Abstract
Objectives
Pulsus paradoxus is one of the few objective, bedside measures of acute asthma exacerbation severity but is difficult to measure in tachypneic and tachycardic patients and in noisy clinical environments. Our primary objective was to examine whether pulse oximeter plethysmograph-estimate-of-pulsus-paradoxus (PEP) is associated with physiologic and symptom measures of acute exacerbation severity (airway resistance by impulse oscillometry [%IOS] and the Acute Asthma Intensity Research Score [AAIRS]). Secondary objectives were to validate the previous association of PEP with %FEV1 and to examine associations of change of PEP with change of these outcomes after 2 hours of treatment.
Methods
This was a secondary analysis of data from a prospective observational study of patients aged 5–17 years with acute asthma exacerbations. The predictor variable, PEP, was measured using a dedicated pulse oximeter and waveform analysis program. Outcome measures included the AAIRS, %IOS and %FEV1 at baseline and after 2-hours of treatment. We examined associations of PEP with %IOS and the AAIRS at baseline using multiple linear regression models adjusted for age, gender and race. As secondary analyses we similarly examined the association of PEP with %FEV1 at baseline and change of PEP with change of %IOS, the AAIRS, and %FEV1 after 2-hours of treatment using multiple linear regression models adjusted for the baseline value of the outcome measure and the AAIRS.
Results
Amongst 684 participants (61% males; 61% African-American) there were associations of baseline PEP with %IOS, the AAIRS, and %FEV1 (P < 0.001). Change of PEP after 2-hours of treatment was associated with change of %FEV1 (P < 0.001) and change of the AAIRS (P = 0.01) but not with change of %IOS (P = 0.60).
Conclusions
PEP demonstrates criterion validity in predicting baseline %IOS, the AAIRS, %FEV1 and responsiveness to change of the AAIRS and %FEV1. Data contained in the oximeter plethysmograph waveform might be utilized as a continuous, objective measure of acute asthma exacerbation severity and real-time response to treatment.
Introduction
Asthma is one of the most prevalent chronic diseases of childhood, the most common reason for hospitalization of children in North America, and a frequent reason for emergency department (ED) visits for children and adults.1–4 However, there are limited objective and continuous measures of acute asthma exacerbation severity available to guide treatment and disposition decisions in acute care environments such as the ED.5,6
Pulsus paradoxus is one of the few objective bedside measures for assessment of exacerbation severity and response to treatment, and expert guidelines recommend measuring this physical sign to guide management and hospitalization decisions.5–9 Pulsus paradoxus was first observed in severe asthma by Floyer (1850) and carefully described by Kussmaul (1873) in a patient with pericardial effusion as a pulse that was “simultaneously slight and irregular, disappearing during inspiration and returning upon expiration.”10 However, pulsus paradoxus is infrequently measured in the clinical environment, in part because the traditional technique of manual measurement using a sphygmomanometer and stethoscope is difficult in tachypneic and tachycardic patients and in noisy clinical settings.11–14
This traditional technique of pulsus paradoxus measurement is an indirect means of estimating dynamic change of left ventricular stroke volume during the respiratory cycle, the underlying physiologic derangement that results in pulsus paradoxus.15 This physiologic derangement is most accurately represented by change of the arterial waveform rather than with manual measurement that uses a sphygmomanometer and stethoscope. However, intra-arterial catheters are invasive and infrequently used in patients with acute asthma exacerbations.
A finger arterial blood pressure (FINAP, Finapres Medical Systems, Amsterdam, The Netherlands) device has been shown to provide accurate representation of the arterial waveform. Pulsus paradoxus calculated from the FINAP correlates highly (r = 0.96) with pulsus paradoxus calculated from an intra-arterial waveform as did pulsus paradoxus measured as percentage pulse waveform decrease on inspiration (r = 0.59).16 However, the FINAPRES device is not widely available in clinical settings.
Studies from our group and others have demonstrated that the pulse oximeter plethysmograph waveform accurately represents the peripheral arterial waveform and support its use to estimate pulsus paradoxus.17–25 We have measured change in area-under-the-curve of the oximeter plethysmograph waveform during the respiratory cycle to estimate pulsus paradoxus physiology (PEP). We first examined this measure in healthy adults in whom pulsus paradoxus was induced using an airway circuit with applied inspiratory and expiratory resistance, and then in adults with obstructive airways disease during routing pulmonary function testing.21,22
We subsequently demonstrated that PEP correlates with percent-predicted forced expiratory volume in 1-second (%FEV1) and airway resistance by the interrupter technique before treatment, and change of PEP correlated with change of %FEV1 after 2 and 4 hours of treatment. This prior report included data from the first 249 of 933 children with acute asthma exacerbations recruited for a 5-year parent study to develop an asthma prediction rule.23,26 The present report includes data from participants 250 to 933. The purpose of the present report is to examine the performance of PEP as a measure of acute asthma exacerbation severity and response to treatment using data from this second portion of our cohort participants.
Objective
Our primary objective was to examine the criterion validity and responsiveness of PEP to predict the Acute Asthma Intensity Research Score (AAIRS) and percent-predicted airway resistance by impulse oscillometry (%IOS) using data from participants in the second portion of the study cohort for whom we have not previously reported PEP analyses.27–30 Secondary objectives were to examine the criterion validity of PEP to predict %FEV1 using data from these participants and to examine the responsiveness of PEP in the subsequently enrolled participants in the Acute Asthma Severity Assessment Protocol (ASAP) to predict change of each of the primary and secondary outcome measures after the first 2 hours of treatment.31
Methods
Study Design, Setting and Population
We performed a secondary analysis of data from the Acute Asthma Severity Assessment Protocol, a prospective study of pediatric patients aged 5 – 17 years with doctor-diagnosed asthma who presented to our tertiary children’s hospital pediatric emergency department (ED) with acute asthma exacerbations.31 As noted, during the recruitment period April, 2008 to February, 2013, 933 participants were enrolled. The present analyses include data from participants 250 to 933 that were not included in our previous report.23 Because race might have an independent association with the outcomes of interest, race was classified as American Indian or Alaska Native, Asian, African-American, Native Hawaiian or Pacific Islander or White, reported by the parent.
Prior to enrollment of participant 740 we acquired a MasterScreen IOS system (CareFusion, Yorba Linda, CA) for measurement of airway resistance by impulse oscillometry (IOS), and participants 740 to 933 attempted measurement of airway resistance using this device.27,28 The study was approved by our Institutional Review Board (protocol #080058), and written informed consent was obtained from each parent and written assent from each participant.
Predictor Variable
The predictor variable was PEP, measured using a Novametrix oxypleth pulse oximeter (Respironics Novametrix, Wallingford, CT) configured to output the raw, high-resolution plethysmograph waveform light signal. The signal was fed to a laptop computer via a serial cable and processed with a dedicated waveform analysis algorithm using code developed within graphical measurement and analysis software (LabVIEW 7.1, National Instruments, Austin, TX). Further processing of this signal by the software program to output PEP (measured in percent) is detailed in our previous report.21 We measured PEP at baseline before initiation of treatment with systemic corticosteroid and bronchodilators and again at 2 and 4 hours after initiation of treatment if the participant remained in the ED at that time. We have found that all clinically meaningful change of %FEV1 occurs in the first 2 hours of treatment.23 For this reason we did not include 4 hour data in analyses for this report.
Outcome Measures
We used the AAIRS and %IOS at baseline as the primary outcome measures to assess criterion validity of PEP as a measure of acute exacerbation severity.23 The AAIRS is a bedside acute asthma severity score that was available for all participants and that has been validated against %FEV1.29,30 The 7-component AAIRS includes accessory muscle (suprasternal-sternocleidomastoid, intercostal, subcostal) retractions, air entry, wheezing, oxygen saturation on room air and expiratory phase prolongation, with a total score range of 0 – 16 (16 most severe). %IOS requires tidal breathing for approximately 10 seconds and does not require the forced vital-capacity maneuver necessary for spirometry (FEV1). %IOS correlates with %FEV1 in children and has demonstrated validity and reliability as a measure of lung function in children as young as 3 years.27,32–35 The IOS system reports %IOS based on normative values for ages 3 – 17 years.32,36
FEV1 is the widely accepted criterion measure of severity of airway obstruction occurring during asthma exacerbations.8,37 Because we have previously reported the association of PEP with %FEV1 in a separate portion of our cohort, %FEV1 was a secondary outcome measure for this study.37 Each participant was instructed in performance of spirometry for %FEV1 in accordance with ATS guidelines.37,38 Additional outcome measures included change of the AAIRS, %IOS, and %FEV1 during the first 2 hours of treatment to assess responsiveness of PEP to change of exacerbation severity.
Data Analysis
We present descriptive statistics as mean (standard deviation, SD) or median [interquartile range, IQR], as appropriate. In order to assess whether our cohort was representative of the overall population of patients aged 5 – 17 years who presented to our pediatric ED with acute exacerbations, we compared the demographic characteristics or our sample to all patients admitted to the pediatric ED with a final diagnosis code of acute asthma exacerbation (ICD 493).
We examined associations of PEP with the AAIRS, %IOS, and %FEV1 before treatment using pre-specified multivariable linear regression models adjusted for age, gender and race because these demographic characteristics may confound the associations of interest. Associations of change of PEP with change of each of these outcome measures after 2 hours of treatment were examined with similar models additionally adjusted for the baseline value of the outcome measure and the AAIRS. We examined whether each model fulfilled the assumptions of multiple linear regression, including constant variance of errors and a linear relationship between predictor and outcome measures. Transformation of the outcome variables were performed, if necessary, to satisfy this assumption. We included all data, including apparent outliers, and did not adjust for multiple comparisons. Statistical significance was defined as P < 0.05.
%IOS and %FEV1 were not available for all participants, and multiple imputation would not be appropriate for these variables.39 With this in mind, we used complete-case analyses. IOS values were the least-available outcome variable (n = 168), and the total degrees of freedom (df) for the predictor variables age, gender, race, baseline AAIRS and baseline value of the outcome variable was 6 df. This would result in 28 outcomes per df for the models with the greatest number of predictor variables, satisfying the widely recognized requirement for ≥ 15 df per outcome to avoid overfitting.40
Some patients were enrolled in the study on more than one occasion and were assigned a unique participant identification number for each enrollment. We used robust standard error estimation with the patient medical record number for these participants as a cluster in order to adjust the variance in our model to account for these repeated measurements. In addition we performed a sensitivity analyses for each primary and secondary outcome measure using only data from the most recent enrollment for these participants. The most recent enrollment was chosen because these were most likely to include IOS measurement. All analyses were performed using open-source R statistical software v.3.1.2. (www.r-project.org. R Foundation for Statistical Computing, Vienna, Austria).
To assess the accuracy and completeness of our report in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD), we used the STARD checklist and generated the recommended flow diagram.41 The funding organization (National Institutes of Health) had no role in the conduct or reporting of the study.
Results
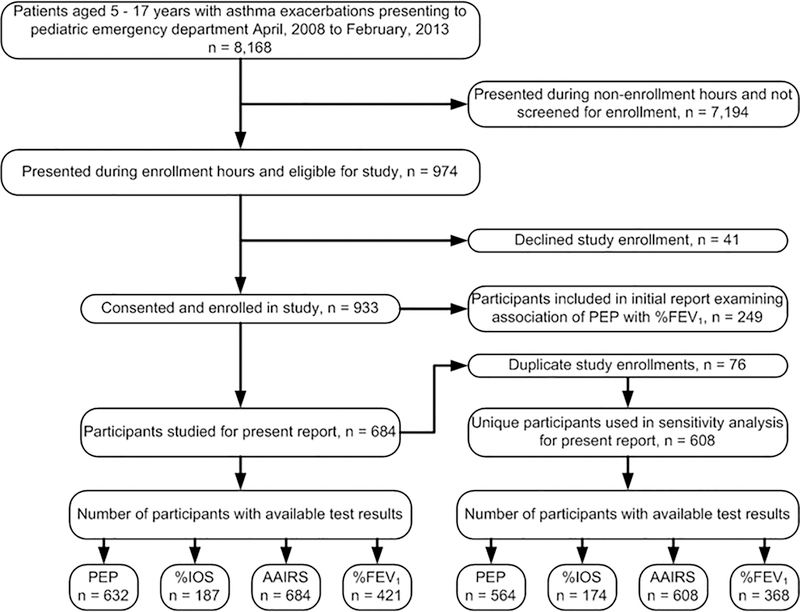
The 684 participant included in this report were enrolled during the period June, 2009 to February, 2013. (Figure 1). Demographic and asthma characteristics of the 684 study participants included for analyses are presented in Table 1. Participants were primarily in middle childhood, African-American, male, publicly insured and with high rates of chronic symptoms and poor disease control. Values for PEP and each of the outcome variables are displayed in Table 2.
Figure 1:
Participant recruitment
Abbreviations: PEP, Plethysmograph Estimate of Pulsus Paradoxus; %IOS, %-predicted airway resistance by impulse oscillometry; AAIRS, Acute Asthma Intensity Research Score; %FEV1, %-predicted forced expiratory volume in 1-second.
Table 1.
Characteristics of 684 participants and of population aged 5 – 17 years with asthma exacerbations seen in pediatric emergency department during study period.
| Characteristic | Study Participants (n = 684) | PED Asthma Patients a (n = 8,168 ) |
|---|---|---|
| Demographic | ||
| Age | 8.8 [6.9,11.2] | 7.1 [4.7, 10.3] |
| Male | 425 (61) | 5,135 (63) |
| Race | ||
| African-American | 418 (61) | 4,623 (57) |
| White | 260 (38) | 2,792 (34) |
| Asian | 5 (1) | 86 (1) |
| Medicaid insurance | 440 (64) | 5,198 (64) |
| Asthma | NA c | |
| Asthma control and future risk b | ||
| Daytime symptoms > 2/week | 347 (51) | |
| Nocturnal symptoms or awakening | 388 (57) | |
| Activity limitation | 337 (49) | |
| Need for rescue treatment > 2/week | 327 (48) | |
| Exacerbation in past year | 420 (61) | |
| Using inhaled corticosteroid | 319 (47) | |
| Second-hand smoke exposure | 225 (33) | |
| Prior PCCU asthma admission | 154 (23) | |
| Prior ETI for asthma | 31 (5) | |
Values are n (%) or median [interquartile range].
Abbreviations: PED, pediatric emergency department; PCCU, pediatric critical care unit admission; ETI, endotracheal intubation; PEP, plethysmograph estimate of pulsus paradoxus; %FEV1, %-predicted forced expiratory volume in 1-second; %IOS, airway resistance by impulse oscillometry; AAIRS, acute asthma intensity research score.
Baseline Population: all patients aged 5 to 17 years presenting to pediatric emergency department during study period with final primary diagnosis of asthma exacerbation by ICD code 493, including study sample
Global Initiative for Asthma (GINA) chronic control characteristics for preceding 3-month period.39
Table 2.
Values of PEP, %FEV1, %IOS and the AAIRS before treatment and after 2 hours of treatment
| Measure | Value [IQR] |
|
|---|---|---|
| Before treatment | After treatment | |
| PEP a | 41 [34, 48] | 38 [32, 43] |
| %IOS b | 161 [124, 212] | 127 [104, 159] |
| AAIRS c | 5 [2, 7] | 2 [0, 5] |
| %FEV1 d | 47 [34, 66] | 58 [42, 75] |
Abbreviations: PEP, plethysmograph estimate of pulsus paradoxus; %FEV1, %-predicted forced expiratory volume in 1-second; %IOS, %-predicted airway resistance by impulse oscillometry; AAIRS, acute asthma intensity research score; IQR, interquartile range.
n = 632 before and n = 462 after treatment
n = 187 before and n = 140 after treatment
n = 684 before and n = 498 after treatment
n = 421 before and n = 320 after treatment
The distribution for %IOS values at baseline was skewed. We log transformed this variable because it improved the model fitting assumptions assessed by the residual plot and normal Q-Q plot. The statistical assumptions for multiple linear regression were satisfied for each model.
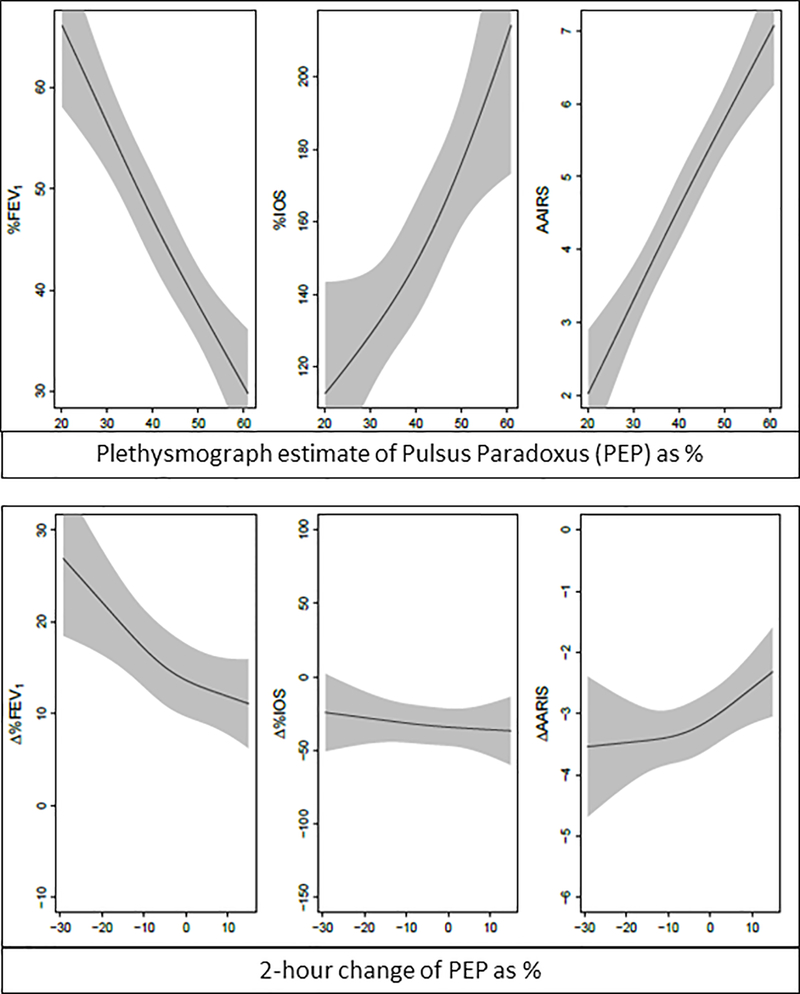
There were significant associations between PEP and each outcome of interest before treatment (P < 0.001, Table 3 and Figure 2). Adjusted R2 and 95% confidence intervals (CI) for each model are displayed in Table 4. The before treatment effect sizes are interpreted as follows. The interquartile range for PEP before treatment was 34.4% to 48.1%. Before treatment, a patient with a PEP value of 48.1% would be expected to have a %FEV1 12.1% lower than a patient with a PEP value of 34.4% after adjustment for age, gender and race.
Table 3.
Effect sizes for outcome measures associated with PEP before and after 2-hours of treatment in children aged 5 – 17 years with acute asthma exacerbations.
| Outcome measure | R2 (95% CI) Before treatment a, b | P | R2 (95% CI) After treatment c, d | P |
|---|---|---|---|---|
| %IOS | 1.2 (1.1, 1.4) | <0.001 | −3.3 (−10.4, 3.7) | 0.598 |
| AAIRS | 1.7 (1.4, 2.0) | <0.001 | 0.4 (0.1, 0.7) | 0.01 |
| %FEV1 | −12.1 (−14.9, −9.3) | <0.001 | −4.2 (−6.3, −2.0) | <0.001 |
Abbreviations: PEP, plethysmograph estimate of pulsus paradoxus; %FEV1, %-predicted forced expiratory volume in 1-second; %IOS, %-predicted airway resistance by impulse oscillometry; AAIRS, acute asthma intensity research score; CI, confidence interval.
Effect size for outcome measure across interquartile range of PEP of 34.4% to 48.1%.
Multivariable linear regression models adjusted for age, gender, race.
Effect size estimates for change of outcome measure after 2-hr of treatment across interquartile range of change of PEP (−10.7% to 1.7%) during this interval.
Multivariable linear regression models adjusted for age, gender, race, baseline AAIRS and baseline value of the outcome measure.
Figure 2:
Associations of Plethysmograph Estimate of Pulsus Paradoxus (PEP) with %-predicted FEV1 (%FEV1), %-predicted airway resistance by impulse oscillometry (%IOS), and the Acute Asthma Intensity Research Score (AAIRS) before treatment (top panel) and associations of change of PEP with change of each outcome after 2 hours of treatment (Δ%FEV1, Δ%IOS, ΔAAIRS, bottom panel). Grey bands are 95% confidence intervals.
Table 4.
Adjusted coefficients of variation (R2) for multivariable models before and after 2-hours of treatment in children aged 5 – 17 years with acute asthma exacerbations.
| Outcome Measure | R2 (95% CI) Before treatment a, b | R2 (95% CI) After treatment a, c |
|---|---|---|
| %IOS | 0.160 (0.101, 0.310) | 0.595 (0.502, 0.778) |
| AAIRS | 0.189 (0.142, 0.258) | 0.213 (0.175, 0.280) |
| %FEV1 | 0.186 (0.137, 0.273) | 0.155 (0.119, 0.261) |
Abbreviations: PEP, plethysmograph estimate of pulsus paradoxus; R2, coefficient of variation; CI, confidence interval. %IOS, %-predicted airway resistance by impulse oscillometry; AAIRS, acute asthma intensity research score; %FEV1, %-predicted forced expiratory volume in 1-second.
Values are adjusted R2 (95% CI) for each multivariable model.
Multivariable linear regression models adjusted for age, gender, race.
Multivariable linear regression models adjusted for age, gender, race, baseline AAIRS and baseline value of the outcome measure.
Whereas change of PEP after 2 hours of treatment was associated with change of %FEV1 (P < 0.001) and change of the AAIRS (P = 0.01), we did not find such an association with change of %IOS (P = 0.60). For after-treatment effect size, the interquartile range for change of PEP was −10.7% to 1.7%. A patient with an increase of PEP of 1.7% would be expected to have a 4.17% smaller change of %FEV1 and a 0.38 point greater change of the AAIRS compared to a patient with a PEP decrease of 10.75%, after adjustment for age, gender, race, baseline AAIRS and baseline %FEV1.
For the sensitivity analysis, 76 duplicate patient enrollments were excluded by using only participant data from the most recent enrollment, resulting in 608 unique participants. The adjusted associations of baseline PEP with %FEV1, %IOS and the AAIRS remained (P < 0.001) as did the 2-hour change of PEP with change of %FEV1 (P < 0.001) and the AAIRS (P = 0.017). There was again no association of change of PEP with change of %IOS (P = 0.64).
Of the 25 items included in the STARD checklist, this report conforms to 23 of the STARD items relevant to the study design.41 Figure 1 presents a flow diagram conforming to STARD recommendations.
Discussion
The results of this study suggest that PEP has criterion validity in predicting a comprehensive bedside severity score (the AAIRS) and %IOS and %FEV1 before treatment, as well as responsiveness to change of the AAIRS and %FEV1 but not to change of %IOS. In addition the results confirm that plethysmograph estimate of pulsus paradoxus (PEP) has criterion validity in predicting %FEV1 and responsiveness to change of %FEV1.
We were surprised at the low values for coefficient of variation for explained variability of each outcome measure by PEP, although this is consistent with a prior investigation by Wright and colleagues in which pulsus paradoxus measurement made using change in height of the FINAP waveform correlated with %-predicted peak expiratory flow (r = −31).25 We used the unprocessed plethysmograph waveform to capture AUC-variability during the respiratory cycle as a measure of pulsus paradoxus; a potential disadvantage of this approach was introduction of movement artifact and other sources of unwanted variability. This may have decreased the ability of PEP to explain variability of our outcomes of interest. As well, there is likely variability in measurement of %IOS, the AAIRS, and %FEV1 that may also contribute to decreased ability of PEP to statistically explain variability of these outcomes.42
There are no objective continuous measures of asthma exacerbation severity that are available at the bedside in most acute care settings, although expert panel guidelines recommend a management approach to exacerbations that is based on assessment of severity, including measurement of FEV1.8 The oximeter plethysmograph waveform might be utilized for this purpose, as it correlates with accepted measures of acute exacerbation severity.
The pulse oximeter plethysmograph waveform has been demonstrated to accurately represent the peripheral arterial waveform.17,25 As such, information in the plethysmograph waveform might be used to quantify physiologic events that can be measured using arterial waveform data, including pulsus paradoxus.
This is an evolving technology that will require further validation of PEP correlation with lung function and other severity metrics of disorders that result in elevated pulsus paradoxus. In addition, further elucidation of normative and percent-predicted values for PEP according to age, gender and other patient characteristics are next stages in developing this technology.
Although PEP does not correlate with change of %IOS, the latter may itself have limited validity as a measure of change of asthma exacerbation severity in response to treatment. Further, the AAIRS, %IOS and %FEV1 each measure different domains of exacerbation severity, and it is reasonable to consider pulsus paradoxus and PEP as measures of additional severity domains. Emergency Medicine clinicians frequently consider multiple data points of disease severity, and each of these measures may be similarly regarded as data points that assist in decision-support for treatment and disposition decisions in patients with acute asthma exacerbations. PEP might contribute to this decision-making as a continuous bedside measure of acute asthma exacerbation severity and response to treatment.
Limitations
Limitations of our study include that we have examined PEP in only one condition that results in abnormally elevated PP, acute asthma exacerbations. In addition we did not employ further signal processing to minimize waveform artifact; our aim was to use the unprocessed waveform to capture all variability during the respiratory cycle. Finally, there are potential limitations in using IOS, the AAIRS and FEV1 as outcome measures because these measures represent different domains of acute asthma exacerbations. Alternatively, this might be viewed as a study strength because the primary pathophysiological event, airway inflammation, results in complex derangements of lung function that are heterogeneous between individuals with asthma.43 Additionally, FEV1 is widely recognized as the criterion measure of airway obstruction, and IOS and the AAIRS have been demonstrated to correlate with FEV1.27–30
Conclusions
Information contained in the oximeter plethysmograph waveform (PEP) might be utilized as a continuous, objective and effort-independent measure of acute asthma exacerbation severity, responsiveness to treatment, and possibly of other pathophysiologic conditions resulting in pulsus paradoxus. This measurement warrants further validation and could be incorporated in next-generation pulse oximeters.
At a Glance.
There are limited objective bedside measures of acute asthma exacerbation severity and response to treatment.
Pulse oximeter plethysmograph estimate of pulsus paradoxus (PEP) might provide a continuous, noninvasive measure of exacerbation severity.
PEP is significantly associated with three criterion measures of exacerbation severity: airway resistance by impulse oscillometry, forced expiratory-volume in 1-second, and the Acute Asthma Intensity Research Score.
Acknowledgments
Financial support
This research was supported by the National Institutes of Health [Grant K23 HL80005] (Dr. Arnold), [NCRR UL1 RR024975] (Vanderbilt CTSA/REDCap database), and NIAID [K24 AI77930] (Dr. Hartert).
Footnotes
Potential conflict of interest
Dr. Arnold holds a patent (US 6,869,402 B2) related to the method of estimating pulsus paradoxus described in this study; he and Vanderbilt University have potential to benefit financially from this intellectual property. Ms. Wang has no conflicts of interest to disclose. Dr. Hartert has no conflicts of interest to disclose.
Prior presentation: Pediatric Academic Societies; Vancouver, BC; April, 2014.
Contributor Information
Donald H Arnold, Department of Pediatrics, Division of Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA.; Center for Asthma Research, Vanderbilt University School of Medicine, Nashville, TN, USA.
Li Wang, Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN, USA..
Tina V Hartert, Department of Medicine, Division of Allergy, Pulmonary & Critical Care Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA.; Center for Asthma Research, Vanderbilt University School of Medicine, Nashville, TN, USA.
BIBLIOGRAPHY & REFERENCES CITED
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ 2002;51:1–13. [PubMed] [Google Scholar]
- 2.Newacheck PW, Halfon N. Prevalence, impact, and trends in childhood disability due to asthma. Archives of pediatrics & adolescent medicine 2000;154:287–93. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. National health statistics reports 2011:1–14. [PubMed] [Google Scholar]
- 4.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2010. Vital and health statistics Series 10, Data from the National Health Survey 2011:1–80. [PubMed] [Google Scholar]
- 5.Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet 1988;1:873–5. [DOI] [PubMed] [Google Scholar]
- 6.Holleman DR Jr., Simel DL. Does the clinical examination predict airflow limitation? JAMA : the journal of the American Medical Association 1995;273:313–9. [PubMed] [Google Scholar]
- 7.McFadden ER Jr. Acute severe asthma. AmJRespirCrit Care Med 2003;168:740–59. [DOI] [PubMed] [Google Scholar]
- 8.National Heart L, and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Asthma Education and Prevention Program: National Institutes of Health; 2007. [Google Scholar]
- 9.Moons KGM. Diagnostic Research, Theory and Application: Erasmus Universiteit Rotterdam; 1996. [Google Scholar]
- 10.Bilchick KC, Wise RA. Paradoxical physical findings described by Kussmaul: pulsus paradoxus and Kussmaul’s sign. Lancet 2002;359:1940–2. [DOI] [PubMed] [Google Scholar]
- 11.Jay GD, Onuma K, Davis R, Chen MH, Mansell A, Steele D. Analysis of physician ability in the measurement of pulsus paradoxus by sphygmomanometry. Chest 2000;118:348–52. [DOI] [PubMed] [Google Scholar]
- 12.Galant SP, Groncy CE, Shaw KC. The value of pulsus paradoxus in assessing the child with status asthmaticus. Pediatrics 1978;61:46–51. [PubMed] [Google Scholar]
- 13.Shim C, Williams MH Jr. Pulsus paradoxus in asthma. Lancet 1978;1:530–1. [DOI] [PubMed] [Google Scholar]
- 14.Pearson MG, Spence DP, Ryland I, Harrison BD. Value of pulsus paradoxus in assessing acute severe asthma. British Thoracic Society Standards of Care Committee. BMJ 1993;307:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamzaoui O, Monnet X, Teboul JL. Pulsus paradoxus. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology 2013;42:1696–705. [DOI] [PubMed] [Google Scholar]
- 16.Steele DW, Wright RO, Lee CM, Jay GD. Continuous noninvasive determination of pulsus paradoxus: a pilot study. AcadEmergMed 1995;2:894–900. [DOI] [PubMed] [Google Scholar]
- 17.Cannesson M, Besnard C, Durand PG, Bohe J, Jacques D. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care 2005;9:R562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. ArchPediatrAdolescMed 2004;158:48–51. [DOI] [PubMed] [Google Scholar]
- 19.Wisely NA, Cook LB. Arterial flow waveforms from pulse oximetry compared with measured Doppler flow waveforms apparatus. Anaesthesia 2001;56:556–61. [DOI] [PubMed] [Google Scholar]
- 20.Rayner J, Trespalacios F, Machan J, et al. Continuous noninvasive measurement of pulsus paradoxus complements medical decision making in assessment of acute asthma severity. Chest 2006;130:754–65. [DOI] [PubMed] [Google Scholar]
- 21.Arnold DH, Spiro DM, Desmond RA, Hagood JS. Estimation of airway obstruction using oximeter plethysmograph waveform data. RespirRes 2005;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold D, LaFleur B, Spiro D, King P, Hartert T. Correlation of pulse oximeter estimated pulsus paradoxus with severity of airflow obstruction: a prospective, observational analysis. ProcAmThorSoc 2005;2:A626. [Google Scholar]
- 23.Arnold DH, Jenkins CA, Hartert TV. Noninvasive assessment of asthma severity using pulse oximeter plethysmograph estimate of pulsus paradoxus physiology. BMC pulmonary medicine 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartert TV, Wheeler AP, Sheller JR. Use of pulse oximetry to recognize severity of airflow obstruction in obstructive airway disease: correlation with pulsus paradoxus. Chest 1999;115:475–81. [DOI] [PubMed] [Google Scholar]
- 25.Wright RO, Steele DW, Santucci KA, Natarajan R, Jay GD. Continuous, noninvasive measurement of pulsus paradoxus in patients with acute asthma. ArchPediatrAdolescMed 1996;150:914–8. [DOI] [PubMed] [Google Scholar]
- 26.Arnold DH, Gebretsadik T, Moons KG, Harrell FE, Hartert TV. Development and internal validation of a pediatric acute asthma prediction rule for hospitalization. The journal of allergy and clinical immunology In practice 2015;3:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song TW, Kim KW, Kim ES, Kim KE, Sohn MH. Correlation between spirometry and impulse oscillometry in children with asthma. Acta paediatrica (Oslo, Norway : 1992) 2008;97:51–4. [DOI] [PubMed] [Google Scholar]
- 28.Vink GR, Arets HG, van der LJ, van der Ent CK. Impulse oscillometry: a measure for airway obstruction. PediatrPulmonol 2003;35:214–9. [DOI] [PubMed] [Google Scholar]
- 29.Arnold DH, Saville BR, Wang W, Hartert TV. Performance of the Acute Asthma Intensity Research Score (AAIRS) for acute asthma research protocols. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2012;109:78–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold DH, O’Connor MG, Hartert TV. Acute Asthma Intensity Research Score: updated performance characteristics for prediction of hospitalization and lung function. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2015;115:69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold DH, Gebretsadik T, Abramo TJ, Sheller JR, Resha DJ, Hartert TV. The Acute Asthma Severity Assessment Protocol (AASAP) study: objectives and methods of a study to develop an acute asthma clinical prediction rule. Emergency medicine journal : EMJ 2012;29:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology 2003;22:1026–41. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld M, Allen J, Arets BH, et al. An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Annals of the American Thoracic Society 2013;10:S1–s11. [DOI] [PubMed] [Google Scholar]
- 34.Ducharme FM, Davis GM, Ducharme GR. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest 1998;113:1322–8. [DOI] [PubMed] [Google Scholar]
- 35.Ducharme FM, Davis GM. Measurement of respiratory resistance in the emergency department: feasibility in young children with acute asthma. Chest 1997;111:1519–25. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie SA, Chan E, Dundas I, et al. Airway resistance measured by the interrupter technique: normative data for 2–10 year olds of three ethnicities. ArchDisChild 2002;87:248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. AmRevRespirDis 1991;144:1202–18. [DOI] [PubMed] [Google Scholar]
- 38.Arnold DH, Gebretsadik T, Hartert TV. Spirometry and PRAM Severity Score Changes During Pediatric Acute Asthma Exacerbation Treatment in a Pediatric Emergency Department. The Journal of asthma : official journal of the Association for the Care of Asthma 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. Journal of clinical epidemiology 2006;59:1087–91. [DOI] [PubMed] [Google Scholar]
- 40.Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 41.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. FamPract 2004;21:4–10. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor MG, Berg K, Stack LB, Arnold DH. Variability of the Acute Asthma Intensity Research Score in the pediatric emergency department. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2015;115:244–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemanske RF Jr., Busse WW. Asthma: clinical expression and molecular mechanisms. JAllergy ClinImmunol 2010;125:S95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]