Abstract
Study objective
Montelukast sodium is a leukotriene-receptor antagonist approved as a controller medication for chronic asthma and allergic rhinitis in children and adults. We sought to characterize adverse events associated with single montelukast exposures in children ages 5 – 17 years and to determine whether adverse events were dose related for all-dose and for ultra-high-dose (≥ 50 mg) exposures.
Methods
This is a retrospective analysis of data from the National Poison Data System for exposures that included montelukast in individuals aged 5 – 17 years for calendar years 2000 – 2016. Filters were applied to identify exposure events in which montelukast was the primary exposure and for which the exact or lowest-possible ingested dose was recorded. Characteristics of adverse events were examined using descriptive statistics and multivariable logistic models were used to examine whether associations of montelukast and adverse events were dose related.
Results
During the 17-year study period there were 17,069 montelukast exposures available for analyses. Patients were median [interquartile range, IQR] age 7 [5, 9] years, and 10,907 (64%) male gender. Abdominal pain was the most common adverse event (0.23%). There were 618 ultra-high-dose exposures (≥ 50 mg). These patients had median age 6 [5, 8] years, and 347 (56%) male gender. Abdominal pain was the most common adverse event (1.46%). Increasing ingested dose was associated with abdominal pain (adjusted odds ratio, aOR 1.01, 95% CI 1.01, 1.02) after adjustment for age and gender. No serious or life-threatening events were reported.
Conclusions
Single-dose exposures of montelukast up to 445 mg are rarely associated with any adverse events and are not associated with serious or life-threatening adverse events in children aged 5 – 17 years.
Keywords: Montelukast exposure, montelukast ingestion, pediatric, leukotriene receptor antagonist, overdose, adverse event
Introduction
Cysteinyl leukotrienes (CysLTs) are produced by airway inflammatory cells from arachidonic acid via the 5-lipoxygenase pathway, and contribute to the bronchospasm, eosinophil chemotaxis, mucus secretion and airway edema of acute asthma exacerbations.[1, 2, 3, 4]
Montelukast sodium is a potent leukotriene-receptor antagonist (LTRA). It is U.S. Food and Drug Administration (FDA) approved in the U.S. for relief of symptoms of perennial allergic rhinitis in patients 6 months of age and older, for prophylaxis and chronic treatment of asthma in patients 12 months of age and older and for prevention of exercise-induced bronchoconstriction in patients 6 years of age and older.[5] Dosing for daily use is age based, with the highest daily dose being 10 mg as used in adults >17 years.
An adult dose-ranging study of montelukast examined doses of 10 mg to 200 mg daily for 6 weeks and found no dose-related adverse events or laboratory abnormalities in comparison with placebo.[6] Further, a dose of 600 mg (200mg three times daily) for 10.3 days in adults was well tolerated with no serious adverse events.[7] In an adult pharmacokinetic and safety study, montelukast was well tolerated at single doses up to 800 mg.[8] Montelukast was well tolerated in clinical studies that included 2,751 children aged 6 months to 14 years; the most frequent adverse experiences were similar between placebo and montelukast and included fever, upper respiratory infection, worsening asthma, vomiting and diarrhea.[9] Finally, limited case series and poison control center reports of montelukast exposures in children have noted a low risk of adverse effects.[10, 11, 12]
However, in 2008 the U.S. FDA issued an alert for increased risk of suicidal thoughts and other neuropsychiatric events reported during post-marketing surveillance of LTRAs in pediatric patients, and in 2009 manufacturers of montelukast updated the Precautions section of the prescribing information to reflect this concern.[13] In response to an FDA request, a systematic review of double-blind randomized controlled trials (DBRCTs) was performed to examine the frequency of behavior-related adverse events (AEs) and included 35 adult and 11 pediatric trials of daily montelukast for asthma or rhinitis.[14] The frequency of behavior-related AEs in 11,673 patients receiving montelukast was 2.73% and in 8,827 placebo-group patients was 2.27% (OR 1.12; 95% CI 0.93 – 1.36); behavior-related AEs leading to medication discontinuation occurred in 0.07% of montelukast and 0.11% of placebo recipients (OR 0.52; 95% CI 0.17, 1.51). Again in response to an FDA request, two reviews were performed that examined suicidality-related adverse events in 20,131 patients in 116 adult and pediatric studies of daily montelukast.[15] The first examined investigator-reported suicidality-related AEs in 20,131 patients receiving montelukast and found that these events were rare and similar between montelukast and placebo. The second review included retrospective adjudication of possible suicidality-related AEs amongst 22,433 patients and identified 1 event amongst 9,929 patients taking montelukast. No completed suicide was reported in either review. Finally, a review of 14,670 World Health Organization (WHO) Individual Case Safety Reports for montelukast from the time of drug availability in 1998 to January 1, 2015 amongst individuals aged <18 years reported psychiatric events in 18% and ‘nervous system disorders’ in 8% of patients receiving daily montelukast.[16]
There is limited knowledge whether single doses exceeding those recommended for control of asthma and allergic rhinitis (4 – 10 mg) are safe and well tolerated in children and adolescents. In particular, there is limited knowledge of adverse events following ingestion of doses far exceeding recommended daily doses.
Objective
We sought to examine demographic characteristics and adverse events associated with montelukast exposures in patients aged 5 – 17 years and to examine whether adverse events are dose related. Additionally, we sought to examine adverse events after ultra-high-dose exposures (≥ 50 mg). We chose to examine this age range and to define ultra-high-dose exposures in this way because clinical trials of montelukast for acute asthma exacerbations are being planned in this age group using doses approaching 50 mg.
Importance
Understanding adverse events after ingestions of montelukast in pediatric patients is essential to acute exposure management. Moreover, knowledge of potential adverse events following single, large doses will inform the design of future clinical trials of high-dose oral montelukast for other conditions such as acute asthma exacerbations or infectious diseases.
Methods
This study was a 17-year (January 1, 2000 – December 31, 2016) retrospective analysis of data recorded by Specialists in Poison Information at U.S. Poison Control Centers on montelukast exposures among children ages 5–17 from the American Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS) database for reported exposures in individuals 5 – 17 years of age inclusive for product codes corresponding to montelukast sodium The dataset included over 70 primary variables in addition to 9 adverse event categories by organ system and over 150 individual adverse events, all of which are catalogued in a Coding Users’ Manual.
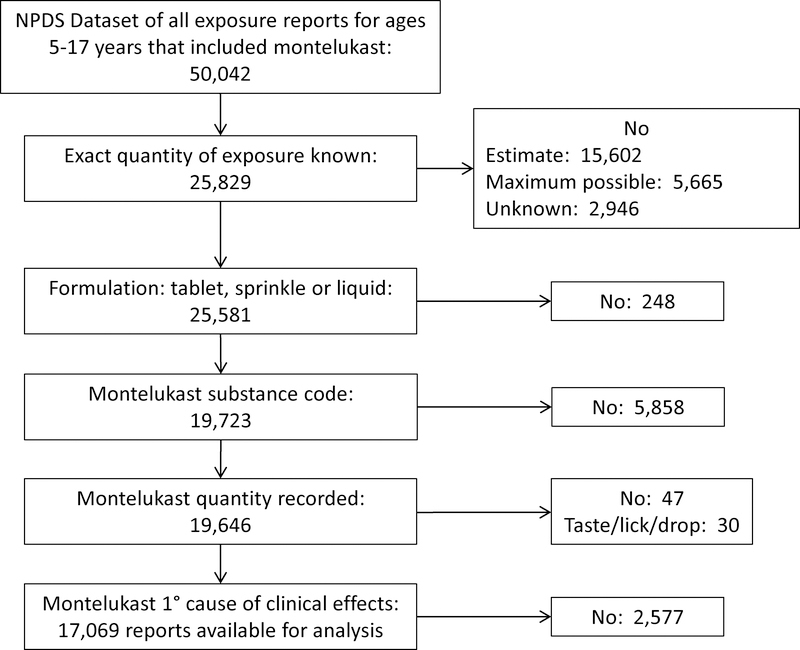
We filtered the report according to the following sequence of database variables (Figure 1). Substance Certainty, in which confidence of the substance quantity is recorded as exact, an estimate or the maximum possible ingested dose was used to exclude all scenarios in which there was not certainty of the exact ingested dose. Substance Formulation was then used to exclude all scenarios in which formulation was not solid (tablet, sprinkle) or liquid. Generic Code Name was then used to exclude all non-montelukast exposures. Substance Quantity was used to exclude scenarios in which the quantity of ingested drug was not recorded. Substance Sequence Number designates the rank of each substance relative to the individual’s adverse events, and was then used to exclude scenarios in which montelukast was not designated as the primary cause of adverse events. Using Substance Quantity and Substance Quantity Unit, we then calculated the ingested amount of montelukast in milligrams. If the number of tablets was specified but the size of tablet was not, we assumed that the tablet size was 4 mg in order to calculate the lowest dose ingested. Scenarios in which quantity was designated as “taste/lick/drop” were excluded from further analysis. This decision was based on the desire to identify the lowest dose of montelukast that resulted in adverse events. Finally, we did not impute missing data.[17] Finally, for analysis of ultra-high-dose montelukast exposures of ≥ 50 mg, we created a second dataset that included only these exposures.
Figure 1.
Flow chart indicating filtering of National Poison Data System (NPDS) dataset for exposure reports that included montelukast in pediatric patients aged 5 – 17 years.
Analytic Approach
We used descriptive statistics to present demographic characteristics and adverse events, termed clinical effects by the NPDS, after montelukast exposures in pediatric patients aged 5 to 17 years inclusive. Continuous data are presented as median [interquartile range, IQR] for non-normally distributed variables and as mean (standard deviation, SD) for normally distributed variables. Categorical variables are presented as percentages. The NPDS registry includes diverse and comprehensive adverse events by body organ system. Many of these adverse events are clearly not relevant to montelukast exposure (e.g., corneal abrasion) and for this reason were not included in data analyses. This report includes the 22 items of The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.[18] The study protocol was reviewed and approved by our Institutional Review Board (protocol #170484).
Because adverse events were rare, multivariable models to examine whether these outcomes were associated with individual characteristics could include a very limited number of explanatory variables to avoid overfitting.[19] With this limitation in mind, we examined whether there was an association of adverse events only for the outcome abdominal pain.
Results
During the 17-year study period, there were a total of 50,042 reported exposures among children aged 5–17 years. The exact amount of ingested montelukast was known and considered to be the primary cause of any adverse events in 17, 069 of these children. (Figure 1). Amongst these 17,069 reports, age was 7 [5, 9] years, 10,907 (64%) were male gender, 3% of exposures were intentional, and 96% were managed outside a healthcare facility (Table 1). Most children were treated and released with no adverse effects or rare moderate (0.42%) or major (0.22%) adverse effects. No patient required cardiopulmonary resuscitation, and there were no deaths.
Table 1.
Patient characteristics for reports montelukast primary exposures to NPDS for in children aged 5 – 17 years for calendar years 2000 – 2016.
| Patient characteristic | All ingested doses | Ingested doses ≥ 50mg |
|---|---|---|
| Age (median [IQR], years) | 7 [5, 9] | 6 [5, 6] |
| Male gender | 10,907 (64) | 347 (56) |
| Amount ingested | ||
| Median [IQR], mg | 8 [8, 10] | 80 [60, 112] |
| Range, mg | 4 – 445 | 50 – 445 |
| Reason for ingestion | ||
| Unintentional | 16,374 (96) | 481 (78) |
| Intentional | 513 (3) | 130 (21) |
| Management site | ||
| Non-healthcare facility | 16,312 (96) | 390 (63) |
| Patient already en route to healthcare facility | 490 (3) | 175 (28) |
| Referred to a healthcare facility | 138 (1) | 46 (7) |
| Level of care for those referred to healthcare facility | ||
| Treated, evaluated and released | 481 (77) | 170 (77) |
| Admitted to critical care unit | 7 (1) | 4 (2) |
| Admitted to noncritical care unit | 15 (2) | 7 (3) |
| Admitted to psychiatric care unit | 45 (7) | 21 (10) |
| Patient refused referral | 25 (4) | 5 (2) |
| Patient lost to follow up / left AMA | 55 (9) | 14 (6) |
| Treatments applied, | ||
| Cathartic | 5 (0.03) | 2 (0.32) |
| Charcoal, multiple dose | 0 | 0 |
| Charcoal, single dose | 19 (0.11) | 15 (2.4) |
| Cardiopulmonary resuscitation | 0 | 0 |
| Observation only | 17,069 (100) | 618 (100) |
| Medical outcome | ||
| No effect | 16,853 (99) | 283 (46) |
| Minor effect | 52 (0.30) | 42 (7) |
| Moderate effect | 71 (0.42) | 1 (7) |
| Major effect | 38 (0.22) | 0 |
| Death | 0 | 0 |
| Not followed, judged as nontoxic exposure | 6,189 (36) | 73 (12) |
| Not followed, minimal clinical effects possible | 7,610 (45) | 195 (32) |
| Clinical effect judged not due to montelukast | 202 (1) | 9 (1) |
| Clinical effect duration | ||
| Unknown | 16,853 (99) | 575 (93) |
| ≤2 hours | 52 (0.30) | 12 (2) |
| >2 hours to ≤8 hours | 71 (0.42) | 15 (2) |
| >8 hours to ≤24 hours | 38 (0.22) | 10 (2) |
| >24 hours to ≤3 days | 10 (0.06) | 3 (0.5) |
| >3 days to ≤1 week | 2 (0.01) | 3 (0.5) |
Values are n (%) unless otherwise specified.
Percent values may not sum to 100 due to rounding or because they are not mutually exclusive.
Abbreviations: AMA, against medical advice; IQR, interquartile rance; NPDS, National Poison Data System
Amongst 618 patients with exposures of ≥ 50 mg montelukast, age was 6 [5, 8] years, 347 (56%) were male, with median ingested dose 80 [60, 112] mg and range 50 – 445 mg. More (21%) of these exposures were intentional in comparison with all ingested doses, with 10% admitted to a psychiatric care unit. Fewer (63%) of these exposures were managed in a non-healthcare facility in comparison with all ingested doses, with 28% en route to a healthcare facility at the time of the NPDS report. These patients had infrequent adverse events, most of which were judged as minor (7%) or moderate (7%).
Adverse effects following these montelukast exposures were rare, with abdominal pain and vomiting representing the most frequent effects amongst all reports and amongst exposures ≥ 50mg (Table 2). Yet even these adverse events were reported in < 2% of exposures. Neither serious or life-threatening adverse events (e.g., transaminitis, cardiac arrest, renal failure) nor deaths were reported. Additionally, none of the major adverse events listed in Table 3 were reported after any of these 17,069 exposures. In a logistic multivariable regression model, there was an association between a report of abdominal pain and increasing ingested doses for all exposures (adjusted odds ratio, aOR 1.01, 95% confidence interval, CI 1.01, 1.02) and for exposures ≥ 50 mg (aOR 1.01, 95% CI 1.0, 1.02), adjusted for age and gender. There was no association of adverse events with age or gender for all exposures.
Table 2.
Adverse events reported in patients aged 5 – 17 years after montelukast primary exposures reported to NPDS for calendar years 2000 – 2016.
| Clinical effect | All ingested doses (n=17,069) | Ingested doses ≥ 50mg (n=618) |
|---|---|---|
| Abdominal pain | 40 (0.23) | 9 (1.46) |
| Vomiting | 29 (0.17) | 7 (1.13) |
| Headache | 20 (0.12) | 4 (0.65) |
| Nausea | 18 (0.11) | 5 (0.81) |
| Dizziness or vertigo | 13 (0.08) | 2 (0.32) |
| Rash | 12 (0.07) | 0 |
| Agitation / irritability | 11 (0.06) | 1 (0.16) |
| Diarrhea | 1 (0.05) | 2 (0.32) |
| Tachycardia | 6 (0.04) | 1 (0.16) |
| Hallucinations or delusions | 5 (0.03) | 0 |
| Confusion | 4 (0.02) | 0 |
| Tremor | 3 (0.02) | 0 |
| Syncope | 1 (0.01) | 0 |
| Throat irritation | 1 (0.01) | 0 |
Values are n (%) and specify those clinical effects recorded as related to the exposure to montelukast, listed in decreasing order of frequency for all ingested doses.
Abbreviation: NPDS, National Poison Data System
Table 3.
Adverse events not reported in any of 17,069 patients aged 5 – 17 years after montelukast primary exposures reported to NPDS for calendar years 2000 – 2016.
| AST or ALT elevation > 100 |
| Ataxia |
| Cardiac arrest |
| Dysrhythmia (Ventricular tachycardia/fibrillation/other) |
| Esophageal injury |
| Hyperventilation or tachypnea |
| Melena |
| Muscle weakness |
| Renal failure |
| Respiratory arrest |
| Rhabdomyolysis |
| Seizure (singe, multiple or status epilepticus) |
Abbreviation: AST, aspartate aminotransferase; ALT, alanine aminotransferase; NPDS, National Poison Data System
Discussion
Adverse events are rare after single exposures of montelukast in children aged 5 – 17 years, with no medical effects noted in 99% of all exposures and moderate effects in 7% of ultra-high-dose exposures. The most common adverse event was abdominal pain, occurring in < 1% of all exposures and in approximately 1.5% of ultra-high-dose exposures. Adverse events in our study occurred much less frequently than reported in studies of children enrolled in clinical trials of daily montelukast dosing for control of asthma and allergic rhinitis.[9] Moreover, our study provides further evidence that neuropsychiatric and behavioral AEs are rarely associated with montelukast in children and adolescents.[14, 15]
Most exposures were in males in middle childhood and were unintentional. Almost all of these exposures were managed outside a healthcare facility with only 7% of ultra-high-dose exposures referred to a healthcare facility. Although there was an association of montelukast dose with abdominal pain after adjustment for age and gender, the aOR of 1.01 was not clinically meaningful and reflects the large cohort under analysis.
There are limitations to our study. We used the NPDS data registry that includes data entered over a 17-year period. Although Specialists in Poison Information receive training in variable definition and data entry and use the highly-specified Coding Users’ Manual, we could not ascertain the accuracy of the data used for this analysis. The dose of an exposure may have not been coded correctly. Moreover, ascertainment of data accuracy is a potential limitation with all public health registries.[20, 21] This is because data accuracy relies upon a report of exposure and adverse effects from an observer, patient or clinician to a coding specialist. However, it is unlikely that adverse effects occurring in this cohort were not reported because NPDS Specialists establish a line of communication with the reporter and follow a patient through a sufficient time period until “medical outcome can be documented with reasonable certainty” as specified in the NPDS Coding User’s Manual.[22] Notwithstanding this potential limitation, registries are amongst the most comprehensive and robust sources of data to answer questions pertaining to rare medical exposures and outcomes such as montelukast exposures. Because Specialists are trained to follow the case until outcome can be reasonably ascertained and to perform highly specified data entry, we believe inaccurate or unreported adverse effects are unlikely.
An additional limitation is that the data registry may not include whether a patient is receiving chronic medications, including montelukast. Because most patients were managed as outpatients, routine laboratory testing was not performed in the majority of these children. In addition, the database was not designed for research such as this and does not include all adverse events of interest. For example, there have been concerns of neuropsychiatric events in patients receiving daily montelukast for asthma control.[16] While our objective was to examine adverse events associated with single-dose ingestions of montelukast and though we were able to examine some neuropsychiatric outcomes (i.e., confusion, dizziness or vertigo, hallucinations or delusions, slurred speech), suicidal ideation was not included amongst adverse events in the database.
Strengths of the study include the large population under study over a 17-year period, data acquisition and recording by trained Specialists using the comprehensive and highly-specified Coding Users’ Manual, and real-time data entry at the time of each exposure call to U.S. Poison Control Centers. In addition, we were able to apply filters to the 50,042 exposure events that included montelukast to identify 17,069 events comprising single-drug montelukast exposure with known or lowest-possible ingested dose and adverse effects attributable solely to montelukast. This strengthens the validity of our conclusion that single-dose ingestions of montelukast up to 445 mg are rarely associated with any adverse events and not associated with serious or life-threatening adverse events.
Conclusion
In conclusion, single-dose exposures to montelukast up to 445 mg are rarely associated with any adverse events. Furthermore, serious or life-threatening adverse events in children aged 5 – 17 years have not been reported. The rarity of adverse events following single, large doses might inform the design of future clinical trials of high-dose oral montelukast for other conditions such as acute asthma exacerbations or infectious diseases.
Footnotes
Disclosure of competing interest or conflicts of interest: The authors have no financial interests or other competing or conflicting interests to declare.
References
- 1.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. The New England journal of medicine. 1999;340:197–206. Epub 1999/01/23. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MK, Weinberg JB. Eicosanoids and respiratory viral infection: coordinators of inflammation and potential therapeutic targets. Mediators of inflammation. 2012;2012:236345 Epub 2012/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004;59:100–4. Epub 2004/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiss TF, Hill JB, Harman E, Zhang J, Tanaka WK, Bronsky E, Guerreiro D, Hendeles L. Increased urinary excretion of LTE4 after exercise and attenuation of exercise-induced bronchospasm by montelukast, a cysteinyl leukotriene receptor antagonist. Thorax. 1997;52:1030–5. Epub 1998/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singulair Highlights of prescribing information.1998; Accessed May 2, 2017 Available from: https://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf.
- 6.Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, Reiss TF. A placebo-controlled, dose-ranging study of montelukast, a cysteinyl leukotriene-receptor antagonist. Montelukast Asthma Study Group. The Journal of allergy and clinical immunology. 1998;102:50–6. Epub 1998/07/29. [DOI] [PubMed] [Google Scholar]
- 7.Reiss TF, Altman LC, Chervinsky P, Bewtra A, Stricker WE, Noonan GP, Kundu S, Zhang J. Effects of montelukast (MK-0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. The Journal of allergy and clinical immunology. 1996;98:528–34. Epub 1996/09/01. [DOI] [PubMed] [Google Scholar]
- 8.Schoors DF, De Smet M, Reiss T, Margolskee D, Cheng H, Larson P, Amin R, Somers G. Single dose pharmacokinetics, safety and tolerability of MK-0476, a new leukotriene D4-receptor antagonist, in healthy volunteers. British journal of clinical pharmacology. 1995;40:277–80. Epub 1995/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisgaard H, Skoner D, Boza ML, Tozzi CA, Newcomb K, Reiss TF, Knorr B, Noonan G. Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr Pulmonol. 2009;44:568–79. Epub 2009/05/19. [DOI] [PubMed] [Google Scholar]
- 10.McKee DB, Rose SR. Safety of montelukast in accidental ingestions by children. North American Congress of Clinical Toxicology, Congress; 2002. 2002. [Google Scholar]
- 11.Cobb DB, Abbott CL, Watson WA, Fernandez MC. High-dose montelukast exposures in a 3-year-old and a 5-year-old child. Veterinary and human toxicology. 2002;44:91–2. Epub 2002/04/05. [PubMed] [Google Scholar]
- 12.Forrester MB. Pediatric montelukast ingestions reported to Texas poison control centers, 2000–2005. Journal of toxicology and environmental health Part A. 2007;70:1792–7. Epub 2007/10/16. [DOI] [PubMed] [Google Scholar]
- 13.Updated Information on Leukotriene Inhibitors: Montelukast (marketed as Singulair), Zafirlukast (marketed as Accolate), and Zileuton (marketed as Zyflo and Zyflo CR).2009 April 17, 2017. April 17, 2017]. Available from: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htm#end_of_text.
- 14.Philip G, Hustad CM, Malice MP, Noonan G, Ezekowitz A, Reiss TF, Knorr B. Analysis of behavior-related adverse experiences in clinical trials of montelukast. The Journal of allergy and clinical immunology. 2009;124:699–706.e8. Epub 2009/10/10. [DOI] [PubMed] [Google Scholar]
- 15.Philip G, Hustad C, Noonan G, Malice M-P, Ezekowitz A, Reiss TF, Knorr B. Reports of suicidality in clinical trials of montelukast. Journal of Allergy and Clinical Immunology. 2009;124:691–6.e6. [DOI] [PubMed] [Google Scholar]
- 16.Aldea Perona A, Garcia-Saiz M, Sanz Alvarez E. Psychiatric Disorders and Montelukast in Children: A Disproportionality Analysis of the VigiBase((R)). Drug safety. 2016;39:69–78. Epub 2015/12/02. [DOI] [PubMed] [Google Scholar]
- 17.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. Journal of clinical epidemiology. 2006;59:1087–91. Epub 2006/09/19. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of clinical epidemiology. 2008;61:344–9. Epub 2008/03/04. [DOI] [PubMed] [Google Scholar]
- 19.Harrell F Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. 2nd ed. New York: Springer; 2015. [Google Scholar]
- 20.Richesson R, Vehik K. Patient registries: utility, validity and inference. Advances in experimental medicine and biology. 2010;686:87–104. Epub 2010/09/09. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer EA, Stausberg J. [What can and cannot be achieved by registries : Perspective of the registry working group of the German Network of Health Services Research]. Der Unfallchirurg. 2016;119:493–500. Epub 2016/05/14. [DOI] [PubMed] [Google Scholar]
- 22.American Association of Poison Control Centers, National Poison Data System (NPDS), NPDS Coding User’s Manual. February 21, 2016. [Google Scholar]