Abstract
Magnesium hydroxide nanoparticles (Mg(OH)2NPs) have recently attracted significant attention due to their wide applications as environmentally friendly antimicrobial nanomaterials, with potentially low toxicity and low fabrication cost. Here, we describe the synthesis and characterisation of a range of surface modified Mg(OH)2NPs, including particle size distribution, crystallite size, zeta potential, isoelectric point, X-ray diffraction (XRD), dynamic light scattering (DLS), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), energy dispersive X-ray analysis (EDX), Fourier transform infrared spectroscopy (FTIR), and transmission electron microscopy (TEM). We explored the antimicrobial activity of the modified Mg(OH)2NPs on the microalgae (C. reinhardtii), yeast (S. cerevisiae) and Escherichia coli (E. coli). The viability of these cells was evaluated for various concentrations and exposure times with Mg(OH)2NPs. It was discovered that the antimicrobial activity of the uncoated Mg(OH)2NPs on the viability of C. reinhardtii occurred at considerably lower particle concentrations than for S. cerevisiae and E. coli. Our results indicate that the antimicrobial activity of polyelectrolyte-coated Mg(OH)2NPs alternates with their surface charge. The anionic nanoparticles (Mg(OH)2NPs/PSS) have much lower antibacterial activity than the cationic ones (Mg(OH)2NPs/PSS/PAH and uncoated Mg(OH)2NPs). These findings could be explained by the lower adhesion of the Mg(OH)2NPs/PSS to the cell wall, because of electrostatic repulsion and the enhanced particle-cell adhesion due to electrostatic attraction in the case of cationic Mg(OH)2NPs. The results can be potentially applied to control the cytotoxicity and the antimicrobial activity of other inorganic nanoparticles.
Keywords: Mg(OH)2NPs, magnesium hydroxide, polyelectrolytes, poly (styrene sulfonate), poly (allyl amine) hydrochloride, antimicrobial nanoparticles, algae, yeast, bacteria, C. reinhardtii, S.cerevisiae, E. coli
1. Introduction
The increased proliferation of infectious illnesses that are caused by microorganisms found in food packaging, medical devices, water treatment systems, and domestic appliances has elicited increased interest [1,2,3]. The increased resistance of microorganisms against current biocides has caused great concern, particularly for individuals of compromised immune systems [4,5]. This has prompted expanded efforts to investigate new types of nanomaterials as antibacterial agents [6,7,8], which do not rely on the existing pathways of antimicrobial resistance. Recent studies have been concentrated on antibacterial inorganic nanoparticles, for example, metal oxide nanoparticles, like ZnO, MgO, CuO, Cu2O, Al2O3, TiO2, CeO2, and Y2O3; metals, e.g., copper, silver, gold etc., metal hydroxides, such as Mg(OH)2 as well as colloids made from biodegradable materials, such as chitosan, lignin, and dextran, loaded with antibacterial agents [9]. Mg(OH)2NPs have been successfully deployed as antifungal and antibacterial agents towards different microorganisms [10,11,12] and there are indications that they can be highly effective [13]. Mg(OH)2NPs have attracted significant attention over the years due to their wide applications in different fields as an environmentally friendly material with low cost of production [14,15,16,17] and they may be potentially used in pharmaceutical formulations [18,19,20,21]. However, a limited number of studies have investigated the antimicrobial effects of Mg(OH)2NPs and reported that the in vivo toxicity values are low, thus demonstrating that it has a non-toxic effect on humans in sensible amounts [22]. Recently, it has been reported that Mg(OH)2NPs were effective as antibacterial agents towards several bacteria, including E. coli, S. aureus, P. aeruginosa, and B. phytofirmans [23,24,25,26,27,28], and a number of studies have been focused on this new and effective antimicrobial agent [17]. Dong et al. have investigated the antibacterial action of Mg(OH)2NPs on Burkholderia phytofirmans and Escherichia coli [24]. Their results indicated that the Mg(OH)2NPs suspensions are effective towards B. phytofirmans and E. coli. Their study has also examined the role of the OH- and Mg2+ ions, which are naturally present in the Mg(OH)2NPs suspension, on the antimicrobial action. They showed that an alkaline medium of pH 10.4, as well as an equivalent amount of Mg2+ ions in the aqueous solution. could not kill the bacteria [24]. They have also indicated that Mg(OH)2NPs can kill E. coli, even in dark conditions, which suggested that no photocatalytic properties are involved in their antibacterial action [23]. Hence, the antibacterial mechanism of Mg(OH)2NPs seems to be very different to those of other metal and metal-based compounds [28,29,30,31]. Pan et al. synthesised Mg(OH)2NPs from three different precursors (MgCl2, MgSO4, and MgO) and tested their antibacterial efficiency towards E. coli as a model Gram-negative bacteria [17]. Bactericidal examinations indicated that the antibacterial activity of Mg(OH)2NPs was inversely related to particle size. Their results also revealed that the ability of Mg(OH)2NPs to adhere on the bacterial cell walls decreased in the order: Mg(OH)2_MgCl₂ > Mg(OH)2_MgSO₄ > Mg(OH)2_MgO, which showed that the toxicity of the produced Mg(OH)2NPs may be caused by the electrostatic interaction induced by secondary adsorption of counter-ions. This means that the type of precursor magnesium salt that is used to produce the Mg(OH)2NPs by hydrolysis can greatly influence their antimicrobial properties by the secondary absorption of counter-ions on the particles surface. These authors propose that Mg(OH)2NPs adsorb on the negatively charged bacterial cell wall and somehow disrupt its integrity, and increase its permeability, which kills the bacteria [17].
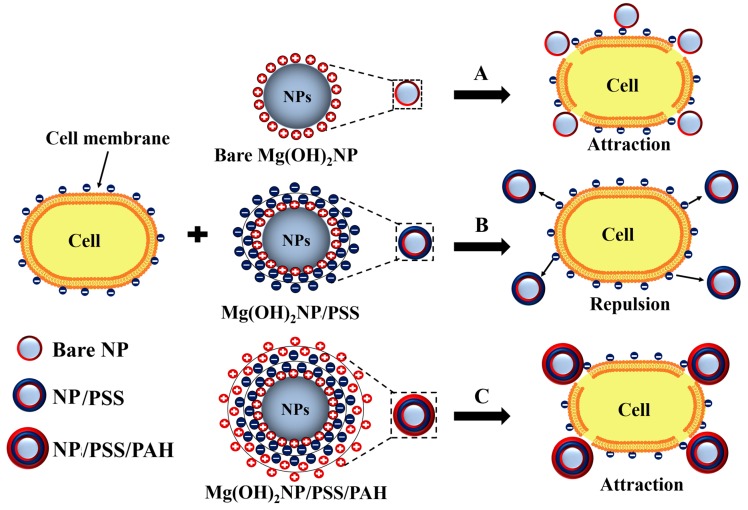
In this article, we investigate the role of the polymer coating in the antimicrobial activity of Mg(OH)2 nanoparticles that were synthesised by the direct precipitation method. Three different types of microorganisms, C. reinhardtii, S.cerevisiae cells, and Gram-negative E. coli were used to examine the antimicrobial activity of the surface modified Mg(OH)2NPs. In this study, we are interested in using the surface functionalized Mg(OH)2NPs as innovative anti-algal, anti-bacterial, and antifungal agents. As C. reinhardtii is a typical representative of the algae genre and S. cerevisiae is a fungal microorganism, they are a good proxy for these assessments. We explored the relationship between the antifungal and antibacterial effect of the particle size, surface charge, in addition to their adhesion to the microbial cell wall. The size of the Mg(OH)2NPs is likewise essential for their potential activity, as smaller particles have higher portability to relocate between the biological compartments [32]. Moreover, the surface charge of the Mg(OH)2NPs determines their ability to electrostatically interact with the biological membranes. The present study investigates the impact of the Mg(OH)2NPs concentration, their zeta potential, and particle size on the viability of C. reinhardtii, S.cerevisiae, and E. coli at different exposure times. We explore the antimicrobial activity and the nanoparticle internalisation between C. reinhardtii, S.cerevisiae, and E. coli. In our experiments with surface functionalized Mg(OH)2NPs on microbial cells, which were systematically done on C. reinhardtii, S.cerevisiae, and E. coli, we have tested their effect in the absence of growth media whose components may potentially interfere with the particle surface charge. This would lead to ambiguity in the results, depending of the media composition and concentration. To avoid this, we remove the microbial cells from the media prior to testing the effect of the surface functionalized Mg(OH)2NPs on them. We investigate the antibacterial activity of Mg(OH)2NPs that are coated with anionic and cationic polyelectrolytes. Our working hypothesis is that coating the Mg(OH)2NPs with cationic polyelectrolytes may enhance their antimicrobial activity, while coating them with anionic polyelectrolytes as an outer layer may lead to decreased antibacterial activity because of their electrostatic repulsion from the bacterial cell wall (Figure 1).
Figure 1.
Schematic diagram showing the various contacting patterns between the bare and polyelectrolyte-coated Mg(OH)2NPs on cells. (A and C) The adhesion of the uncoated and cationic polyelectrolyte-coated Mg(OH)2NPs to the cell wall surfaces is favoured due to their opposite surface charges. (B) The interaction between the anionic outer surface of the cell membrane and the Mg(OH)2NPs coated with anionic polyelectrolyte is repulsive. The cationic Mg(OH)2NPs and Mg(OH)2NPs/PSS/PAH nanoparticles are expected to be more effective against the microbial cells than the anionic Mg(OH)2NPs/PSS particles.
2. Materials and Methods
2.1. Materials
Magnesium chloride (98%, Sigma Aldrich, UK), sodium hydroxide (99.6%, Fisher, UK), and fluorescein diacetate (FDA, 98%, Fluka, UK) were used as supplied. Poly(sodium 4-styrene sulfonate) sodium salt (PSS), average M.W. 70 kDa, and poly(allylamine hydrochloride) (PAH), average M.W. 15 kDa, were purchased from Sigma Aldrich, UK. Deionized water that was produced by a Milli-Q reverse osmosis system (Millipore, UK) was used in all of the experiments. S.cerevisiae (from Sigma-Aldrich, UK) was cultured, as follows. 10 mg of lyophilised S.cerevisiae (baker’s yeast) was hydrated in 10 mL of pre-autoclaved deionized water at room temperature. Afterwards, 1 mL of the hydrated yeast suspension was added to 100 mL of the pre-autoclaved YPD culture media (yeast extract, peptone, and dextrose) and incubated for 48 h at 30 °C [33]. Escherichia coli, which was sourced from Thermofisher (Invitrogen MAX Efficiency™ DH10B™), was kindly provided for our antibacterial tests by Prof. Rotchell’s group at the University of Hull, UK. E. coli was grown up using Luria-Bertani medium (LB medium) [34], which can be prepared from 1 g tryptone, 0.5 g yeast extract, and 1 g sodium chloride in 100 mL deionized water. Subsequently, these components were autoclaved for one hour at 1.5 bar at 125 °C. A few microlitres of the stock suspension of E. coli were dispersed in the autoclaved culture media next to the Bunsen burner once the culture media was cooled down to room temperature. The cultured E. coli was incubated with shaking at 25 °C for 48 h to yield 5×105–1×106 colony forming units per mL (CFU/mL). Flickinger’s group from North Carolina University, USA kindly provided Chlamydomonas reinhardtii (cc-124 strain). This microalgae culture was grown in Tris-Acetate-Phosphate (TAP) culture medium and incubated at a temperature of 30 °C. The C. reinhardtii culture media consisted of TAP salts (NH4Cl; MgSO4 · 7H2O; and, CaCl2 · 2H2O), phosphate buffer solution (PBS), and Hutner’s trace elements solution (EDTA disodium salt, ZnSO4 · 7H2O, H3BO3, MnCl2 · 4H2O, CoCl2 · 6H2O, CuSO4 · 5H2O, FeSO4 · 7H2O, (NH4)6Mo7O24 · 4H2O), all purchased from Sigma-Aldrich, UK. The microalgae batch was grown in TAP media at pH 7 while being illuminated for 72 h by a white luminescent lamp with a light intensity of 60 W m−2 under constant stirring on a magnetic stirrer.
2.2. Characterisation
The Mg(OH)2NPs size distribution and the zeta potential were characterised by a Zetasizer Nano ZL instrument (Malvern, UK). A digital sonicator (Branson LTD) was utilized for dispersing the Mg(OH)2NPs samples at 40% amplitude for 15 min at 2.0 s ON/2.0 s OFF pulse time. The thermogravimetric analysis (TGA) of Mg(OH)2NPs was done using a Mettler Toledo TGA/DSC instrument under N2 atmosphere. The crystallite size of Mg(OH)2NPs at various temperatures was studied by X-ray diffraction (Siemens D5000 X-Ray Diffractometer at 0.15418 nm wavelength). A JEM 2011 (JEOL, Japan) Transmission Electron Microscopy (TEM) machine was used to characterise the particle size and the morphology of Mg(OH)2NPs on the microbial cells surface. The JEOL JSM-6480 LV SEM instrument was utilized for characterising the morphology of Mg(OH)2NPs with bacterial.
2.3. Synthesis of Mg(OH)2NPs
The Mg(OH)2NPs were prepared from magnesium chloride (MgCl2) as a source of magnesium ions and sodium hydroxide (NaOH) aqueous solutions. Precipitation was induced by a dropwise addition of 0.4 M NaOH into the 0.2 M MgCl2 solution under continuous stirring at different reaction temperatures (i.e., 25 °C, 50 °C, 75 °C and 100 °C) for 1 h. The white product was centrifuged and washed with copious amounts of high purity water and ethanol for the effective removal of impurities. The final product was dried at 80 °C for 24 h [6]. Aqueous dispersions of the Mg(OH)2NPs were then prepared by dispersing 0.025 g of the Mg(OH)2 sample in 100 mL deionized water by using a digital sonicator (Branson Ltd.) at 40% of the maximum power for 15 min at 2 s ON/2 s OFF pulse time. Figure S1 shows a schematic diagram of the synthesis method of Mg (OH)2NPs.
2.4. Preparation of Polyelectrolyte-Coated Mg(OH)2NPs
Polyelectrolyte-coated Mg(OH)2NPs were prepared by using the Mg(OH)2NPs synthesised at a reaction temperature of 75 °C. 50 mL of 1000 µg mL−1 Mg(OH)2NPs dispersion in deionized water were added dropwise to an equal amount of 50 mg mL−1 PSS (M.W. ~70 kDa) solution in 1 mM NaCl. The samples were washed three times by centrifugation for 1 h at 10,000 rpm to remove the excess of PSS after shaking for 1 h on orbital shaker. Finally, the PSS-coated Mg(OH)2NPs were re-dispersed in 50 mL deionized water [35] and the particle size and zeta potential measured by a Zetasizer Nano ZL instrument. To prepare the PAH-coated nanoparticles, the PSS-coated Mg(OH)2NP suspension was mixed dropwise with 50 mL of 50 mg mL−1 PAH (M.W. 15 kDa) that was dissolved in 1 mM NaCl solution. The mixture was shaken for 20 min and centrifuged three times at 10,000 rpm for one hour to yield Mg(OH)2NPs/PSS/PAH.
2.5. Antimicrobial Assay of Polyelectrolyte-Coated Mg(OH)2NPs on S.cerevisiae
The effect of Mg(OH)2NPs on S.cerevisiae cells was examined after removing the cells from the culture media. 30 mL dispersion of S.cerevisiae cells were washed by centrifugation with deionized water three times, and then re-dispersed in 30 mL deionized water. 5 mL of S.cerevisiae cell dispersion in deionized water were incubated with 5 mL Mg(OH)2NPs aqueous suspension upon increasing total particle concentrations (0, 250, 500, 1000, 2500, and 5000 μg mL−1) for 10 min, 6 h, 12 h, and 24 h. 1 mL of each incubated S.cerevisiae sample was centrifuged at 3500 rpm for 4 min and washed with deionized water to remove the excess of Mg(OH)2NPs. The S.cerevisiae cells were re-suspended in 1 mL of deionized water, then two drops of 1 mM FDA solution in acetone were added to each sample and then mixed together for 15 min. After that, the samples were washed three times with deionized water by centrifugation at 3500 rpm for 4 min. Finally, the viability of the cells was examined by fluorescence microscopy and an automatic cell counter (Nexcelom Cellometer Auto X4 Fluorescence). Similar experiments were utilized to test the effect of Mg(OH)2NPs that were coated with polyelectrolytes on the viability of S.cerevisiae.
2.6. Antibacterial Assay of Polyelectrolyte-Coated Mg(OH)2NPs on E. coli
10 mL of the E. coli culture grown in LB medium was washed, centrifuged three times with deionized water at 5000 rpm for three minutes, and redispersed in 100 mL deionized water. Subsequently, 5 mL of the washed E. coli suspension were incubated with a series of 5 mL aliquots of aqueous dispersions of Mg(OH)2NPs of different concentrations (0, 250, 500, 750, 1000, 2500, 5000, and 6000 µg mL−1). After each incubation, 1 mL of each E. coli suspension sample was washed and re-suspended in 1 mL deionized water. Afterwards, 100 μL of culture media free E. coli bacteria were incubated with 100 μL of the BacTiter-Glo Microbial cell viability reagent in a white opaque 96-well solid flat bottom microplate, shaken for 30 s, and then incubated for five minutes at 25 °C. The relative luminance was measured as a function of incubation time to find out the cell viability upon incubation with different concentration of Mg(OH)2NPs. The same experiments were also repeated with polyelectrolyte-coated Mg(OH)2NPs. This was done by incubating an aliquot of the E. coli suspension (diluted 10 times) with Mg(OH)2NPs that were coated with poly(sodium-4-styrenesolfonate and poly(allylamine hydrochloride) for up to 24 h.
2.7. Antialgal Activity of Polyelectrolyte-Coated Mg(OH)2NPs to C. reinhardtii
50 mL of C. reinhardtii culture were washed from the culture media three times by using deionized water and centrifugation, and finally re-dispersed in 30 mL deionized water. 5 mL of the washed C. reinhardtii cells were incubated with a series of 5 mL aliquots of aqueous Mg(OH)2NPs dispersions at different concentrations at 25 °C. A control sample of the same cells was treated in a similar way without any exposure to Mg(OH)2NPs. After that, 1 mL of the C. reinhardtii suspension was taken from each treated sample and washed with deionized water to remove the excess of nanoparticles by centrifugation at 3500 rpm for 4 min. The C. reinhardtii cells were re-suspended in 1 mL deionized water, and two drops of 1 mM FDA solution in acetone were added to each sample and mixed together for 15 min. After that, the samples were washed three times with deionized water by centrifugation at 3500 rpm for 4 min. Finally, the automatic cell counter was utilized to assay the microalgae cell viability. The same method was used to test the effect of Mg(OH)2NPs that were coated with PSS and PAH on the viability of C. reinhardtii, which were incubated at different particle concentrations for various exposure times.
2.8. SEM and TEM Sample Preparation Protocol for C. reinhardtii, S.cerevisiae and E. coli after Exposure to Bare- and Polyelectrolyte-Coated Mg(OH)2NPs
The C. reinhardtii, S.cerevisiae and E. coli were washed by centrifugation and then fixed with 2.5% glutaraldehyde at room temperature for two hours in 0.1M cacodylate buffer pH 7.2 after incubation with the bare- or polyelectrolyte-coated Mg(OH)2NPs of various concentrations. These samples were then post-fixed in 1% osmium tetroxide for 1 h, and then dehydrated in a range of ethanol-water mixtures with an increasing ethanol content from 50 vol% up to 100 vol%, followed by a critical point drying. After incubation with Mg(OH)2NPs, the microbial cells were prepared for TEM imaging while using the following procedure. The cells were washed with deionized water to remove the excess of Mg(OH)2NPs by centrifugation at 500 rpm and then fixed in 2 wt% glutaraldehyde for one hour at room temperature. This was followed by a treatment with 1 wt% osmium tetroxide for one hour. Subsequently, the samples were incubated for one hour with 2.5% uranyl acetate and washed with aqueous ethanol solutions of increasing concentration, as described above. After standard dehydration, the microbial samples were embedded in fresh epoxy/Araldite at 60 °C for two days, left for two days at room temperature, and sectioned with an ultra-microtome. SEM and TEM imaged microbial samples before and after the nanoparticle treatment.
2.9. Zeta Potential Measurements of the C. reinhardtii, S.cerevisiae and E. coli cells after treatment with Mg(OH)2NPs
The changes of surface charge of C. reinhardtii, S.cerevisiae, and E. coli after exposure to Mg(OH)2NPs at different concentrations (0, 250, 500, 750, 1000, 2500, 5000, and 6000 µg mL−1) were determined by a Zetasizer Nano ZL instrument (Malvern, UK). The cells were removed from the excess of Mg(OH)2NPs in the aqueous phase by centrifugation, and then dispersed in deionized water. For each sample, an appropriate amount of undiluted solution was placed into the cuvette, and an average zeta potential value was obtained from three individual measurements. The solution media was deionized water in all of the zeta potential measurements.
2.10. MIC of Non-modified and PSS/PAH-coated Mg(OH)2NPs on Microbial Cells
The following protocol was used to determine the Minimal Inhibitory Concentration (MIC) of Mg(OH)2NPs and PSS/PAH-coated Mg(OH)2NPs on cells. A negative control of 100 µL of LB medium was added to the first line of wells of a 96 well plate. 50 µL of LB medium were added to the treatment wells and the positive bacteria control wells. A stock solution of Mg(OH)2NPs and PSS/PAH-coated Mg(OH)2NPs was created in fresh LB medium to a total volume of 10 mL. 50 µL of this formulation ware added to the first line of treatment wells, and serial diluted 1:2 across the 96 well plate, which ensured that it was mixed by pipetting up and down within each well. An overnight culture of E. coli was diluted into a sterilised 0.85% saline until an absorbance reading between 0.08 and 0.12 at 625 nm was obtained on a spectrophotometer (0.5 Mcfarland Standard). The saline diluted bacteria were further diluted 1:150 into LB (10 mL LB + 66.67 µL of bacteria in saline solution) yielding a 10 mL stock containing 5 × 105–1 × 106 cells per mL. 50 µL from this bacteria stock were added to each treatment and positive bacteria control wells, seeding with 2.5 × 104–5 × 104 cells per well. Each well contained a final volume of 100 µL, with decreasing concentrations of treatment on equal amounts of bacteria. The plate was incubated for 24 h at 37 °C. After incubation, 20 µL of resazurin solution were added to each well. The MIC was determined from the lowest concentration treatment, which inhibited growth.
3. Results and Discussion
3.1. Preparation and Characterisation of Mg(OH)2NPs
The mean hydrodynamic diameter and zeta potential of the Mg(OH)2NPs in deionized water were measured by the dynamic light scattering instrument (DLS) of suspensions that were prepared by dispersing 0.025 g of Mg(OH)2NPs sample in 100 mL of deionized water by a digital sonicator. The average hydrodynamic diameter of Mg(OH)2NPs was found to be 69 ± 10 nm and their zeta potential was +30 ± 6 mV, as shown in Figure S2 and Figure S3. The thermo gravimetric analysis (TGA) that was carried out between 50 and 1000 °C, as shown in Figure S4A, indicated that the Mg(OH)2 sample was stable up to 270 °C. Subsequently, the endothermic peak corresponding to the removal of the adsorbed water molecules took place between 270–300 °C, as also reported by other authors [36]. The major weight loss step has been found in the temperature range of 300–450 °C, which is due to the transition phase, corresponding to the decomposition of Mg(OH)2NPs to MgO. The TGA curve exhibits a total mass loss equal to 29.46%, which is slightly lower than the calculated mass loss (30.8%) that is attributed to the complete dehydroxylation process of Mg(OH)2. This result also agrees with previous studies [36,37,38]. An Energy dispersive X-ray Diffraction (EDX) analysis was carried out on the synthesised Mg(OH)2NPs to verify the elemental composition. The EDX data in Figure S4B confirm the presence of magnesium and oxygen signals in the Mg(OH)2NPs sample. The elemental analysis of the Mg(OH)2NPs yielded 36.59% of magnesium and 59.94% of oxygen, which indicates that the formed Mg(OH)2NPs were in its highly purified form and in agreement with previous studies [24].
We studied the effect of annealing temperature on the particle size of Mg(OH)2NPs for the synthesis that was done at various reaction temperature (25 °C, 50 °C, 75 °C, and 100 °C). Figure S4C shows the impact of the temperature of the reaction mixture on the size of Mg(OH)2NPs for one hour. Mg(OH)2NPs of lower average size were produced at 75 °C and 100 °C, while larger particles were created at 25 °C and 50 °C. Therefore, 75 °C and 100 °C were the optimal temperatures for the production of Mg(OH)2NPs.
The zeta potential and particle size of the produced Mg(OH)2NPs was measured at pH in the range 3–12 and the results are shown in Figure S4D. The isoelectric point of the non-coated Mg(OH)2NPs was approximately at pH 11.7, i.e., the bare Mg(OH)2NPs are cationic at neutral pH. As it can be seen from Figure S4D, the zeta potential decreases and the particle size increases upon the increase of pH. The aggregation of Mg(OH)2NPs occurs above pH 8.5. The Fourier transform infrared spectroscopy (FTIR) spectrum of the Mg(OH)2NPs synthesised using a magnesium chloride solution at different reaction temperatures is shown in Figure S5 (ESI). The sharp and intense 3700 cm−1 FTIR peak corresponds to the Mg(OH)2 asymmetric O–H stretching. The band at 1400 cm−1 is due to the water O–H stretch. The strong and wide 570 cm−1 peak is due to Mg−O stretching. No other absorption peaks from impurities were detected. This result indicates that the Mg(OH)2 that was obtained had higher purity and it is also in agreement with previous studies [39,40].
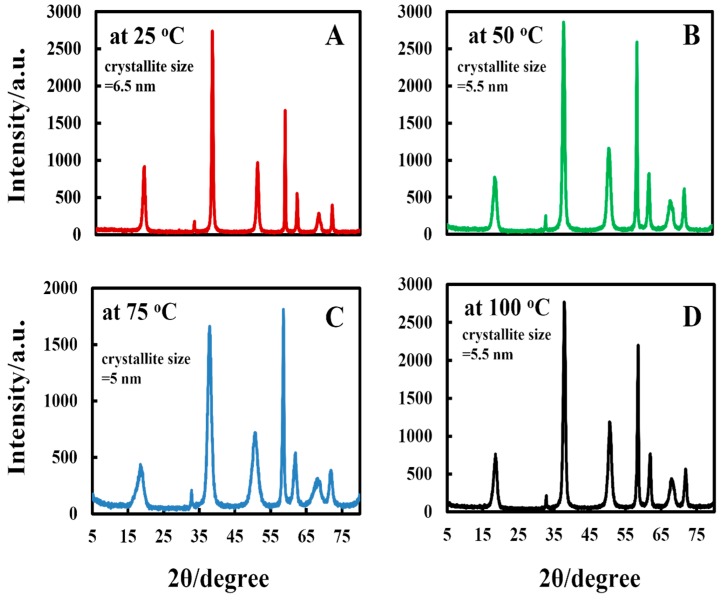
3.2. Effect of the Precipitation Temperature on the Crystallite Size of the Synthesised Mg(OH)2NPs
Figure 2 shows the XRD pattern of Mg(OH)2NPs samples that were obtained at various reaction temperatures 25 °C, 50 °C, 75 °C, and 100 °C while using magnesium chloride as a precursor. The diffraction peaks are in agreement with the hexagonal structure of Mg(OH)2NPs according to Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 00-044-1482, which indicates that no apparent impurities are detected. The average crystallite size of Mg(OH)2NPs was calculated by using the Scherrer equation, D = Kλ/βcosθ, were D is the crystallite size in nm, K is a dimensionless shape constant taken as 0.94, 2θ is the diffraction angle, λ is the wavelength of the X-ray radiation (CuKα = 0.15406 nm), and β is the full width at the half-maximum (FWHM) of the diffraction peak.
Figure 2.
Comparison of the X-ray pattern of Mg(OH)2NPs precipitated at 25 °C (A), 50 °C (B), 75 °C (C), and 100 °C (D).
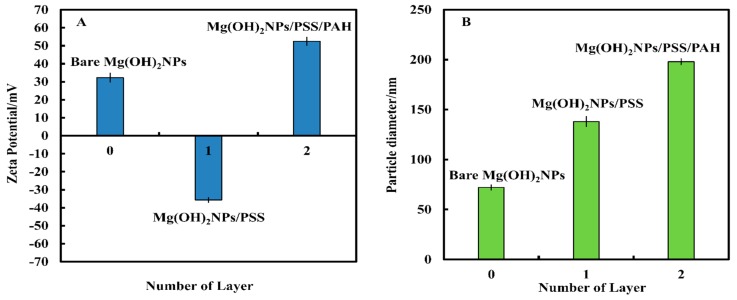
3.3. Polyelectrolyte-Functionalized Mg(OH)2NPs
We coated 69 nm Mg(OH)2NPs with two subsequent layers of PSS and PAH via the procedures explained above [35]. Figure 3A shows the zeta potential of the coated Mg(OH)2NPs as a function of the number of polyelectrolyte layers. The zeta potential of the Mg(OH)2NPs changed from approximately +30 mV to −36 mV for Mg(OH)2NPs/PSS. Further coating with PAH yielded positively charged Mg(OH)2NPs/PSS/PAH with a zeta potential of +51 mV. As expected, the particle surface charge alternates with the addition of the oppositely charged polyelectrolyte layer. Figure 3B shows that the coated NPs size increases after every subsequent polyelectrolyte coating due to partial aggregation.
Figure 3.
(A) The zeta potential and (B) particle size of bare-and polyelectrolyte-coated Mg(OH)2NPs.
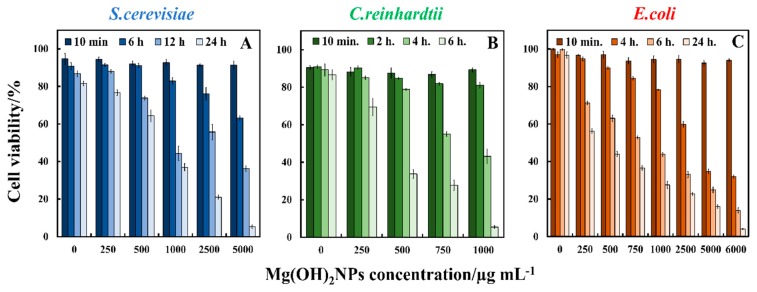
3.4. Antimicrobial Assay of Bare Mg(OH)2NPs on S.cerevisiae, C. reinhardtii and E. coli
We compared the antimicrobial activity of bare Mg(OH)2NPs on C. reinhardtii, S. cerevisiae, and E. coli, and Figure 4 shows the cell viability. We incubated samples of S.cerevisiae cells with dispersed Mg(OH)2NPs at different particle concentrations (0, 250, 500, 1000, 2500, and 5000 μg mL−1) for different periods of time, up to one day. Subsequently, an aliquot of every sample was taken to examine the number of viable S.cerevisiae cells while using an automatic cell counter by FDA cell viability assay. Figure 4A shows the percentage of viable S.cerevisiae cells at various incubation times (10 min., 6 h, 12 h, and 24 h). One can see that the percentage of viable S.cerevisiae after 10 min incubation is at the same level as in the untreated sample. After 6 h of incubation, no measurable antimicrobial effect is noticed up to 500 µg mL−1 Mg(OH)2NPs; however, antimicrobial activity is observed at 1000, 2500, and 5000 µg mL−1 Mg(OH)2NPs. After 12 h, the viability of S.cerevisiae sharply decreases at particle concentrations in the range 500–5000 µg mL−1. After one day of incubation, concentrations higher than 250 µg mL−1 bare Mg(OH)2NPs showed measurable antimicrobial activity towards S.cerevisiae. The optical microscopy examination of these samples suggests that the S.cerevisiae cells aggregate at a high particle concentration. Our results indicate a strong decline of the S.cerevisiae cell viability at concentrations above 1000 µg mL−1 bare Mg(OH)2NPs. Figure S15 (ESI) shows the same data, as in Figure 4A–C in CFU mL−1.
Figure 4.
The antimicrobial activity of bare Mg(OH)2NPs on (A) S.cerevisiae (B) C. reinhardtii and (C) E. coli at various particle concentrations. The cells were incubated with the Mg(OH)2NPs at different periods of time shown.
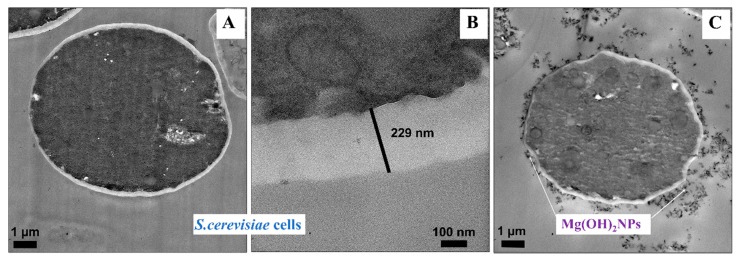
The data in Figure 4A suggest that, at high concentrations of bare Mg(OH)2NPs, they electrostatically adhere to the negatively charged cell membranes, which subsequently kills the cells. The attachment of Mg(OH)2NPs to the cells was also examined by TEM imaging. Figure 5A,C show TEM images of the S.cerevisiae cells before and after treatment with 1000 µg mL−1 Mg(OH)2NPs solution for 24 h. Those are compared with the untreated samples of S.cerevisiae that are shown in Figure 5A,B. The TEM images show that, before the treatment (Figure 5A), the membrane of the S.cerevisiae cells is regular and smooth, the treatment with 1000 µg mL−1 Mg(OH)2NPs leads to a significant accumulation of Mg(OH)2NPs on the external wall of S.cerevisiae at such a high particle concentration (Figure 5C).
Figure 5.
Transmission electron microscopy (TEM) images of S. cerevisiae after being incubated for one day with bare Mg(OH)2NPs: (A) A control sample without Mg(OH)2NPs. (B) A high-resolution TEM image of the S. cerevisiae wall without Mg(OH)2NPs. (C) S. cerevisiae cells incubated with 1000 µg mL−1 Mg(OH)2NPs showing the attachment of Mg(OH)2NPs to the outer cell surface.
We also confirmed these results by EDX of the treated S. cerevisiae cells, which showed the presence of Mg on the outer part of the cell membrane (Figure S6, ESI). Although the exposure of the S. cerevisiae cells to bare Mg(OH)2NPs at concentration 1000 µg mL−1 caused a cytotoxicity effect (Figure 5C), it did not cause an internalisation of Mg(OH)2NPs, as the cell wall of S. cerevisiae cells is very thick (200 nm, Figure 5B) when compared to other microbial cells. We envisage two probable mechanisms for the antimicrobial effect of Mg(OH)2NPs on yeast. There is a significant accumulation of Mg(OH)2NPs on the cell membranes due to their cationic nature at neutral pH. As these particles have very irregular morphology and they consist of smaller crystallites, their adhesion to the cell membrane in large amounts can potentially cause its dislocation and cracking. Local damage of the membrane may lead to cell viability loss although we do not observe a straight permeation of Mg(OH)2NPs to the S.cerevisiae wall. Another possible mechanism of membrane damage can be caused by the counter-ion atmosphere of the Mg(OH)2NPs, which consists of highly concentrated hydroxyl ions (OH-) of very high local pH which can cause lipid hydrolysis on the membrane surface and killing the cell. Some of these mechanisms have been commented on by other authors for uncoated Mg(OH)2NPs [17,24,28].
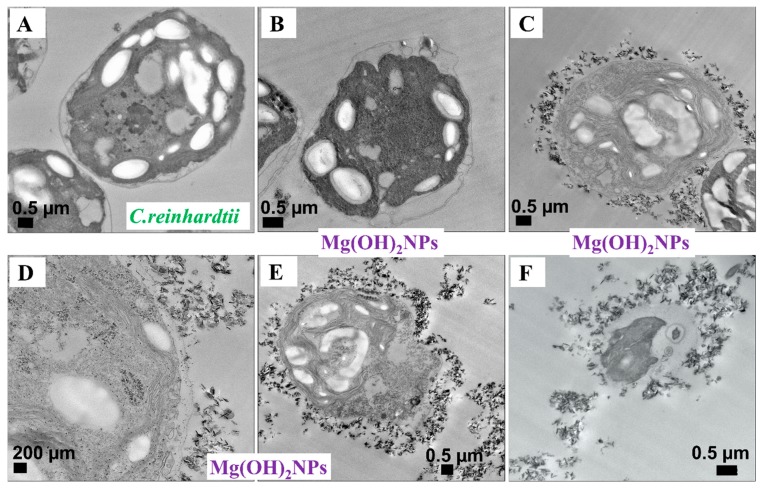
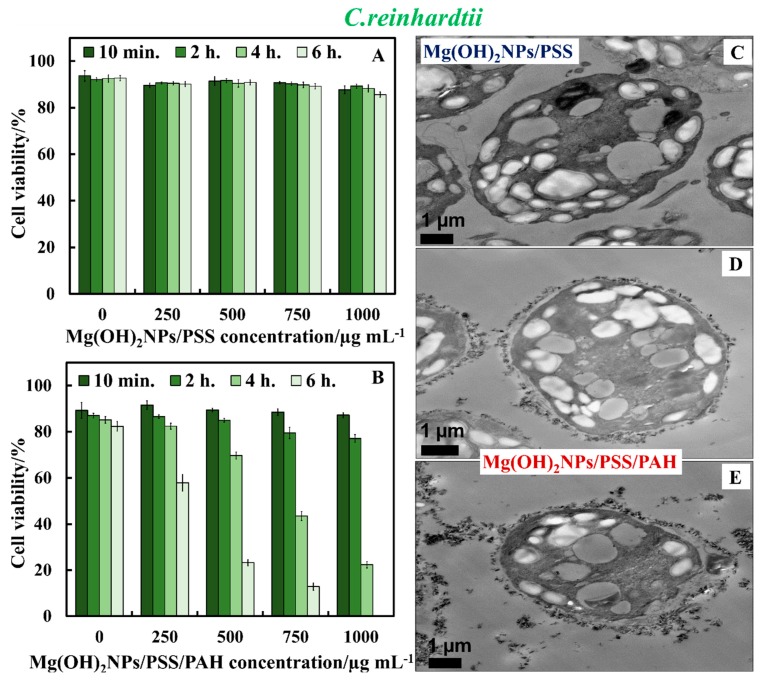
We also examined the antimicrobial activity of Mg(OH)2NPs towards C. reinhardtii under similar conditions for various exposure times, as shown in Figure 4B. At a 10 min exposure time, all of the C. reinhardtii were viable at the similar level as the untreated sample. After two hours of incubation, the C. reinhardtii viability declined for Mg(OH)2NPs concentrations from 250 µg mL−1 to 1000 µg mL−1. After 4 h, the C. reinhardtii viability was reduced to 40% at 1000 µg mL−1 of Mg(OH)2NPs, while, after 6 h, it sharply declined for 250 µg mL−1 to 1000 µg mL−1 concentrations of Mg(OH)2NPs, which led to the complete loss of cell viability at concentrations above 750 µg mL−1 Mg(OH)2NPs. Figure 6 shows TEM images of C. reinhardtii exposed to Mg(OH)2NPs at various concentrations. TEM images of the C. reinhardtii after 6 h of incubation with Mg(OH)2NPs indicate the localization of the Mg(OH)2NPs with respect to the cell membrane. One can see that the outer cell wall of C. reinhardtii obtained a thick layer of associated NPs after treatment with 750 µg mL−1, 1000 µg mL−1 and 5000 µg mL−1 concentrations of Mg(OH)2NPs (Figure 6C–F). The internalization of Mg(OH)2NPs in the C. reinhardtii was not observed, even at 5000 μg mL−1 Mg(OH)2NPs, as shown in Figures S7 and S8. The EDX shows the absence of Mg in the inside of C. reinhardtii, but confirms its presence on the outer wall.
Figure 6.
TEM images of C. reinhardtii after being exposed to 0, 250, 750, 1000 and 5000 µg mL−1 Mg(OH)2NPs for six hours (fixed, embedded in resin and sectioned). (A) An untreated sample without Mg(OH)2NPs; (B) C. reinhardtii treated with 250 μg mL−1 Mg(OH)2NPs; (C) and (D) C. reinhardtii incubated with 750 μg mL−1 Mg(OH)2NPs at different magnifications. (E) and (F) C. reinhardtii treated with 1000 μg mL−1 and 5000 μg mL−1 Mg(OH)2NPs, respectively.
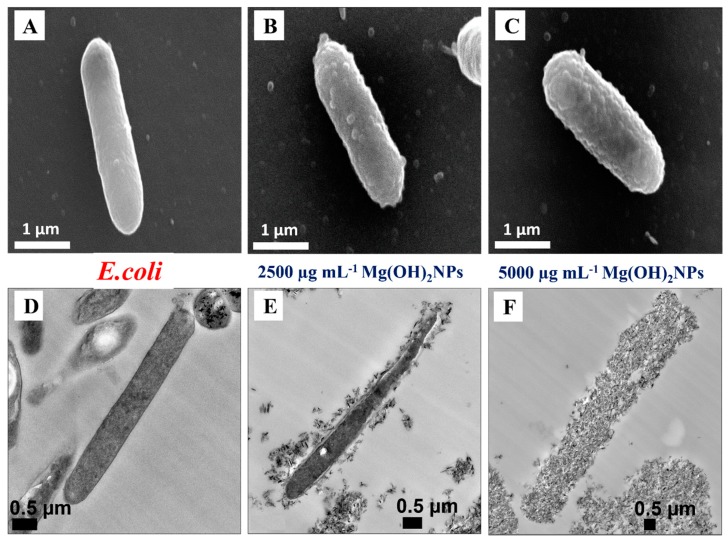
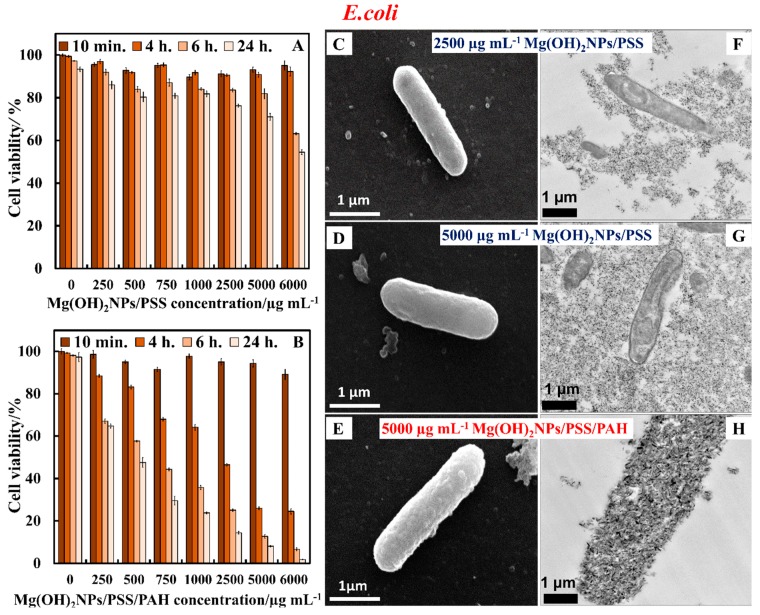
Figure 4C shows the results for the effect of bare Mg(OH)2NPs towards E. coli at various incubation times. The data demonstrate that the bare Mg(OH)2NPs have excellent antimicrobial effects on the E. coli at 6000 µg mL−1 for one day. The E. coli viability sharply decreases for treatment with 6000 µg mL−1 Mg(OH)2NPs after four hours of exposure. The viability decreases further after six hours and, after one day, it resulted in approximately 97% loss of viability. Past research suggested that the antibacterial effect might be credited to multiple factors: (i) the cellular internalization of NPs where they could potentially interfere with the bacterial DNA and cellular organelles [41,42,43,44,45]; (ii) immediate contacts with the bacterial cell wall [23,24]; and, (iii) the increased local dissolution of metal ions of the nanoscale metal oxide [46,47]. Usually, the antimicrobial impact is dependent on the size of the nanoparticles and a better antimicrobial effect is achieved with smaller nanoparticles [17,48,49,50].
The Mg(OH)2NPs with the smallest size (about 70 nm) had the highest antimicrobial activity. We undertook TEM imaging and EDX analysis to examine the location of magnesium in the E. coli after treating them with non-coated Mg(OH)2NPs. Magnesium was not detected by the EDX in many randomly selected regions inside the E. coli, but it was primarily found on the outer side of the cell wall, as shown in Figure S9 (ESI). This showed that the dissolved magnesium ions and Mg(OH)2NPs did not go to the inside of E. coli. Nevertheless, evident changes of the E. coli cell structure were seen after incubation with Mg(OH)2NPs. Figure 7D shows the images of untreated E. coli, where the bacteria have preserved the integrity of their cell walls. After incubation with 2500 µg mL−1 (7E) and 5000 µg mL−1 (7F) Mg(OH)2NPs for one day, the cell profile became fussy and the walls of E. coli appear as disintegrated. Therefore, the antimicrobial activity of Mg(OH)2NPs might be expressed more via their adsorption on the outer side of the cell wall, rather than through internalization in the cell, which leads to the decay of the cell walls of E. coli. Furthermore, SEM imaging was used to study the presence of Mg(OH)2NPs on the surfaces of the bacteria. Figure 7A–C show E. coli samples after being treated with 2500 µg mL−1 and 5000 µg mL−1 Mg(OH)2NPs for one day. They indicate that the cell wall has a build-up of a dense layer of nanoparticles. Moreover, EDX indicated that the samples contain magnesium, and confirmed that the Mg(OH)2NPs have the ability to adhere on the bacterial cell wall with occasional penetration on the inside. Consequently, the E. coli lack of viability is associated with the compromised integrity of bacteria walls, which is seen by SEM and TEM for these samples. This is consistent with the mechanisms outlined above which indicates that the antimicrobial action of Mg(OH)2NPs on the cells is likely to be due to the cationic character of Mg(OH)2NPs that adsorb on the negatively charged bacterial cell wall by electrostatic attraction. The adsorbed Mg(OH)2NPs disrupt the integrity of the bacterial cell wall, which then increases its permeability and kills the bacteria.
Figure 7.
Scanning electron microscopy (SEM) and TEM images of E. coli after being incubated for 24 h with a suspension of bare Mg(OH)2NPs: (A) SEM and (D) TEM images of an untreated sample. (B) SEM and (E) TEM images of E. coli incubated with 2500 µg mL−1 Mg(OH)2NPs. (C) SEM and (F) TEM images of E. coli incubated with 5000 µg mL−1 Mg(OH)2NPs.
3.5. Zeta Potential Measurements of Cells after Treatment with Mg(OH)2NPs
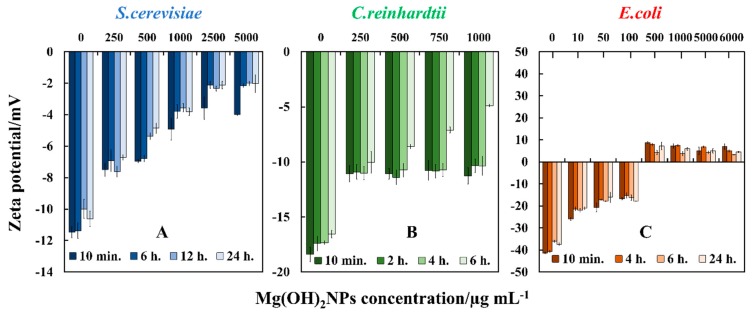
We further explored the effect of the particles attachment on the outer cell wall, as it may play a significant role on their antimicrobial action [17,24,28]. We studied the zeta potential of the S.cerevisiae, C. reinhardtii, and E. coli after treatment with Mg(OH)2NPs in solution. The cells were incubated with Mg(OH)2NPs suspensions at different particle concentrations. Subsequently, an aliquot of every suspension was taken to examine the cells average zeta potential value by a Zetasizer instrument. We found that Mg(OH)2NPs have an average zeta potential of +30 ± 6 mV. Upon incubation with bare Mg(OH)2NPs, the S.cerevisiae cells, which are negatively charged (zeta potential of −12 ± 5 mV), still were shown to be negative, but significantly reduced by magnitude zeta potential due to a build–up of cationic NPs, as shown in Figure 8A. Note that the zeta potential of threated cells does not vary much with the duration of the treatment.
Figure 8.
The zeta potential of cells treated with different concentrations of Mg(OH)2NP suspensions at various incubation times: (A) S.cerevisiae, (B) C. reinhardtii, and (C) E. coli cells.
The C. reinhardtii cells that have a negative zeta potential of −18 ± 5mV also reduced their zeta potential by magnitude after treatment with the cationic Mg(OH)2NPs, but did not charge reverse, even at high particle concentrations, as presented in Figure 8B. Figure 8C shows the impact of bare Mg(OH)2NPs on the E. coli zeta potential. E. coli cells, which carried negative charge (zeta potential −41±5 mV), remained negatively charged when treated with up to 100 µg mL−1 Mg(OH)2NPs. At higher Mg(OH)2NPs concentration, the zeta potential of E. coli cells turned positive when exposed to 500 µg mL−1 to 6000 µg mL−1 Mg(OH)2NPs. These results show that the adhesion of Mg(OH)2NPs to cells might indeed be primarily driven by electrostatic interactions [17,51]. It can be concluded that the positive charge of Mg(OH)2NPs has high impact on the adsorption of particles on the cells membrane. The SEM and TEM images confirmed this (Figure 6).
3.6. Antimicrobial Assay of Polyelectrolyte-Coated Mg(OH)2NPs on S.cerevisiae, C. reinhardtii, and E. coli
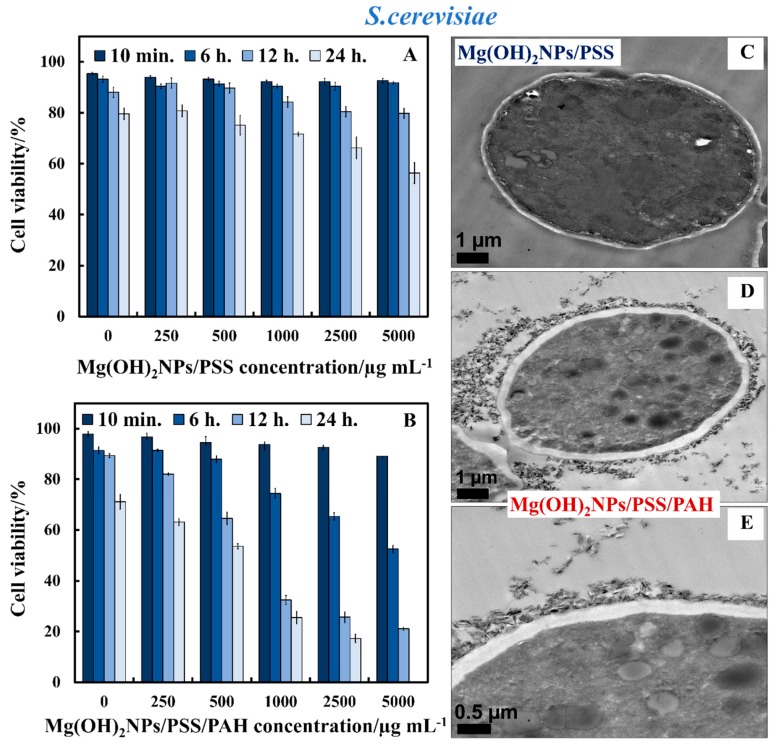
We studied the antimicrobial activity of Mg(OH)2NPs that were coated with multilayers of polyelectrolytes on S.cerevisiae, C. reinhardtii, and E. coli. We functionalized Mg(OH)2NPs with PSS and PAH and compared their antimicrobial effect with that of the bare Mg(OH)2NPs. The aqueous suspensions of S.cerevisiae were incubated with Mg(OH)2NPs/PSS and Mg(OH)2NPs/PSS/PAH suspensions at various particle concentrations (0, 250, 500, 1000, 2500, and 5000 μg mL−1) for up to one day. The results represented in Figure 9A show that the anionic Mg(OH)2NPs/PSS have a lower antimicrobial activity on S.cerevisiae when compared to the cationic bare Mg(OH)2NPs (c.f. Figure 4A). No change in the S.cerevisiae viability was registered for Mg(OH)2NPs/PSS, even at high particle concentrations, at incubating times up to six hours. The same treatment with the cationic Mg(OH)2NPs/PSS/PAH showed a significant antimicrobial activity on S.cerevisiae at particle concentrations of 1000, 2500, and 5000 μg mL−1, as shown in Figure 9B. A very strong effect of the Mg(OH)2NPs/PSS/PAH on S.cerevisiae viability was observed upon their incubation with high particle concentrations of 5000 μg mL−1 for up to 24 h. In contrast, upon incubation with the anionic Mg(OH)2NPs/PSS at high particle concentrations of 5000 μg mL−1, we observed a moderate impact on S.cerevisiae viability for up to one day of incubation (Figure 9A). Figure S12 compares the anti-yeast activity bare Mg(OH)2NPs with Mg(OH)2NPs/PSS and Mg(OH)2NPs/PSS/PAH as a function of the nanoparticle concentration for 24 h of exposure.
Figure 9.
S. cerevisiae cell viability as a function of nanoparticle concentration after incubation for up to 24 h with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH. TEM images of S. cerevisiae cells incubated for 24 h with (C) Mg(OH)2NPs/PSS and (D,E) Mg(OH)2NPs/PSS/PAH at different magnifications.
Hence, by coating the Mg(OH)2NPs with an outer layer of anionic polyelectrolyte, their antimicrobial activity is significantly decreased because of the electrostatic repulsion between the anionic Mg(OH)2NPs/PSS and the anionic surface of S. cerevisiae cells. Figure 9C–E show TEM images of S. cerevisiae cells after their incubation with Mg(OH)2NPs that were coated with PSS and PAH layers.
The TEM image in Figure 9C indirectly confirms the lack of nanoparticle accumulation due to the electrostatic repulsion among the anionic Mg(OH)2NPs/PSS and the negatively charged S. cerevisiae cell wall. Figure 9D,E show the great accumulation of Mg(OH)2NPs/PSS/PAH on the cell walls, which corresponds to a much higher activity towards S. cerevisiae. One can conclude that coating Mg(OH)2NPs with PSS as an external layer significantly diminishes their ability to attach on the treated cells, as shown in Figure 9C. S. cerevisiae cell viability tests revealed that Mg(OH)2NPs/PSS were much less effective in killing the cells than the Mg(OH)2NPs/PSS/PAH or bare Mg(OH)2NPs, which strongly accumulate on the cell membrane due to electrostatic attraction. These results were also supported by the TEM images of S. cerevisiae. Figure S16 shows the same data as Figure 9A,B in CFU ml−1. Figure 10A,B compares the antimicrobial activity of multilayer-coated Mg(OH)2NPs with PSS and PAH polyelectrolytes at various NPs concentrations on the C. reinhardtii. Figure 10A shows that, for incubating times of up to 6 h, no measurable variation in the C. reinhardtii cell viability was detected for Mg(OH)2NPs/PSS, even at high particle concentrations. However, at similar conditions, the cationic Mg(OH)2NPs/PSS/PAH displayed a marked antimicrobial activity on C. reinhardtii, even at 250 µg mL−1. A very strong effect of the Mg(OH)2NPs/PSS/PAH on the C. reinhardtii cells viability was observed for exposure times of up to six hours at 1000 µg mL−1 particle concentrations (Figure 10B). One can conclude that, by coating the Mg(OH)2NPs with an external layer of anionic polyelectrolyte, their antimicrobial activity decreased for both S. cerevisiae and C. reinhardtii, because of the electrostatic repulsion between the Mg(OH)2NPs/PSS and the cells walls. Figure 10C–E confirm this hypothesis with TEM images of C. reinhardtii exposed into the polyelectrolyte-coated Mg(OH)2NPs. We also conducted similar tests with Gram-negative bacteria (E. coli) and polyelectrolyte-coated Mg(OH)2NPs when the bacterial cells were removed from their culture media. Figure S17 (ESI) shows the same data as Figure 10A,B in CFU mL−1. Figure S13 compares the anti-yeast activity bare Mg(OH)2NPs with Mg(OH)2NPs/PSS and Mg(OH)2NPs/PSS/PAH as a function of the nanoparticle concentration for 24 h of exposure.
Figure 10.
C. reinhardtii cell viability as a function of nanoparticle concentration after incubation for up to 6 h with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH. TEM images of C. reinhardtii after being incubated for 6 h with (C) 1000 μg mL−1 Mg(OH)2NPs/PSS, (D) 750 μg mL−1 Mg(OH)2NPs /PSS/PAH, and (E) 1000 μg mL−1 of Mg(OH)2NPs /PSS/PAH.
Figure 11A,B show the effect of polyelectrolyte multilayer-coated Mg(OH)2NPs against E. coli. Similarly to S. cerevisiae and C. reinhardtii, we found no pronounced antibacterial effect of Mg(OH)2NPs/PSS on E. coli for various exposure times. Figure 11A shows that the antibacterial activity of Mg(OH)2NPs/PSS against E. coli is also much lower than the one of the bare Mg(OH)2NPs. We envisage that this result is due to a similar decrease of the NPs accumulation on the bacterial cell wall after the functionalization of the Mg(OH)2NPs with an anionic PSS layer (see Figure 11C,D,F,G). The subsequent deposition of a cationic polyelectrolyte layer of PAH, yields Mg(OH)2NPs/PSS/PAH, which showed excellent antibacterial properties against E. coli, as seen in Figure 11B. Note that the PAH-coated NPs have even stronger antibacterial activity than the uncoated Mg(OH)2NPs towards E. coli. Hence, the antibacterial activity of the polyelectrolyte coated Mg(OH)2NPs appears to alternate with their surface charge. Figure S18 (ESI) shows the same data as in Figure 10A,B in CFU mL−1.
Figure 11.
The E. coli cell viability after treatment with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH for various incubation times as a function of the NPs concentration. (C) SEM and (F) TEM images of E. coli after incubation with 2500 µg mL−1 Mg(OH)2NPs/PSS; (D) SEM and (G) TEM images of E. coli after incubation with 5000 µg mL−1 Mg(OH)2NPs/PSS; (E) SEM and (H) TEM images of E. coli after incubation with 5000 µg mL−1 Mg(OH)2NPs/PSS/PAH. The cells were removed from the particle suspension before the sample preparation for TEM and SEM imaging.
The E. coli Gram-negative cell wall is composed of an organized triple membrane that contains a thin inner layer of peptidoglycan between an outer membrane consisting of porins [41], phospholipids molecules, lipopolysaccharides (LPS), lipoproteins, surface proteins, and a cytoplasmic membrane consisting of phospholipids molecules and porins (see Figure S10) [41]. Figure 11C–H show the SEM and TEM images of E. coli after treatment for 24 h with Mg(OH)2NPs coated with a single layer of PSS and ones with additional layer of PAH. Note that there are a very few Mg(OH)2NPs/PSS that are attached to the bacteria, as shown in Figure 11C,D,F,G. On the other hand, we found a significant accumulation of Mg(OH)2NPs/PSS/PAH onto the surface of the bacteria, as shown in Figure 11E,H. These SEM and TEM images are consistent with the antibacterial activity profile of the polyelectrolyte-coated Mg(OH)2NPs against E. coli, as reported in Figure 11B. It can be argued that the weak attachment of the anionic particles Mg(OH)2NPs/PSS to the bacteria, as supported via the SEM and TEM images, causes little damage of the bacteria wall. Figure 7B,C,E,F for the bare Mg(OH)2NPs and Figure 11E,H for the Mg(OH)2NPs/PSS/PAH, show that there is a substantial build-up of cationic NPs (uncoated and PAH-coated Mg(OH)2NPs) onto the anionic bacterial cell surface, which corresponds to a greater local increase of the NPs concentration that successively disrupts the bacteria. Figure S14 compares the anti-yeast activity bare Mg(OH)2NPs with Mg(OH)2NPs/PSS and Mg(OH)2NPs/PSS/PAH as a function of the nanoparticle concentration for 24 h of exposure. We also examined the antibacterial activity of MgCl2 solutions of various concentrations for 24 h on E. coli in order to investigate if this is due to higher local concentration of Mg2+ ions, where the bacterial cells were extracted from the culture media in a similar processes, as explained above for the Mg(OH)2NPs treatment with E. coli. We found that MgCl2 did not have significant antibacterial action when compared to the Mg(OH)2NPs, even at high concentrations, as shown in Figure S11 (ESI). The effect of the pH on the bacterial cell viability is similar—we found that the incubation of bacteria with NaOH solution of pH 10.4 (corresponding to the pH of bare Mg(OH)2NPs suspension) did not produce a comparable effect. Hence, the analysis of these results suggests that magnesium ions in the Mg(OH)2NPs and the basic pH of 10.4 is unlikely to be responsible for the killing of E. coli [28]. The most likely explanation is the rough surface morphology of the clustered Mg(OH)2NPs, which, when electrostatically attracted towards the cell membrane, cause membrane disruptions that kill the bacteria.
Note that neither Mg2+ ions nor reactive oxygen species (ROS) generation can explain the antimicrobial properties of the Mg(OH)2NPs. The solubility of Mg(OH)2NPs is too low for the free Mg2+ ions to have any measurable cytotoxic effect, as their concentration is limited by the solubility product of Mg(OH)2NPs (1.8 × 10−11 M3). The Mg(OH)2NPs are not a photoactive material, which means that it is not producing ROS upon illumination. Hence, the antimicrobial effect is likely coming from the surface roughness of the Mg(OH)2NPs, which electrostatically stick onto the negatively charged microbial cells due to their cationic character and pierce their cell membrane. The effect is also amplified by the high concentration of OH- ions in their electric double layers, which they bring in close contact with the microbial cell surface upon adhesion. This can potentially lead to partial hydrolysis of the lipids in their cell membranes and cell death.
We also determined the MIC of free Mg(OH)2NPs and PAH-coated Mg(OH)2NPs on E. coli, S. cerevisiae, and C. reinhardtii. We found that, at the same conditions, the MIC of the PAH-coated Mg(OH)2NPs is two times lower than that of free Mg(OH)2NPs (see Table 1).
Table 1.
Minimum inhibitory concentration (MIC) of bare Mg(OH)2NPs and PSS/PAH-coated Mg(OH)2NPs against C. reinhardtii, S. cerevisiae and E. coli.
| Mg(OH)2NPs | PSS/PAH-coated Mg(OH)2NPs | |
|---|---|---|
| MIC | MIC | |
| C. reinhardtii | 1000 µg mL−1 | 750 µg mL−1 |
| S.cerevisiae | 5000 µg mL−1 | 2500 µg mL−1 |
| E. coli | 5000 µg mL−1 | 2500 µg mL−1 |
3.7. Effect of the bare Mg(OH)2NPs and PSS/PAH-coated Mg(OH)2NPs on human cells
Figure S19 (ESI) shows the cytotoxicity assay of the bare Mg(OH)2NPs and Mg(OH)2NPs/PSS/PAH on human embryonic kidney cells (HEK 293 cell line) for up to 24 h of exposure. The cells were removed from the original culture media and then transferred to PBS before exposure to the nanoparticles. This was done to avoid the adsorption of the media components on the particles. Both runs were done at the varying overall nanoparticles concentration and at different incubation times. One can see a very small effect on the presence of free Mg(OH)2NPs and Mg(OH)2NPs/PSS/PAH on the cells viability over a period of up to 24 h. Note that the control sample of HEK 293 cells has lost a minor fraction of their viability over this period of time due to the removal of the culture media. One can conclude that the nanoparticle does not measurably impact the HEK 292 cell viability up to 2500 µg mL−1. However, the effect on yeast, algae, and E. coli is very significant at these concentrations of free Mg(OH)2NPs and Mg(OH)2NPs/PSS/PAH—see Figure 4, Figure 9B, Figure 10B and Figure 11B, respectively. Therefore, one may conclude that the Mg(OH)2NPs show excellent biocompatibility with this human cell line. More research will be conducted in the future regarding the effects of the nanoparticles on different type of other cell lines.
4. Conclusions
When compared with other inorganic nanoparticles that were studied in the literature, Mg(OH)2NPs have high antimicrobial activity at moderate to high particle concentrations. However, the Mg(OH)2NPs have great application potential as a new antimicrobial agent, since Mg(OH)2 is a nontoxic material and has been broadly used in medical industries and food. Here, we studied various ways to control the antimicrobial activity and cytotoxicity of a range of bare and surface-modified Mg(OH)2NPs on three different types of microbial cells: microalgae, yeast, and bacteria. The antimicrobial activity of the Mg(OH)2NPs on S. cerevisiae, C. reinhardtii, and E. coli was examined. This work suggests that bare Mg(OH)2NPs are effective antimicrobial agents. The results from TEM and SEM analysis showed that the direct contact between the Mg(OH)2NPs and the cell membrane of S. cerevisiae, C. reinhardtii, and E. coli is very important for their effective antimicrobial action. A series of polyelectrolyte-coated Mg(OH)2NPs were likewise synthesised while using the layer by-layer technique and their antimicrobial activity towards S. cerevisiae, C. reinhardtii, and E. coli was compared with that of bare Mg(OH)2NPs in order to evaluate the role of the surface coating. It was discovered that the antimicrobial activity of the coated Mg(OH)2NPs alternates with their surface charge. The anionic nanoparticles (Mg(OH)2NPs/PSS) have much lower antibacterial activity than the cationic ones (Mg(OH)2NPs/PSS/PAH and bare Mg(OH)2NPs). We also show that Mg(OH)2NPs/PSS/PAH and bare Mg(OH)2NPs have only very minor impact on selected human cells line (HEK293), which implies good biocompatibility. This can bring important insights as to how the antmicrobial properties of Mg(OH)2NPs and other inorganic nanoparticles can be controlled by designing nanoparticle surface coatings that promote their adhesion to the microbial cell walls, as well as by taking into account the nanoparticles surface morphology.
Acknowledgments
The authors appreciate the technical help from Tony Sinclair and Ann Lowry at the University of Hull Microscopy Suite with the SEM and TEM sample preparation and imaging.
Supplementary Materials
The following are available online at https://www.mdpi.com/2313-7673/4/2/41/s1, Figure S1. A schematic overview summarizing the synthesis method of Mg(OH)2NPs, Figure S2. Particle size of Mg(OH)2NPs made from magnesium chloride at 75°C, Figure S3. The zeta potential of Mg(OH)2NPs made from magnesium chloride at 75°C, Figure S4. (A) Thermal gravimetric analysis pattern of Mg(OH)2NPs powder. (B) The EDX spectra of the uncoated Mg(OH)2NPs. (C) The impact of reaction temperature on the size of the produced Mg(OH)2NPs. (D) Variations in particle size and zeta potential of Mg(OH)2NPs suspensions with pH, Figure S5. FTIR spectra of the as prepared Mg(OH)2NPs at different reaction temperatures, Figure S6. EDX diagram of S. cerevisiae cells at 1000 µg mL−1, Figure S7. EDX chart of the C. reinhardtii with Mg(OH)2NPs at 1000 µg mL−1, Figure S8. EDX chart of the C. reinhardtii with Mg(OH)2NPs at 5000 µg mL−1, Figure S9. EDX diagram of E. coli cells incubated with Mg(OH)2NPs at 2500 µg mL−1 and 5000 µg mL−1, Figure S10. Schematic overview of the bacterial cell wall, Figure S11. The antibacterial impact of various concentration of MgCl2 towards E. coli for various exposure times, Figure S12. S. cerevisiae cell viability after incubation as a function of nanoparticle concentration for up to 24 h with uncoated and polyelectrolyte-coated Mg(OH)2NPs; Figure S13. The antialgal activity of uncoated and polyelectrolyte-coated Mg(OH)2NPs, Figure S14. Relationship between the antibacterial efficiency of uncoated and polyelectrolyte-coated Mg(OH)2NPs on the viability of E. coli., Figure S15. Colony forming unit (CFU) count of bare Mg(OH)2NPs on (A) S. cerevisiae (B) C. reinhardtii and (C) E. coli at various particle concentrations, Figure S16. Colony forming unit (CFU) count of S. cerevisiae as a function of nanoparticle concentration after incubation for up to 24 hours with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH, Figure S17. Colony forming unit (CFU) count of C. reinhardtii as a function of nanoparticle concentration after incubation for up to 6 h with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH, Figure S18. Colony forming unit (CFU) count of E. coli after treatment with (A) Mg(OH)2NPs/PSS and (B) Mg(OH)2NPs/PSS/PAH for various incubation times as a function of the NPs concentration, Figure S19. Comparison of the cell viability of human embryonic kidney cells (HEK 293 cell line) upon incubation as a function of nanoparticle concentration for up to 24 h with bare Mg(OH)2NPs and Mg(OH)2NPs/PSS/PAH, Figure S20. Antibacterial activity of SiO2NPs at various concentrations on E. coli., Figure S21. The anti-yeast, anti-algal and antibacterial activity of free PAH at various concentrations on (A) S. cerevisiae, (B) C. reinhardtii (C) E. coli.
Author Contributions
The manuscript was written through contributions of all authors. V.N.P. gave the idea of the study, A.F.H. did the experimental work co-supervised on equal basis by V.N.P. and T.S.H., A.F.H. produced the figures and the initial draft which was edited and further improved by V.N.P. and T.S.H. All authors have given approval to the final version of the manuscript.
Funding
A.F.H. thanks the Iraqi Government, the Higher Committee for Education Development of Iraq and the University of Babylon, Iraq for the financial support for his PhD study during the work on this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lee D., Cohen R.E., Rubner M.F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir. 2005;21:9651–9659. doi: 10.1021/la0513306. [DOI] [PubMed] [Google Scholar]
- 2.Rawlinson L.A.B., Ryan S.M., Mantovani G., Syrett J.A., Haddleton D.M., Brayden D.J. Antibacterial effects of poly (2-(dimethylamino ethyl) methacrylate) against selected gram-positive and gram-negative bacteria. Biomacromolecules. 2009;11:443–453. doi: 10.1021/bm901166y. [DOI] [PubMed] [Google Scholar]
- 3.Muñoz-Bonilla A., Fernández-García M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012;37:281–339. doi: 10.1016/j.progpolymsci.2011.08.005. [DOI] [Google Scholar]
- 4.Halbus A.F., Horozov T.S., Paunov V.N. Strongly enhanced antibacterial action of copper oxide nanoparticles with boronic acid surface functionality. ACS Appl. Mater. Inter. 2019;11:12232–12243. doi: 10.1021/acsami.8b21862. [DOI] [PubMed] [Google Scholar]
- 5.Halbus A.F., Horozov T.S., Paunov V.N. Self-grafting copper oxide nanoparticles show a strong enhancement of their anti-algal and anti-yeast action. Nanoscale Adv. 2019;1:2323–2336. doi: 10.1039/C9NA00099B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vatsha B., Tetyana P., Shumbula P.M., Ngila J.C., Sikhwivhilu L.M., Moutloali R.M. Effects of precipitation temperature on nanoparticle surface area and antibacterial behaviour of Mg(OH)2 and MgO nanoparticles. J. Biomater. Nanobiotechnol. 2013;4:365–373. doi: 10.4236/jbnb.2013.44046. [DOI] [Google Scholar]
- 7.Al-Obaidy S.S., Halbus A.F., Greenway G.M., Paunov V.N. Boosting the antimicrobial action of vancomycin formulated in shellac nanoparticles of dual-surface functionality. J. Mater. Chem. B. 2019;7:3119–3133. doi: 10.1039/C8TB03102A. [DOI] [Google Scholar]
- 8.Weldrick P.J., Iveson S., Hardman M.J., Paunov V.N. Breathing new life into old antibiotics: Overcoming antibacterial resistance by antibiotic-loaded nanogel carriers with cationic surface functionality. Nanoscale. 2019;11:10472–10485. doi: 10.1039/C8NR10022E. [DOI] [PubMed] [Google Scholar]
- 9.Halbus A.F., Horozov T.S., Paunov V.N. Colloid particle formulations for antimicrobial applications. Adv. Colloid Interface Sci. 2017;249:134–148. doi: 10.1016/j.cis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Chen B., Banfield J.F. Particle Size and pH Effects on Nanoparticle Dissolution. J. Phys. Chem. C. 2010;114:14876–14884. doi: 10.1021/jp1060842. [DOI] [Google Scholar]
- 11.Liu J., Aruguete D.M., Murayama M., Hochella M.F., Jr. Influence of size and aggregation on the reactivity of an environmentally and industrially relevant nanomaterial (PbS) Environ. Sci. Technol. 2009;43:8178–8183. doi: 10.1021/es902121r. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Chen Y., Westerhoff P., Hristovski K., Crittenden J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008;42:2204–2212. doi: 10.1016/j.watres.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Petosa A.R., Jaisi D.P., Quevedo I.R., Elimelech M., Tufenkji N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010;44:6532–6549. doi: 10.1021/es100598h. [DOI] [PubMed] [Google Scholar]
- 14.Sada E., Kumazawa H., Sawada Y., Kondo T. Simultaneous absorption of dilute nitric oxide and sulfur dioxide into aqueous slurries of magnesium hydroxide with added iron (II)-EDTA chelate. Ind. Eng. Chem. Process Des. Dev. 1982;21:771–774. doi: 10.1021/i200019a037. [DOI] [Google Scholar]
- 15.Gui H., Zhang X., Dong W., Wang Q., Gao J., Song Z., Lai J., Liu Y., Huang F., Qiao J. Flame retardant synergism of rubber and Mg(OH)2 in EVA composites. Polymer. 2007;48:2537–2541. doi: 10.1016/j.polymer.2007.03.019. [DOI] [Google Scholar]
- 16.Cao H., Zheng H., Yin J., Lu Y., Wu S., Wu X., Li B. Mg(OH)2 complex nanostructures with superhydrophobicity and flame-retardant effects. J. Phys. Chem. C. 2010;114:17362–17368. doi: 10.1021/jp107216z. [DOI] [Google Scholar]
- 17.Pan X., Wang Y., Chen Z., Pan D., Cheng Y., Liu Z., Lin Z., Guan X. Investigation of antibacterial activity and related mechanism of a series of nano-Mg(OH)2. ACS Appl. Mater. Interfaces. 2013;5:1137–1142. doi: 10.1021/am302910q. [DOI] [PubMed] [Google Scholar]
- 18.Martin P.D., Schneck D.W., Dane A.L., Warwick M.J. The effect of a combination antacid preparation containing aluminium hydroxide and magnesium hydroxide on rosuvastatin pharmacokinetics. Curr. Med. Res. Opin. 2008;24:1231–1235. doi: 10.1185/030079908X280662. [DOI] [PubMed] [Google Scholar]
- 19.Scott G., Reynolds C.V., Milosavljev S., Langholff W., Shenouda M., Rordorf C. Lack of effect of omeprazole or of an aluminium hydroxide/magnesium hydroxide antacid on the pharmacokinetics of lumiracoxib. Clin. Pharmacokinet. 2004;43:341–348. doi: 10.2165/00003088-200443050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kang J., Schwendeman S.P. Comparison of the effects of Mg (OH)2 and sucrose on the stability of bovine serum albumin encapsulated in injectable poly (d,l-lactide-co-glycolide) implants. Biomaterials. 2002;23:239–245. doi: 10.1016/S0142-9612(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 21.Tong K., Song X., Xiao G., Yu J. Colloidal processing of Mg (OH)2 aqueous suspensions using sodium polyacrylate as dispersant. Ind. Eng. Chem. Res. 2014;53:4755–4762. doi: 10.1021/ie5002857. [DOI] [Google Scholar]
- 22.Waaijers S.L., Kong D., Hendriks H.S., de Wit C.A., Cousins I.T., Westerink R.H., Leonards P.E., Kraak M.H., Admiraal W., de Voogt P. Reviews of Environmental Contamination and Toxicology. Springer; New York, NY, USA: 2013. Persistence, bioaccumulation, and toxicity of halogen-free flame retardants; pp. 1–71. [DOI] [PubMed] [Google Scholar]
- 23.Dong C., Song D., Cairney J., Maddan O.L., He G., Deng Y. Antibacterial study of Mg(OH)2 nanoplatelets. Mater. Res. Bull. 2011;46:576–582. doi: 10.1016/j.materresbull.2010.12.023. [DOI] [Google Scholar]
- 24.Dong C., Cairney J., Sun Q., Maddan O.L., He G., Deng Y. Investigation of Mg (OH)2 nanoparticles as an antibacterial agent. J. Nanopart. Res. 2010;12:2101–2109. doi: 10.1007/s11051-009-9769-9. [DOI] [Google Scholar]
- 25.Dong C., He G., Li H., Zhao R., Han Y., Deng Y. Antifouling enhancement of poly (vinylidene fluoride) microfiltration membrane by adding Mg(OH)2 nanoparticles. J. Membr. Sci. 2012;387:40–47. doi: 10.1016/j.memsci.2011.10.007. [DOI] [Google Scholar]
- 26.Wang Z., Li C., Mu Y., Lin Z., Yi A., Zhang Q., Yan B. Nanoadduct relieves: Alleviation of developmental toxicity of Cr(VI) due to its spontaneous adsorption to Mg(OH)2 nanoflakes. J. Hazard. Mater. 2015;287:296–305. doi: 10.1016/j.jhazmat.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Pilarska A., Bula K., Myszka K., Rozmanowski T., Szwarc-Rzepka K., Pilarski K., Chrzanowski Ł., Czaczyk K., Jesionowski T. Functional polypropylene composites filled with ultra-fine magnesium hydroxide. Open Chem. 2015;13:161–171. doi: 10.1515/chem-2015-0024. [DOI] [Google Scholar]
- 28.Dong C., He G., Zheng W., Bian T., Li M., Zhang D. Study on antibacterial mechanism of Mg(OH)2 nanoparticles. Mater. Lett. 2014;134:286–289. doi: 10.1016/j.matlet.2014.07.110. [DOI] [Google Scholar]
- 29.Li Q., Mahendra S., Lyon D.Y., Brunet L., Liga M.V., Li D., Alvarez P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008;42:4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Mendonca A.F., Amoroso T.L., Knabel S.J. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 1994;60:4009–4014. doi: 10.1128/aem.60.11.4009-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A., Vemula P.K., Ajayan P.M., John G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008;7:236–241. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino A., Fujioka K., Oku T., Suga M., Sasaki Y.F., Ohta T., Yasuhara M., Suzuki K., Yamamoto K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. doi: 10.1021/nl048715d. [DOI] [Google Scholar]
- 33.Mudunkotuwa I.A., Grassian V.H. Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: Surface coverage, surface speciation, and its impact on nanoparticle− nanoparticle interactions. J. Am. Chem. Soc. 2010;132:14986–14994. doi: 10.1021/ja106091q. [DOI] [PubMed] [Google Scholar]
- 34.LB (Luria-Bertani) liquid medium. Cold Spring Harb. Protoc. 2006 doi: 10.1101/pdb.rec8141. [DOI] [Google Scholar]
- 35.Al-Awady M.J., Greenway G.M., Paunov V.N. Nanotoxicity of polyelectrolyte-functionalized titania nanoparticles towards microalgae and yeast: Role of the particle concentration, size and surface charge. RSC Adv. 2015;5:37044–37059. doi: 10.1039/C5RA05577F. [DOI] [Google Scholar]
- 36.Yu J.C., Xu A., Zhang L., Song R., Wu L. Synthesis and characterization of porous magnesium hydroxide and oxide nanoplates. J. Phys. Chem. B. 2004;108:64–70. doi: 10.1021/jp035340w. [DOI] [Google Scholar]
- 37.Dhaouadi H., Chaabane H., Touati F. Mg(OH)2 nanorods synthesized by a facile hydrothermal method in the presence of CTAB. Nano Micro Lett. 2011;3:153–159. doi: 10.1007/BF03353666. [DOI] [Google Scholar]
- 38.Meshkani F., Rezaei M. Facile synthesis of nanocrystalline magnesium oxide with high surface area. Powder Technol. 2009;196:85–88. doi: 10.1016/j.powtec.2009.07.010. [DOI] [Google Scholar]
- 39.Pang H., Ning G., Gong W., Ye J., Lin Y. Direct synthesis of hexagonal Mg(OH)2 nanoplates from natural brucite without dissolution procedure. Chem. Commun. 2011;47:6317–6319. doi: 10.1039/c1cc10279f. [DOI] [PubMed] [Google Scholar]
- 40.Wu X.F., Hu G.S., Wang B.B., Yang Y.F. Synthesis and characterization of superfine magnesium hydroxide with monodispersity. J. Cryst. Growth. 2008;310:457–461. doi: 10.1016/j.jcrysgro.2007.10.025. [DOI] [Google Scholar]
- 41.Brayner R., Ferrari-Iliou R., Brivois N., Djediat S., Benedetti M.F., Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6:866–870. doi: 10.1021/nl052326h. [DOI] [PubMed] [Google Scholar]
- 42.Lin W., Xu Y., Huang C.C., Ma Y., Shannon K.B., Chen D.R., Huang Y.W. Toxicity of nano-and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009;11:25–39. doi: 10.1007/s11051-008-9419-7. [DOI] [Google Scholar]
- 43.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Al-Awady M.J., Fauchet A., Greenway G.M., Paunov V.N. Enhanced antimicrobial effect of berberine in nanogel carriers with cationic surface functionality. J. Mater. Chem. B. 2017;5:7885–7897. doi: 10.1039/C7TB02262J. [DOI] [PubMed] [Google Scholar]
- 45.Al-Awady M.J., Weldrick P.J., Hardman M.J., Greenway G.M., Paunov V.N. Amplified antimicrobial action of chlorhexidine encapsulated in PDAC-functionalized acrylate copolymer nanogel carriers. Mater. Chem. Front. 2018;2:2032–2044. doi: 10.1039/C8QM00343B. [DOI] [Google Scholar]
- 46.Franklin N.M., Rogers N.J., Apte S.C., Batley G.E., Gadd G.E., Casey P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 47.Heinlaan M., Ivask A., Blinova I., Dubourguier H.C., Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 48.Choi O., Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 49.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 50.Al-Obaidy S.S., Greenway G.M., Paunov V.N. Dual-functionalised shellac nanocarriers give a super-boost of the antimicrobial action of berberine. Nanoscale Adv. 2019;1:858–872. doi: 10.1039/C8NA00121A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P., Powell B.A., Mortimer M., Ke P.C. Adaptive interactions between zinc oxide nanoparticles and Chlorella sp. Environ. Sci. Technol. 2012;46:12178–12185. doi: 10.1021/es303303g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.