Abstract
Series of multivalent α-l-fucoside containing glycoclusters and variously decorated l-fucosides were synthesized to find potential inhibitors of fucose-specific lectins and study the structure-binding affinity relationships. Tri- and tetravalent fucoclusters were built using copper-mediated azide-alkyne click chemistry. Series of fucoside monomers and dimers were synthesized using various methods, namely glycosylation, an azide-alkyne click reaction, photoinduced thiol-en addition, and sulfation. The interactions between compounds with six fucolectins of bacterial or fungal origin were tested using a hemagglutination inhibition assay. As a result, a tetravalent, α-l-fucose presenting glycocluster showed to be a ligand that was orders of magnitude better than a simple monosaccharide for tested lectins in most cases, which can nominate it as a universal ligand for studied lectins. This compound was also able to inhibit the adhesion of Pseudomonas aeruginosa cells to human epithelial bronchial cells. A trivalent fucocluster with a protected amine functional group also seems to be a promising candidate for designing glycoconjugates and chimeras.
Keywords: l-fucosides, multivalency, lectins, glycoclusters, hemagglutination, cystic fibrosis
1. Introduction
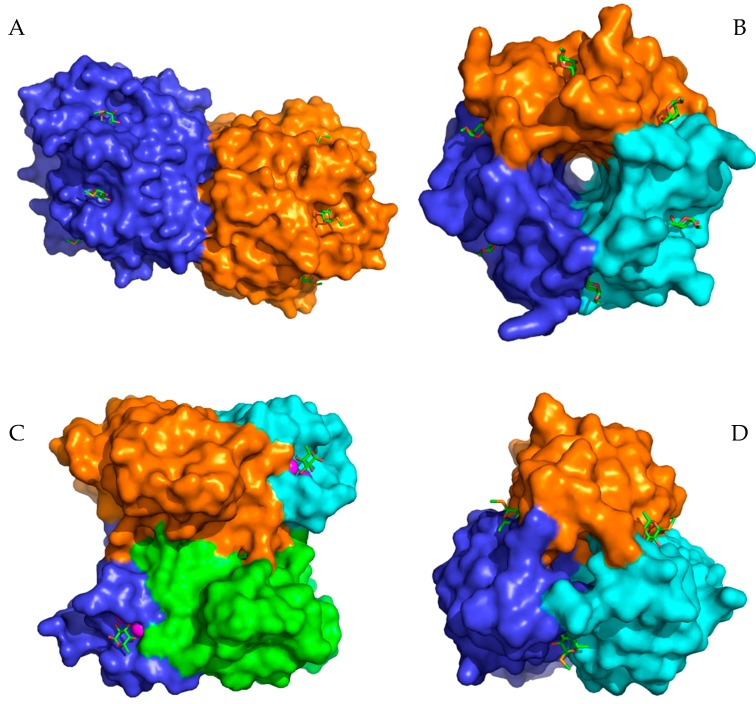
Lectins are specific carbohydrate-binding proteins of a non-immune origin. A common role of these proteins is their involvement in recognition and adhesion processes between pathogens and hosts. For example, the chronic infection and colonization of lungs by opportunistic microorganisms is the main cause of mortality among people suffering from cystic fibrosis (CF) [1]. It was demonstrated that major changes in CF glycosylation are represented by increased fucosylation and decreased sialylation [2]. Therefore, lectins from the pathogens could be important virulence factors also representing a suitable therapeutic target [3]. Three of the most widespread pathogens associated with cystic fibrosis—Pseudomonas aeruginosa, Burkholderia cenocepacia, and Aspergillus fumigatus—produce fucose-specific lectins considered to be involved in pathogenesis. The PA-IIL lectin (LecB) from the Gram-negative bacterium P. aeruginosa is involved in the adhesion of the bacterium to the host cells via interactions with host glycoconjugates and in the formation of biofilm [4]. PA-IIL was also shown to block epithelial cells’ ciliary beating [5]. The BC2L-C lectin from the bacterium B. cenocepacia (closely related to P. aeruginosa) has two distinct domains with unique selectivity [6]. The N-terminal domain is a TNF-α-like l-fucose binding domain, while the C-terminal part displays selectivity for d-mannose and l-glycero-d-manno-heptose in a calcium-dependent manner. The N-terminal domain has a strong pro-inflammatory effect and is expected to bind fucosylated epitopes on human glycolipids [6]. Aspergillus fumigatus lectin AFL from the fungus A. fumigatus stimulates human bronchial cells to produce IL-8 and is supposed to contribute to the inflammatory response observed upon the exposure of a patient to A. fumigatus [7]. Although these lectins are all fucose-specific and their supposed functions are similar, they differ significantly in terms of their structural arrangement and binding modes (Figure 1). The N-terminal domain of BC2L-C forms a trimer with binding sites located between neighboring monomers. PA-IIL is a homotetramer with a single binding site per monomer. Two calcium ions mediate binding of the sugar in each binding site [8]. AFL is a dimer, where each monomer forms a six-bladed β-propeller with six non-equivalent binding sites all located on the opposite side of the molecule to the N- and C-termini [9].
Figure 1.
Structures of selected lectins from pathogenic microorganisms used for the inhibition studies. (A) AFL dimer in a complex with seleno fucopyranoside (PDB 4AGI). (B) RSL trimer in a complex with methyl α-l-fucopyranoside (PDB 2BT9). (C) PA-IIL tetramer in a complex with α-l-fucopyranoside (PDB 1UZV). (D) Trimer of the BC2L-C N-terminal domain in a complex with seleno fucopyranoside (PDB 2WQ4). Carbohydrates in the binding sites are depicted as sticks. The magenta spheres represent calcium ions in the binding sites of lectin PA-IIL.
In this study, we focused on potential inhibitors of fucose-specific lectins from pathogens associated with cystic fibrosis mentioned above. To evaluate common rules for their inhibition, we further included three AFL homologues, known as AAL (Aleuria aurantia lectin), AOL (Aspergillus oryzae lectin), and RSL (Ralstonia solanacearum lectin), from the AAL lectin family, sharing the same six-bladed β-propeller fold, but differing in subtle carbohydrate specificities. The involvement of AOL from Aspergillus oryzae in the allergic responses to the fungus was proposed with the suggested mechanism of AOL binding to fucose residues of IgE [10]. AAL from the orange peel fungus Aleuria aurantia was the first characterized representative of this lectin family. Both AOL and AAL form dimers similar to AFL. In contrast to the AFL dimer interface, which is formed by loops of all six blades and only the N-terminus is involved, only the loops of blades 6, 1, and 2 come into contact upon dimerization in AAL and both the N- and C-termini are crucial [7,11]. AAL contains only five binding sites, with the sixth believed to be inactive [11]. RSL from Ralstonia solanacearum, a dangerous phytopathogen of important agricultural plants (e.g., potatoes, tomatoes) [12], may be involved in adhesion of the bacteria, possibly via binding to terminal fucosides of plant xyloglucans [13]. In contrast to the others, RSL forms the six-bladed β-propeller fold by trimerization, where the monomers present two binding sites each, one formed by oligomerization and the second in between the blades of the same monomer (Figure 1) [13].
Lectins are usually multivalent proteins frequently displaying an avidity effect resulting in a significantly increased affinity towards their ligands. Consequently, the multivalent inhibitors with several carbohydrate moieties attached are generally considered to be among the most efficient molecules [14]. Several classes of inhibitors were tested against some of the selected lectins. The known multivalent inhibitors of AFL include cyclopeptide-based hexavalent compounds with a terminal fucose residue and multivalent compounds based on cyclodextrin or octameric silsesquioxane scaffolds [15,16]. C-hexopyranosyl calix[4]arene conjugates were used as potential multivalent inhibitors of BC2L-C and AFL [17]. A broad variety of potential monovalent and multivalent inhibitors were designed and tested against PA-IIL, including C-glycosidic glycomimetics, cinnamide and sulfonamide carbohydrate derivatives, fucofullerenes, glycopeptide dendrimers, pentavalent pillar[5]arene-based glycoclusters, perylenediimide-based glycoclusters, and photoswitchable Janus glycodendrimer micelles [18,19,20,21,22,23,24]. Fucofullerenes and C-hexopyranosyl calix[4]arene conjugates were examined as potential multivalent inhibitors of RSL [17,18,19,20].
In our current work, we focused on multivalent (tri- and tetravalent) glycoclusters with different aglycons (spacers) to exploit their avidity effects. Several thio-α/β-l-fucopyranosides were synthesized to explore the influence of the presence of the anomeric sulfur on the binding, which are more stable in vivo due to the resistance to the degradative enzymes. We further aimed to find a common inhibitor with a reasonable efficiency for all tested lectins from the pathogens associated with cystic fibrosis. The inhibitory potency of all used inhibitors against selected lectins was determined by a hemagglutination inhibition assay with microscope detection, whose robustness and applicability have been demonstrated previously [25,26].
2. Results and Discussion
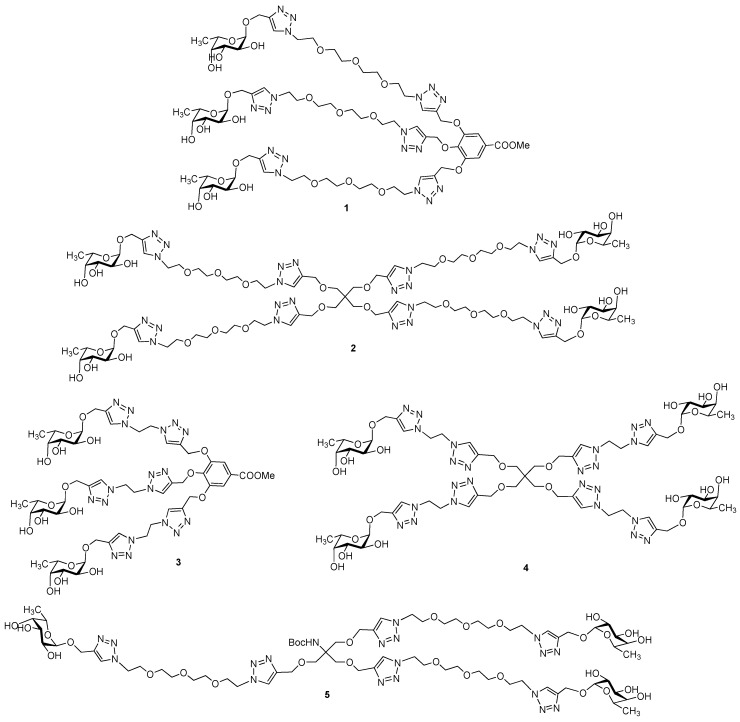
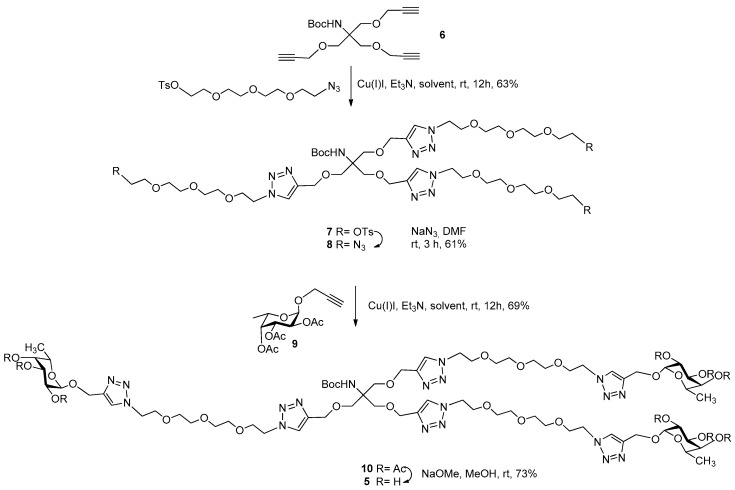
Tri- and tetravalent α-l-fucose-presenting glycoclusters 1, 2, 3, and 4 were synthesized previously [26] (Scheme 1). Similarly to our recent work [26,27], the fucocluster 5 was synthesized starting from the propargylated NHBoc-Tris scaffold 6 [28] by coupling with tosylated O-(2-azidoethyl)-triethylene glycol [29] using a Cu(I)-catalyzed azide-alkyne cycloaddition reaction (CuAAC) to get tosylated compound 7 (Scheme 2). The tosyl groups of the scaffold were converted into azido functions using sodium azide, resulting in compound 8, which was also suitable for a subsequent click reaction and was coupled with acetylated propargyl α-l-fucopyranoside 9 [30] to produce the protected fucocluster 10 with a 69% yield. Finally, protecting groups were removed by Zemplén deacetylation to get 5, containing a protected NH-group, which is suitable for further conjugation to synthesize, e.g., chimera compounds and glycoconjugates.
Scheme 1.
Chemical structures of multivalent glycoclusters containing α-l-fucosides.
Scheme 2.
Synthesis of multivalent fucoside 5.
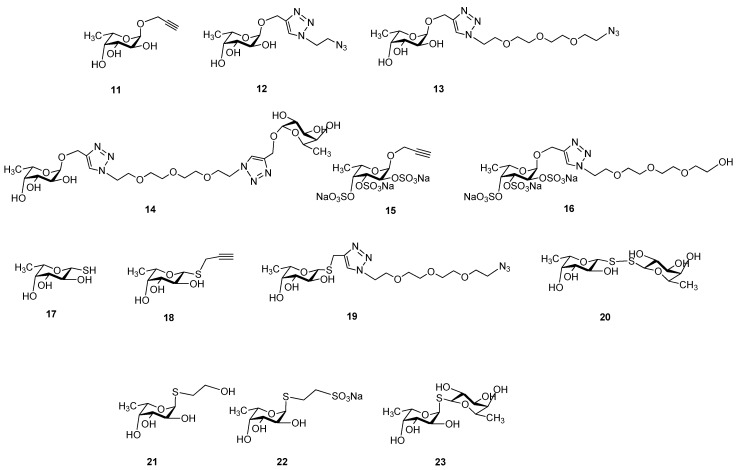
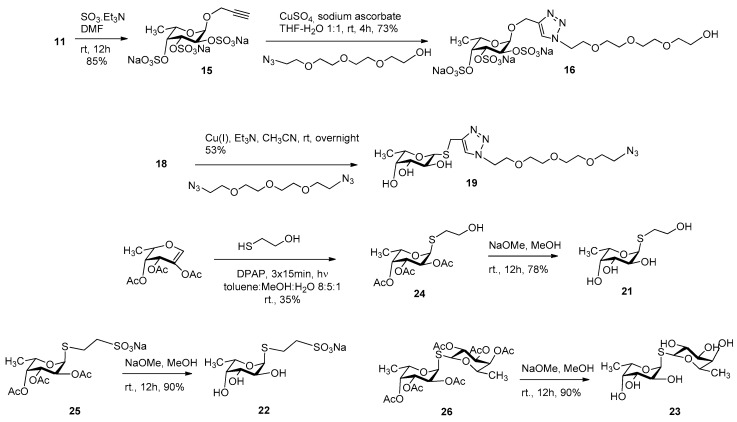
Other series of already described (11, 12, 13, 14, 17, 18, and 20) or newly synthesized fucosides (15, 16, 19, 21, 22, and 23) were used for an investigation of lectin-carbohydrate interactions that consisted of α-O-fucosides (11 [31], 12 [26], 13 [26], 14 [26]); sulfated α-O-fucosides (15 and 16); β-1-thiofucose derivatives 17 [32], 18 [33], and 19 and disulfide 20 [34]; as well as α-1-thiofucosides 21, 22, and 23 (Scheme 3).
Scheme 3.
Chemical structures of l-fucoside series.
Persulfation of 11 using the SO3.Et3N complex resulted in 15 with an 85% yield, which was then coupled with O-(2-azidoethyl)triethylene glycol by a click reaction using CuSO4-sodium ascorbate to get 16 with a 73% yield (Scheme 4). Compound 19 was prepared from 18 and 1,11-diazido-3,6,9-trioxaundecaneglycol by a Cu(I)-catalyzed click reaction with a 53% yield. Finally, 1,2-cis-α-linked thioglycoside 21 was created in a stereoselective manner by a photoinduced addition reaction [34,35] between 2-acetoxy-3,4-di-O-acetyl-l-fucal [36] and 2-mercaptoethanol. The radical-mediated addition provided 24, which was deacetylated by the Zemplén method, resulting in 21. S-fucoside 22 and thiodisaccharide 23 were prepared by Zemplén deacetylation from their protected forms 25 and 26 [34], respectively.
Scheme 4.
Synthesis of persulfated fucosides 15 and 16 and thioglycosides 19, 21, 22, and 23.
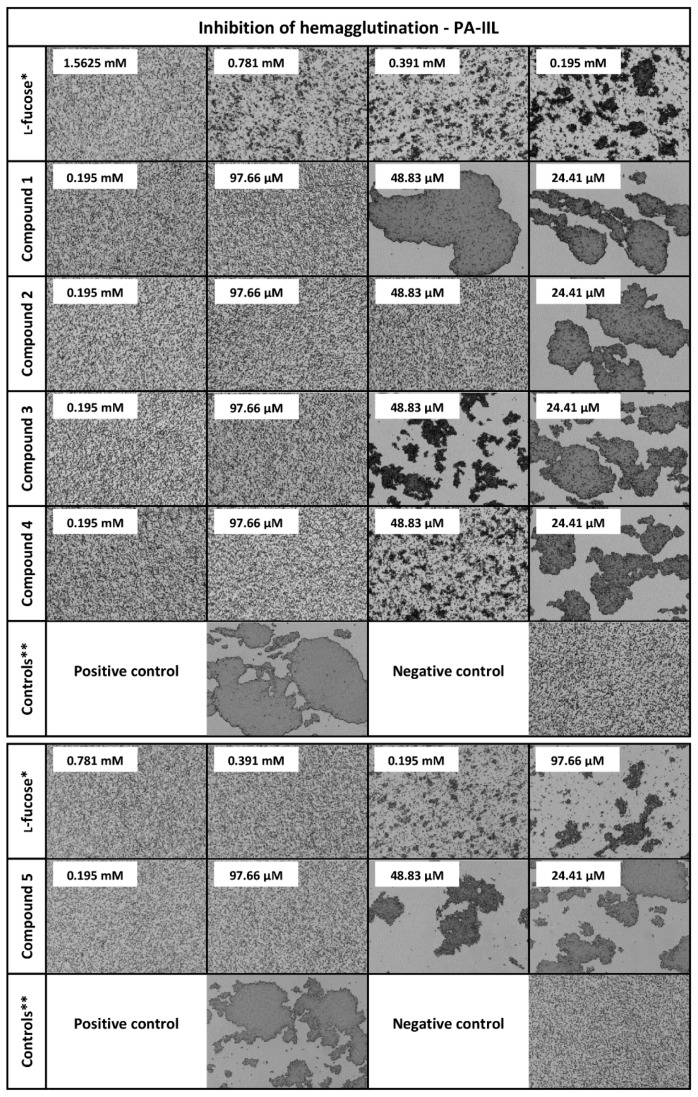
The ability of glycoclusters and l-fucosides to inhibit selected lectins was determined by a hemagglutination inhibition assay with microscope detection (see Table 1 and Figure 2). The inhibitory activity of the tested compounds was semi-quantitatively evaluated by a comparison with the standard (l-fucose). Compounds 15 and 16 were either not able to inhibit any lectin or only very weakly. Although the binding modes of the AAL family, PA-IIL, and BC2L-C differ, the persulfated compounds were not functional inhibitors for any of these proteins, probably due to the steric hindrances and/or requirements of free OH groups for the binding. Compound 17 (thio analogue of l-fucose) was a slightly better inhibitor of PA-IIL, probably due to its small size, but in general, it performed poorly. Lectin BC2L-C was not able to recognize 17 at all. Considering β-thio-l-fucopyranosides, compounds 18 and 19 were generally weak inhibitors with a potency lower than or similar to free l-fucose. The divalent compound 20 was also not an efficient inhibitor of any lectin, confirming the known fact that the simple effect of increasing the number of fucose units is not sufficient for the inhibition [37]. The α-thio-l-fucopyranosides (21, 22, and 23) were generally better inhibitors than l-fucose for all lectins. It indicated that the weaker binding of 17, 18, and 19 was most likely caused by the β configuration and not by the presence of sulfur itself. Therefore, the thioglycosides could be potentially used in the inhibitors to achieve a higher stability towards glycoside hydrolase enzymes. The derivatization of α-l-fucopyranose with different aglycons did not interfere with the inhibitory ability of the tested compounds. The α-O-l-fucopyranosides (11, 12, 13, and 14) were equal or better inhibitors than l-fucose for all lectins. The used linkers are therefore suitable for usage in multivalent α-l-fucoside-containing glycoclusters. Compounds 12 and 14 were surprisingly very efficient inhibitors of RSL, with a potency of 32 and 128, respectively. This effect was not observed for any other tested lectin, even though AFL, AAL, and AOL are close homologues of RSL. However, RSL differs from other members of the AAL family by displaying a higher affinity to l-fucose. Compound 12 can probably form additional interactions with RSL via its side-chain and the divalent nature of 14 could be enough to exploit the avidity effect of RSL. Considering the multivalent α-l-fucoside-containing glycoclusters, they proved to be efficient inhibitors of lectins from the AAL family. The best inhibitor for all lectins was 2, the tetravalent compound with longer tetraethylene-glycol spacers. The length of the spacers appeared to be more crucial than the number of branches or their topology. For the majority of the AAL family lectins, the compounds 1, 2, and 5 (longer spacers) worked better than compounds 3 and 4 (shorter spacers). The exception to this observation was again for RSL, where 5 represented the least preferred multivalent inhibitor. Considering PA-IIL, compound 2 was also the best inhibitor, even though the binding mode of PA-IIL significantly differs from that of the AAL family. However, the increment of inhibitory potency of multivalent inhibitors compared to the monovalent compounds was lower than for the AAL family. Taking into account the simple effect of the increased concentration of fucose units in the multivalent compound (β, potency/valency), 2 only showed a two times better inhibitory potency than the majority of monovalent compounds (Table 1). 2 was also the best inhibitor of BC2L-C, but its efficiency was even lower. Generally, in the case of BC2L-C, the multivalent compounds used in this study did not display a significantly higher inhibitory potency than monovalent compounds when comparing their β factors. These data may suggest that BC2L-C does not employ a strong avidity effect during interaction. However, as previously tested C-hexopyranosyl calix[4]arene-based inhibitors displayed up to a 256-fold increase in inhibitory potency compared to monovalent l-fucose [17], the currently used multivalent inhibitors may be simply unable to bind to several binding sites simultaneously and further optimization will be necessary. Nevertheless, the tetravalent α-O-glycoside 2 with terminal l-fucose with longer spacers surprisingly proved to be the best inhibitor of all lectins used in this study. This universality could be attributed to its flexibility and the sufficient length of the spacers. Although the rigid spacers or scaffolds are generally considered to be more effective for the binding due to the stabilization of optimized sugar conformations and/or due to the lower entropic cost during interaction [37,38], flexible linkers are probably more suitable for the non-specialized inhibition of lectins with different binding modes.
Table 1.
Potencies of tested inhibitors against fucose-specific lectins determined by a hemagglutination inhibition assay.
| AFL | RSL | AAL | AOL | PA-IIL | BC2L-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | Valency | Potency | β# | Potency | β# | Potency | β# | Potency | β# | Potency | β# | Potency | β# |
| l-fucose* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Compound 1 | 3 | 64 | 21.3 | 512 | 170.7 | 32 | 10.7 | 64 | 21.3 | 16 | 5.3 | 8 | 2.7 |
| Compound 2 | 4 | 128 | 32 | 1024 | 256 | 128 | 32 | 256 | 64 | 32 | 8 | 16 | 4 |
| Compound 3 | 3 | 16 | 5.3 | 512 | 170.7 | 16 | 5.3 | 8 | 2.7 | 16 | 5.3 | 4 | 1.3 |
| Compound 4 | 4 | 16 | 4 | 256 | 64 | 16 | 4 | 8 | 2 | 16 | 4 | 4 | 1 |
| Compound 5 | 3 | 32 | 10.7 | 128 | 42.7 | 64 | 21.3 | 128 | 42.7 | 4 | 1.3 | 8 | 2.7 |
| Compound 11 | 1 | 4 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 1 |
| Compound 12 | 1 | 8 | 8 | 32 | 32 | 8 | 8 | 8 | 8 | 4 | 4 | 2 | 2 |
| Compound 13 | 1 | 4 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 2 |
| Compound 14 | 2 | 4 | 2 | 128 | 64 | 8 | 4 | 4 | 2 | 8 | 4 | 2 | 1 |
| Compound 15 | 1 | 0.125 | 0.125 | ND | - | ND | - | 0.5 | 0.5 | 0.5 | 0.5 | ND | - |
| Compound 16 | 1 | 0.125 | 0.125 | ND | - | ND | - | ND | - | 0.125 | 0.125 | 0.25 | 0.25 |
| Compound 17 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | ND | - |
| Compound 18 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.03125 | 0.03125 | 0.5 | 0.5 |
| Compound 19 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 0.0625 | 0.0625 | 0.5 | 0.5 |
| Compound 20 | 2 | 0.5 | 0.25 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | 0.125 | 0.0625 | 1 | 0.5 |
| Compound 21 | 1 | 2 | 2 | 4 | 4 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | 4 |
| Compound 22 | 1 | 2 | 2 | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | 4 |
| Compound 23 | 2 | 8 | 4 | 8 | 4 | 4 | 2 | 8 | 4 | 4 | 2 | 8 | 4 |
* Standard # Potency/Valency ND Not detected.
Figure 2.
Influence of l-fucose, compounds 1, 2, 3, 4, and 5, on hemagglutination caused by lectin PA-IIL. Used as an illustrative example of the data (images) acquired by a hemagglutination inhibition assay with microscope detection. The images for lectins AFL, RSL, AAL, AOL, and BC2L-C are included in the supplementary material. * Standard. Standard experiment was done anew for every used batch of protein or red blood cells. ** Controls were done anew for every used batch of protein or red blood cells. Positive control: experiment without any inhibitor. Negative control: experiment without lectin PA-IIL.
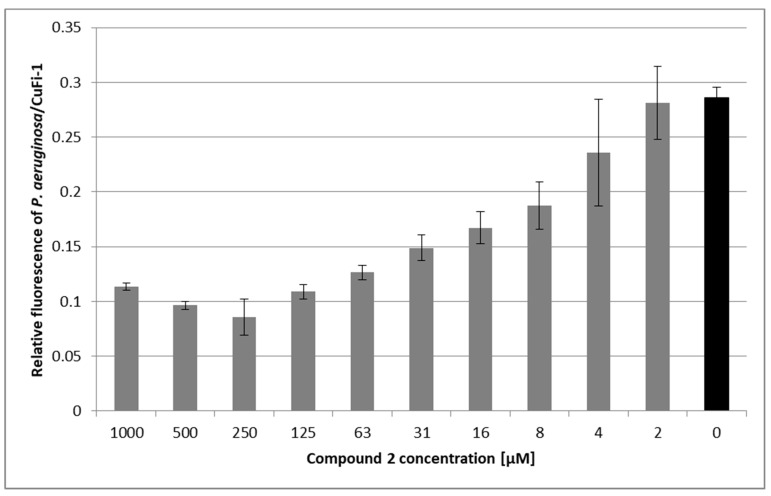
As Pseudomonas aeruginosa is the most common pathogen associated with cystic fibrosis and involvement of its lectin PA-IIL (LecB) in pathogenesis is continually studied [5,39,40], the inhibitory potential of compound 2 was further evaluated using an ex vivo bacterial adhesion assay. P. aeruginosa strain 1763 isolated from a cystic fibrosis patient was incubated with epithelial bronchial cells derived from a cystic fibrosis patient (CuFi-1) in the presence or absence of compound 2. Ten concentrations of this inhibitor were tested. Compound 2 had a significant protecting effect against P. aeruginosa adhesion (see Figure 3). Compared to an untreated control, the inhibitor was able to reduce bacterial adhesion to ∼30% when used in the most effective concentration (0.25 mM). Paradoxically, the inhibitory effect of compound 2 decreased in concentrations above 0.25 mM. This behaviour was attributed to the cross-linking of bacterial cells by a multivalent inhibitor. The subsequent adhesion of bacterial clusters to the epithelial cells resulted in an increased fluorescence signal. Cross-linking of bacterial cells by multivalent carbohydrate-based compounds has been reported previously [17]. In contrast to the low inhibitory activity against lectin PA-IIL observed in vitro by a hemagglutination inhibition assay, the results obtained on a cell-cell level indicate that compound 2 has the potential to reduce the interactions of P. aeruginosa with epithelial cells of humans suffering from cystic fibrosis.
Figure 3.
P. aeruginosa adhesion to human bronchial epithelial cells (CuFi-1) in the presence of compound 2. Monolayers of CuFi-1 cells stained with PKH67 were exposed to suspensions containing P. aeruginosa stained with PKH26 and compound 2 (grey bars) or PBS (Phosphate Buffered Saline) as a control (black bar). After 2-h incubation, non-adhered bacteria were removed by washing and the relative fluorescence of adhered bacteria and epithelial cells was quantified using Tecan Infinite M200 Pro. Results are expressed as a relative fluorescence ratio of P. aeruginosa/CuFi-1 plotted as a function of compound 2’s concentration. Plotted data are means ± SD of three independent incubations.
Therefore, it could be used for prophylaxis against the bacterial colonization of bronchial cells, similarly to, e.g., chicken immunoglobulins [41], because once the airway cells are colonized, any aid interfering with bacterium adherence is of limited use. As follows from ex vivo experiments with confluent cell layers and a heavy load of Pseudomonas aeruginosa (8.4 × 106 CFU/well) presented in this paper, the protective concentration of compound 2 is rather high. It is disputable whether such a concentration is relevant to in vivo scenarios. However, it should be noted that for cystic fibrosis patients, it is feasible to reach high doses of medication applied directly to lungs (inhalation). On a daily basis, they inhale antibiotics, e.g., 300 mg of tobramycin in 5 mL, two times a day, for 28 days [42]. In another study, patients were treated with the inhalation of 10 mL of 0.1 M fucose/0.1 M galactose two times a day for 21 days [43]. Of course, a closer look at in vivo conditions is necessary. A mouse cystic fibrosis model, which is currently being developed [44], is intended to be used.
3. Experiment
3.1. General Methods
Optical rotations were measured at room temperature with a Perkin-Elmer 241 automatic polarimeter. TLC analysis was performed on Kieselgel 60 F254 (Merck, Kenilworth, NJ, USA) silica gel plates with visualization by immersion in a sulfuric-acid solution (5% in EtOH) followed by heating. Column chromatography was performed on silica gel 60 (Merck 0.063–0.200 mm), and flash column chromatography was performed on silica gel 60 (Merck 0.040–0.063 mm). Gel filtration was performed on Sephadex G-25 resin, using water as the eluent. Organic solutions were dried over MgSO4 and concentrated under vacuum. The 1H (400 MHz) and 13C NMR (100.28 MHz) spectra were recorded with a Bruker DRX-400 spectrometer. Chemical shifts are referenced to Me4Si or DSS (0.00 ppm for 1H) and to solvent signals (CDCl3: 77.00 ppm, CD3OD: 49.15 ppm, DMSO-d6: 39.51 ppm for 13C). MS (MALDI-TOF) analysis was carried out in positive reflectron mode with a BIFLEX III mass spectrometer (Bruker, Germany) with delayed-ion extraction. The matrix solution was a saturated solution of 2,5-dihydroxy-benzoic acid (DHB) in N,N-dimethylformamide.
3.2. Synthesis
Compound 8
Et3N (190 µL, 1 equiv./alkyne) and Cu(I)I (2.5 mg, 0.1 equiv./alkyne) were added to a stirred solution of 6 (466 mg, 1.39 mmol) and 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate [29] (2.34 g, 6.25 mmol, 1.3 equiv./alkyne) in CH3CN (5 mL) under an argon atmosphere and the mixture was stirred overnight at room temperature. The reaction mixture was evaporated, and the crude product was purified by flash column chromatography (CH2Cl2:MeOH 97:3) to give 7 (1.24 g, 63%) as a yellowish syrup. Subsequently, NaN3 (333 mg, 6 equiv.) was added to a stirred solution of 7 (1.24 g, 0.85 mmol) in DMF (10 mL) under an argon atmosphere and stirred overnight at room temperature. The reaction mixture was diluted with water (5 mL), stirred for a further 5 min, and evaporated. The residue was dissolved in CH2Cl2 (300 mL) and extracted with water (2 × 50 mL) and brine (50 mL), dried over MgSO4, filtered, and evaporated. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 97:3) to give 8 (557 mg, 61%) as a colorless syrup. Rf = 0.52 (CH2Cl2:MeOH 95:5). 1H NMR (400 MHz, CDCl3) δ 7.70 (s, 3H, 3 × CH triazole), 4.57 (s, 6H), 4.52 (t, J = 5.1 Hz, 6H), 3.87-3.85 (t, J = 5.1 Hz, 6H), 3.72 (s, 6H, 3 × CH2), 3.65–3.60 (m, 28H, 12 × CH2), 3.37–3.35 (m, 4H), 2.88 (d, 2H), 2.30–2.25 (m, 2H) 1.37 (s, 9H, 3 × CH3). 13C NMR (101 MHz, CDCl3) δ 154.7 (1C, CO), 144.6 (3C, C=CH), 123.6 (3C, C=CH), 70.5 (3C, 3 × CH2), 70.4, 69.9, 69.3 (18C, 18 × CH2 TEG), 69.1, 64.7 (3C, 3 × CH2), 58.3, 50.5, 50.0 (6C, 6 × NCH2 TEG), 28.2 (3C, 3 × CH3 Boc); MALDI-TOF MS: m/z 1090.46 [M + Na]+ (calcd for C42H73N19O14 1090.55).
Compound 10
Et3N (73 µL, 1 equiv./alkyne) and Cu(I)I (10 mg, 0.1 equiv./alkyne) were added to a stirred solution of 9 (770 mg, 2.34 mmol) and azide 8 (557 mg, 0.52 mmol, 1.3 equiv./alkyne) in CH3CN (5 mL) under an argon atmosphere and the mixture was stirred overnight at room temperature. The reaction mixture was evaporated, and the crude product was purified by flash column chromatography (CH2Cl2:MeOH 97:3) to give 10 (734 mg, 69%) as a colorless syrup. [α]24D -68.18 (c = 0.22, CHCl3), Rf = 0.38 (CH2Cl2:MeOH 95:5). 1H NMR (360 MHz, CDCl3) δ 7.73 (s, 6H, 6 × CH triazole), 5.37-4.10 (m, 15H, 3 × H-1, H-2, H-3, H-4), 4.82 (d, J = 12.4 Hz, 3H) 4.66 (d, J = 12.4 Hz, 3H), 4.60-4.53 (m, 15H), 4.25-4.19 (m, 3H) 3.89 (t, J = 5.2 Hz, 14H,), 3.75 (s, 7H), 3.60 (d, J = 4.0 Hz, 20H, CH2), 2.14, 2.04, 1.9 (s, 27H, 9 × CH3,ac), 2.10-2.01 (m, 2H), 1.40 (s, 9H, 3 × CH3,Boc), 1.1 (d, J = 6.4 Hz, 9H, 3 × C-6).13C NMR (91 MHz, CDCl3) δ 196.7 (1C, COBoc) 170.4, 170.2, 169.8 (9C, 9 × COac), 144.3 (6C, 6 × C=CH triazol) 123.8, (6C, 6 × C=CH triazol) 95.4 (3C, C-1), 70.9, 67.8, 67.7, 64.5 (12C, Cskeleton), 70.3 (18C, 18 × OCH2), 70.2 (3C, 3 × OCH2), 69.2, 64.5, 61.0 (6C, 6 × OCH2), 50.1 (6C, 6 × NCH2 TEG), 28.2 (3C, 3 × CH3,Boc), 20.6, 20.5 (9C, 9 × CH3,ac), 15.7 (3C, 3 × C-6). MALDI-TOF MS: m/z 2075.16 [M + Na]+ (calcd for C87H133N19O38 2074.90).
Compound 5
To the stirred solution of 10 (90 mg, 0.04 mmol) in dry MeOH (1 mL), a catalytic amount of NaOMe was added (pH ~ 9). The reaction mixture was stirred overnight at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered, and evaporated, and the crude product was then purified by flash column chromatography (CH3CN:H2O 7:3) to give compound 5 (53 mg, 73%) as a white powder. [α]24D −27.3 (c = 0.22, MeOH), Rf = 0.37 (CH3CN:H2O 7:3). 1H NMR (360 MHz, D2O) δ 8.03 (s, 3H, 3 × CH), 7.98 (s, 3H, 3 × CH), 4.95 (bs, 2H), 4.84–4.73 (m, 8H), 4.73–4.63 (m, 8H, 3 × CH2), 4.62–4.74 (m, 18H, 3 × H-2, H-3, H-4, H-5), 3.89 (d, J = 6.4 Hz, 15H), 3.82–3.69 (m, 9H), 3.52 (dt, J = 24.8, 5.0 Hz, 31H), 1.26 (s, 9H, 3 × CH3), 1.07 (d, J = 6.5 Hz, 9H, 3 × C-6). 13C NMR (91 MHz, D2O) δ 197.2 (1C, COBoc), 143.8 (6C, 6 × Cq triazol), 125.1 (6C, 6 × CH triazol), 98.5 (3C, C-1), 71.7, 69.5, 67.9, 66.7 (12C, C-skeleton), 69.6 (18C, 18 × CH2), 69.4 (3C, 3 × CH2), 68.6 (1C, Cq), 63.4, 60.5 (6C, 6 × CH2), 58.3 (1C, Cq), 49.9 (6C, 6 × NCH2), 27.5 (3C, 3 × CH3,Boc), 15.2 (3C, 3 × C-6). MALDI-TOF MS: m/z 1696.91 [M + Na]+ (calcd for C69H115N19O29 1696.80).
Compound 15
Propargyl α-l-fucopyranoside 11 [31] (50 mg, 0.25 mmol) was dissolved in dry DMF (3 mL) and SO3.Et3N (270 mg, 2 equiv./−OH) was then added under argon atmosphere, and it was stirred overnight. The reaction mixture was neutralized with aqueous solution of NaHCO3 (250 mg, 6 equiv.) and it was concentrated. The crude product was suspended in MeOH (5 mL) and filtered to remove salts, and was then purified by gel filtration to give compound 15 (107 mg, 85%) as a colorless syrup. [α]24D −94.6 (c = 0.13, H2O), Rf = 0.56 (CH2Cl2:MeOH:H2O 7:6:1). 1H NMR (360 MHz, D2O) δ 5.39 (d, J = 3.8 Hz, 1H), 4.93 (d, J = 3.1 Hz, 1H), 4.60–4.51 (m, 2H), 4.40–4.24 (m, 3H), 2.93 (t, J = 2.4 Hz, 1H), 1.28 (d, J = 6.5 Hz, 3H).13C NMR (91 MHz, D2O) δ 100.9, 96.6, 79.9, 73.4, 72.9, 67.6, 56.6, 16.6. MALDI-TOF MS: m/z 531.18 [M + Na]+ (calcd for C9H11Na3O14S3 530.89).
Compound 16
Persulfated propargyl fucoside 15 (50 mg, 0.10 mmol) and O-(2-azidoethyl)triethylene glycol (26 mg, 0.4 mmol) were dissolved in THF:H2O = 1:1 (1 mL) and then CuSO4 (4 mg, 0.2 equiv.) and Na-ascorbate (20 mg, 1.0 equiv.) were added under argon atmosphere and it was stirred for 4 h. The reaction mixture was concentrated and the crude product was purified by flash column chromatography (CH2Cl2:MeOH:H2O 7:6:1) and by gel filtration to give compound 16 (54 mg, 73%) as a colorless syrup. [α]24D −77.0 (c = 0.10, H2O), Rf = 0.32 (CH2Cl2:MeOH:H2O 7:6:1). 1H NMR (400 MHz, D2O) δ 8.16 (d, J = 3.2 Hz, 1H), 5.32 (dd, J = 3.5, 1.5 Hz, 1H), 4.91 (ddq, J = 3.1, 2.4, 0.8 Hz, 1H), 4.87–4.82 (m, 2H), 4.71–4.61 (m, 3H), 4.56–4.49 (m, 1H), 4.19–4.11 (m, 1H), 4.01–3.94 (m, 2H), 3.71 (dd, J = 6.2, 3.0 Hz, 2H), 3.69–3.59 (m, 11H), 1.19 (dd, J = 6.7, 2.9 Hz, 3H). 13C NMR (101 MHz, D2O) δ 144.6, 126.6, 97.5, 79.8, 73.4, 73.0, 72.6, 70.6, 70.6, 70.4, 69.7, 67.2, 61.9, 61.3, 51.1, 16.6. MALDI-TOF MS: m/z 749.95 [M + Na]+ (calcd for C17H28N3Na3O18S3 750.01).
Compound 19
To a stirred solution of 1,11-diazido-3,6,9-trioxaundecaneglycol (293 mg, 1.2 mmol) and alkyne 18 (138 mg, 0.4 mmol) dissolved in CH3CN (5 mL), Et3N (60 µL, 1 equiv.) and Cu(I)I (8 mg, 0.1 equiv.) were added under argon atmosphere and it was stirred overnight. The reaction mixture was concentrated and the crude product was purified by flash column chromatography (CH2Cl2/MeOH 95:5) and gel filtration to give compound 19 (123 mg, 52%) as a colorless syrup. [α]24D +29.1 (c = 0.11, MeOH), Rf = 0.23 (CH2Cl2/MeOH 95:5). 1H NMR (400 MHz, Methanol-d4) δ 7.77 (s, 1H), 4.50 (t, J = 5.0 Hz, 2H), 4.28 (d, J = 9.5 Hz, 1H), 4.20 (s, 3H), 3.95 (m, 2H), 3.86 (t, J = 5.1 Hz, 2H), 3.65 (dd, J = 16.2, 7.2 Hz, 12H), 3.48 (dd, J = 9.0, 3.4 Hz, 1H), 3.37 (t, J = 5.0 Hz, 1H), 3.33 (s, 2H), 1.28 (d, J = 6.4 Hz, 3H).13C NMR (101 MHz, Methanol-d4) δ 145.0, 123.4, 84.8, 74.6, 71.3, 70.2, 70.2, 70.1, 70.1, 69.7, 69.1, 68.9, 50.3, 50.0, 22.7, 16.0. MALDI-TOF MS: m/z 485.26 [M + Na]+ (calcd for C17H30N6O7S 485.18).
Compound 21
A catalytic amount of NaOMe (pH ~9) was added to a stirred solution of 24 (122 mg, 0.2 mmol) in dry MeOH (3 mL) and stirred overnight at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered, and evaporated, and the crude product was then purified by flash column chromatography (CH2Cl2/MeOH 95:5) and gel filtration to give compound 21 (154 mg, 78%) as a colorless syrup. [α]24D −331.9 (c = 0.16, MeOH), Rf = 0.23 (CH2Cl2/MeOH 95:5). 1H NMR (400 MHz, Methanol-d4) δ 5.36 (d, J = 5.6 Hz, 1H), 4.31 (qd, J = 6.7, 1.1 Hz, 1H), 4.06 (dd, J = 10.1, 5.6 Hz, 1H), 3.80–3.54 (m, 4H), 2.78 (ddd, J = 13.5, 7.3, 6.2 Hz, 1H), 2.65 (ddd, J = 13.6, 7.3, 6.5 Hz, 1H), 1.25 (d, J = 6.6 Hz, 4H). 13C NMR (101 MHz, Methanol-d4) δ 88.05 (C-1), 73.39, 72.29, 69.47, 68.07 (C-2,3,4,5), 62.67 (-CH2), 33.70 (-SCH2), 16.57 (C-6). MALDI-TOF MS: m/z 247.32 [M + Na]+ (calcd for C8H16O5S 247.06).
Compound 22
A catalytic amount of NaOMe (pH ~9) was added to a stirred solution of 25 (109 mg, 0.25 mmol) in dry MeOH (10 mL) and stirred overnight at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered, and evaporated, and the crude product was then purified by flash column chromatography (CH3CN/H2O 95:5) to give compound 22 (70 mg, 90%) as white crystals. Mp.: 248–249 °C. [α]24D −268.3 (c = 0.06, MeOH), Rf = 0.53 (CH3CN/H2O 9:1). 1H NMR (360 MHz, Methanol-d4) δ 5.39 (d, J = 5.6 Hz, 1H, H-1), 4.38–4.19 (m, 1H), 4.06 (dd, J = 10.1, 5.6 Hz, 1H), 3.75–3.54 (m, 2H), 3.18–3.03 (m, 2H), 3.05–2.79 (m, 2H), 1.25 (d, J = 6.5 Hz, 3H, CH3). 13C NMR (91 MHz, Methanol-d4) δ 88.0 (C-1), 73.4, 72.3, 69.4, 68.1 (C-2,3,4,5), 53.2 (-CH2SO3Na), 26.2 (-SCH2), 16.5 (C-6). MALDI-TOF MS: m/z 333.11 [M + Na]+ (calcd for C8H15NaO7S2 333.01).
Compound 23
A catalytic amount of NaOMe (pH ~9) was added to a stirred solution of 26 (210 mg, 0.36 mmol) in dry MeOH (10 mL) and stirred overnight at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered, and evaporated, and the crude product was then purified by flash column chromatography (CH2Cl2/MeOH 7:3) to give compound 23 (107 mg, 90%) as a white powder. [α]24D −179.3 (c = 0.30, MeOH), Rf = 0.15 (CH2Cl2/MeOH 8:2). 1H NMR (360 MHz, Methanol-d4) δ 5.60 (d, J = 5.5 Hz, 2H, H-1, H-1′), 4.42 – 4.38 (m, 4H), 4.07 (dd, J = 10.0, 5.5 Hz, 2H), 3.72–3.35 (m, 8H), 1.26 (d, J = 6.4 Hz, 3H), 1.22 (d, J = 6.5 Hz, 3H). 13C NMR (91 MHz, Methanol-d4) δ 88.1, 87.6 (C-1, C-1′), 76.3, 76.1, 73.4, 73.1, 72.4, 71.8, 69.7, 68.9 (C-2,3,4,5,2′,3′,4′,5′), 17.2, 16.6 (C-6,6′). MALDI-TOF MS: m/z 349.19 [M + Na]+ (calcd for C12H22NaO8S 349.09).
Compound 24
2-Acetoxy-3,4-di-O-acetyl-l-fucal (272 mg, 1.0 mmol) and 2-mercaptoethanol (140 µL, 2 mmol) were dissolved in toluene:methanol:water = 8:5:1 (5 mL) and 2,2-dimethoxy-2-phenylacetophenone (DPAP, 25 mg, 0.10 mmol) was added. The solution was irradiated with UV-light at room temperature for 3 × 15 min. Then, the solution was concentrated and the residue was purified using column chromatography (n-hexane: aceton 9:1) to give compound 24 (122 mg, 35%) as a colorless syrup. [α]24D −135.9 (c = 0.02, CH3Cl), Rf = 0.50 (n-hexane: aceton 7:3). 1H NMR (400 MHz, CDCl3) δ 5.74 (d, J = 5.5 Hz, 1H), 5.37–5.13 (m, 3H), 4.59–4.49 (m, 1H), 3.80 (dt, J = 9.0, 4.6 Hz, 2H), 2.94–2.65 (m, 3H), 2.22–2.15 (m, 4H), 2.11 (d, J = 1.0 Hz, 3H), 2.20, 2.11, 2.04 (s, 3 × 3H), 1.20 (d, J = 6.5 Hz, 3H).
MALDI-TOF MS: m/z 373.54 [M + Na]+ (calcd for C14H22O8S 373.09).
3.3. Lectins Production and Purification
Lectins BC2L-C, AFL, PA-IIL (LecB), and RSL in recombinant forms were produced and purified as previously described [6,7,8,13]. Briefly, transformed Escherichia coli cells bearing a plasmid for the particular lectin were cultured in LB (Luria-Bertani) broth medium containing an appropriate antibiotic at 37 °C. When the culture reached an OD600 of ≈ 0.5, cells were induced by isopropyl 1-thio-β-d-galactopyranoside (IPTG) added to a final concentration of 0.5 mM. Cells were incubated at 30 °C for 3 h, harvested by centrifugation, and resuspended in a suitable buffer. Cells were then disintegrated by sonication and the cytosolic fraction containing soluble lectin was separated by centrifugation. Lectins were then purified by affinity chromatography on a d-mannose-agarose column, dialyzedm and further processed according to the previously published procedures [6,7,8,13]. Freeze-dried lectins were stored at −20 °C. Lectins AAL (Vector Laboratories) and AOL (TCI) were purchased in a freeze-dried form.
3.4. Hemagglutination Inhibition Assay
Lectins AFL, RSL, AAL, and AOL were dissolved in the PBS buffer to the concentration 0.1 mg·mL−1. Lectins were mixed with carbohydrate inhibitors serially diluted in PBS buffer in a 5 µL:5 µL ratio. The final (working) concentration of lectins was therefore 0.05 mg·mL−1. A total of 10 µL of 20% papain-treated, acid-stabilized red blood cells 0+ in PBS buffer was then added, and the mixture was thoroughly mixed and incubated for 5 min at room temperature. After incubation, the mixture was mixed again, transferred to a microscope slide, and examined. The examination was conducted using the Levenhuk D2L NG Digital Microscope (Levenhuk, Tampa, FL, USA). Images were obtained with a Levenhuk D2L digital camera (Levenhuk, Tampa, FL, USA) using the software ToupView for Windows (Levenhuk, Tampa, FL, USA). The positive (experiment without an inhibitor) and negative (experiment without a lectin) control were prepared and processed in the same way using an appropriate volume of dissolving buffer instead of the omitted components. The minimal inhibitory concentration (MIC) of the inhibitor able to inhibit hemagglutination was determined and compared with the standard (l-fucose), and the potency of the inhibitor was calculated (MIC of the standard/MIC of the inhibitor). MIC of the standard was determined every time a new batch of lectins or red blood cells were used for experiments to diminish the biological variability.
The PA-IIL and BC2L-C lectins were dissolved in the buffer containing calcium ions necessary for their activity (20 mM Tris/HCl, 150 mM NaCl, 5 mM CaCl2, pH 7.5) to the concentration 2.5 mg·mL−1 and 0.2 mg·mL−1, respectively. Lectins were mixed with carbohydrate inhibitors serially diluted in the Tris buffer in a 5 µL:5 µL ratio. The final (working) concentration of the lectins was therefore 1.25 mg·mL−1 and 0.1 mg·mL−1. A total of 10 µL of 20% papain-treated, acid-stabilized red blood cells 0+ in the Tris buffer was then added, and the mixture was thoroughly mixed and incubated for 5 min at room temperature. The examination was conducted and evaluated as mentioned above.
The above-mentioned concentrations of all lectins resulted from optimization, i.e., concentrations enabling the formation of observable hemagglutinates in 5 min at room temperature were used.
3.5. Pseudomonas Aeruginosa Adherence Assay
3.5.1. Cell Labelling
Epithelial CuFi-1 cells (an immortalized epithelial cell line derived from cystic fibrosis human lungs, ATCC, Poland) were labelled with PKH67 (Fluorescent Cell Linker Kit, Sigma-Aldrich, Taufkirchen, Germany), a green fluorescent membrane marker, as follows. An aliquot of cell suspension containing 7 × 106 cells was washed with PBS buffer and centrifuged (100× g for 5 min). The cell pellet was resuspended in 250 µL of Diluent C (Fluorescent Cell Linker Kit, Sigma-Aldrich, Taufkirchen, Germany). Immediately, an equal volume of 8 µM PKH67 in Diluent C (2 µL of PKH67 in 248 µL of Diluent C) was added to the cell suspension. After 5 min incubation at room temperature with periodic mixing, the staining reaction was stopped by adding an equal volume of 1% Fetal Bovine Serum (GibcoTM Invitrogen, Paisley, UK) in PBS. After 1 min incubation with FBS, the suspension was centrifuged (100× g for 10 min). Finally, the cells were washed three times with Bronchial Epithelial Cell Growth Basal Medium (Lonza, Basel, Switzerland), followed by centrifugation (100× g for 5 min).
Pseudomonas aeruginosa strain 1763 (University Hospital Motol, Prague, Czech Republic) was stained with PKH26 (Fluorescent Cell Linker Kit Sigma-Aldrich, Germany), a red fluorescent membrane marker. Bacteria were grown in a suspension culture in PS medium (peptone/casein digest) in an Erlenmeyer flask for 14 h. The culture was washed with PBS buffer and centrifuged (13,400× g for 5 min). The pellet containing 1.5 × 109 bacteria was resuspended in 125 µL of Diluent C (Fluorescent Cell Linker Kit, Sigma-Aldrich, Germany) and an equal volume of 16 µM PKH26 in Diluent C (2 µL of PKH26 in 123 µL of Diluent C) was added to the bacterial suspension. The bacteria/dye suspension was incubated for 30 min at room temperature with periodic mixing. The staining was quenched by adding an equal volume of 1% BSA (Bovine Serum Albumin) (Merck, Germany) in PBS. The suspension was incubated with BSA for 1 min to allow the binding of excess dye and then centrifuged (15,700× g for 10 min). Bacteria were washed two times with PBS, followed by centrifugation (13,400× g for 7.5 and 5 min).
3.5.2. Inhibition of Pseudomonas aeruginosa Adhesion on Epithelial Cells
Epithelial cells CuFi-1 stained with PKH67 were seeded at 8.4 × 104 cells/well onto 96-well plates (CellBIND® 96-well Flat Clear Bottom Black Polystyrene Microplates, Corning Incorporated, Corning, NY, USA) and incubated for 42–45 h at 37 °C, 5% CO2, to form a confluent monolayer and regenerate. Prior to the assay, wells were washed with PBS. Compound 2 was diluted with PBS to desired concentrations (2 µM–1 mM). PKH26-stained bacteria P. aeruginosa were added to the diluted inhibitor solutions. Bacterial suspensions were immediately added to the wells (50 µL/well). The input ratio was about 100 bacteria per epithelial cell (8.4 × 106 bacteria/well). A suspension without an inhibitor was used as a control. After 2 h incubation at room temperature, the wells were extensively washed three times with PBS to remove non-adhered bacteria The fluorescence of adhered P. aeruginosa cells (Ex 522 nm, Em 569 nm for PKH26) on epithelial cells (Ex 470 nm, Em 505 for PKH67) was quantified using the spectrofluorometer Tecan Infinite M200 Pro (Tecan Group Ltd., Männedorf, Switzerland). Results were expressed as a relative fluorescence ratio of P. aeruginosa/CuFi-1. Three independent incubations were performed.
4. Conclusions
The binding of a broad number of newly synthesized l-fucosides of varying structures, including α- and β-linked mono- and multivalent O- and S-fucosides, as well as persulfated fucoside derivatives, were tested against six lectins of bacterial and fungal origin. The tetravalent, α-l-fucoside-containing compound with tetraethylene-glycol bridges (2) was shown to be a universal inhibitor of fucose-specific lectins from pathogens associated with cystic fibrosis, i.e., P. aeruginosa, B. cenocepacia, and A. fumigatus. This inhibitor was also able to significantly inhibit the adhesion of P. aeruginosa cells to epithelial bronchial cells derived from a cystic fibrosis patient in an ex vivo bacterial adherence assay. This molecule also displayed the best inhibitory potency against the RSL lectin from the plant pathogen R. solanacearum, suggesting that it could be used to prevent wilting infections of plants. All multivalent inhibitors worked also well against lectins AAL and AOL.
Supplementary Materials
The following are available online: Figure S1: Influence of l-fucose, compounds 1, 2, 3, 4 and 5 on hemagglutination caused by lectin AFL. Figure S2. Influence of l-fucose, compounds 1, 2, 3, 4, 14 and 5 on hemagglutination caused by lectin RSL. Figure S3. Influence of l-fucose, compounds 1, 2, 3, 4 and 5 on hemagglutination caused by lectin AAL. Figure S4. Influence of l-fucose, compounds 1, 2, 3, 4 and 5 on hemagglutination caused by lectin AOL. Figure S5. Influence of l-fucose, compounds 1, 2, 3, 4 and 5 on hemagglutination caused by lectin BC2L-C.
Author Contributions
Investigations, S.L.T., L.M., M.V., E.M. and V.K.; conceptualization, P.H., A.B., M.W. and M.C.; supervision, M.W. and M.C.; funding acquisition, A.B., M.C. and M.W.
Funding
The synthetic work was supported by the National Research and Development and Innovation Office of Hungary (K119509, M. Csávás and TÉT_15_IN-1-2016-0071, A. Borbás) and the EU and co-financed by the European Regional Development Fund under the project GINOP-2.3.2-15-2016-00008. The project was supported by the János Bolyai Fellowship of the Hungarian Academy of Sciences (M. Csávás). The research was also supported by the New National Excellence Program (ÚNKP-18-4, Bolyai +, M. Csávás) of the Ministry of Human Capacities. This work was supported by the Czech Science Foundation (15-17572S) and by the Ministry of Education, Youth and Sports (CIISB research infrastructure LM2015043).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 2, 3, 4, 5, 11–23 are available from the authors.
References
- 1.Gibson R.L., Burns J.L., Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Scanlin T.F., Glick M.C. Terminal glycosylation in cystic fibrosis. Biochim. Biophys. Acta. 1999;1455:241–253. doi: 10.1016/S0925-4439(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 3.Sharon N., Lis H. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 4.Imberty A., Wimmerová M., Mitchell E.P., Gilboa-Garber N. Structures of the lectins from Pseudomonas aeruginosa: Insight into the molecular basis for host glycan recognition. Microbes Infect. 2004;6:221–228. doi: 10.1016/j.micinf.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Chemani C., Imberty A., de Bentzmann S., Pierre M., Wimmerová M., Guery B.P., Faure K. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect. Immun. 2009;77:2065–2075. doi: 10.1128/IAI.01204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Šulák O., Cioci G., Lameignère E., Balloy V., Round A., Gutsche I., Malinovská L., Chignard M., Kosma P., Aubert D.F., et al. Burkholderia cenocepacia BC2L-C is a super lectin with dual specificity and proinflammatory activity. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houser J., Komarek J., Kostlanova N., Cioci G., Varrot A., Kerr S.C., Lahmann M., Balloy V., Fahy J.V., Chignard M., et al. A soluble fucose-specific lectin from Aspergillus fumigatus conidia-structure, specificity and possible role in fungal pathogenicity. PLoS ONE. 2013;8:1–15. doi: 10.1371/journal.pone.0083077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell E.P., Sabin C., Šnajdrová L., Pokorná M., Perret S., Gautier C., Hofr C., Gilboa-Garber N., Koča J., Wimmerová M., et al. High affinity fucose binding of Pseudomonas aeruginosa lectin PA-IIL: 1.0 A resolution crystal structure of the complex combined with thermodynamics and computational chemistry approaches. Proteins. 2005;58:735–746. doi: 10.1002/prot.20330. [DOI] [PubMed] [Google Scholar]
- 9.Houser J., Komarek J., Cioci G., Varrot A., Imberty A., Wimmerova M. Structural insights into Aspergillus fumigatus lectin specificity: AFL binding sites are functionally non-equivalent. Acta Cryst. 2015;D71:442–453. doi: 10.1107/S1399004714026595. [DOI] [PubMed] [Google Scholar]
- 10.Yamaki K., Yoshino S. Aspergillus oryzae lectin induces anaphylactoid oedema and mast cell activation through its interaction with fucose of mast cell–bound non-specific IgE. Scand. J. Immun. 2011;74:445–453. doi: 10.1111/j.1365-3083.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 11.Wimmerova M., Mitchell E., Sanchez J.F., Gautier C., Imberty A. Crystal structure of fungal lectin: Six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J. Biol. Chem. 2003;278:27059–27067. doi: 10.1074/jbc.M302642200. [DOI] [PubMed] [Google Scholar]
- 12.Denny T.P. Ralstonia solanacearum—A plant pathogen in touch with its host. Trends Microbiol. 2000;8:486–489. doi: 10.1016/S0966-842X(00)01860-6. [DOI] [PubMed] [Google Scholar]
- 13.Kostlánová N., Mitchell E.P., Lortat-Jacob H., Oscarson S., Lahmann M., Gilboa-Garber N., Chambat G., Wimmerová M., Imberty A. The fucose-binding lectin from Ralstonia solanacearum. A new type of beta-propeller architecture formed by oligomerization and interacting with fucoside, fucosyllactose, and plant xyloglucan. J. Biol. Chem. 2005;280:27839–27849. doi: 10.1074/jbc.M505184200. [DOI] [PubMed] [Google Scholar]
- 14.Cecioni S., Imberty A., Vidal S. Glycomimetics versus Multivalent Glycoconjugates for the Design of High Affinity Lectin Ligands. Chem. Rev. 2015;115:525–561. doi: 10.1021/cr500303t. [DOI] [PubMed] [Google Scholar]
- 15.Goyard D., Baldoneschi V., Varrot A., Fiore M., Imberty A., Richichi B., Renaudet O., Nativi C. Multivalent Glycomimetics with Affinity and Selectivity toward Fucose-Binding Receptors from Emerging Pathogens. Bioconjug. Chem. 2018;29:83–88. doi: 10.1021/acs.bioconjchem.7b00616. [DOI] [PubMed] [Google Scholar]
- 16.Lehot V., Brissonnet Y., Dussouy C., Brument S., Cabanettes A., Gillon E., Deniaud D., Varrot A., Le Pape P., Gouin S.G. Multivalent fucosides with nanomolar affinity for the aspergillus fumigatus lectin flea prevent spore adhesion to pneumocytes. Chemistry. 2018;24:19243–19249. doi: 10.1002/chem.201803602. [DOI] [PubMed] [Google Scholar]
- 17.Kašaková M., Malinovská L., Klejch T., Hlaváčková M., Dvořáková H., Fujdiarová E., Rottnerová Z., Maťátková O., Lhoták P., Wimmerová M., et al. Selectivity of original C-Hexopyranosyl Calix[4]arene conjugates towards lectins of different origin. Carbohydr. Res. 2018;469:60–72. doi: 10.1016/j.carres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Sommer R., Wagner S., Rox K., Varrot A., Hauck D., Wamhoff E.C., Schreiber J., Ryckmans T., Brunner T., Rademacher C., et al. Glycomimetic, orally bioavailable lecb inhibitors block biofilm formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018;140:2537–2545. doi: 10.1021/jacs.7b11133. [DOI] [PubMed] [Google Scholar]
- 19.Hauck D., Joachim I., Frommeyer B., Varrot A., Philipp B., Möller H.M., Imberty A., Exner T.E., Titz A. Discovery of two classes of potent glycomimetic inhibitors of pseudomonas aeruginosa lecb with distinct binding modes. ACS Chem. Biol. 2013;8:1775–1784. doi: 10.1021/cb400371r. [DOI] [PubMed] [Google Scholar]
- 20.Buffet K., Gillon E., Holler M., Nierengarten J.F., Imberty A., Vincent S.P. Fucofullerenes as tight ligands of RSL and LecB, two bacterial lectins. Org. Biomol. Chem. 2015;13:6482. doi: 10.1039/C5OB00689A. [DOI] [PubMed] [Google Scholar]
- 21.Reymond J.L., Bergmann M., Darbre T. Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors. Chem. Soc. Rev. 2013;42:4814. doi: 10.1039/c3cs35504g. [DOI] [PubMed] [Google Scholar]
- 22.Galanos N., Gillon E., Imberty A., Matthews S.E., Vidal S. Pentavalent pillar[5]arene-based glycoclusters and their multivalent binding to pathogenic bacterial lectins. Org. Biomol. Chem. 2016;14:3476. doi: 10.1039/C6OB00220J. [DOI] [PubMed] [Google Scholar]
- 23.Donnier-Maréchal M., Galanos N., Grandjean T., Pascal Y., Ji D., Dong L., Gillon E., He X., Imberty A., Kipnis E., et al. Perylenediimide-based glycoclusters as high affinity ligands of bacterial lectins: Synthesis, binding studies and anti-adhesive properties. Org. Biomol. Chem. 2017;15:10037. doi: 10.1039/C7OB02749D. [DOI] [PubMed] [Google Scholar]
- 24.Hua Y., Beshr G., Garvey C.J., Tabor R.F., Titz A., Wilkinson B.L. Photoswitchable Janus glycodendrimer micelles as multivalent inhibitors of LecA and LecB from Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces. 2017;159:605–612. doi: 10.1016/j.colsurfb.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Adamová L., Malinovská L., Wimmerová M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014;77:841–849. doi: 10.1002/jemt.22407. [DOI] [PubMed] [Google Scholar]
- 26.Jančaříková G., Herczeg M., Fujdiarová E., Houser J., Kövér K.E., Borbás A., Wimmerová M., Csávás M. Synthesis of α-L-fucopyranoside-presenting glycoclusters and investigation of their interaction with recombinant Photorhabdus asymbiotica lectin (PHL) Chem. Eur. J. 2018;24:4055–4068. doi: 10.1002/chem.201705853. [DOI] [PubMed] [Google Scholar]
- 27.Herczeg M., Mező E., Molnár N., Ng S.-K., Lee Y.-C., Dah-Tsyr Chang M., Borbás A. Inhibitory effect of multivalent rhamnobiosides on recombinant horseshoe crab plasma lectin interactions with Pseudomonas aeruginosa PAO1. Chem. Asian J. 2016;11:3398–3413. doi: 10.1002/asia.201601162. [DOI] [PubMed] [Google Scholar]
- 28.Chabre Y.M., Contino-Pepin C., Placide V., Shiao T.C., Roy R. Expeditive synthesis of glycodendrimer scaffolds based on versatile TRIS and mannoside derivatives. J. Org. Chem. 2008;73:5602–5605. doi: 10.1021/jo8008935. [DOI] [PubMed] [Google Scholar]
- 29.Leaver D.J., Dawson R.M., White J.M., Polyzos A., Hughes A.B. Synthesis of 1,2,3-triazole linked galactopyranosides and evaluation of cholera toxin inhibition. Org. Biomol. Chem. 2011;21:8465–8474. doi: 10.1039/c1ob06317k. [DOI] [PubMed] [Google Scholar]
- 30.Deguise I., Lagnoux D., Roy R. Synthesis of glycodendrimers containing both fucoside and galactoside residues and their binding properties to Pa-IL and PA-IIL lectins from Pseudomonas aeruginosa. New J. Chem. 2007;31:1321–1331. doi: 10.1039/b701237c. [DOI] [Google Scholar]
- 31.Guchhait G., Misra A.K. Efficient glycosylation of unprotected sugars using sulfamic acid: A mild eco-friendly catalyst. Catal. Commun. 2011;14:52–57. doi: 10.1016/j.catcom.2011.07.016. [DOI] [Google Scholar]
- 32.Matta K.L., Girotra R.N., Barlow J.J. Synthesis of p-nitrobenzyl and p-nitrophenyl 1-thioglycopyranosides. Carbohydr. Res. 1975;43:101–109. doi: 10.1016/S0008-6215(00)83976-2. [DOI] [PubMed] [Google Scholar]
- 33.Adinolfi M., Capasso D., Di Gaetano S., Iadonisi A., Leone L., Pastore A. A straightforward synthetic access to symmetrical glycosyl disulfides and biological evaluation thereof. Org. Biomol. Chem. 2011;9:6278–6283. doi: 10.1039/c1ob05619k. [DOI] [PubMed] [Google Scholar]
- 34.Eszenyi D., Kelemen V., Balogh F., Bege M., Csávás M., Herczegh P., Borbás A. Promotion of a reaction by cooling—Stereoselective 1,2-cis-alpha-Thioglycoconjugation by Thiol-Ene coupling at −80 °C. Chem. Eur. J. 2018;24:18–4532. doi: 10.1002/chem.201800668. [DOI] [PubMed] [Google Scholar]
- 35.Lázár L., Csávás M., Herczeg M., Herczegh P., Borbás A. Synthesis of S-linked glycoconjugates and S-disaccharides by thiol-ene coupling reaction of enoses. Org. Lett. 2012;14:4650–4653. doi: 10.1021/ol302098u. [DOI] [PubMed] [Google Scholar]
- 36.Varela O., De Fina G.M., De Lederkremer R.M. The reaction of 2-hydroxyglycal esters with alcohols in the presence of N-iodosuccinimide, stereoselective synthesis of α anomers of alkyl 3-deoxyhex-2-enopyranosides and 3,4-dideoxyhex-3-enopyranosid-2-uloses. Carbohydr. Res. 1987;167:187–196. doi: 10.1016/0008-6215(87)80278-1. [DOI] [Google Scholar]
- 37.Cecioni S., Praly J.-P., Matthews S.E., Wimmerová M., Imberty A., Vidal S. Rational design and synthesis of optimized glycoclusters for multivalent lectin–carbohydrate interactions: influence of the linker arm. Chem. Eur. J. 2012;18:6250–6263. doi: 10.1002/chem.201200010. [DOI] [PubMed] [Google Scholar]
- 38.Deniaud D., Julienne K., Gouin S.G. Insights in the rational design of synthetic multivalent glycoconjugates as lectin ligands. Org. Biomol. Chem. 2011;9:966–979. doi: 10.1039/C0OB00389A. [DOI] [PubMed] [Google Scholar]
- 39.Tielker D., Hacker S., Loris R., Strathmann M., Wingender J., Wilhelm S., Rosenau F., Jaeger K.-E. Pseudomonas aeruginosa LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151:1313–1323. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- 40.Cott C., Thuenauer R., Landi A., Kühn K., Juillot S., Imberty A., Madl J., Eierhoff T., Römer W. Pseudomonas aeruginosa lectin LecB inhibits tissue repair processes by triggering β-catenin degradation. Biochim. Biophys. Acta. 2016;1863:1106–1118. doi: 10.1016/j.bbamcr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson E., Kollberg H., Johannesson M., Wejåker P.E., Carlander D., Larsson A. More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: A case report. J. Med. Food. 2007;10:375–378. doi: 10.1089/jmf.2006.214. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann G., Yang L., Wu H., Song Z., Wang H., Høiby N., Ulrich M., Molin S., Riethmüller J., Döring G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010;202:1585–1592. doi: 10.1086/656788. [DOI] [PubMed] [Google Scholar]
- 43.Hauber H.P., Schulz M., Pforte A., Mack D., Zabel P., Schumacher U. Inhalation with fucose and galactose for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. Int. J. Med. Sci. 2008;5:371–376. doi: 10.7150/ijms.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubíčková B., Hadrabová J., Vašková L., Mandys V., Stiborová M., Hodek P. Susceptibility of airways to Pseudomonas aeruginosa infection: Mouse neuraminidase model. Monatsh. Chem. 2017;148:1993–2002. doi: 10.1007/s00706-017-2035-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.