Abstract
PRAME (PReferentially expressed Antigen in MElanoma) is a melanoma-associated antigen that was isolated by autologous T cells in a melanoma patient. While frequent PRAME mRNA expression is well documented in cutaneous and ocular melanomas, little is known about PRAME protein expression in melanocytic tumors. in this study we examined the immunohistochemical expression of PRAME in 400 melanocytic tumors, including 155 primary and 100 metastatic melanomas, and 145 melanocytic nevi. Diffuse nuclear immunoreactivity for PRAME was found in 87% of metastatic and 83.2% of primary melanomas. Among melanoma subtypes, PRAME was diffusely expressed in 94.4% of acral melanomas, 92.5% of superficial spreading melanomas, 90% of nodular melanomas, 88.6% of lentigo maligna melanomas, and 35% of desmoplastic melanomas. When in situ and nondesmoplastic invasive melanoma components were present, PRAME expression was seen in both. Of the 140 cutaneous melanocytic nevi, 86.4% were completely negative for PRAME. immunoreactivity for PRAME was seen, albeit usually only in a minor subpopulation of lesional melanocytes, in 13.6% of cutaneous nevi, including dysplastic nevi, common acquired nevi, traumatized/recurrent nevi, and Spitz nevi. Rare isolated junctional melanocytes with immunoreactivity for PRAME were also seen in solar lentigines and benign nonlesional skin. Our results suggest that immunohistochemical analysis for PRAME expression may be useful for diagnostic purposes to support a suspected diagnosis of melanoma. it may also be valuable for margin assessment of a known PRAME-positive melanoma, but its expression in nevi, solar lentigines, and benign nonlesional skin can represent a pitfall and merits further investigations to better assess the potential clinical utility of this marker.
Keywords: PRAME, immunohistochemistry, melanoma, nevus
PRAME (PReferentially expressed Antigen in MElanoma) is a tumor-associated antigen that was first identified through analysis of the specificity of tumor-reactive T-cell clones derived from a patient with metastatic cutaneous melanoma.1 It was subsequently found that PRAME is not only expressed in cutaneous melanoma, but also ocular melanoma and various nonmelanocytic malignant neoplasms, including non-small cell lung cancer, breast carcinoma, renal cell carcinoma, ovarian carcinoma, leukemia, synovial sarcoma, and myxoid liposarcoma.2–10 Normal healthy tissues are not known to express PRAME except for testis, ovary, placenta, adrenals, and endometrium.1,11 Because of its expression profile, PRAME is a member of the family of cancer testis antigens (CTA), and an attractive target for immunotherapy. A number of clinical trials are underway trying to exploit CTAs, including PRAME, for cancer treatment.7,12
PRAME is not only of interest as treatment target for patients with metastatic melanoma. It has also been identified as an important biomarker for metastatic risk in class 1 uveal melanoma.13,14 Furthermore, PRAME is part of a 12-gene array prognostic assay for uveal melanoma.13 It is also a component of a 23-gene array diagnostic assay for cutaneous melanoma,15,16 and one of the 2 genes used in a noninvasive molecular assay for guiding clinicians on the need for biopsy of a melanocytic lesion.17
Because of the clinical interest in targeting PRAME for treatment and exploring PRAME as a potential biomarker for diagnosis or prognosis there is a need for confirmation of PRAME expression by immunohistochemistry (IHC). In this study we sought to determine the frequency of PRAME expression in primary and metastatic melanomas. We were also interested in examining PRAME expression in melanocytic nevi to explore whether IHC for this marker could be valuable as adjunct information for the distinction of nevi from melanomas. Furthermore we tested 20 lesions of solar lentigo for PRAME expression, as the results would be relevant if PRAME was used as a marker for the diagnosis of lentigo maligna melanoma in situ.
MATERIALS AND METHODS
Case Selection
A total of 145 melanocytic nevi, 155 primary, and 100 metastatic melanomas were retrieved from the institutional pathology archive under an IRB-approved protocol. Only cases with unequivocal diagnoses were included for this study to determine the frequency of PRAME expression using IHC. The tumors were reviewed by at least 2 dermatopathologists (C.L., K.J.B.) with agreement on the diagnoses. Several melanomas were also seen by pathologists at other institutions. Only cases with agreement on the diagnosis were included herein. The primary melanomas included 48 in situ lesions. Of the 107 primary invasive melanomas, 14 (13.1%) had documented subsequent metastases assessed with PRAME IHC. We also retrieved 20 lesions of solar lentigo and 10 sections of sun-damaged skin with junctional melanocyte hyperplasia. For the latter, we required a slight increase in the density of cytologically bland melanocytes at the dermoepidermal junction in association with marked solar elastosis. Cases were included only when we were certain that they did not represent melanoma in situ.
Immunohistochemical Analysis
Five micrometer thick tissue sections were cut from formalin-fixed and paraffin-embedded tissue blocks. A commercially available antibody to PRAME (MAb EPR20330; Abcam, #219650) was used on an automated Leica-Bond stainer platform. The staining result was recorded as the percentage of immunoreactive tumor cells with nuclear labeling per total number of tumor cells. Zero indicated no staining at all. Staining of 1% to 25% of tumor cells was scored as 1+. Labeling of 26% to 50% of tumor cells was scored as 2+. If 51% to 75% of tumor cells were positive, it was designated as 3+. If 76% or more of the tumor cells were positive, it was recorded as 4+ or “diffuse.”
RESULTS
Metastatic Melanoma
A total of 100 lesions of metastatic cutaneous melanoma were examined. Ninety-two of them (92%) were immunoreactive for PRAME. Eighty-seven metastatic melanomas (87%) stained diffusely (4+ labeling score) in all or nearly all tumor cell nuclei (Fig. 1). In 5 metastatic lesions, the labeling was inhomogenous, with immunoreactivity for PRAME seen in <75% of the tumor cells. Eight tumors (8%) were completely negative for PRAME.
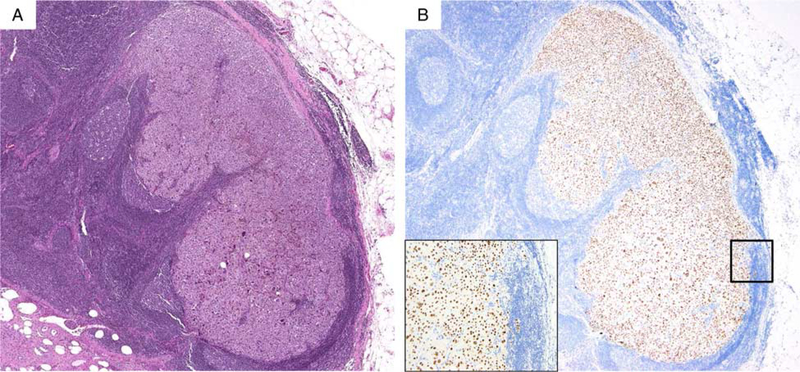
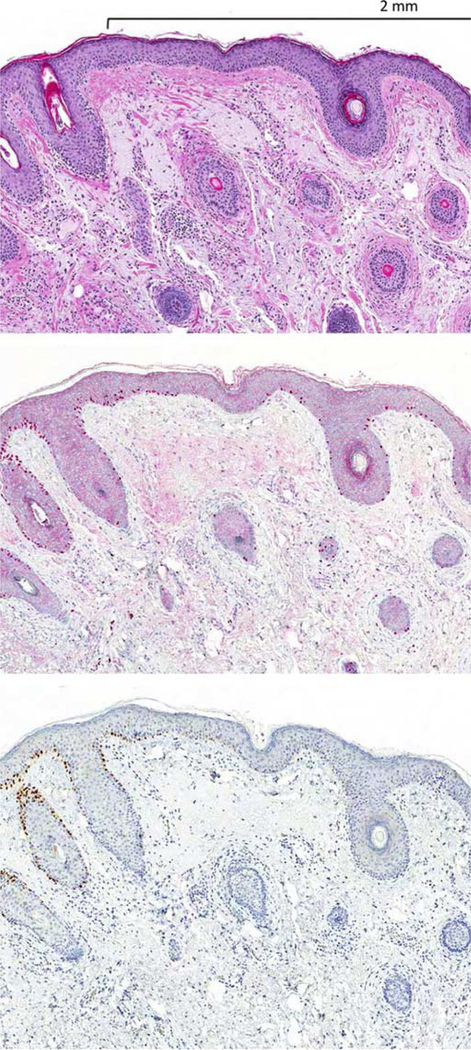
FIGURE 1.
A, Metastatic melanoma in lymph node (H&E-stain). B, The tumor cells are diffusely immunopositive for PRAME (nuclear labeling). inset highlights PRAME labeling is nuclear.
Primary Cutaneous Melanoma
In all, 155 primary cutaneous melanomas were analyzed. They included 53 superficial spreading melanomas, 44 lentigo maligna melanomas, 18 acral melanomas, 20 desmoplastic melanomas, and 10 nondesmoplastic nodular melanomas. Additional 10 cases included cutaneous paramucosal and nevoid melanomas. The patients ages ranged from 17 to 100 years (mean = 68 y, median = 70 y). Eighty-nine were male, 66 were female. The thickness of the invasive tumors ranged from 0.1 to 20 mm (mean Breslow thickness = 3.3 mm; median Breslow thickness = 1.7 mm). Of the 155 melanomas tested, 107 were invasive. Of the 155 primary melanomas tested, 129 (83.2%) were diffusely immunoreactive for PRAME. If desmoplastic melanomas were excluded, 122/135 (90.4%) of primary tumors were diffusely positive for PRAME. Forty-five (93.8%) of all in situ melanomas expressed PRAME in nearly all tumor cells (4+ labeling) (Table 1). All nondesmoplastic melanomas with both in situ and invasive tumor showed equal expression of PRAME in both components (Figs. 2, 3). In terms of histologic subtype, PRAME was diffusely expressed (4+ labeling) in 94.4% of acral melanomas, 92.5% of superficial spreading melanomas, 90% of nodular melanomas, and 88.6% of lentigo maligna melanomas. PRAME IHC expression was lowest for desmoplastic melanomas: only 7/20 tumors (35%) showed 4+ PRAME IHC labeling. Our series of desmoplastic melanomas included tumors with and without associated melanoma in situ. PRAME was preferentially expressed in the associated melanoma in situ, if present, and in the case of mixed desmoplastic melanomas, predominantly in the nondesmoplastic invasive tumor component. Pure pauci-cellular desmoplastic melanoma components were either negative for PRAME or expressed PRAME only weakly and focally. For melanomas found to be associated with a melanocytic nevus, only the melanoma component was PRAME-positive (Fig. 4). Cases of primary invasive melanoma with corresponding metastases showed concordance in the extent of PRAME expression in 12 of 14 pairs of tumors—all diffusely (4+) positive. In the 2 patients with discordant staining scores between primary and metastatic lesions, the primary tumors were a mixed desmoplastic melanoma with 1+ PRAME expression and a primary lentigo maligna melanoma with 2+ PRAME labeling score, respectively. Both subsequent metastases diffusely expressed PRAME (4+). With regard to adjacent normal skin, no nuclear labeling was seen in normal nonmelanocytic tissues, but cytoplasmic labeling of sebaceous epithelial cells was noted (Fig. 3).
TABLE 1.
Primary Cutaneous Melanomas With Diffuse (4+) PRAME IHC Expression
| Melanoma Type | In Situ Only | Invasive | Total |
|---|---|---|---|
| Superficial spreading | 12/12 | 37/41 | 49/53 |
| Lentigo maligna | 24/27 | 15/17 | 39/44 |
| Acral | 7/7 | 10/11 | 17/18 |
| Nodular | NA | 9/10 | 9/10 |
| Other* | 2/2 | 6/8 | 8/10 |
| Subtotal† | 45/48 | 77/87 | 122/135 |
| Desmoplastic‡ | NA | 7/20 | 7/20 |
| Total | 45/48 | 84/107 | 129/155 |
This category includes (proportion of cases with 4+ PRAME): lentiginous vulvar in situ melanomas (2/2), nevoid melanoma (2/2), malignant melanoma exblue nevus (0/1), cutaneous paramucosal (3/3), and unclassified invasive melanomas (1/2).
Subtotal = all melanomas except for desmoplastic melanomas.
This category comprises (proportion of cases with 4+ PRAME): spindle cell melanomas with variable desmoplasia, including pure (0/4) and mixed (6/14) desmoplastic melanomas, and spindle cell neurotropic (1/2) melanomas.
NA indicates not available.
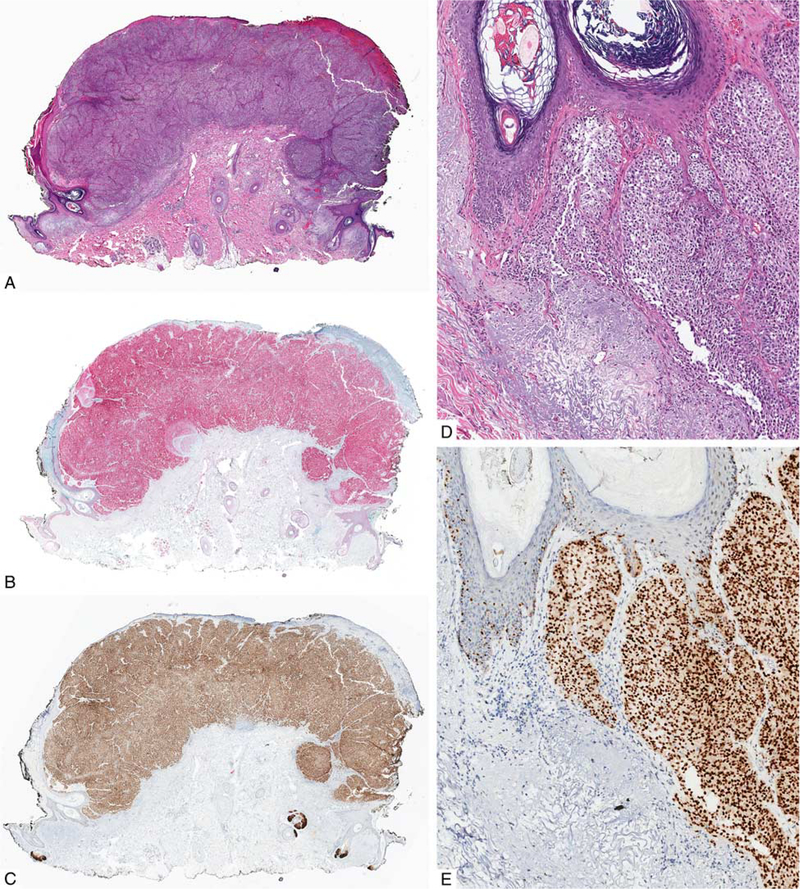
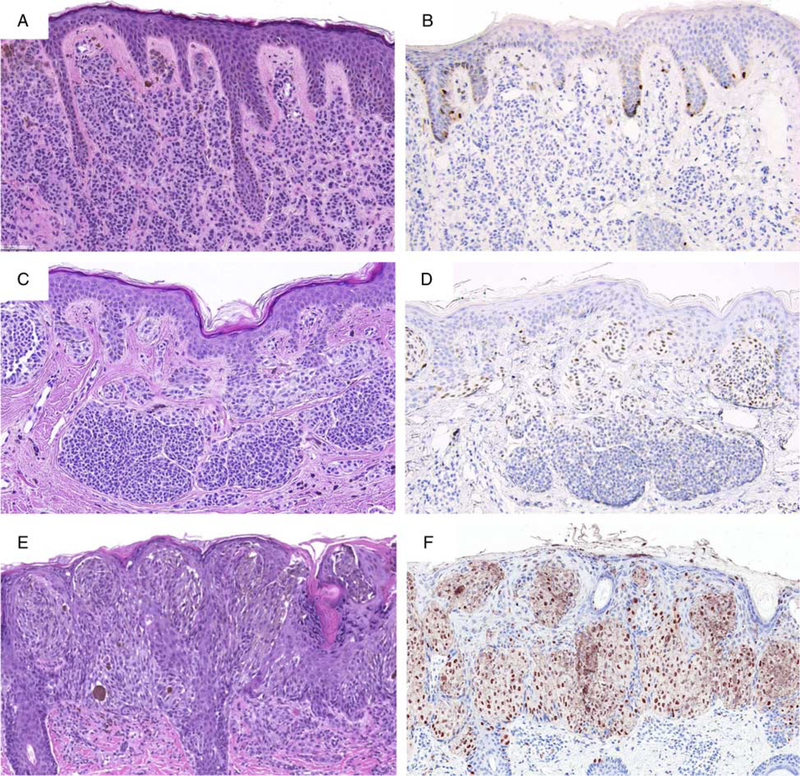
FIGURE 2.
Primary melanoma. A, Ulcerated polypoid tumor from the neck of a 73-year-old man. B, IHC for Sox10: the tumor cells are homogenously immunoreactive for Sox10. C, IHC for PRAME: the tumor cells diffusely express PRAME. D, Melanoma is present in both the epidermis and dermis (H&E-stained section). E, IHC for PRAME highlights both in situ and invasive tumor cells.
FIGURE 3.
Primary melanoma from the scalp of a 75-year-old man. A, Both in situ and invasive melanoma are equally strongly immunoreactive for PRAME. There is prominent follicular involvement by melanoma. B, The melanocytes show nuclear labeling for PRAME. The sebaceous glands show cytoplasmic labeling.
FIGURE 4.
Melanoma associated with a melanocytic nevus in the ear of a 63-year-old man. A, Nodular silhouette of the lesion with a more densely cellular tumor cell population on the right side of the lesion. B, IHC for PRAME stains only the densely cellular nodule. C, The less cellular area shows cytologic features of a melanocytic nevus. D, The PRAME-positive tumor cells are cytologically atypical. Cytogenetic analysis of the tumor cells revealed a number of chromosomal aberrations, including loss of 9p and gain of 8q (not shown).
Margin Assessment for Melanoma In Situ
Ten excisions for primary melanomas were specifically evaluated for margin assessment to determine the clearance between melanoma in situ and the nearest side margin using in parallel hematoxylin and eosin (H&E)-stained sections and immunostains for PRAME and Sox10 expression. Assessment of margins using IHC for PRAME correlated well with margin status and margin clearance based on routine H&E-stained sections and IHC for Sox10 (Table 2). An example of margin assessment is illustrated in Figure 5, which corresponds to lesion 3 from Table 2. The nuclei of the melanocytes of the melanoma in situ are immunoreactive for PRAME, while the melanocytes of the adjacent tumor-negative skin are not.
TABLE 2.
Margin Assessment of Melanoma In Situ Using PRAME IHC
| Case | Age (y) | Sex | Site | Margin Clearance Assessed by H&E (mm) |
Clearance Assessed by Sox10—IHC (mm) |
Clearance Assessed by PRAME—IHC (mm) |
|---|---|---|---|---|---|---|
| 1 | 68 | Female | Cheek | 0.5 | 0.5 | 0.45 |
| 2 | 83 | Female | Cheek | 2 | 1.8 | 2 |
| 3 | 69 | Male | Finger | 2 | 2 | 2 |
| 4 | 72 | Male | Ear | 3.9 | 4 | 4 |
| 5 | 78 | Female | Cheek | 3 | 3 | 3.3 |
| 6 | 69 | Male | Neck | 1.7 | 1.6 | 1.6 |
| 7 | 73 | Female | Foot | 0.3 | 0.3 | 0.3 |
| 8 | 69 | Male | Chest | 4.5 | 4.5 | 4.5 |
| 9 | 50 | Male | Neck | 2.5 | 2.5 | 2.5 |
| 10 | 57 | Male | Forehead | 0.3 | 0.2 | 0.2 |
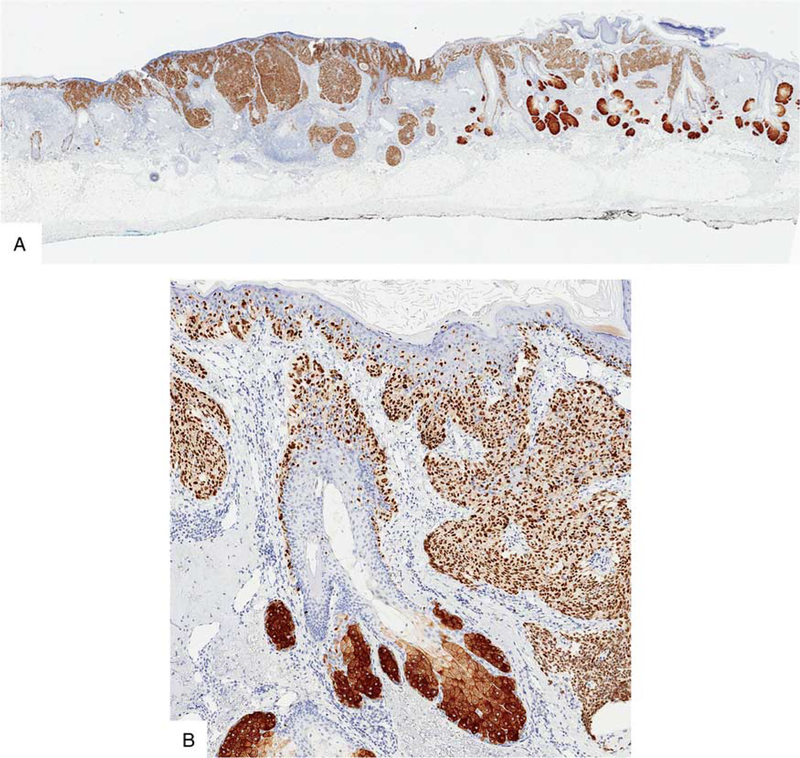
FIGURE 5.
Section from a staged excision of a lentigo maligna melanoma in situ with a rim of normal skin. A, H&E-stained section. The left blue-inked section edge faced the tumor debulk. The right side represents the outer rim—margin—of the excision. B, IHC for Sox10. C, IHC for PRAME. While the melanoma in situ strongly labels for PRAME, the melanocytes of the adjacent benign skin are negative.
Melanocytic Nevi
A total of 140 cutaneous and 5 nodal melanocytic nevi were studied for PRAME expression (Table 3). Most cutaneous melanocytic nevi (86.4%) lacked any staining for PRAME. However, a small subset of cutaneous nevi showed focal PRAME immunoreactivity in a minor population of lesional melanocytes (1+ labeling score) (Fig. 6). One dysplastic nevus and one recurrent nevus showed immunoreactivity in nearly half of the lesional melanocytes (2 + labeling score). One lesion of a pigmented junctional Spitz nevus from the cheek of a 6-year-old child showed diffuse staining. This was the only benign lesion in this series with a 4 + labeling score. None of the 5 nodal melanocytic nevi expressed PRAME.
TABLE 3.
PRAME IHC Expression in Melanocytic Nevi
| Type of Melanocytic Nevus |
Diffuse (4+) IHC PRAME Expression |
Focal (1 or 2+) IHC PRAME Expression |
|---|---|---|
| Common acquired nevus | 0/40 | 4/40 (1+) |
| Dysplastic (Clark’s) nevus | 0/60 | 10/60 (1+) |
| 1/60 (2+) | ||
| Blue nevus | 0/10 | 0/10 |
| Spitz nevus | 1/10 | 1/10 (1+) |
| Deep penetrating nevus | 0/3 | 0/3 |
| Traumatized/ recurrent nevus | 0/15 | 1/15 (2+) |
| 1/15 (1+) | ||
| Congenital nevus | 0/2 | 0/2 |
| Nodal nevus | 0/5 | 0/5 |
| Total | 1/145 | 18/145 |
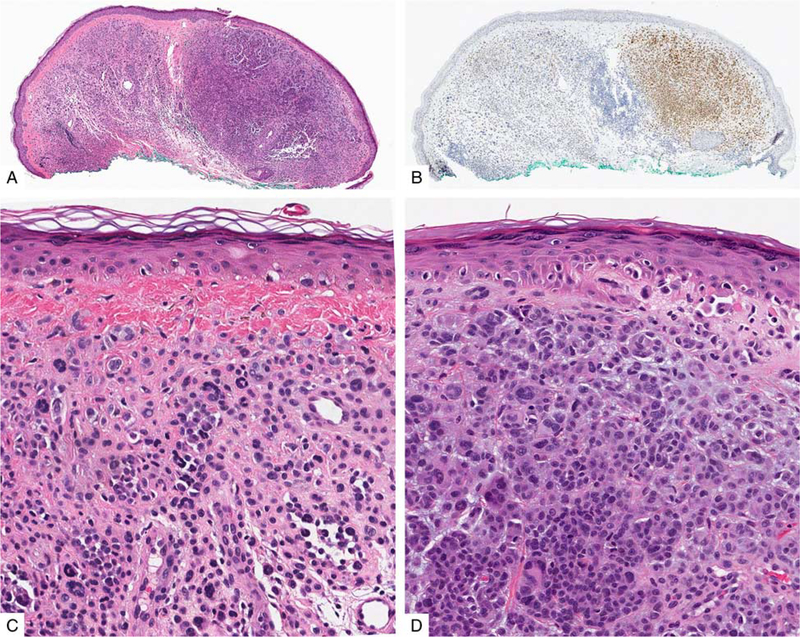
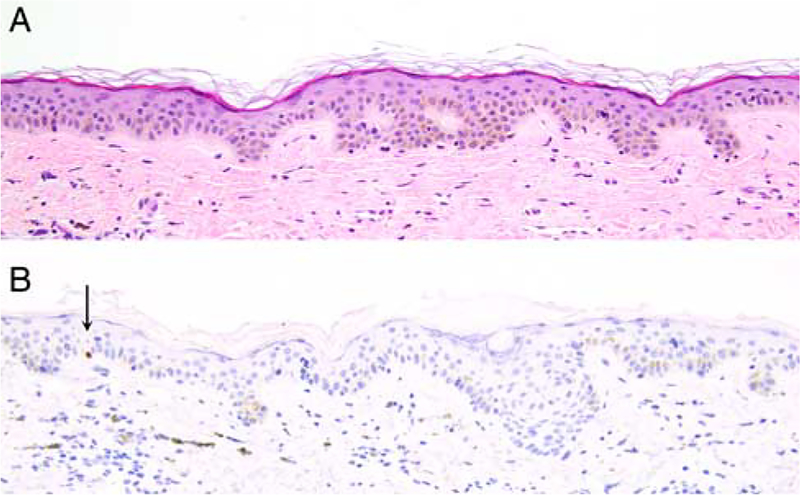
FIGURE 6.
PRAME immunoreactivity in nevi. A, Ordinary melanocytic nevus (H&E-stain). B, A few junctional melanocytes express PRAME (1+). C, Compound dysplastic nevus (H&E-stain). D, The center of the lesion contains a number of PRAME-positive melanocytes at the dermoepidermal junction and in the superficial dermis (2+). E, Predominantly junctional Spitz nevus on the cheek of a child (H&E-stain). F, The intraepidermal lesional melanocytes diffusely label for PRAME (4+).
Solar Lentigo
Twenty lesions of solar lentigo were also analyzed to further assess for potential pitfalls in using IHC for PRAME, as not infrequently both clinically and histo-pathologically lentigo maligna needs to be distinguished from solar lentigo for diagnosis and/or margin assessment. The lesions were from 20 patients ranging in age from 42 to 83 years, with a medium age of 66 years. Twelve of the lesions were from the head and neck area, 6 from extremities, and 2 from the back. Rare isolated melanocytes immunoreactive for PRAME were seen in 3 of 20 (15%) solar lentigines (Fig. 7).
FIGURE 7.
A, Solar lentigo (H&E). B, A rare isolated junctional melanocyte labels for PRAME (arrow).
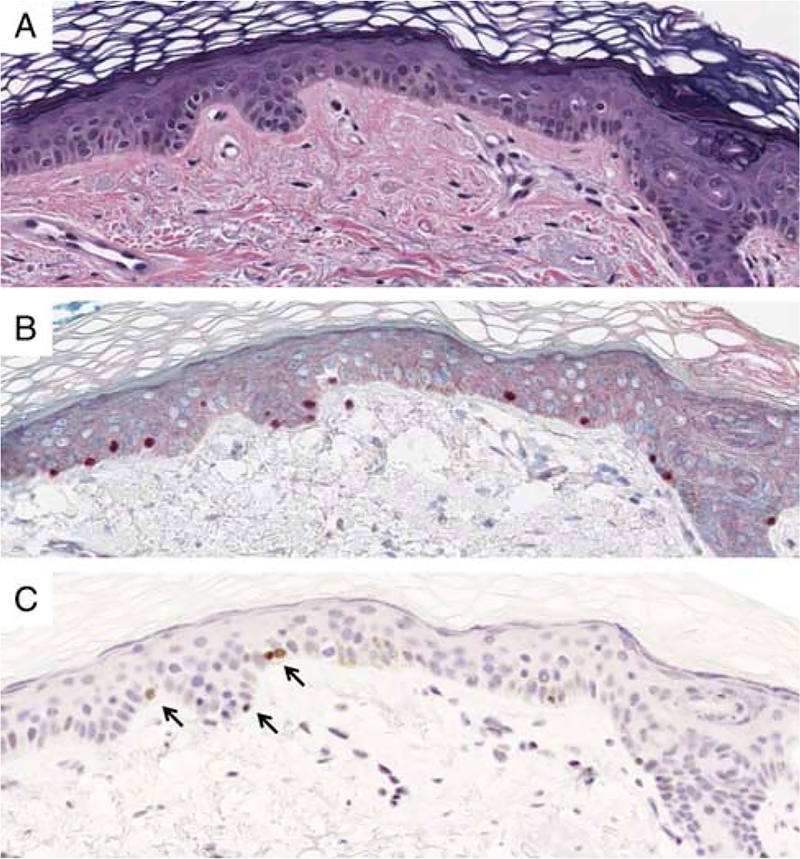
Benign Nonlesional Sun-damaged Skin With Junctional Melanocyte Hyperplasia
As an additional step to evaluate possible diagnostic problems for margin assessment of lentigo maligna melanoma in situ we also examined 10 sections of benign nonlesional portions of lentigo maligna reexcisions with variable junctional melanocyte hyperplasia. The ages of the patients ranged from 51 to 79 years (mean = 67 y, median = 63 y). They included 5 men and 5 women. Five of the excisions were from the upper arm, 4 from the cheek, and one from the upper back. Solar elastosis was seen in all cases. Five of the 10 sections contained rare junctional melanocytes, which were immunoreactive for PRAME (Fig. 8).
FIGURE 8.
A, Benign sun-damaged skin (H&E). B, An immunostain for Sox10 documents cytologically bland benign junctional melanocytes. C, Rare junctional melanocytes show nuclear labeling for PRAME (arrows). Most melanocytes are negative for PRAME.
DISCUSSION
CTA represent an opportunity for targeted cancer immunotherapy, given their predominantly restricted expression to cancer cells.7,12,18 Their expression in non-neoplastic cells appears to be significantly lower than in neoplastic cells and limited to the testis, ovary, placenta, adrenals, and endometrium.1,19,20 PRAME is a CTA that has been found in melanoma and is currently explored as a potential therapeutic target.7,12,18,21 With clinical interest in PRAME expression for treatment purposes we thought it would be useful to have an IHC assay available for rapid documentation of PRAME protein expression in melanoma. Having an antibody to visualize PRAME expression would also be helpful to assess limitations in the sensitivity and specificity of various molecular assays that include PRAME gene expression as a test parameter.15–17 To this end we sought to determine the feasibility of IHC for PRAME in formalin-fixed and paraffin-embedded tissue and to determine the frequency of PRAME expression in metastatic melanoma. Our results document that PRAME is frequently expressed in metastatic melanomas. In the cases reported herein ~87% of metastatic melanomas expressed PRAME in a diffuse homogenous pattern, with additional 5% of cases (total of 92% of cases) showing at least focal immunoreactivity. Thus, the frequency of PRAME expression in our series of melanomas based on immunohistochemical detection is similar to the reported frequency of 91% that was based on mRNA studies.1 The common expression of PRAME in melanoma makes it an attractive target for immunotherapy.
Given prior evidence of PRAME as biomarker and its expression profile (expected to be present in malignant tumors only except for low levels in some non-neoplastic tissues) we also reasoned that IHC for PRAME could be useful for selected diagnostic problems: the distinction of nevus from melanoma (in skin or lymph nodes) and for the assessment of margin clearance of melanomas. Therefore, we sought to determine first the frequency of PRAME expression in primary melanomas of different histopathologic subtypes, and then to assess its specificity by examining various melanocytic nevi. We found a high frequency of PRAME expression in primary cutaneous melanomas. The frequency varied depending on the melanoma subtype. The frequency of PRAME expression was high (around 90%) in conventional (acral, superficial spreading, nodular, and lentigo maligna) melanomas. The frequency of expression was low in desmoplastic melanomas. In the latter, diffuse PRAME expression was seen only in 35% of cases.
With regard to melanocytic nevi, most of them were completely negative for PRAME. However, focal staining was seen in a few lesions. For a CTA like PRAME mRNA or protein expression is not expected in most normal tissues. However, in testis, ovary, placenta, adrenals, and endometrium PRAME RNA expression has already been reported at fractions of levels encountered in tumors.1,20 Furthermore, limitations in the specificity of PRAME-based gene expression assays for the distinction of nevi from melanoma suggested that low levels of PRAME expression could be found in cutaneous melanocytic nevi.15,16 Our study confirms that PRAME expression can be seen in benign melanocytic neoplasms.22 PRAME expression in nevi was usually focal and preferentially seen in junctional nests and/or rare solitary units of junctional melanocytes. Occasionally a few intradermal melanocytes were also PRAME-positive. Nevi with focal PRAME expression included common acquired, dysplastic, traumatized or recurrent melanocytic nevi. Among the 140 cutaneous nevi of this series, diffuse PRAME expression was seen only in a single case of a pigmented predominantly junctional Spitz nevus from the cheek of a 6-year-old child. The biology underlying PRAME expression in benign melanocytic nevi remains to be determined, but for practical diagnostic purposes, it is important for dermatopathologists to know that PRAME expression per se does not equal malignancy. Nonetheless, our results suggest that diffuse labeling for PRAME in melanocytic neoplasms is strongly associated with melanoma. Thus, when other findings (microscopic features, clinical setting, dermoscopy, cytogenetic data) favor melanoma, positive expression for PRAME would be additional supportive evidence for melanoma. While our observations are encouraging, a large study of melanocytic proliferations with ambiguous microscopic features and/or associated diagnostic controversy is needed to assess the clinical utility of PRAME immunoreactivity for problematic lesions. On the basis of preliminary observations comparing PRAME expression with cytogenetic test results, diffuse labelling for PRAME correlates strongly with cytogenetic aberrations that would support a diagnosis of melanoma (K.J.B., unpublished data, 2018).
Given the high frequency of PRAME expression in melanoma and general absence of PRAME in normal tissues, we anticipate that PRAME IHC will likely be useful in the evaluation of margin status, particularly in cases with a lentiginous in situ component such as seen in acral and lentigo maligna melanomas. In contrast to immunostains for Melan-A or Sox10, IHC for PRAME gives a “cleaner” microscopic picture as to where a lesion ends, as unlike Melan-A and Sox10, PRAME usually does not stain normal melanocytes (Fig. 5). In our hands PRAME-positivity tends to correlate well with the histologic assessment of margin clearance. In some cases it may be superior to H&E or immunostains for melanocyte differentiation antigens, if inflammation, cautery artifacts or background melanocyte hyperplasia make it difficult to determine the end of a lesion. The use of PRAME IHC for margin assessment, however, is not without pitfalls, as rare isolated non-neoplastic melanocytes may be PRAME-positive as our observations on solar lentigines and sections from chronically sun-damaged skin indicate (Figs. 7, 8). This finding warrants caution when using IHC for PRAME for the distinction of a solar lentigo from a lentigo maligna or lentigo maligna from “background” junctional melanocyte hyperplasia. In our experience so far benign PRAME-positive junctional melanocytes can usually be distinguished from melanoma in situ by their lower cell density and bland cytology. Furthermore, they tend to occupy only a small area of epidermis and are flanked by stretches of epidermis with PRAME-negative melanocytes. However, on a small biopsy sample the detection of a few PRAME-positive junctional cells may pose a diagnostic challenge, which is why it is important to interpret the immunohistochemical findings in context with clinical and other histopathologic features. The presence of rare PRAME-positive melanocytes in association with some solar lentigines and sun-damaged skin constitutes an interesting observation that merits further investigations to determine the significance of this phenomenon. While we did not find any nuclear labeling for PRAME in any cells of the skin other than melanocytes, cytoplasmic labeling of sebaceous glands is a persistent phenomenon using the antibody EPR20330. We suspect it most likely reflects an artifactual phenomenon and does not imply protein expression in the cytoplasm of sebaceous epithelium.
In conclusion, we have documented herein that PRAME is diffusely expressed in the vast majority of primary and metastatic cutaneous melanomas, with the exception of desmoplastic melanomas. As most PRAME-positive melanomas homogenously express PRAME and normal skin usually lacks PRAME-positive cells, IHC for PRAME can be used as an ancillary tool for melanoma margin assessment. Furthermore, as diffuse PRAME expression is rare in melanocytic nevi, testing for diffuse PRAME expression has potential value as an ancillary method for the distinction of nevi from melanoma, including the pathologic staging of melanomas associated with a nevus remnant. The detection of PRAME expression in some nevi and solar lentigines points to a significant limitation in the use of this marker, and helps explain occasional “false-positive” results of gene expression arrays, which rely on PRAME. More work is clearly needed, especially on PRAME expression in diagnostically problematic melanocytic tumors. Studies to further address the specificity of PRAME expression in various nonmelanocytic cutaneous and extracutaneous tumors are currently under way. While we have already found some limitations in the diagnostic use of PRAME expression, we find the observations so far encouraging and anticipate that IHC for PRAME will become a useful addition to the diagnostic armamentarium of practicing pathologists.
ACKNOWLEDGMENTS
The authors wish to thank Denise Frosina for excellent technical assistance with the characterization of the antibody. They also thank Fernando Garcia and Maria Sanchez for their assistance with digital images.
Conflicts of Interest and Source of Funding:
Supported in part by the Cancer Center Support Grant of the National institutes of Health/National Cancer Institute under award number P30CA008748. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Ikeda H, Lethe B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. [DOI] [PubMed] [Google Scholar]
- 2.Hemminger JA, Toland AE, Scharschmidt TJ, et al. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod Pathol. 2014;27:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iura K, Kohashi K, Hotokebuchi Y, et al. Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma. J Pathol Clin Res. 2015;1:144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iura K, Maekawa A, Kohashi K, et al. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum Pathol. 2017;61:130–139. [DOI] [PubMed] [Google Scholar]
- 5.Neumann E, Engelsberg A, Decker J, et al. Heterogeneous expression of the tumor-associated antigens RAGE-1, PRAME, and glycoprotein 75 in human renal cell carcinoma: candidates for T-cell-based immunotherapies? Cancer Res. 1998;58:4090–4095. [PubMed] [Google Scholar]
- 6.Oberthuer A, Hero B, Spitz R, et al. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004;10:4307–4313. [DOI] [PubMed] [Google Scholar]
- 7.Pujol JL, De Pas T, Rittmeyer A, et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in patients with resected non-small cell lung cancer: a phase I dose escalation study. J Thorac Oncol. 2016;11:2208–2217. [DOI] [PubMed] [Google Scholar]
- 8.Roszik J, Wang W-L, Ravi V, et al. Expression and clinical correlations of PRAME in sarcoma subtypes. J Clin Oncol. 2016;34:11067–11067. [Google Scholar]
- 9.Sanchez MI, Field MG, Kuznetsov JN, et al. The role of PRAME in promoting uveal melanoma metastasis [abstract] In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; Washington, DC. Philadelphia, PA: AACR; Cancer Res; 2017; 77(13 Suppl):Abstract nr 4861. [Google Scholar]
- 10.Zhang W, Barger CJ, Eng KH, et al. PRAME expression and promoter hypomethylation in epithelial ovarian cancer. Oncotarget. 2016;7:45352–45369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodison S, Urquidi V. The cancer testis antigen PRAME as a biomarker for solid tumor cancer management. Biomark Med. 2012;6:629–632. [DOI] [PubMed] [Google Scholar]
- 12.Gutzmer R, Rivoltini L, Levchenko E, et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: results of a phase I dose escalation study. ESMO Open. 2016;1:e000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field MG, Durante MA, Decatur CL, et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget. 2016;7:59209–59219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke LE, Flake DD II, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107–1113. [DOI] [PubMed] [Google Scholar]
- 17.Ferris LK, Jansen B, Ho J, et al. Utility of a noninvasive 2-gene molecular assay for cutaneous melanoma and effect on the decision to biopsy. JaMa Dermatol. 2017;153:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gezgin G, Luk SJ, Cao J, et al. PRAME as a potential target for immunotherapy in metastatic uveal melanoma. JAMA Ophthalmol. 2017;135:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang AY, Dao T, Gejman RS, et al. A therapeutic T cell receptor mimic antibody targets tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest. 2017;127:2705–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadelin F, Fulton J, McEwan PA, et al. Leucine-rich repeat protein PRAME: expression, potential functions and clinical implications for leukaemia. Mol Cancer. 2010;9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmaninejad A, Zamani MR, Pourvahedi M, et al. Cancer/testis antigens: expression, regulation, tumor invasion, and use in immunotherapy of cancers. Immunol Invest. 2016;45:619–640. [DOI] [PubMed] [Google Scholar]
- 22.Westekemper H, Karimi S, Susskind D, et al. Expression of MCSP and PRAME in conjunctival melanoma. Br J Ophthalmol. 2010; 94:1322–1327. [DOI] [PubMed] [Google Scholar]