Abstract
An enantioselective copper-catalyzed azide− alkyne cycloaddition (E-CuAAC) is reported by kinetic resolution. Chiral triazoles were isolated in high yield with limiting alkyne (up to 97:3 enantiomeric ratio (er)). A range of substrates were tolerated (>30 examples), and the reaction was scaled to >1 g. The er of a triazole product could be enhanced by recrystallization and the recovered scalemic azide could be racemized and recycled. Recycling the azide allows efficient use of the undesired azide enantiomer.
Graphical abstract
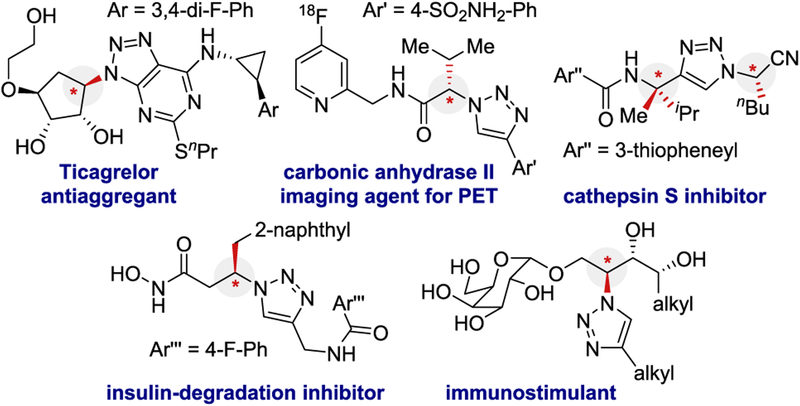
The copper(I) catalyzed azide−alkyne cycloaddition reaction (CuAAC)1,2 is an important transformation. Applications for CuAAC penetrate numerous subdisciplines, including chemical biology, medicinal chemistry, and polymer chemistry.3–5 This click reaction6 is operationally simple, functional-group-tolerant, high yielding, and broad in scope. Furthermore, the triazole ring is gaining recognition as a protease-resistant peptidomimetic.4 Biologically active α-chiral triazoles have been reported (Figure 1).7–12 Because of the prevalence of α-amino acid-derived amides, an enantioselective CuAAC (E-CuAAC) reaction would be useful for accessing α-N-chiral triazoles. However, E-CuAAC has proven challenging in part because (i) both cycloaddition components have linear geometries, (ii) CuAAC does not require a ligand, and (iii) the product triazole is a competent CuAAC ligand.13 Therefore, ECuAAC must kinetically outcompete the background CuAAC reaction.
Figure 1.
Representative bioactive α-N-chiral triazoles.
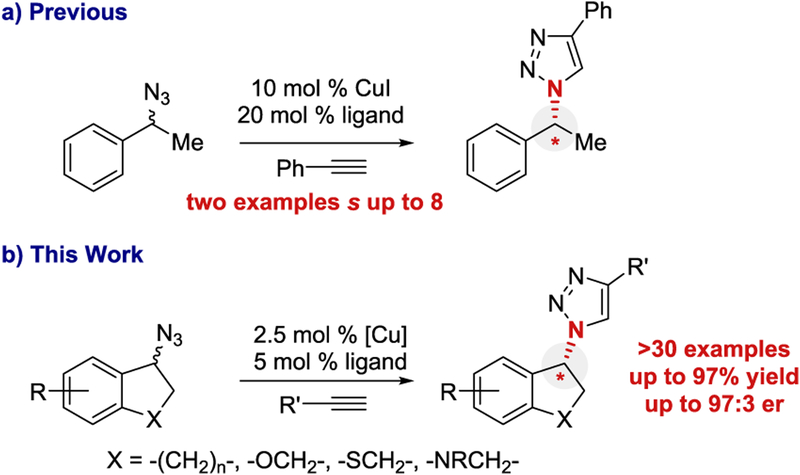
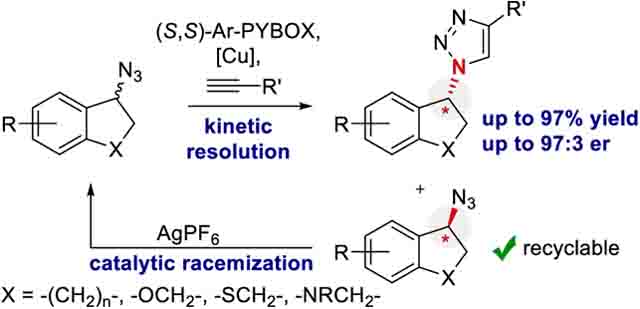
A few reports describe kinetic resolution,14–16 dynamic kinetic resolution,17 or desymmetrization18–21 for E-CuAAC.22 The majority of successful E-CuAAC reactions generate α-C-chiral triazoles, where the new stereocenter is alkyne-derived.15,16,18,20,21 The original E-CuAAC reported by Fokin and Finn is the state-of-the-art E-CuAAC kinetic resolution for α-N-chiral triazole formation, where the new stereocenter is derived from the azide component (Scheme 1a).14 The authors provided only two examples of kinetic resolution with a selectivity factor (s) of 3.2 and 8, respectively (ca. 70:30 er and 84:16 er, assuming 40% conversion of the azide). Using the same conditions, the desymmetrization of two bis-azides were reported to result predominantly in the formation of bistriazole (not shown).
Scheme 1.
Azide Kinetic Resolution by E-CuAAC
Our laboratory became interested in using the unique properties of allylic azides to establish α-N-chiral centers though dynamic kinetic resolution.23–25 Recently, we reported the first E-CuAAC reaction that proceeded by dynamic kinetic resolution.17 This work prompted an exploration of E-CuAAC by azide kinetic resolution for α-N-chiral triazole synthesis (Scheme 1b). Reported herein is a successful expansion of the kinetic resolution by E-CuAAC.
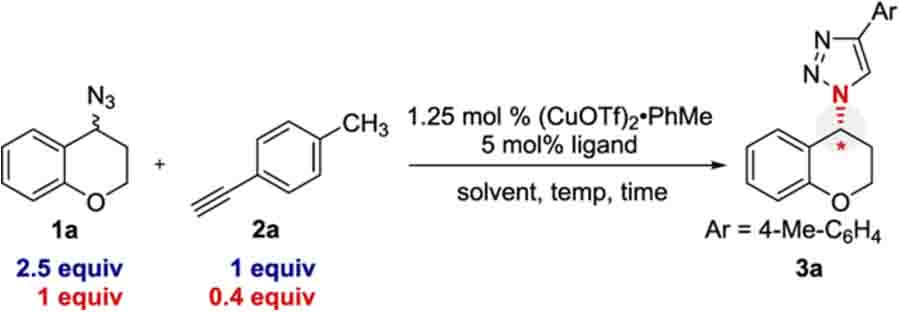
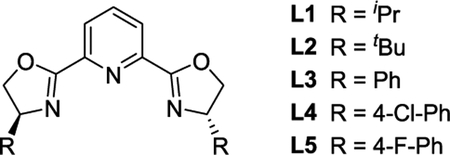
Our investigation began with azide 1a, alkyne 2a, and commercially available PYBOX ligands (Table 1, entries 1−3). Minimal enantioselectivity was observed with these ligands, confirming the report from Fokin and Finn.14 A wide variety of chiral ligands were screened consisting of numerous ligand classes (see Table 1, entries 4 and 5, as well as the Supporting Information). Only the aryl-PYBOX ligands provided reasonable enantioselectivity, with ligand L5 providing the best selectivity among the ligands screened. The data obtained are reported here with er instead of the selectivity parameter s because (i) er is more synthetically meaningful, (ii) er is directly obtained by chiral HPLC, and (iii) several kinetic assumptions are made when deriving s, which are likely faulty for E-CuAAC, based on reported CuAAC kinetics.26,27 One could convert the reported er to a presumed s by assuming 40% conversion for reactions approaching completion. Lowering the reaction temperature resulted in an increase in enantioselectivity, albeit at the cost of conversion (Table 1, entries 6−8). The reaction was faster in DME or PhCF3 as the solvent, relative to CH2Cl2, and the enantioselectivity was maintained. When using PhCF3 as the solvent, the reaction was complete within 48 h (entry 11; see the Supporting Information for additional optimization).
Table 1.
E-CuAAC Optimization by Kinetic Resolutiona
 | |||||
|---|---|---|---|---|---|
| entry | ligand | solvent | Temperature (°C) | yieldb (%) | enantiomeric ratio, erc |
| 1 | (R)-L1 | CH2Cl2 | rtd | 88 (35) | 55:45 |
| 2 | (R)-L2 | CH2Cl2 | rtd | 55 (22) | 50:50 |
| 3 | (R)-L3 | CH2Cl2 | rtd | 92 (37) | 22:78 |
| 4 | (R)-L4 | CH2Cl2 | rtd | 95 (38) | 21:79 |
| 5 | (S)-L5 | CH2Cl2 | rtd | 96 (38) | 80:20 |
| 6e | (S)-L5 | CH2Cl2 | 0 | 84 (34) | 84:16 |
| 7e,f | (S)-L5 | CH2Cl2 | −15 | 64 (26) | 86:14 |
| 8e,g | (S)-L5 | CH2Cl2 | −20 | 34 (14) | 89:11 |
| 9e,f | (S)-L5 | DME | −15 | 87 (35) | 86:14 |
| 10e,f | (S)-L5 | PhCF3 | −15 | 92 (37) | 88:12 |
| 11e,h | (S)-L5 | PhCF3 | −15 | 92 (37) | 88:12 |
Reactions conducted with azide 1a (0.125 mmol) and alkyne 2a(0.05 mmol) at 0.1 M in varying solvent with (CuOTf)2·PhMe (0.62 μmol) and varying ligand (2.5 μmol). All yield and er values reflect the average of duplicate trials.
Yield determined using calibrated GC with triphenylmethane as an internal standard. The yield is calculated based on either the alkyne (limiting reagent) or azide (kinetic resolution component).
Chiral HPLC was used to determine er.
rt = room temperature.
Concentration was 0.125 M.
Time = 72 h.
Time = 96 h.
Time = 48 h.
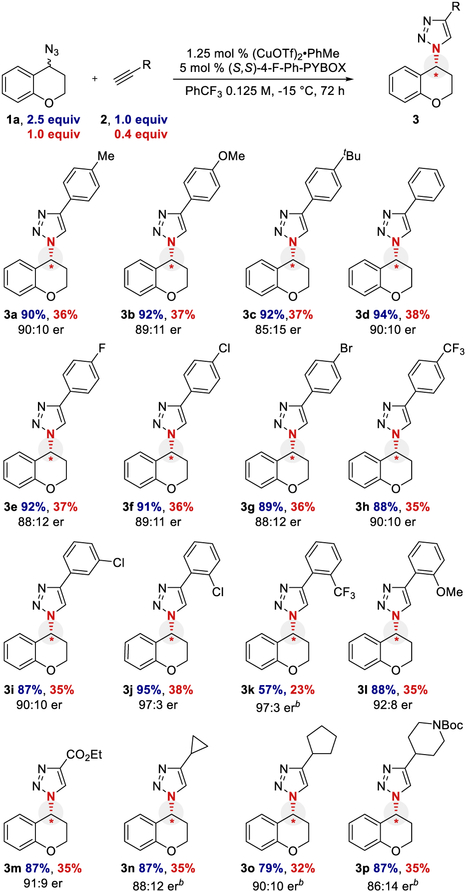
With the optimized conditions in hand, the scope of the alkyne coupling partner was investigated (Scheme 2). The model substrate was isolated in comparable yield and er as expected (3a). Other aryl alkynes were tolerated with electron-rich (3b and 3c), electron-neutral (3d and 3e), and electron-deficient substituents (3f−3h) on the arene. Substituents positioned meta and ortho on the aryl alkyne provided good enantioselectivity (3i−3l). Ethyl propiolate (3m), cycloalkyl alkynes (3n and 3o), and a heterocyclic alkyne (entry 3p) were tolerated, although several substrates required higher catalyst loadings or longer reaction times to reach higher conversion. Note that long reaction times are not uncommon for other ECuAAC reactions.15,18,19
Scheme 2. Substrate Scope of Alkyne Coupling Partnera.
aIsolated yields are reported. The yield is calculated based on either the alkyne (limiting reagent) or azide (kinetic resolution component). Enantiomeric ratio (er) was determined by chiral HPLC. Yield and er values are the average of duplicate trials. b2.5 mol % (CuOTf)2·PhMe, 10 mol % (S,S)-4-F-Ph-PYBOX, and 0.1 M in PhCF3.
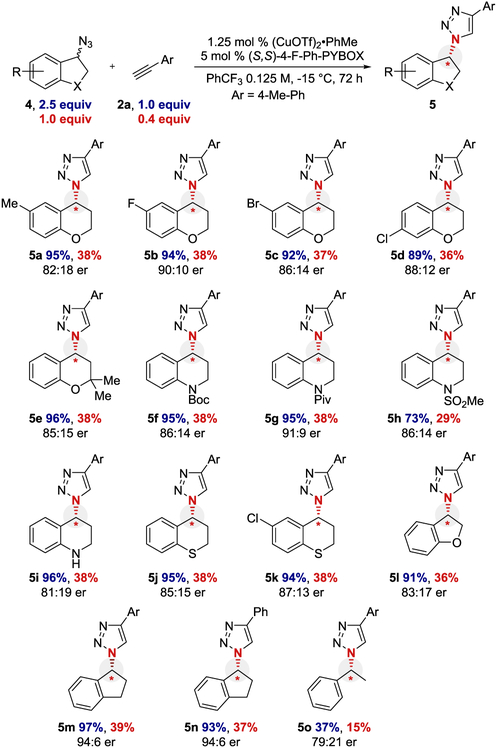
The scope of the azide component that could be kinetically resolved was explored (Scheme 3). Substituents on the chromane arene core were tolerated, including methyl (5a) and halogens (5b−5d). Groups could also be added α to the oxygen atom (5e). Azido-tetrahydroquinolines with various N-protecting groups (5f−5h) and the free NH (5i) provided triazoles in high yield and acceptable enantioselectivity. Azidothiochromanes (5j and 5k), azido-dihydrobenzofuran (5l), and azido-indane (5m and 5n) could also be resolved. Substrates 5m and 5n are noteworthy because the original E-CuAAC reported by Fokin and Finn described this as a problematic substrate (s < 1.3).14 Acyclic substrate 5o provided slower conversion and slightly reduced selectivity.
Scheme 3. Substrate Scope of Azide Coupling Partnera.
aIsolated yields are reported. The yield is calculated based on either the alkyne (limiting reagent) or azide (kinetic resolution component). Enantiomeric ratio (er) was determined by chiral HPLC. Yield and er values are the average of duplicate trials.
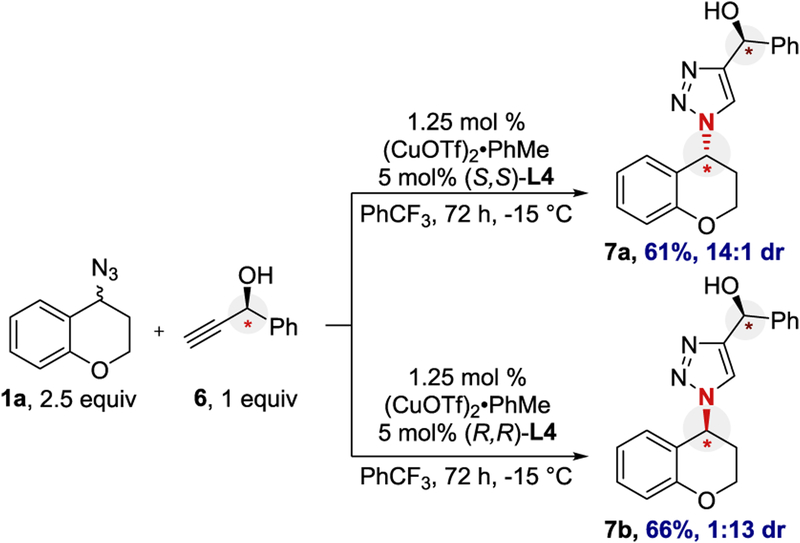
Chiral alkyne 6 was used to test for matched/mismatched behavior (Scheme 4). Treating azide rac-1a with chiral alkyne 6 and either enantiomer of ligand L4 resulted in moderate yield with opposite diastereoselectivity (14:1 and 1:13). This reversal of diastereoselectivity indicates this reaction is under catalyst control.
Scheme 4.
Matched/Mismatched Experiment
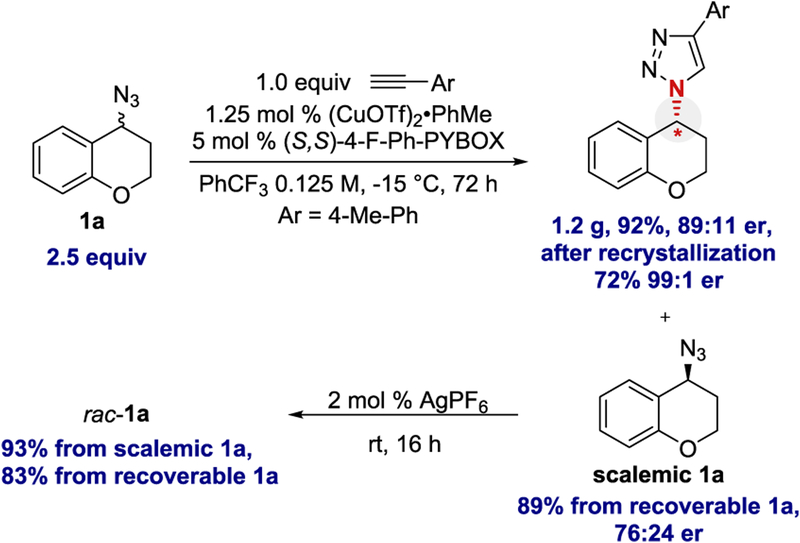
The kinetic resolution could be successfully scaled to provide more than 1 g of triazole product (Scheme 5). The initial enantioselectivity was 89:11 er, which corresponds to an s = 13.5. The enantiopurity could be enhanced upon recrystallization (99:1 er). The excess azide was recovered (76:24 er) and racemized upon exposure to catalytic AgPF6.28 The azide could then be recycled and used in a subsequent reaction. The ability to recycle the recovered azide improves the overall efficiency of the reaction. The (R)-1a enantiomer preferentially reacted with the catalyst derived from ligand (S,S)-L5. This was determined by analyzing scalemic azide recovered from the reaction. The scalemic azide was compared to a sample of (S)-1a which was accessed via diazo transfer from commercially available (S)-3,4-dihydro-2H-chromen-4-amine. The same analysis was conducted with azide recovered in the synthesis of triazoles 3j and 3p, which confirmed the absolute configuration of the product. The configuration of the other triazole products were assigned based on analogy.
Scheme 5.
Gram-Scale Reaction and Racemization of Recovered Azide
This report describes an expanded scope for both the azide and alkyne coupling partners in an E-CuAAC. The products of this kinetic resolution are α-N-chiral triazoles and can be obtained in up to 97% yield and up to 97:3 er. The reaction can be conducted to isolate more than 1 g of product and the excess azide can be recovered, racemized, and recycled. The er of the product triazoles can be readily improved to 99:1 with a single recrystallization.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health, under Award No. R35GM124718. We also acknowledge NIH Shared Instrumentation Grant No. S10OD011952.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b01556.
Procedures, characterization, and spectral and chromatographic data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Tornøe CW; Christensen C; Meldal M Peptidotriazoles on Solid Phase : [1,2,3]-Triazoles by Regiospecific Copper (I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem 2002, 67, 3057–3064. [DOI] [PubMed] [Google Scholar]
- (2).Rostovtsev VV; Green LG; Fokin VV; Sharpless KB A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- (3).Meldal M; Tornøe CW Cu-Catalyzed Azide - Alkyne Cycloaddition. Chem. Rev 2008, 108, 2952–3015. [DOI] [PubMed] [Google Scholar]
- (4).Pedersen DS; Abell A 1,2,3-Triazoles in Peptidomimetic Chemistry. Eur. J. Org. Chem 2011, 2011, 2399–2411. [Google Scholar]
- (5).Finn MG; Fokin VV Click Chemistry: Function Follows Form. Chem. Soc. Rev 2010, 39, 1231–1232. [DOI] [PubMed] [Google Scholar]
- (6).Kolb HC; Finn MG; Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- (7).Ellman JA; Jain RK; Wood WJL; Tsuruoka H; Patterson AW Substrate Activity Screening: A Fragment-Based Method for the Rapid Identification of Nonpeptidic Protease Inhibitors. J. Am. Chem. Soc 2005, 127, 15521–15527. [DOI] [PubMed] [Google Scholar]
- (8).Lee T; Cho M; Ko S-Y; Youn H-J; Baek DJ; Cho W-J; Kang C-Y; Kim S Synthesis and Evaluation of 1,2,3-Triazole Containing Analogues of the Immunostimulane Alpha-GalCer. J. Med. Chem 2007, 50, 585–589. [DOI] [PubMed] [Google Scholar]
- (9).Patterson AW; Wood WJL; Hornsby M; Lesley S; Spraggon G; Ellman JA Identification of Selective, Nonpeptidic Nitrile Inhibitors of Cathepsin S Using the Substrate Activity Screening Method. J. Med. Chem 2006, 49, 6298–6307. [DOI] [PubMed] [Google Scholar]
- (10).Huber K; Hamad B; Kirkpatrick P Fresh from the Pipeline: Ticagrelor. Nat. Rev. Drug Discovery 2011, 10, 255–256. [DOI] [PubMed] [Google Scholar]
- (11).Deprez-Poulain R; Hennuyer N; Bosc D; Liang WG; Enee E; Marechal X; Charton J; Totobenazara J; Berte G; Jahklal J; Verdelet T; et al. Catalytic Site Inhibition of Insulin-Degrading Enzyme by a Small Molecule Induces Glucose Intolerance in Mice. Nat. Commun 2015, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Mocharla VP; Walsh JC; Padgett HC; Su H; Fueger B; Weber WA; Czernin J; Kolb HC From In Situ to In Vivo: An In Situ Click-Chemistry-Derived Carbonic Anhydrase II Imaging Agent for Positron Emission Tomography. ChemMedChem 2013, 8, 43–48. [DOI] [PubMed] [Google Scholar]
- (13).Rodionov VO; Fokin VV; Finn MG Mechanism of the Ligand-Free CuI-Catalyzed Azide-Alkyne Cycloaddition Reaction. Angew. Chem., Int. Ed 2005, 44, 2210–2215. [DOI] [PubMed] [Google Scholar]
- (14).Meng JC; Fokin VV; Finn MG Kinetic Resolution by Copper-Catalyzed Azide-Alkyne Cycloaddition. Tetrahedron Lett 2005, 46, 4543–4546. [Google Scholar]
- (15).Brittain WDG; Buckley BR; Fossey JS Kinetic Resolution of Alkyne-Substituted Quaternary Oxindoles via Copper Catalysed Azide-Alkyne Cycloadditions. Chem. Commun 2015, 51, 17217–17220. [DOI] [PubMed] [Google Scholar]
- (16).Brittain WDG; Chapin BM; Zhai W; Lynch VM; Buckley BR; Anslyn EV; Fossey JS The Bull-James Assembly as a Chiral Auxiliary and Shift Reagent in Kinetic Resolution of Alkyne Amines by the CuAAC Reaction. Org. Biomol. Chem 2016, 14, 10778–10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Liu E-C; Topczewski JJ Enantioselective Copper Catalyzed Alkyne-Azide Cycloaddition by Dynamic Kinetic Resolution. J. Am. Chem. Soc 2019, 141, 5135–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhou F; Tan C; Tang J; Zhang YY; Gao WM; Wu HH; Yu YH; Zhou J Asymmetric Copper(I)-Catalyzed Azide-Alkyne Cycloaddition to Quaternary Oxindoles. J. Am. Chem. Soc 2013, 135, 10994–10997. [DOI] [PubMed] [Google Scholar]
- (19).Page P; Martin J; Stephenson G; Gaumont A-C; Deschamps D; Buttress J; Lancelot M; Sheldon A; Alayrac C An Investigation of the Asymmetric Huisgen ‘Click’ Reaction. Synlett 2013, 24, 2723–2729. [Google Scholar]
- (20).Zheng Z-J; Song T; Xu L-W; Deng Y; Xu Z; Zhou W; Li L Enantioselective Copper-Catalyzed Azide-Alkyne Click Cyclo-addition to Desymmetrization of Maleimide-Based Bis(Alkynes). Chem. - Eur. J 2015, 21, 554–558. [DOI] [PubMed] [Google Scholar]
- (21).Osako T; Uozumi Y Enantioposition-Selective Copper-Catalyzed Azide-Alkyne Cycloaddition for Construction of Chiral Biaryl Derivatives. Org. Lett 2014, 16, 5866–5869. [DOI] [PubMed] [Google Scholar]
- (22).Brittain WDG; Buckley BR; Fossey JS Asymmetric Copper-Catalyzed Azide-Alkyne Cycloadditions. ACS Catal 2016, 6, 3629–3636. [Google Scholar]
- (23).Ott AA; Goshey CS; Topczewski JJ Dynamic Kinetic Resolution of Allylic Azides via Asymmetric Dihydroxylation. J. Am. Chem. Soc 2017, 139, 7737–7740. [DOI] [PubMed] [Google Scholar]
- (24).Porter MR; Shaker RM; Calcanas C; Topczewski JJ Stereoselective Dynamic Cyclization of Allylic Azides : Synthesis of Tetralins, Chromanes, and Tetrahydroquinolines. J. Am. Chem. Soc 2018, 140, 1211–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ott AA; Packard MH; Ortuño MA; Johnson A; Suding VP; Cramer CJ; Topczewski JJ Evidence for a Sigmatropic and an Ionic Pathway in the Winstein Rearrangement. J. Org. Chem 2018, 83, 8214–8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Keith JM; Larrow JF; Jacobsen EN Practical Considerations in Kinetic Resolution Reactions. Adv. Synth. Catal 2001, 343, 5–26. [Google Scholar]
- (27).Worrell BT; Malik JA; Fokin VV Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ott AA; Topczewski JJ Catalytic Racemization of Activated Organic Azides. Org. Lett 2018, 20, 7253–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.