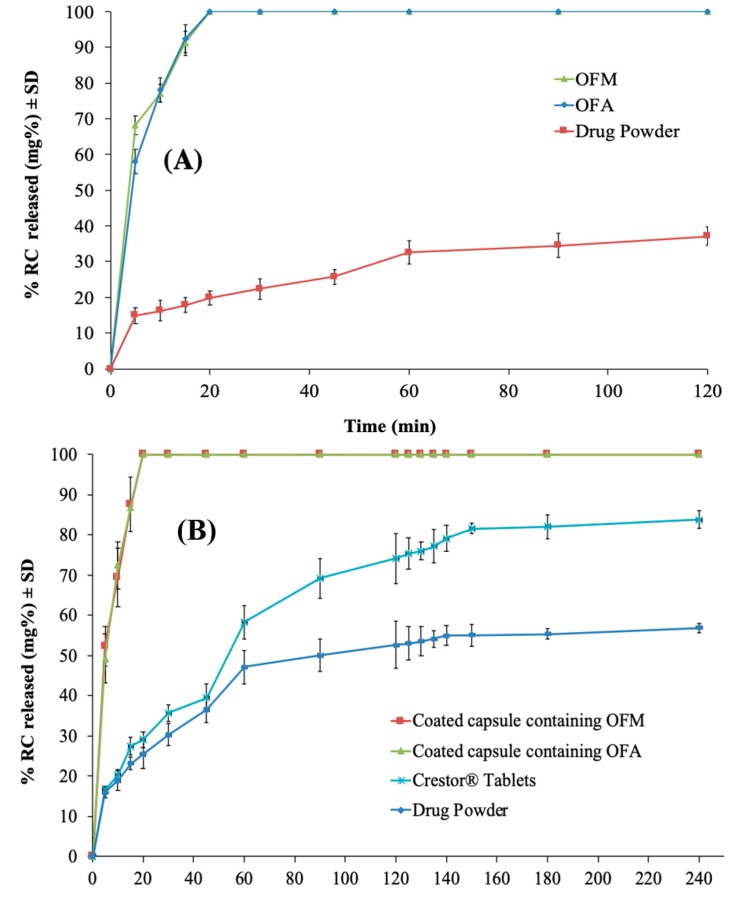
Figure 7.
Release profile of RC from the optimized IFN formulations in 0.1N HCl (A), in comparison with the drug powder, and from the enteric-coated capsules containing the optimized formulations in citrate buffer (pH 6.6) (B), in comparison with the market product (Crestor® Tablets) and the drug powder.