2018): Duration of untreated psychosis and neurocognition in first-episode psychosis: A meta-analysis. Schizophr Res. 193:3–10. [DOI] [PubMed] [Google Scholar]
- 15.Sarpal DK, Robinson DG, Fales C, Lencz T, Argyelan M, Karlsgodt KH, et al. (2017): Relationship between Duration of Untreated Psychosis and Intrinsic Corticostriatal Connectivity in Patients with Early Phase Schizophrenia. Neuropsychopharmacology. 42:2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. (2015): Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E, et al. (2016): Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophr Bull. 42:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL (2009): Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 34:2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. (2016): Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. Am J Psychiatry. 173:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Esposito M, Postle BR (2015): The cognitive neuroscience of working memory. Annu Rev Psychol. 66:115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A 3rd, Noll DC, et al. (2001): Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 58:280–288. [DOI] [PubMed] [Google Scholar]
- 22.Van Snellenberg JX, Girgis RR, Horga G, van de Giessen E, Slifstein M, Ojeil N, et al. (2016): Mechanisms of Working Memory Impairment in Schizophrenia. Biol Psychiatry. 80:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD (2009): Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 108:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalbrzikowski M, Murty VP, Stan PL, Saifullan J, Simmonds D, Foran W, et al. (2017): Differentiating between clinical and behavioral phenotypes in first-episode psychosis during maintenance of visuospatial working memory. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company. [Google Scholar]
- 27.Woerner MG, Mannuzza S, Kane JM (1988): Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 24:112–117. [PubMed] [Google Scholar]
- 28.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010): Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.<j/>Hollingshead A, and Redlich F. (1958): Social Class and Mental Illness. New York: John Wiley and Sons. [Google Scholar]
- 30.Patel AX, Kundu P, Rubinov M, Jones PS, Vertes PE, Ersche KD, et al. (2014): A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011): Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauli WM, O’Reilly RC, Yarkoni T, Wager TD (2016): Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci U S A. 113:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. (2008): Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- 34.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H (2012): Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anticevic A, Repovs G, Barch DM (2013): Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 39:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins DO, Gu H, Boteva K, Lieberman JA (2005): Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 162:1785–1804. [DOI] [PubMed] [Google Scholar]
- 37.Norman RM, Townsend L, Malla AK (2001): Duration of untreated psychosis and cognitive functioning in first-episode patients. Br J Psychiatry. 179:340–345. [DOI] [PubMed] [Google Scholar]
- 38.Verstynen TD, Badre D, Jarbo K, Schneider W (2012): Microstructural organizational patterns in the human corticostriatal system. J Neurophysiol. 107:2984–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD (2012): Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 169:1203–1210. [DOI] [PubMed] [Google Scholar]
- 40.Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. (2009): Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 35:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. (2015): Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roffman JL, Tanner AS, Eryilmaz H, Rodriguez-Thompson A, Silverstein NJ, Ho NF, et al. (2016): Dopamine D1 signaling organizes network dynamics underlying working memory. Sci Adv. 2:e1501672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. (2013): Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 18:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazy TE, Frank MJ, O’Reilly RC (2006): Banishing the homunculus: making working memory work. Neuroscience. 139:105–118. [DOI] [PubMed] [Google Scholar]
- 45.Chatham CH, Frank MJ, Badre D (2014): Corticostriatal output gating during selection from working memory. Neuron. 81:930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murty VP, Sambataro F, Radulescu E, Altamura M, Iudicello J, Zoltick B, et al. (2011): Selective updating of working memory content modulates meso-cortico-striatal activity. Neuroimage. 57:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloemendaal M, van Schouwenburg MR, Miyakawa A, Aarts E, D’Esposito M, Cools R (2015): Dopaminergic modulation of distracter-resistance and prefrontal delay period signal. Psychopharmacology (Berl). 232:1061–1070. [DOI] [PubMed] [Google Scholar]
- 48.Collins AGE, Ciullo B, Frank MJ, Badre D (2017): Working Memory Load Strengthens Reward Prediction Errors. J Neurosci. 37:4332–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, et al. (2015): Ventral Striatal Activation During Reward Processing in Psychosis: A Neurofunctional Meta-Analysis. JAMA Psychiatry. 72:1243–1251. [DOI] [PubMed] [Google Scholar]
- 50.Ermakova AO, Knolle F, Justicia A, Bullmore ET, Jones PB, Robbins TW, et al. (2018): Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geier CF, Garver K, Terwilliger R, Luna B (2009): Development of working memory maintenance. J Neurophysiol. 101:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.