Abstract
In order to improve the indoor air quality, volatile organic compounds (VOCs) can be removed via an efficient approach by using catalysts. This review proposed a comprehensive summary of various nanomaterials for thermal/photo-catalytic removal of VOCs. These representative materials are mainly categorized as carbon-based and metallic oxides materials, and their morphologies, synthesis techniques, and performances have been explained in detail. To improve the indoor and outdoor air quality, the catalytic nanomaterials can be utilized for emerging building applications such as VOC-reduction coatings, paints, air filters, and construction materials. Due to the characteristics of low cost, non-toxic and high chemical stability, metallic oxides such as TiO2 and ZnO have been widely investigated for decades and dominate the application market of VOC-removal catalyst in buildings. Since other catalysts also showed brilliant performance and have been theoretically researched, they can be potential candidates for applications in future healthy buildings. This review will contribute to further knowledge and greater potential applications of promising VOC-reducing catalytic nanomaterials on healthier buildings for a better indoor and outdoor environment well-being.
Keywords: nanomaterials, VOCs removal, photocatalysis, thermal oxidation, catalytic oxidation, healthy buildings, green application, photocatalytic reactor
1. Introduction
Healthy buildings aim to provide a healthy built environment for occupants inside buildings, and indoor air quality can significantly impact occupants’ health [1]. Since people spend most of their time inside buildings, indoor air quality has become an increasing concern. Indoor air quality can be affected by various factors including toxicological, microbiological, physical systems, indoor, and outdoor ventilations [2]. The advances in construction technology have produced numerous applications of synthetic building materials, but have also brought some adverse effects on the indoor environment, including the major pollution from volatile organic compounds (VOCs). VOCs are defined as having a boiling point that ranges between 50 °C and 260 °C [3]. They can contribute to the formation of ozone and fine particulates in the atmosphere [4] and work directly as toxic substances to the environment and human being. The exposure to VOCs can result in both acute and chronic health effects including respiratory diseases, impaired neurobehavioral function, and sick building syndrome, etc. [2].
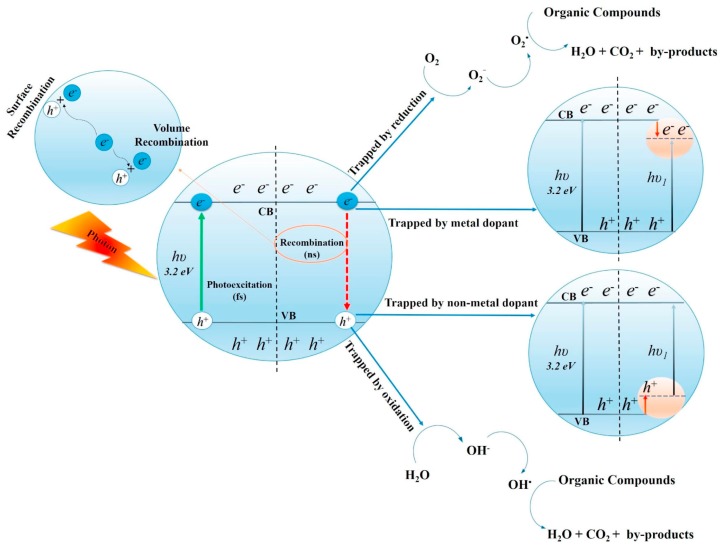
In order to remove the VOCs, many methods have been proposed and can be roughly divided into two main groups according to their mechanisms: adsorption techniques and oxidation techniques [5], or the combination of them [6]. The former one is a conventional method by transferring VOCs from the air to the solid phase via adsorbents, e.g., activated carbon [7], biochar [8] and fibre [9], etc. which faces challenges like saturation and pore blockages. The latter oxidation techniques provide a better approach to cost-effectively remove VOCs, which show higher degradation activity towards polar VOCs (OVOCs > Ahs > AlHs) [10]. Photo and thermal catalytic oxidation are two of the most common oxidation techniques. Thermal oxidation reactions require a high temperature above 600 °C, and the oxidation efficiency grows with the temperature increase. The technique of photocatalytic oxidation commonly uses nano-semiconductor catalysts and ultraviolet (UV) light to convert organic compounds in indoor air into benign and odourless constituents—water vapor (H2O) and carbon dioxide (CO2) for air purification [5,11,12]. Figure 1 shows the basic principle of photocatalytic oxidation for the removal of VOCs. An electron in an electron-filled valence band (VB) is excited by photoirradiation to a vacant conduction band (CB), leaving a positive hole in the VB. Later, the photogenerated electrons and holes can react with H2O and O2 molecules to reduce and oxidate VOCs on the surface of a photocatalyst [12,13,14].
Figure 1.
Mechanisms of photocatalytic oxidation for the removal of VOCs [14]. (reproduced from [14], with permission from Elsevier, 2019).
Most of the VOCs in indoor air are aromatics, aldehydes, and halocarbons, and they are rich in the established, new and renovated buildings [15]. Measurements indicate that similar exposure level is shared by VOCs in various indoor and building materials, and coverings are the major sources of VOCs [16]. VOCs are common in industry [5] and widely used in construction projects. Since many VOCs will off-gas a significant proportion of their volume in a relatively short time, the VOC concentrations could be much higher than typical ambient levels in newly-constructed or decorated buildings [2]. To reduce the VOC emissions and indoor concentrations inside the buildings, some proposed to bake-out the housing unit with a radiant floor-heating system [17], and a long-enough bake-out could deplete solvents and reduce VOC emissions [18]. While a more efficient method is to adopt photocatalysis such as TiO2, these materials can be applied on buildings as a coating or paint, or synthesised with mortar, which will further affect the aesthetics, sanitation and efficiency of the buildings [19,20,21]. Therefore, special attention should be paid to the construction materials.
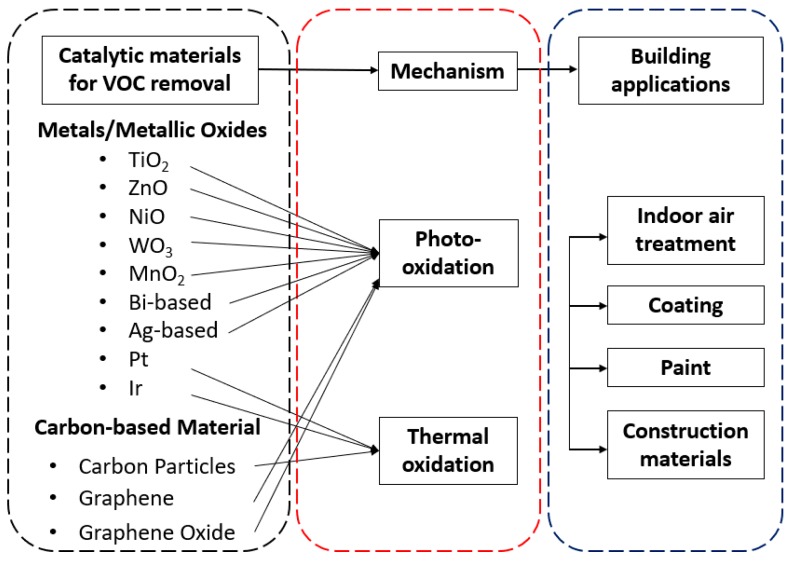
Since the topic has been investigated for decades, many researchers have summarized the materials for the removal [5,22] and sensing [23,24] of VOCs. Most references have been reviewed based on a specific category of material, such as TiO2 [13,25,26], graphene-based materials [27], zinc indium sulphide [28] and silica-nanosphere-based materials [29] etc., or focusing on the catalytic oxidation processes in a specific situation such as low temperature [30], visible light [31,32], or based on a summary from the perspective of various VOCs [5]. However, few have been done from a material perspective together with the consideration of applications on buildings. In this review paper, we do not discuss adsorption-based materials because there are already existing review papers [5,9]. Since the performances of the catalytic oxidations are different for various materials, it is reasonable to categorize by their physiochemical characteristics, which will further affect their applications on buildings. Therefore, this review proposed a novel summary on the materials for catalytic removal of VOCs and their applications on buildings (Figure 2). Morphology, synthesis, performance, and their applications will be explained in detail. This review will further contribute to the applications of photocatalysis materials on buildings.
Figure 2.
Outline of this review paper.
2. Materials
This review will summarize the materials used for catalytic oxidation of VOCs. The commonly used materials and their characteristics have been listed in Table 1. For this study, catalysts used for the oxidation of VOCs can be classified into two major groups: metallic oxide catalysts and carbon-cased materials.
Table 1.
Commonly used materials for catalytic removal of VOCs.
| No. | Catalytic | Category | VOC | Nanomaterial | Morphology | Medium | Doping Concentration | Synthesis | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Photo- | TiO2 | Trichloro-ethylene | nanostructured TiO2 particles | Primary particle size: 2.3–30 nm, secondary particle size: 100–900 nm | titanium isopropoxide | water concentrations: 2.3, 0.3, 0.27, and 0.18 M | low-temperature synthesis, modified sol–gel method | [33] |
| 2 | Photo- | TiO2 | Toluene | Titanium isopropoxide | Primary particle size:11 nm | isopropanol–water solution | 2.5 mL H2O, 25 mL ethanol, 150-mL (hydrothermal) | sol–gel synthesis, thermal & hydrothermal methods | [34] |
| 3 | Photo- | TiO2 | Toluene | TiO2 thin films | particle sizes less than 100 nm, monocrystalline nanodiamond | Titanium (IV) tetraisopropoxide (TTIP) (Ti(OCH(CH3)2)4) and water | detonation method (purchased from microdiamant) | [35] | |
| 4 | Photo- | TiO2 | Toluene, acetaldehyde | TiO2 nanotubes (TNT) & nanopartcles (TNP) film; commercial TiO2 (P25) | average surface area of 50 m2 g−1, primary particle size: 20–30 nm, channel pores diameter: 40–60 nm, tube length: 9.5 (±0.9) μm. | [TNP] Ethanol [TNT] ethylene glycol electrolyte |
[TNP] 0.15 g/mL [TNT] 1st anodization: 0.5 wt% NH4F and 3 wt% H2O; 2nd: 0.3 wt% NH4F and 1 wt% H2O. |
[TNP] doctor-blade method [TNT]two-step electrochemical anodization |
[36] |
| 5 | Photo- | TiO2 | Toluene | Ti-foil (99.7%,0.25 mm, Aldrich, USA) | top and bottom opened structure of which thediameters are 100 nm and 50 nm, respectively NP@DNT films of 15 (±2) μm | ethylene glycol solution containing 0.25 wt% NH4F and 0.3 vol% distilled water | potentiostatic anodization method | [37] | |
| 6 | Photo- | TiO2 | Hexane, methanol | anatase and rutile TiO2 (0.1 mol) | Surface area between 39 to 84 m2/g (given in table) | 1.5 mol anhydrous Ethanol, water–ethanol solution containing 1 mol ethanol with a ratio of water:butoxide = 50:1. | aqueous HNO3 solution of various concentration (0.1–1.0 mol/L) with the ratio of solid (g): liquid (mL) = 1:10 | hydrothermal method | [38] |
| 7 | Photo- | TiO2 | Toluene | Anatase/brookite/rutile tricrystalline TiO2 | amorphous TiO2 suspension | HNO3 solution (65%) | The molar ratios of HNO3 to TBOT (RHNO3) were varied from 0.2 to 1.2 at intervals of 0.2 by varying the volume of HNO3 solution. | low-temperature hydrothermal method | [39] |
| 8 | Photo- | TiO2 | Toluene | co-alloying TiO2 | fine bright yellow powder, primary particles diameter: 1–2 μm | TiCl4 reacted with NbCl5 and urea in an ethanol solution | toluene concentrations: 1~5 ppm; relative humidity: 25~65%; air velocity: 0.78~7.84 cm/s; irradiancy: 42~95 W/m2. | urea-glass synthesis | [40] |
| 9 | Photo- | TiO2 | Isopropyl alcohol | Hybrid CuxO/TiO2 Nanocomposites | Commercial TiO2 (rutile phase, 15 nm grain size, 90 m2/g specific surface area) | CuCl2 solution, NaOH and glucose solutions (reduce & control the CuI/CuII ratio | 10 mL of CuCl2 solution. Weight fraction of Cu: TiO2 is 1 × 103: 2 × 102. |
simple impregnation method | [41] |
| 10 | Photo- | TiO2 | Toluene | commercial TiO2 (P25) | Platinum nanoparticles in the size of 1–3 nm were clearly deposited on the surface of TiO2 | 0.5 wt% Pt and 30 mM fluoride for VOC degradation |
sodium fluoride (10, 30, and 50 mM) and Pt (0.1, 0.5, and 1 wt%) | photo deposition method | [42] |
| 11 | Photo- | TiO2 | Toluene | hybrid nanomaterial Pt-rGO-TiO2 | TiO2 nanopowder: commercial P25 (Degussa). | ethanol-water | 0.1, 0.5, 1 and 2 wt% Pt-rGO-TiO2 nanocomposite catalysts | solvothermal method | [43] |
| 12 | Photo- | TiO2 | Toluene | Composites ACFF 0.5 mL tetra-butyl titanate (97 wt%) |
Diameter: 12 μm, pore size: 32 μm. | Polytetrafluoroethylene (Teflon)-lined stainless-steel autoclaves | 1.0, 2.0, 3.5 and 5.0 l of toluene were injected into the above reactor | Purchased ACFF, | [6] |
| 13 | Photo- | TiO2 | Formaldehyde, trichloro-ethylene | TiO2 nanoparticles | BET area:392 m2 g−1, micro mean pore size: 0.6 nm | 8 wt% DAPs | incipient wetness impregnation, freeze-drying, or mechanical mixing | [44] | |
| 14 | Photo- | Zinc oxide | Toluene | ZnAl2O4 nanoparticles | commercial P25 powder (reference) TiO2 nanoballs in anatase phase |
[solvothermal synthetic] Al(NO3)3·9H2O (2 mmol), Zn(NO3)2·6H2O (1 mmol), ethylene glycol (30 mL) [citrate precursors] 0.01 M Zn(NO3)2·6H2O, 0.02 M Al(NO3)3·9H2O, 100 mL DI water [hydrothermal] an equimolar amount of Zn(NO3)3·6H2O (2 mmol), Al(NO3)3·9H2O (4 mmol), urea[CO(NH2)2] (20 mmol) and deionized water (80 mL) |
solvothermal, citrate precursor, hydrothermal methods | [45] | |
| 15 | Photo- | Ni oxide | Toluene | Nitrogen-doped carbon nanotubes (NCNTs) supported NiO(NiO/NCNTs) | NCNTs: tubular structure, 20 nm-diameter; NiO: crystallite, 3–10 nm | catalyst and pyridine and/or 3-(aminomethyl)pyridine | volume ratio of pyridine to 3-(aminomethyl)pyridine: 5, 3, 1 and 0 | Chemical vapor deposition method | [46] |
| 16 | Photo- | WO3 | H2O2 | Nano-diamonds combined with WO3 | ND: ca. 4–6 nm diameter | WO3 (Aldrich) | 0.5–16 wt% ND contents | Simple dehydration condensation | [47] |
| 17 | Photo- | Manganese Oxide | Benzene, Toluene, Ethylbenzene, Xylenes | Manganese Oxide and Copper | KMnO4 solution (OMS-2); Mn(CH3COO)2 4H2O (AMO) |
Mn(CH3COO)2 solution (OMS-2); KMnO4 (AMO); |
a simple refluxing method | [48] | |
| 18 | Photo- | Manganese Oxide | Formaldehyde indoors | manganese oxide | Shown in SEM images | ethanol solution of manganese acetate tetrahydrate (Mn(CH3COO)2·4H2O |
Mn(CH3COO)2·4H2O:PAN-ACNF 0.5–20 wt.% | [49] | |
| 19 | Photo- | Bi-based compounds | Acetone, toluene | Bi2WO6 | CQDs: high dispersion, uniform size of 3–5 nm in diameter | carbon quantum dots (CQDs) | adding 1.0–6.0 g of CQDs | Hydrothermal synthesis | [50] |
| 20 | Photo- | AgBr | methyl orange | AgBr | monoclinic WO3 substrate, face-centered cubic AgBr nanoparticles: crystalline sizes less than 56.8 nm. | WO3 | AgBr contents were respectively obtained and defined as TA-0.05, TB-0.10, TC-0.15, TD-0.20, TE-0.25, TF-0.30 and TG-0.40. | deposition–precipitation method | [51] |
| 21 | Thermal | Platinum | Toluene | Pt/Al2O3–CeO2 nanocatalysts | average size: 5–20 nm. | CeO2(10%)/Al2O3, 2.8 g Ce(NO3)3·6H2O, 100 mL distilled water | ceria loading of 10, 20 and 30% | wet impregnation method | [52] |
| 22 | Thermal | Platinum | benzene | Pt/Al2O3 | Pt particle sizes between 1.2–2.2 nm | H2PtCl6·6H2O | Pt/A l2O3−x, x: pH value of 7.0, 9.0 and 11.0 | modified ethylene glycol (EG) reduction approach | [53] |
| 23 | Thermal | Platinum | Formaldehyde (HCHO) | Pt/TiO2/Al2O3 | BET area from 16.5 to 182.5 m2/g | (NH4)[TiO(C2O4)2] | The platinum loading: 0.62, 1.26,1.19 and 1.25 gm−2 | Electro-deposition technology | [54] |
| 24 | Thermal | Silica-iridium | Toluene | chloride-ion free iridium acetylacetonate, Ir(AcAc)3 | ∼5 to 27 nm | SiO2 Degussa Aerosil 200 | Size of iridium particles: ~5 to 27 nm (calcination temperature 350~750 °C) | incipient wetness impregnation | [55] |
| 25 | Thermal | Carbon | benzene, toluene, ethylbenzene, and oxylene | Pt/carbon nanotube (CNT) Multiwalled carbon nanotubes (MWCNT) | CNTs: 20–50 nm column diameters MWCNTs: 20–50 nm diameters | acid treatment using HF, H2SO4, and HNO3 | Pt content in the catalysts ranging from 10 to 30 wt%. | a molecular-level mixing method | [56] |
| 26 | Photo- | Carbon based | Volatile Aromatic Pollutant | TiO2_graphene | Shown in SEM image | An ethanol-water solvent | P25_GR with weight addition ratios of 0.2, 0.5, 1, 2, 5, 10, and 30% GR. | facile hydrothermal reaction | [57] |
| 27 | Photo- | Carbon-based | methanol | graphene oxide, reduced graphene oxide, and few-layer graphene | BET area (m2/g): rGO+TiO2: 49.34, GO+TiO2: 43.79, G+TiO2: 41.54 |
Polyacrylonitrile | a polymer concentration of 5% (w/w) in N,N-dimethylformamide. | hydrothermal method (reduced graphene oxide); others purchased | [58] |
2.1. Metallic Oxides
2.1.1. Titanium Dioxide (TiO2)
With a wide band-gap energy, durability against photo-corrosion, low toxicity, and low cost, TiO2 is regarded as the most efficient and applicable material [59]. In 1972, the photoelectrochemical decomposition of water under irradiation with light on TiO2 was found for the first time [60]. Photocatalysis performances of TiO2 and its derivatives were studied over decades.
The photocatalyst performance can be affected by the structure and morphology [33] and treatments [34] of the TiO2 particles. Maira et al. [33] investigated the effects of different synthesis parameters on the size and morphology of the TiO2 particles, and the trichloroethylene degradation over TiO2 catalyst exhibits a maximum at a primary particle size of 7 nm. They [34] further compared the treatments applied to an amorphous TiO2 precursor for obtaining nanosized TiO2 particles. Compared to the anatase TiO2 treated with the thermal method, the one with the hydrothermal method can improve the photo activity and showed a higher number of hydrogen-bonded hydroxyl groups that are more stable under RT outgassing and a stronger adsorption ability on Benzaldehyde.
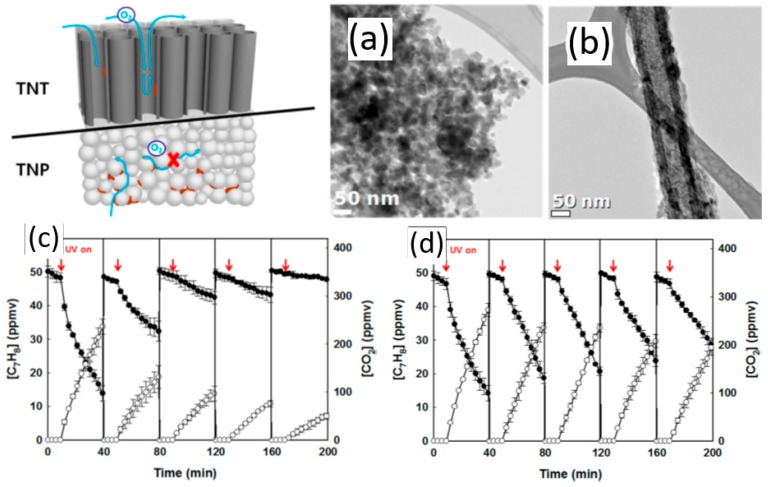
TiO2 with different morphologies showed different photocatalytic performances. Lee et al. [35] developed novel nanostructured gas filtering systems with TiO2 thin films using atomic layer deposition (ALD) for VOCs, which showed a superior efficiency for the toluene adsorption. Weon and Choi [36] compared the photocatalytic activities of TiO2 nanotubes (TNT) and TiO2 nanoparticles (TNP) film during the repeated cycles of photocatalytic degradation of gaseous toluene and acetaldehyde. Figure 3a,b shows the TEM images of fresh TNP and TNT, respectively. The photocatalytic activity of TNT showed only moderate reduction after the five cycles of toluene degradation, whereas TNP underwent rapid deactivation as the photocatalysis cycles were repeated, even in a more oxidizing atmosphere (Figure 3c,d). With a highly-ordered open channel structure, TNT can easily supply O2 molecules to the active sites with less mass transfer limitation, which prevents the TNT surface from carbonaceous residues accumulation. It indicated that the structural characteristics of TNT are highly advantageous in preventing the catalyst deactivation during the photocatalytic degradation of aromatic compounds. Weon et al. [37] later synthesized freestanding doubly open-ended TiO2 nanotubes (DNT) film, which exhibited higher activity and durability for the photocatalytic degradation of gaseous acetaldehyde and toluene than TiO2 nanotubes. If the freestanding DNT film was additionally loaded with TiO2 nanoparticles (NP@DNT) in the inner wall, the activity for VOC degradation will be increased by 1.3 and 1.8 times of those for bare DNT and bare TNT, respectively. However, the loading of TiO2 nanoparticles on TiO2 nanotubes showed a lower activity than bare TNT.
Figure 3.
TEM images of fresh (a) TNP and (b) TNT; repeated photocatalytic degradation cycles of gaseous toluene on (c) TNP and (d) TNT in the air (●: [Toluene], ○: [CO2]) [36]. (adapted from [36], with permission from American Chemical Society, 2019).
TiO2 primarily exists in three crystal phases: anatase, brookite and rutile. Among them, the anatase form appears to be the most photoactive and the most practical for widespread environmental applications [25]; the brookite was once regarded to not be suitable as a photocatalyst [25,61], and later the successful synthesis of nanostructured brookite was showed to greatly enhance the photocatalytic performance [62]. Wu et al. [38] investigated the synergetic effect between anatase and rutile nanoparticles in gas-phase photocatalytic oxidations of hexane and methanol. The synergetic effect could be more significant if anatase and rutile particles are closely contacted. The long-term experiment proves the stability of the photocatalyst activity, and it cannot be improved by sulfation, which works well for the single-phase anatase TiO2.
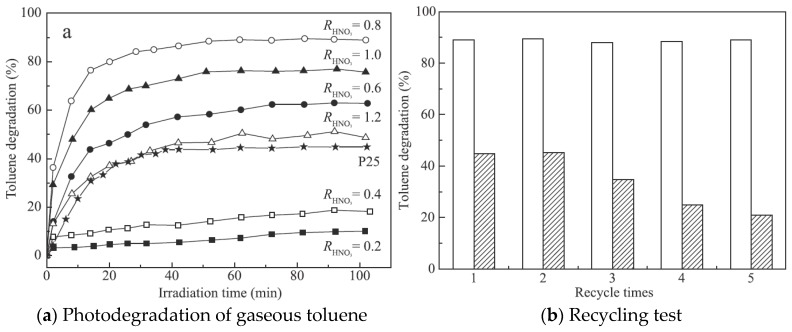
The tricrystalline TiO2 shows higher photocatalytic activity and durability toward gaseous toluene than bicrystalline TiO2 [39]. To remove toluene from the indoor air efficiently and economically, Chen et al. [39] synthesized anatase/brookite/rutile tricrystalline TiO2 by a low-temperature hydrothermal route with HNO3. As shown in Figure 4a, the one with RHNO3 = 0.8 (80.7% anatase, 15.6% brookite and 3.7% rutile) had the highest photocatalytic activity about 3.85-fold higher than that of P25 TiO2, which is a widely-used benchmark model photocatalyst coexisting anatase and rutile phases. Moreover, the high activity did not significantly degrade even after five reuse cycles (Figure 4b).
Figure 4.
Comparison results between TiO2 and P25 for (a) photodegradation rate of gaseous toluene and (b) recycling test over tricrystalline TiO2-0.8 (blank) and P25 (filled) for five repeat uses [39]. (adapted from [39], with permission from Elsevier, 2019).
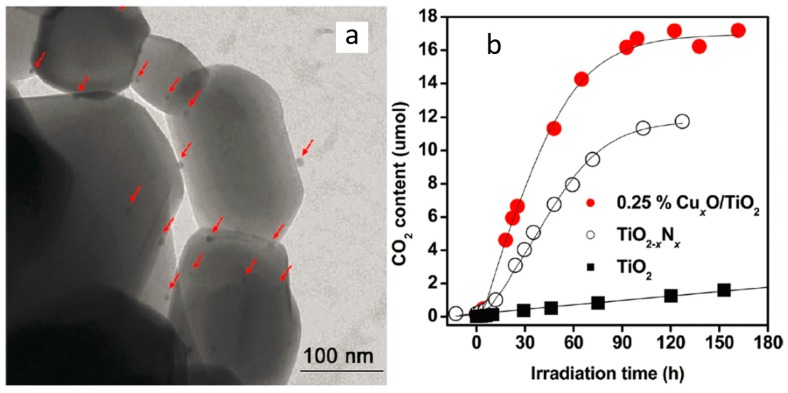
However, the leading semiconductor photocatalyst, TiO2, also suffers from low photocatalytic activity under visible light activation because of its intrinsic wide band gap. Therefore, to increase the efficiency of TiO2 in the visible light region, TiO2 is modified with various nanomaterials including other metal oxides [40,41,42], carbonaceous nanomaterials [43] etc. Zhong et al. [40] developed a TiNbON compound (band energy of 2.3 eV) using urea-glass synthesis as a visible light responsive photocatalytic oxidation material for toluene degradation. Experimental results showed that the visible light-driven catalyst was able to remove up to 58% of the toluene and generated less formaldehyde than the commercial TiO2 with reasonable durability and stability. Qiu et al. [41] grafted nano-CuxO clusters onto TiO2 to generate an excellent risk-reduction material in indoor environments (Figure 5a). The CuII in the CuxO clusters enables TiO2 to protoxidize VOCs under visible light efficiently, and it has the antimicrobial properties under dark conditions due to CuI species. Therefore, the efficient reduction of VOCs and antipathogenic activity could be achieved in hybrid CuxO/TiO2 nanocomposites with a proper proportion of CuI and CuII in CuxO. As shown in Figure 5b, the CuxO/TiO2 sample shows a superior photocatalytic activity over the TiO2−xNx sample with high quantum efficiencies and stability under long-term light irradiation.
Figure 5.
(a) TEM images of the 0.25% CuxO/TiO2 sample. CuxO clusters (marked by red arrows) were highly dispersed on the TiO2 surfaces; (b) comparative studies of CO2 generation over bare TiO2, TiO2−xNx, and 0.25% CuxO/TiO2 samples under the same conditions [41]. (adapted from [41], with permission from American Chemical Society, 2019).
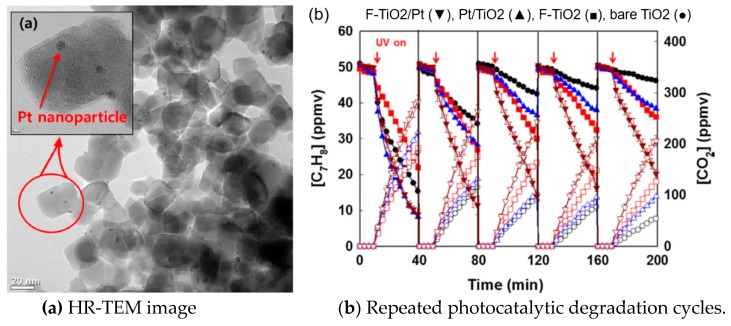
Jo et al. [63] applied an annular reactor coated with unmodified or nitrogen (N)-doped TiO2 for VOCs removal in the indoor environment. The photocatalytic technique using N-doped TiO2 was much superior to that for unmodified TiO2, and it can remove above 90% for four target compounds (ethyl benzene, o,m,p-xylenes) under conditions of less humidified environments, including a typical indoor comfort range (50–60%). Weon et al. [42] modified TiO2 nanoparticles with Pt and fluoride and tested their durability for toluene removal. Figure 6a shows the HR-TEM of the composite. Although Pt/TiO2 had a higher photocatalytic degradation activity than bare TiO2, it could be deactivated rapidly during repeated cycles. Among them, F-TiO2/Pt showed the highest photocatalytic activity and durability for toluene degradation (Figure 6b).
Figure 6.
(a) HR-TEM image and (b) repeated photocatalytic degradation cycles of gaseous toluene on F-TiO2/Pt [42]. (adapted from [42], with permission from Elsevier, 2019).
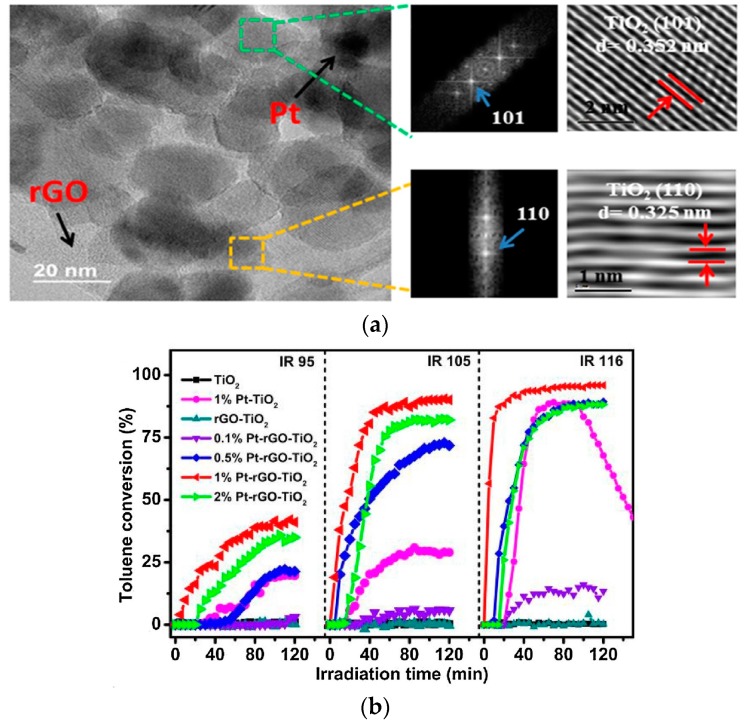
Li et al. [43] introduced a hybrid nanomaterial Pt-rGO-TiO2 (Figure 7a). With a broad light wavelength absorption (800–2500 nm), the highly-active photo-thermal responsive catalyst can decompose VOCs efficiently under IR irradiation. As shown in Figure 7b, the light intensity can affect the efficiency of Pt-rGO-TiO2 composites on the conversion of toluene and the yield of CO2. If the infrared irradiation intensity is 116 mW/cm2, a maximum photo-thermal conversion efficiency of 14.1% would be achieved with a significant toluene conversion of 95% and a CO2 yield of 72%, and a nearly 50 h stability duration.
Figure 7.
(a) High-angle annular dark-field scanning transmission electron microscopy images and HRTEM of 1% Pt-rGO-TiO2; (b) time course of toluene conversion over TiO2, 1% Pt-TiO2 and x% Pt-rGO-TiO2 (x = 0, 0.1, 0.5, 1 and 2) under IR irradiation with various light intensities (95, 106 and 116 mW/cm2) [43]. (adapted from [43], with permission from Elsevier, 2019).
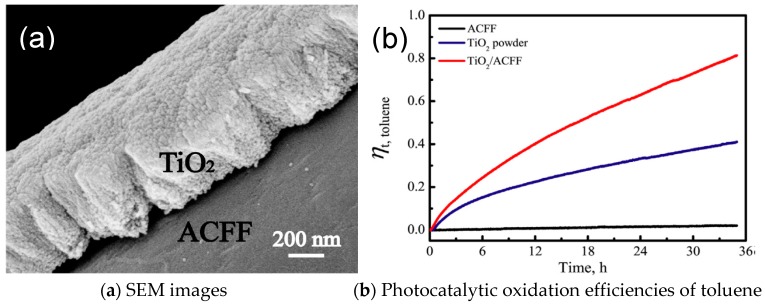
Metal oxides also synthesized with adsorption materials to enhance their photocatalysis performances. Li et al. [6] firstly fabricated nanostructured TiO2/activated carbon fiber-felt (TiO2/ACFF) porous composites by the in-situ deposition of TiO2 microspheres on the carbon fibers in ACFF. Figure 8a shows hierarchical nanostructures constructed by nanocrystals of TiO2 microspheres. Due to the synergetic effects of nanostructured TiO2 and ACFF, the TiO2/ACFF porous composites possess excellent adsorption and photodegradation properties for toluene. The ACFF in the TiO2/ACFF porous composites significantly enhances photocatalytic property for toluene by hindering the recombination of electron-hole pairs, reducing the TiO2 band gap energy to 2.95 eV and accelerating toluene adsorption.
Figure 8.
(a) SEM images and (b) Photocatalytic oxidation efficiencies of toluene as function of photocatalytic time under UV irradiation with TiO2/ACFF porous composites [6]. (adapted from [6], with permission from Elsevier, 2019).
Similar combination can be found in Ref. [44], where the TiO2 nanoparticles (Ti-NP) were synthesized with decahedral anatase particles (DAPs), and their photocatalytic activity of TiO2/zeolite hybrids for VOCs oxidation was analyzed. TiO2 nanoparticles of 5 nm, DAPs of ca. 100 nm, and 1.0 μm clusters of TiO2 made of 15 nm mean particle size characterized the three types of TiO2. The incorporation of TiO2-NP into the zeolitic material led to composites with around 10 times more photoactivity that the single titania particles. Elfalleh et al. [64] used TiO2-impregnated polyester and glass fiber to address the photocatalytic degradation aldehydes (air-solid interface). Also, the TiO2 nanoparticles were fixed by the glass fiber tissue coated with colloidal silica in reactors to photo-catalytically remove isovaleraldehyde [65] and isovaleric acid [66].
2.1.2. Zinc Oxide
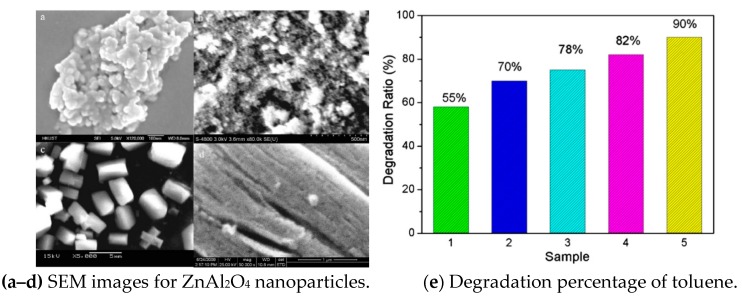
As an alternative to TiO2, zinc oxide is considered to be a fast and efficient chemical decontamination of VOCs [67]. Li et al. [45] compared three synthetic methods to prepare ZnAl2O4 for the photocatalytic degradation of gaseous toluene: solvothermal, citrate precursor and hydrothermal methods, whose SEM figures can be found in Figure 9a–d. The photocatalytic performances of the ZnAl2O4 samples synthesized by facile solvothermal method exhibited about 90% photocatalytic efficiency of toluene (Figure 9e). The photocatalytic oxidation of gaseous pollutant over UV-illuminated ZnAl2O4 is a promising technique for air purification.
Figure 9.
SEM images for ZnAl2O4 nanoparticles synthesized with (a) hydrothermal, (b) citrate precursors, (c,d) solvothermal synthetic methods. (e) The degradation percentage of toluene among 1 (ZnAl2O4 nanoparticles + citrate precursors), 2 (P25 TiO2), 3 (ZnAl2O4 nanoparticles + hydrothermal), 4 (TiO2 nanoballs), and 5 (ZnAl2O4 nanoparticles + solvothermal synthetic) samples under UV illumination [45]. (adapted from [45], with permission from Elsevier, 2019).
2.1.3. Nickel Oxide
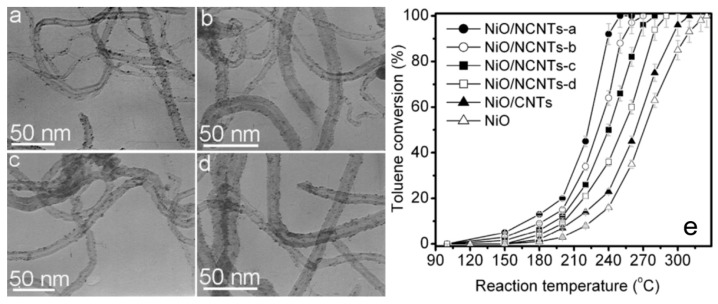
Jiang et al. [46] compared the catalysis performance of nitrogen-doped carbon nanotubes (NCNTs) supported by NiO (NiO/NCNTs) with different pyridine volume ratios (Figure 10a–d). The oxygen adspecies concentration and low-temperature reducibility of NiO/NCNTs increased with increasing the doped graphitic-like N(NG) content of NCNTs (Figure 10e). The optimized NiO/NCNTs-d catalyst with NG content of 6.22 at.% can achieve a completed conversion of toluene at 248 °C, and has a TOF value of nearly 10 times NiO/CNTs at 160 °C.
Figure 10.
TEM images of NiO/NCNT catalysts with the pyridine to 3-(aminomethyl) pyridine volume ratios of (a) 5, (b) 3, (c) 1 and (d) 0; and (e) their toluene conversion vs. reaction temperatures against those of NiO/CNTs [46]. (adapted from [46], with permission from Elsevier, 2019).
2.1.4. Tungsten Triocide
Kim et al. [47] combined nanodiamond (ND) with WO3 (as an alternative co-catalyst for Pt) to degrade VOCs under visible light. NDs-loaded WO3 showed a highly enhanced photocatalytic activity for the degradation of acetaldehyde (~17 times higher than bare WO3), which is more efficient than other well-known co-catalysts (Ag, Pd, Au, and CuO) loaded onto WO3 and comparable to Pt-loaded WO3. the surface conductivity of ND loaded on WO3 plays a critical role in the overall photocatalysis. The photocatalytic activity of NDs/WO3 was higher than that of WO3 loaded with other carbon-based co-catalysts (graphene oxide or reduced graphene oxide).
2.1.5. Manganese Oxide
Genuino et al. [48] synthesized cryptomelane-type octahedral molecular sieve (OMS-2) manganese oxide, amorphous manganese oxide (AMO), and mixed copper manganese oxide (CuO/Mn2O3) nanomaterials, together with commercial MnO2. Due to complex reasons including structure, morphology, hydrophobicity, and redox properties, OMS-2, AMO, and CuO/Mn2O3 showed higher oxidative activities than the commercial MnO2. Miyawaki et al. [49] developed a novel hybrid catalyst for long-lifetime formaldehyde removal, which deposits manganese oxide (MnOx) catalysts on a polyacrylonitril-based activated carbon nanofiber (PAN-ACNF) support. The combination of MnOx with PAN-ACNF induced synergic effects on the formaldehyde removal performance, which doubly improved the performance of PAN-ACNF in either dry or humid conditions without UV light. The manganese oxides were also interacted with cerium oxide [68] and CoAl mixed oxides [69] for a better catalytic removal of gaseous VOCs.
2.1.6. Bi-Based Compounds
Qian et al. [50] incorporated highly stable carbon quantum dots (CQDs) with Bi2WO6 to sufficiently photocatalytic removal of VOCs. The CQDs/Bi2WO6 photocatalyst can extend the absorption into visible light region and improve the photoexcited charge separation in comparison with pristine Bi2WO6. This photocatalyst showed higher photocatalytic oxidation activities towards acetone and toluene under both UV–vis and visible light irradiation.
2.1.7. Ag-Based Compounds
Kobayashi et al. [70] investigated the photocatalytic activity of AgBr. AgBr(N2) prepared at 250 °C showed the highest H2 generation activity, although larger crystallites of Ag were observed. Cao et al. [51] synthesized a novel AgBr/WO3 composite photocatalyst by loading AgBr on WO3 substrate via the deposition–precipitation method. AgBr/WO3 displays good photocatalytic activity under visible light (λ > 420 nm).
2.1.8. Platinum Suported Material
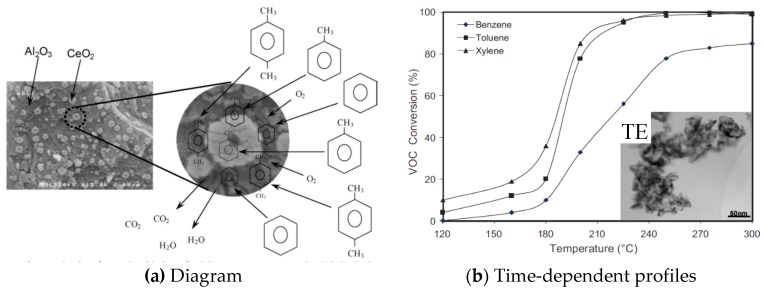
Abbasi et al. [52] prepared Pt/Al2O3–CeO2 nanocatalysts with Pt loading of 1% and ceria loading of 10, 20 and 30% to be utilized in catalytic oxidation of BTX (Figure 11a). The results of toluene oxidation indicated that the synthesized nanocatalysts were highly active and able to remove nearly 100% of toluene and xylene and about 85% of benzene as representative VOCs (Figure 11b).
Figure 11.
(a) Reaction mechanism for total oxidation of VOCs over nanostructured Pt/Al2O3–CeO2 catalysts. (b) Comparison of catalytic performance of synthesized Pt (1 wt%)/Al2O3–CeO2 (30 wt%) nanocatalyst for total oxidation of benzene, toluene and xylene [52]. (adapted from [52], with permission from Elsevier, 2019).
Chen et al. [53] prepared a series of Pt/Al2O3 catalysts by modified ethylene glycol (EG) reduction approach with Pt particle sizes between 1.2–2.2 nm. Pt/Al2O3 catalyst with 1.2 nm Pt size exhibited optimum catalytic oxidation activity of benzene at 145 °C. The catalysts showed excellent adaptability for different VOCs and durability for benzene oxidation during the long-term continuous test, whether in dry air or in the coexistence of CO2, cyclohexane or H2O. Wang et al. [54] prepared a Pt/TiO2/Al2O3 catalyst on an anodic alumite plate for the catalytic decomposition of formaldehyde at ambient temperature. The developed catalyst has good activity on decomposing HCHO at ambient temperature. With quick activation of absorbing oxygen, the Pt/TiO2/Al2O3 catalyst showed a high activity towards the catalytic decomposition of formaldehyde at ambient temperature.
2.1.9. Iridium Particles
Other oxides were also investigated, for example, Schick et al. [55] synthesized iridium particles supported on silica for the total oxidation of VOCs, and the catalytic activity increases when the iridium oxide particle size decreases.
2.2. Carbon-Based Materials
2.2.1. Carbon-Based
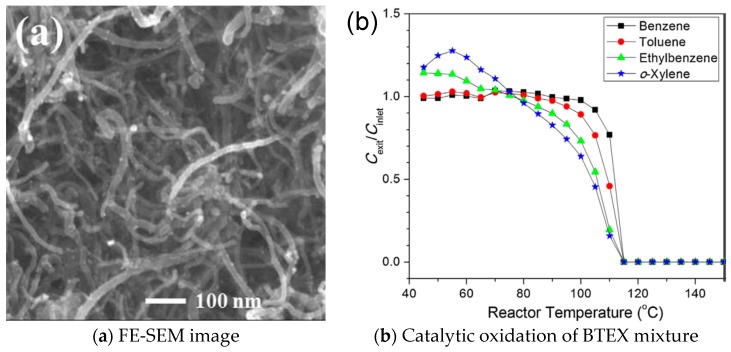
Joung et al. [56] fabricated a novel Pt/carbon nanotube (CNT) catalyst using a molecular-level mixing method (Figure 12a), and its performance on oxidation of benzene, toluene, ethylbenzene, and o-xylene (BTEX) was investigated at temperatures ranging from 40 to 150 °C (Figure 12b). The oxidation activity was presumably promoted because of the higher surface BTEX concentration afforded by the adsorption capability of multiwalled carbon nanotubes. The catalyst was characterized by its unique hydrophobic property, which facilitates the conversion of BTEX with high activity at relatively low temperatures and was unaffected by moisture in the system.
Figure 12.
(a) Field emission scanning electron microscopy image and (b) catalytic oxidation of BTEX mixture as a function of reactor temperature over 30 wt% Pt/CNT catalyst [56]. (adapted from [56], with permission from Elsevier, 2019).
2.2.2. Graphene and Graphene Oxide (GO)
Graphene and graphene oxide (GO) have been considered as a proficient matrix for sorption gaseous pollutants due to their advanced properties including facile synthesis method, high surface area, robust pore structure, lightweight, high chemical stability, and high thermal stability [71]. In recent years, great effort has been put into combining graphene/GO with various metal oxides (including TiO2, WO3, SnO2 and CO3O4 etc.) to improve the efficiency of VOCs’ removal. Even with unique structural and electronic properties, the metal oxide (e.g., TiO2/graphene) was found to be the same in essence as other TiO2/carbon (carbon nanotubes, fullerenes, and activated carbon) composite materials with regards to the enhancement of photocatalytic activity of TiO2 [57]. Since GO can be readily prepared from low-priced graphite materials on a huge scale, the usage of GO-based hybrid multifunctional materials should be much more profitable than that of other expensive nanomaterials such as functionalized carbon nanotubes [71].
Zhang et al. [57] prepared the nanocomposites of TiO2/graphene via a facile hydrothermal reaction of graphene oxide and TiO2 in an ethanol water solvent. This nanocomposite showed much higher photocatalytic activity and stability than bare TiO2 toward the gas-phase degradation of benzene, and the higher weight ratio in TiO2/graphene will decrease the photocatalytic activity. The photocatalytic efficiency can be strongly affected by the structure of graphene-based composites. Roso et al. [58] compared the performances of three graphene-based co-catalysts (graphene oxide, reduced graphene oxide, and few-layer graphene) on methanol gas-phase photooxidation. Among them, the reduced graphene oxide gave the best performance on degrading methanol with a higher rate.
3. Applications on Buildings
Photocatalysts can be applied on construction materials; their superior photoactivated properties can efficiently reduce or abate the harmful VOCs under UV light irradiation. These materials allowed both to degrade polluting compounds at the materials surface and to decrease maintenance costs thanks to their self-cleaning properties. As a main function for the self-cleaning applications, these materials such as TiO2 were coated on the surface or mixed with the building materials including glass, mortars, stone [72], asphalt and concrete [73,74], etc. Table 2 summarized the common applications of photocatalytic materials for VOC removal on buildings.
Table 2.
Applications of photocatalytic materials for VOC removal on buildings.
| No. | Catalytic | Applications | Materials | Comparison & Experiments | Pollutants | Performance | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Thermal | Indoor air purification | Pt/ZnO/SiC | Toluene concentration: 100~500 ppm Loading of Pt nanoparticles:0.030 wt% (Pt/ZnO/SiC) ~0.017 wt% (Pt/SiC). |
Toluene | Toluene was used as a model volatile organic compound and reached complete conversion of up to 100% over the porous tubular Pt/ZnO/SiC material at a filtration velocity of 0.72 m/min within 240 h at 210 °C maintained within 24 h | [75] |
| 2 | Photo- | Indoor air purification | Glass fiber tissue supported TiO2 | Inlet pollutant concentrations (25–300 mg m−3), flow rates (2–8 m3 h−1), relative humidity of effluent (5, 30, 50 and 90%), input of the plasma discharge (9–21 kV) | Trichloromethane (CHCl3) | Combination of plasma DBD and photocatalysis enhances the removal efficiency | [76] |
| 3 | Photo- | Indoor air purification | Ln3+–TiO2 | La3+–TiO2 and Nd3+–TiO2 Lanthanide ion dosage of 0.7%, 1.2%, 1.6% and 2.0% |
benzene, toluene, ethylbenzene and o-xylene (BTEX) | Highest adsorption ability: 0.7% Ln3+–TiO2 catalysts. TiO2 photocatalytic efficiency with the lanthanide ion doping was remarkably enhanced by BTEX removal. The 1.2% Ln3+–TiO2 catalysts achieved the highest photocatalytic activity. Residence time: 72 s using 1.2% La3+–TiO2 catalyst |
[77] |
| 4 | Photo- | Coating | TiO2 thin films | Commercial glasses: Pilkington Activ™ Blue PAB) and Pilkington Activ™ Clear (PAC). | 2-propanol | For the 2-propanol oxidation, PAC was found to be more active under UV light due to the larger surface area and higher TiO2 particle concentration. | [67] |
| 5 | Photo- | Coating | TiO2 coated on fiberglass fibers | TiO2 coated on carbon cloth fibres, a pilot duct system for experiment | polytetrafluoroethylene | The single-pass removal efficiency ranks: alcohols > ketones > aromatics > alkanes. | [69] |
| 6 | Photo- | Coating | TiO2 | single-layer coating & multilayer TiO2 coating | rhodamine B | Degrading self-cleaning ability of analysed coatings caused by ageing processes, and no significant difference between single-layer and multilayer coatings in the long-term | [63] |
| 7 | Photo- | Paint | Three self-cleaning photocatalytic paints | Three white commercial photocatalytic paints; expose to UVC lamp, Xenon lamp, LED and fluorescent lamps for 10 h | methyl red, methylene blue | Limited photocatalytic action under visible light | [74] |
| 8 | Photo- | Paint | commercial AEROXIDE_TiO2 P25 powder | Matrix with nitric acid and H2SO4 | rhodamine b | Nitric acid causes a decrease in crystallinity and photocatalytic activity, which drops by almost 20%; H2SO4 the best candidate for TiO2 nanoparticles acid treatment | [75] |
| 9 | Photo- | Paint | TiO2 microspheres | commercial TiO2 particles P25 | methylene blue solution | MTiO2: more stable and better photoactivity | [73] |
| 10 | Photo- | Paint | TiO2 | 5% P25-TiO2-intermixed and dip-coated SCAM samples | rhodamine b | TiO2/SCAM: high self-cleaning ability and a robust weathering resistance under UV-A and visible light irradiations. | [71] |
| 11 | Photo- | Paint | TiO2 coating (PC-S7, Cristal Active) | TiO2 (P25) intermixed nanopowder. Experiments: air purifying (indicated by NOx removal) and self-cleaning (indicated by Rhodamine b removal). |
Rhodamine b NOx |
TiO2 coating on mortar shows better photocatalytic performances than TiO2 intermixed samples on air purifying and self-cleaning properties under both UV-A and visible light (VL) irradiation conditions. | [70] |
| 12 | Photo- | Paint | TiO2 P25 | ZnO Experiments: Paints were exposed to simulated weathering tests in a QUV panel |
dye Acid Orange 7 | Photocatalytic activity of TiO2 increases with weathering time. ZnO: significantly higher photocatalytic activity for initial photoactivity of the unweathered paints but decreased after weathering. | [72] |
| 13 | Photo- | Mortar | Mortars containing TiO2 and iron oxide pigments | Atmospheric exposure tests and photocatalytic degradation tests were performed. | 2-propanol | Iron oxide pigments caused lower photocatalytic activity compared to white mortars. TiO2 + mortars has lower soiling in atmospheric exposure |
[76] |
3.1. Indoor Air Treatment
Indoor air treatment can be used to filter the VOCs from the air to maintain a good quality of air. The filter can be coated with nanomaterials to achieve a better performance for VOCs oxidation. Li et al. [75] prepared a novel Pt/ZnO/SiC filter for the oxidation of toluene; the ZnO coating can disperse Pt nanoparticles and significantly enhance the photocatalytic performance. This filter can achieve complete conversion of toluene at a filtration velocity of 0.72 m/min within 240 h at 210 °C. TiO2 is more commonly used for application. Zadi et al. [76] evaluated the efficiency of non-thermal plasma and heterogeneous photocatalysis processes for indoor air treatment with glass fiber tissue-supported TiO2. Various impact factors were analyzed and the combination of plasma DBD and photocatalysis is proved to enhance the removal efficiency. Li et al. [77] found that the photocatalytic efficiency of the TiO2 catalysts with the lanthanide ion doping was remarkably enhanced by BTEX removal. By comparing different types of Ln3+–TiO2 (La3+/Nd3+–TiO2) with various lanthanide ion dosage, the 0.7% Ln3+–TiO2 catalysts showed the highest adsorption ability, and 1.2% Ln3+–TiO2 catalysts achieved the highest photocatalytic activity. Boyjoo et al. [78] also reviewed the catalyst used for air purification in 2017 and concluded that the most commonly used materials were TiO2 and ZnO, and only very few studies were using other photocatalysts.
3.2. Coating
Photocatalysts have multi-function when coated on the construction materials. For example, the TiO2 coatings on the glass surface can not only degrade organic dirt and VOCs under light, but also improve the surface hydrophilicity, which can efficiently remove the dust and degraded organic dirt by rainfall [79]. Oladipo et al. [80] reported the photocatalytic activity and energy efficiency of two self-cleaning glasses, Pilkington Activ™ Blue and Pilkington Activ™ Clear. The Clear one was more active than the Blue one towards 2-propanol oxidation under ultraviolet irradiation and showed the best reactivity in the degradation of pre-adsorbed stain under simulated solar irradiation. TiO2-coated exterior paints can contribute to a 90~98% decane degradation [81].
TiO2 can be coated to materials in air filters to enhance the photocatalytic oxidation [82]. Zhong et al. [83] compared the performances of two commercially available photocatalytic oxidation air filters, titanium dioxide (TiO2) coated on fiberglass fibers (TiO2/FGFs) and TiO2 coated on carbon cloth fibers (TiO2/CCFs) in a pilot duct system. The single-pass removal efficiency of these air filters ranks alcohols > ketones > aromatics > alkanes, and various influential factors were analyzed. Jiang et al. [84] coated the ceramic tiles with N, F and Fe ions-doped TiO2 to photo-catalytically remove NO under visible light. Both air pollutions of inorganic NO and organic compounds can be purified by the ceramic tiles. The coating enables ceramic tiles to have enhanced photocatalytic efficiency, low water adsorption performance, good fastness, and antibacterial capability to reduce the risk of bacterial infection.
To reserve the original aesthetic features of historical and monumental architectures, Munafo et al. [72] applied anatase TiO2 colloidal suspensions via spray-coating to deposit transparent self-cleaning coatings on stones. Photocatalytic performances of TiO2-based coatings with one and three spray cycles were compared against that of the untreated travertine. The self-cleaning ability of analyzed coatings was degraded by the ageing processes till reaching low efficiency, and it shows no significant difference between single-layer and multilayer coatings in long-term use. Results seem to encourage the use of nano-structured TiO2 for preserving stone during time, while the undercoat and new and more stable colloidal TiO2 is needed.
To achieve a high photocatalytic efficiency and a robust weathering resistant ability, Guo et al. [85] applied a transparent photocatalytic coating containing TiO2 particles on the architectural mortar; its photocatalytic performances was better than the TiO2 intermixed samples on air purifying and self-cleaning properties under both UV-A and visible light irradiation conditions, and their abilities showed no obvious deterioration during a simulated facade-weathering process.
3.3. Paints
The commercial TiO2, P25 (75% anatase and 25% rutile phases), is commonly applied in paints. Guo et al. [86] directly applied a TiO2-containing paint (clear in colour) on the surface of self-compacting architectural mortars (SCAM). The results showed that the TiO2 paint -oated SCAM sample displayed both a high photocatalytic rhodamine b removal ability and a robust weathering resistance under all conditions. Baudys et al. [87] investigated the photocatalytic properties of self-cleaning acrylic paint containing TiO2 and ZnO using Acid Orange 7 as a model compound. The photocatalytic activity of TiO2 increases with the weathering time. However, even if the initial photoactivity of the unweathered paints with ZnO was significantly higher, the photocatalytic activity decreased after weathering, due to the loss and/or photocorrosion of ZnO particles during the weathering process. Amorim et al. [88] synthesized an efficient and durable self-cleaning acrylic paint containing mesoporous TiO2 (MTiO2). The paint with MTiO2 incorporated showed a better photoactivity and higher durability than the reference paint with P25 added in the cyclic analysis, which indicated that MTiO2 microspheres can provide acrylic paint films with self-cleaning properties without severe degradation of the binder.
The self-cleaning photocatalytic paints could be designed for specific applications for outdoor or indoor environments. Most of these photocatalytic paints are based on titanium dioxide, however, the large band gap of TiO2 requires UV photons for the electrons-holes generation, which makes it difficult to be efficient in the indoor environment. Galenda et al. [89] firstly compared the photocatalytic activity tests of indoor commercial self-cleaning paints under actual indoor light. These paints contain titanium dioxide with different amounts and crystallographic forms. The results of experiments with different lights suggest that all samples are scarcely active under visible light and the pollutant probes are selectively bleached due to their sensitizing effect. Consequently, the pollutants’ ability in injecting electrons in the TiO2 conduction band deeply affects their removal.
Paolini [90] propose a pre-treatment with nitric or sulfuric acid of commercial TiO2 nanopowders to prevent from aging used in coating, mortars, or paints. photocatalytic performances for experiments with various diffuse reflectance of nitric and sulfuric acid were compared. Nitric acid causes a decrease in crystallinity and photocatalytic activity, which drops by almost 20%; and sulfuric acid is the best candidate for TiO2 nanoparticles acid treatment with the aim of improving both their reflectance in a wavelength region unaffected by aging and sustaining their photocatalytic activity over time.
3.4. Construction Materials
Diamanti et al. [91] investigated the photocatalytic and self-cleaning activity of colored mortars containing TiO2, and its possible interaction with iron oxide pigments, which are commonly added to the mixture in the production of colored mortars. Because of the self-cleaning characteristics, TiO2 containing mortars have lower soiling in atmospheric exposure. However, the Iron oxide pigments caused lower photocatalytic activity compared to white mortars. Nath et al. [92] reviewed the photocatalysts used in concrete and found that majority of the work in concrete adopted TiO2 as the photocatalyst. They also regarded the semiconductor oxide LiNbO3, which has been used in electronic instruments to replace TiO2 for artificial photosynthesis, as a promising and relatively new approach to be used in future concrete.
For the applications of photocatalysts on buildings, it is clear that the photocatalyst TiO2 dominates the application market, and very few applications adopt other catalysts such as ZnO. Due to its low cost, high chemical stability and non-toxic properties, TiO2 has been investigated extensively and first applied to the practical applications on buildings. Since various materials have different properties, other materials will also be applied in the near future according to their characteristics.
4. Conclusions
Thermal catalytic and photocatalytic nanomaterials are increasingly being used as an efficient approach to remove the VOCs, and their emerging applications on building and construction materials are promising for their air-cleaning properties. Among these catalysts, TiO2 is the most common, economical and efficient one due to its low cost, high chemical stability, and non-toxic properties. TiO2 has been investigated for decades and dominates the applications of catalytic VOC removal on buildings. Since other thermal catalysts also showed brilliant performance and have been studied continuously, there is expected to be more applications on buildings using other emerging catalysts rather than TiO2. As the photocatalysis performances of metal oxides can be enhanced by the synergy with hybrid adsorption materials [6], more catalysts combining different nanomaterials are expected. Among various applications of catalytic nanomaterials on buildings, the approach of coatings is more efficient and prevalent compared to intermixing [85] on the removal of VOCs. In order to remove the indoor VOCs more efficiently, more researches and applications should be done to increase their efficiencies in specific conditions such as visible light.
Acknowledgments
Acknowledgement is given to Department of Building, National University of Singapore.
Author Contributions
Review proposal, K.W.S.; Information collection, K.W.S. and W.L.; Developing structure, K.W.S. and W.L.; Supervision, K.W.S.; Writing—Original Draft, W.L.; Writing—Review & Editing, K.W.S.
Funding
This research was funded by National Research Foundation (Singapore) grant number NRF2015NRF-POC001-0025 and National University of Singapore grant R-296-000-174-720.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Spengler J.D., Chen Q. Indoor air quality factors in designing a healthy building. Annu. Rev. Energy Environ. 2000;25:567–600. doi: 10.1146/annurev.energy.25.1.567. [DOI] [Google Scholar]
- 2.Jones A.P. Indoor air quality and health. Atmos. Environ. 1999;33:4535–4564. doi: 10.1016/S1352-2310(99)00272-1. [DOI] [Google Scholar]
- 3.Maroni M., Seifert B., Lindvall T. Indoor Air Quality: A Comprehensive Reference Book. Volume 3 Elsevier; New York, NY, USA: 1995. [Google Scholar]
- 4.Weschler C.J. Ozone’s impact on public health: Contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ. Health Perspect. 2006;114:1489–1496. doi: 10.1289/ehp.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C., Miao G., Pi Y., Xia Q., Wu J., Li Z., Xiao J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019;370:1128–1153. doi: 10.1016/j.cej.2019.03.232. [DOI] [Google Scholar]
- 6.Li M., Lu B., Ke Q.-F., Guo Y.-J., Guo Y.-P. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal. J. Hazard. Mater. 2017;333:88–98. doi: 10.1016/j.jhazmat.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 7.González-García P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018;82:1393–1414. doi: 10.1016/j.rser.2017.04.117. [DOI] [Google Scholar]
- 8.Spokas K.A., Novak J.M., Stewart C.E., Cantrell K.B., Uchimiya M., DuSaire M.G., Ro K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere. 2011;85:869–882. doi: 10.1016/j.chemosphere.2011.06.108. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Gao B., Creamer A.E., Cao C., Li Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017;338:102–123. doi: 10.1016/j.jhazmat.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Chen J., Gao Y., Ao Z., Li G., An T., Hu Y., Li Y. A coupled technique to eliminate overall nonpolar and polar volatile organic compounds from paint production industry. J. Clean. Prod. 2018;185:266–274. doi: 10.1016/j.jclepro.2018.03.037. [DOI] [Google Scholar]
- 11.Tompkins D.T., Anderson M.A. Evaluation of Photocatalytic Air Cleaning Capability: A Literature Review & Engineering Analysis. ASHRAE; Atlanta, GA, USA: 2001. [Google Scholar]
- 12.Mo J., Zhang Y., Xu Q., Lamson J.J., Zhao R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009;43:2229–2246. doi: 10.1016/j.atmosenv.2009.01.034. [DOI] [Google Scholar]
- 13.Chen H., Nanayakkara C.E., Grassian V.H. Titanium Dioxide Photocatalysis in Atmospheric Chemistry. Chem. Rev. 2012;112:5919–5948. doi: 10.1021/cr3002092. [DOI] [PubMed] [Google Scholar]
- 14.Shayegan Z., Lee C.-S., Haghighat F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018;334:2408–2439. doi: 10.1016/j.cej.2017.09.153. [DOI] [Google Scholar]
- 15.Cheng M., Brown S. VOCs Identified in Australian Indoor Air and Product Emission Environments; Proceedings of the National Clean Air Conference; Beijing, China. 4–9 September 2005; pp. 23–27. [Google Scholar]
- 16.Wang S., Ang H.M., Tade M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007;33:694–705. doi: 10.1016/j.envint.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Kang D.H., Choi D.H., Lee S.M., Yeo M.S., Kim K.W. Effect of bake-out on reducing VOC emissions and concentrations in a residential housing unit with a radiant floor heating system. Build. Environ. 2010;45:1816–1825. doi: 10.1016/j.buildenv.2010.02.010. [DOI] [Google Scholar]
- 18.Kim S., Choi Y.-K., Park K.-W., Kim J.T. Test methods and reduction of organic pollutant compound emissions from wood-based building and furniture materials. Bioresour. Technol. 2010;101:6562–6568. doi: 10.1016/j.biortech.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L., Shah K.W. RSC Smart Materials. Royal Society of Chemistry; London, UK: 2019. Chapter 16: Electrochromic Smart Windows for Green Building Applications; pp. 494–520. [Google Scholar]
- 20.Huseien G.F., Sam A.R.M., Shah K.W., Asaad M.A., Tahir M.M., Mirza J. Properties of ceramic tile waste based alkali-activated mortars incorporating GBFS and fly ash. Constr. Build. Mater. 2019;214:355–368. doi: 10.1016/j.conbuildmat.2019.04.154. [DOI] [Google Scholar]
- 21.Seh Z.W., Liu S., Zhang S.Y., Shah K.W., Han M.Y. Synthesis and multiple reuse of eccentric Au@TiO2 nanostructures as catalysts. Chem. Commun. 2011;47:6689–6691. doi: 10.1039/c1cc11729g. [DOI] [PubMed] [Google Scholar]
- 22.Hussain M., Akhter P., Iqbal J., Ali Z., Yang W., Shehzad N., Majeed K., Sheikh R., Amjad U., Russo N. VOCs photocatalytic abatement using nanostructured titania-silica catalysts. J. Environ. Chem. Eng. 2017;5:3100–3107. doi: 10.1016/j.jece.2017.06.014. [DOI] [Google Scholar]
- 23.Singh E., Meyyappan M., Nalwa H.S. Flexible Graphene-Based Wearable Gas and Chemical Sensors. ACS Appl. Mater. Interfaces. 2017;9:34544–34586. doi: 10.1021/acsami.7b07063. [DOI] [PubMed] [Google Scholar]
- 24.Andre R.S., Sanfelice R.C., Pavinatto A., Mattoso L.H.C., Correa D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018;156:154–166. doi: 10.1016/j.matdes.2018.06.041. [DOI] [Google Scholar]
- 25.Kwon S., Fan M., Cooper A.T., Yang H. Photocatalytic applications of micro- and nano-TiO2 in environmental engineering. Crit. Rev. Environ. Sci. Technol. 2008;38:197–226. doi: 10.1080/10643380701628933. [DOI] [Google Scholar]
- 26.Tsang C.H.A., Li K., Zeng Y., Zhao W., Zhang T., Zhan Y., Xie R., Leung D.Y.C., Huang H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019;125:200–228. doi: 10.1016/j.envint.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Hu M., Yao Z., Wang X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017;56:3477–3502. doi: 10.1021/acs.iecr.6b05048. [DOI] [Google Scholar]
- 28.Pan Y., Yuan X., Jiang L., Yu H., Zhang J., Wang H., Guan R., Zeng G. Recent advances in synthesis, modification and photocatalytic applications of micro/nano-structured zinc indium sulfide. Chem. Eng. J. 2018;354:407–431. doi: 10.1016/j.cej.2018.08.028. [DOI] [Google Scholar]
- 29.Sharma R., Sharma S., Dutta S., Zboril R., Gawande M.B. Silica-nanosphere-based organic–inorganic hybrid nanomaterials: Synthesis, functionalization and applications in catalysis. Gr. Chem. 2015;17:3207–3230. doi: 10.1039/C5GC00381D. [DOI] [Google Scholar]
- 30.Huang H., Xu Y., Feng Q., Leung D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015;5:2649–2669. doi: 10.1039/C4CY01733A. [DOI] [Google Scholar]
- 31.Pelaez M., Nolan N.T., Pillai S.C., Seery M.K., Falaras P., Kontos A.G., Dunlop P.S.M., Hamilton J.W.J., Byrne J.A., O’Shea K., et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012;125:331–349. doi: 10.1016/j.apcatb.2012.05.036. [DOI] [Google Scholar]
- 32.Truppi A., Petronella F., Placido T., Striccoli M., Agostiano A., Curri M., Comparelli R. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts. 2017;7:100. doi: 10.3390/catal7040100. [DOI] [Google Scholar]
- 33.Maira A.J., Yeung K.L., Lee C.Y., Yue P.L., Chan C.K. Size Effects in Gas-Phase Photo-oxidation of Trichloroethylene Using Nanometer-Sized TiO2 Catalysts. J. Catal. 2000;192:185–196. doi: 10.1006/jcat.2000.2838. [DOI] [Google Scholar]
- 34.Maira A.J., Coronado J.M., Augugliaro V., Yeung K.L., Conesa J.C., Soria J. Fourier Transform Infrared Study of the Performance of Nanostructured TiO2 Particles for the Photocatalytic Oxidation of Gaseous Toluene. J. Catal. 2001;202:413–420. doi: 10.1006/jcat.2001.3301. [DOI] [Google Scholar]
- 35.Lee H.J., Seo H.O., Kim D.W., Kim K.-D., Luo Y., Lim D.C., Ju H., Kim J.W., Lee J., Kim Y.D. A high-performing nanostructured TiO2 filter for volatile organic compounds using atomic layer deposition. Chem. Commun. 2011;47:5605–5607. doi: 10.1039/c1cc10307e. [DOI] [PubMed] [Google Scholar]
- 36.Weon S., Choi W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci.Technol. 2016;50:2556–2563. doi: 10.1021/acs.est.5b05418. [DOI] [PubMed] [Google Scholar]
- 37.Weon S., Choi J., Park T., Choi W. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds. Appl. Catal. B Environ. 2017;205:386–392. doi: 10.1016/j.apcatb.2016.12.048. [DOI] [Google Scholar]
- 38.Wu C., Yue Y., Deng X., Hua W., Gao Z. Investigation on the synergetic effect between anatase and rutile nanoparticles in gas-phase photocatalytic oxidations. Catal. Today. 2004;93–95:863–869. doi: 10.1016/j.cattod.2004.06.087. [DOI] [Google Scholar]
- 39.Chen K., Zhu L., Yang K. Tricrystalline TiO2 with enhanced photocatalytic activity and durability for removing volatile organic compounds from indoor air. J. Environ. Sci. 2015;32:189–195. doi: 10.1016/j.jes.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Zhong L., Brancho J.J., Batterman S., Bartlett B.M., Godwin C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. App. Catal. B Environ. 2017;216:122–132. doi: 10.1016/j.apcatb.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu X., Miyauchi M., Sunada K., Minoshima M., Liu M., Lu Y., Li D., Shimodaira Y., Hosogi Y., Kuroda Y., et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano. 2012;6:1609–1618. doi: 10.1021/nn2045888. [DOI] [PubMed] [Google Scholar]
- 42.Weon S., Kim J., Choi W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound. Appl. Catal. B Environ. 2018;220:1–8. doi: 10.1016/j.apcatb.2017.08.036. [DOI] [Google Scholar]
- 43.Li J.J., Cai S.C., Yu E.Q., Weng B., Chen X., Chen J., Jia H.P., Xu Y.J. Efficient infrared light promoted degradation of volatile organic compounds over photo-thermal responsive Pt-rGO-TiO2composites. Appl. Catal. B Environ. 2018;233:260–271. doi: 10.1016/j.apcatb.2018.04.011. [DOI] [Google Scholar]
- 44.Suárez S., Jansson I., Ohtani B., Sánchez B. From titania nanoparticles to decahedral anatase particles: Photocatalytic activity of TiO2/zeolite hybrids for VOCs oxidation. Catal. Today. 2019;326:2–7. doi: 10.1016/j.cattod.2018.09.004. [DOI] [Google Scholar]
- 45.Li X., Zhu Z., Zhao Q., Wang L. Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: A comparative study. J. Hazard. Mater. 2011;186:2089–2096. doi: 10.1016/j.jhazmat.2010.12.111. [DOI] [PubMed] [Google Scholar]
- 46.Jiang S., Handberg E.S., Liu F., Liao Y., Wang H., Li Z., Song S. Effect of doping the nitrogen into carbon nanotubes on the activity of NiO catalysts for the oxidation removal of toluene. Applied Catal. B Environ. 2014;160–161:716–721. doi: 10.1016/j.apcatb.2014.06.026. [DOI] [Google Scholar]
- 47.Kim H.I., Kim H.N., Weon S., Moon G.H., Kim J.H., Choi W. Robust Co-catalytic performance of nanodiamonds loaded on WO3 for the decomposition of volatile organic compounds under visible light. ACS Catal. 2016;6 doi: 10.1021/acscatal.6b02726. [DOI] [Google Scholar]
- 48.Genuino H.C., Dharmarathna S., Njagi E.C., Mei M.C., Suib S.L. Gas-phase total oxidation of benzene, toluene, ethylbenzene, and xylenes using shape-selective manganese oxide and copper manganese oxide catalysts. J. Phys. Chem. C. 2012;116:12066–12078. doi: 10.1021/jp301342f. [DOI] [Google Scholar]
- 49.Miyawaki J., Lee G.H., Yeh J., Shiratori N., Shimohara T., Mochida I., Yoon S.H. Development of carbon-supported hybrid catalyst for clean removal of formaldehyde indoors. Catal. Today. 2012;185:278–283. doi: 10.1016/j.cattod.2011.09.036. [DOI] [Google Scholar]
- 50.Qian X., Yue D., Tian Z., Reng M., Zhu Y., Kan M., Zhang T., Zhao Y. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B Environ. 2016;193:16–21. doi: 10.1016/j.apcatb.2016.04.009. [DOI] [Google Scholar]
- 51.Cao J., Luo B., Lin H., Chen S. Photocatalytic activity of novel AgBr/WO3 composite photocatalyst under visible light irradiation for methyl orange degradation. J. Hazard. Mater. 2011;190:700–706. doi: 10.1016/j.jhazmat.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 52.Abbasi Z., Haghighi M., Fatehifar E., Saedy S. Synthesis and physicochemical characterizations of nanostructured Pt/Al2O3–CeO2 catalysts for total oxidation of VOCs. J. Hazard. Mater. 2011;186:1445–1454. doi: 10.1016/j.jhazmat.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z., Mao J., Zhou R. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature. Appl. Surf. Sci. 2019;465:15–22. doi: 10.1016/j.apsusc.2018.09.138. [DOI] [Google Scholar]
- 54.Wang L., Sakurai M., Kameyama H. Study of catalytic decomposition of formaldehyde on Pt/TiO2 alumite catalyst at ambient temperature. J. Hazard. Mater. 2009;167:399–405. doi: 10.1016/j.jhazmat.2008.12.129. [DOI] [PubMed] [Google Scholar]
- 55.Schick L., Sanchis R., González-Alfaro V., Agouram S., López J.M., Torrente-Murciano L., García T., Solsona B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs) Chem. Eng. J. 2019;366:100–111. doi: 10.1016/j.cej.2019.02.087. [DOI] [Google Scholar]
- 56.Joung H.-J., Kim J.-H., Oh J.-S., You D.-W., Park H.-O., Jung K.-W. Catalytic oxidation of VOCs over CNT-supported platinum nanoparticles. Appl. Surf. Sci. 2014;290:267–273. doi: 10.1016/j.apsusc.2013.11.066. [DOI] [Google Scholar]
- 57.Zhang Y., Tang Z.-R., Fu X., Xu Y.-J. TiO2−Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2−Graphene Truly Different from Other TiO2−Carbon Composite Materials? ACS Nano. 2010;4:7303–7314. doi: 10.1021/nn1024219. [DOI] [PubMed] [Google Scholar]
- 58.Roso M., Boaretti C., Pelizzo M.G., Lauria A., Modesti M., Lorenzetti A. Nanostructured Photocatalysts Based on Different Oxidized Graphenes for VOCs Removal. Ind. Eng. Chem. Resour. 2017;56:9980–9992. doi: 10.1021/acs.iecr.7b02526. [DOI] [Google Scholar]
- 59.Demeestere K., Dewulf J., Van Langenhove H. Heterogeneous Photocatalysis as an Advanced Oxidation Process for the Abatement of Chlorinated, Monocyclic Aromatic and Sulfurous Volatile Organic Compounds in Air: State of the Art. Crit. Rev Environ. Sci. Technol. 2007;37:489–538. doi: 10.1080/10643380600966467. [DOI] [Google Scholar]
- 60.Fujishima A., Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 61.Di Paola A., Bellardita M., Palmisano L. Brookite, the least known TiO2 photocatalyst. Catalysts. 2013;3:36–73. doi: 10.3390/catal3010036. [DOI] [Google Scholar]
- 62.Monai M., Montini T., Fornasiero P. Brookite: Nothing New under the Sun? Catalysts. 2017;7:304. doi: 10.3390/catal7100304. [DOI] [Google Scholar]
- 63.Jo W.-K., Kim J.-T. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009;164:360–366. doi: 10.1016/j.jhazmat.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 64.Elfalleh W., Assadi A.A., Bouzaza A., Wolbert D., Kiwi J., Rtimi S. Innovative and stable TiO2 supported catalytic surfaces removing aldehydes under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017;343:96–102. doi: 10.1016/j.jphotochem.2017.04.029. [DOI] [Google Scholar]
- 65.Assadi A.A., Bouzaza A., Wolbert D., Petit P. Isovaleraldehyde elimination by UV/TiO2 photocatalysis: Comparative study of the process at different reactors configurations and scales. Environ. Sci. Pollut. Res. 2014;21:11178–11188. doi: 10.1007/s11356-014-2603-7. [DOI] [PubMed] [Google Scholar]
- 66.Palau J., Assadi A.A., Penya-Roja J., Bouzaza A., Wolbert D., Martínez-Soria V. Isovaleraldehyde degradation using UV photocatalytic and dielectric barrier discharge reactors, and their combinations. J. Photochem. Photobiol. A Chemis. 2015;299:110–117. doi: 10.1016/j.jphotochem.2014.11.013. [DOI] [Google Scholar]
- 67.Azzouz I., Habba Y.G., Capochichi-Gnambodoe M., Marty F., Vial J., Leprince-Wang Y., Bourouina T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoengin. 2018;4 doi: 10.1038/micronano.2017.93. [DOI] [Google Scholar]
- 68.Chen J., Chen X., Yan D., Jiang M., Xu W., Yu H., Jia H. A facile strategy of enhancing interaction between cerium and manganese oxides for catalytic removal of gaseous organic contaminants. Appl. Catal. B Environ. 2019;250:396–407. doi: 10.1016/j.apcatb.2019.03.042. [DOI] [Google Scholar]
- 69.Zhao Q., Liu Q., Song C., Ji N., Ma D., Lu X. Enhanced catalytic performance for VOCs oxidation on the CoAlO oxides by KMnO4 doped on facile synthesis. Chemosphere. 2019;218:895–906. doi: 10.1016/j.chemosphere.2018.11.131. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi B., Yamamoto R., Ohkita H., Mizushima T., Hiraishi A., Kakuta N. Photocatalytic Activity of AgBr as an Environmental Catalyst. Top Catal. 2013;56:618–622. doi: 10.1007/s11244-013-0020-7. [DOI] [Google Scholar]
- 71.Samaddar P., Son Y.-S., Tsang D.C.W., Kim K.-H., Kumard S. Progress in graphene-based materials as superior media for sensing, sorption, and separation of gaseous pollutants. Coord. Chem. Rev. 2018;368:93–114. doi: 10.1016/j.ccr.2018.04.013. [DOI] [Google Scholar]
- 72.Munafò P., Quagliarini E., Goffredo G.B., Bondioli F., Licciulli A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014;65:218–231. doi: 10.1016/j.conbuildmat.2014.04.112. [DOI] [Google Scholar]
- 73.Toro C., Jobson B.T., Haselbach L., Shen S., Chung S.H. Photoactive roadways: Determination of CO, NO and VOC uptake coefficients and photolabile side product yields on TiO2 treated asphalt and concrete. Atmos. Environ. 2016;139:37–45. doi: 10.1016/j.atmosenv.2016.05.007. [DOI] [Google Scholar]
- 74.Huseien G.F., Shah K.W., Sam A.R.M. Sustainability of nanomaterials based self-healing concrete: An all-inclusive insight. J. Build. Eng. 2019;23:155–171. doi: 10.1016/j.jobe.2019.01.032. [DOI] [Google Scholar]
- 75.Li L., Zhang F., Zhong Z., Zhu M., Jiang C., Hu J., Xing W. Novel Synthesis of a High-Performance Pt/ZnO/SiC Filter for the Oxidation of Toluene. Ind. Eng. Chem. Res. 2017;56:13857–13865. doi: 10.1021/acs.iecr.7b02793. [DOI] [Google Scholar]
- 76.Zadi T., Assadi A.A., Nasrallah N., Bouallouche R., Tri P.N., Bouzaza A., Azizi M.M., Maachi R., Wolbert D. Treatment of hospital indoor air by a hybrid system of combined plasma with photocatalysis: Case of trichloromethane. Chem. Eng. J. 2018;349:276–286. doi: 10.1016/j.cej.2018.05.073. [DOI] [Google Scholar]
- 77.Li F.B., Li X.Z., Ao C.H., Lee S.C., Hou M.F. Enhanced photocatalytic degradation of VOCs using Ln3+–TiO2 catalysts for indoor air purification. Chemosphere. 2005;59:787–800. doi: 10.1016/j.chemosphere.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Boyjoo Y., Sun H., Liu J., Pareek V.K., Wang S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017;310:537–559. doi: 10.1016/j.cej.2016.06.090. [DOI] [Google Scholar]
- 79.Chabas A., Lombardo T., Cachier H., Pertuisot M.H., Oikonomou K., Falcone R., Verità M., Geotti-Bianchini F. Behaviour of self-cleaning glass in urban atmosphere. Build. Environ. 2008;43:2124–2131. doi: 10.1016/j.buildenv.2007.12.008. [DOI] [Google Scholar]
- 80.Oladipo H., Garlisi C., Al-Ali K., Azar E., Palmisano G. Combined photocatalytic properties and energy efficiency via multifunctional glass. J. Environ. Chem. Eng. 2019;7 doi: 10.1016/j.jece.2019.102980. [DOI] [Google Scholar]
- 81.Monteiro R.A.R., Lopes F.V.S., Silva A.M.T., Ângelo J., Silva G.V., Mendes A.M., Boaventura R.A.R., Vilar V.J.P. Are TiO2-based exterior paints useful catalysts for gas-phase photooxidation processes? A case study on n-decane abatement for air detoxification. Appl. Catal. B Environ. 2014;147:988–999. doi: 10.1016/j.apcatb.2013.09.031. [DOI] [Google Scholar]
- 82.Martinez T., Bertron A., Escadeillas G., Ringot E., Simon V. BTEX abatement by photocatalytic TiO2-bearing coatings applied to cement mortars. Build. Environ. 2014;71:186–192. doi: 10.1016/j.buildenv.2013.10.004. [DOI] [Google Scholar]
- 83.Zhong L., Haghighat F., Lee C.S., Lakdawala N. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation. J. Hazard. Mater. 2013;261:130–138. doi: 10.1016/j.jhazmat.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Jiang Q., Qi T., Yang T., Liu Y. Ceramic tiles for photocatalytic removal of NO in indoor and outdoor air under visible light. Build. Environ. 2019;158:94–103. doi: 10.1016/j.buildenv.2019.05.014. [DOI] [Google Scholar]
- 85.Guo M.-Z., Maury-Ramirez A., Poon C.S. Photocatalytic activities of titanium dioxide incorporated architectural mortars: Effects of weathering and activation light. Build. Environ. 2015;94:395–402. doi: 10.1016/j.buildenv.2015.08.027. [DOI] [Google Scholar]
- 86.Guo M.-Z., Maury-Ramirez A., Poon C.S. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Product. 2016;112:3583–3588. doi: 10.1016/j.jclepro.2015.10.079. [DOI] [Google Scholar]
- 87.Baudys M., Krýsa J., Zlámal M., Mills A. Weathering tests of photocatalytic facade paints containing ZnO and TiO2. Chem. Eng. J. 2015;261:83–87. doi: 10.1016/j.cej.2014.03.112. [DOI] [Google Scholar]
- 88.Amorim S.M., Suave J., Andrade L., Mendes A.M., José H.J., Moreira R.F.P.M. Towards an efficient and durable self-cleaning acrylic paint containing mesoporous TiO2 microspheres. Prog. Org. Coat. 2018;118:48–56. doi: 10.1016/j.porgcoat.2018.01.005. [DOI] [Google Scholar]
- 89.Galenda A., Visentin F., Gerbasi R., Favaro M., Bernardi A., El Habra N. Evaluation of self-cleaning photocatalytic paints: Are they effective under actual indoor lighting systems? Appl. Catal. B Environ. 2018;232:194–204. doi: 10.1016/j.apcatb.2018.03.052. [DOI] [Google Scholar]
- 90.Paolini R., Borroni D., Pedeferri M., Diamanti M.V. Self-cleaning building materials: The multifaceted effects of titanium dioxide. Constr. Build. Mater. 2018;182:126–133. doi: 10.1016/j.conbuildmat.2018.06.047. [DOI] [Google Scholar]
- 91.Diamanti M.V., Del Curto B., Ormellese M., Pedeferri M. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013;46:167–174. doi: 10.1016/j.conbuildmat.2013.04.038. [DOI] [Google Scholar]
- 92.Nath R.K., Zain M.F.M., Jamil M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016;62:1184–1194. doi: 10.1016/j.rser.2016.05.018. [DOI] [Google Scholar]