Abstract
Purpose
Aromatase inhibitors (AI) have been shown to reduce breast cancer-related mortality in women with estrogen positive (ER+) breast cancer. The use of AIs, however, has been associated with higher rates of hypertension, hyperlipidemia, and cardiovascular (CV) events.
Methods
A cross-sectional study of 25 healthy post-menopausal women and 36 women with curative intent breast cancer on an AI was performed to assess endothelial dysfunction, an indicator of risk for CV events. Consented subjects underwent vascular testing using the HDI/Pulse Wave CR-2000 Cardiovascular Profiling System and the EndoPAT2000 system.
Results
Mean age was 61.7 and 59.6 years (cases, controls). Most subjects were Caucasian and overweight. Controls had a lower mean systolic blood pressure (128.6 mmHg vs. 116.2 mmHg, p = 0.004). Median estradiol levels were reduced in cases (2 vs. 15 pg/ml, p < 0.0001). EndoPAT ratio (0.8 vs. 2.7, p < 0.0001) was significantly reduced in cases as compared to controls. Median large artery elasticity (12.9 vs.14.6 ml/mmHg × 10, p = 0.12) and small artery elasticity (5.2 vs. 7.0 ml/mmHg × 100, p = 0.07) were also reduced though not statistically significant. There was no correlation between use of chemotherapy, radiation therapy, type of AI, or duration of AI use and endothelial function. When adjusting for differences in blood pressure, results remained significant.
Conclusion
Breast cancer cases on AIs have reductions in endothelial function, a predictor of adverse CV disease. Impact: Vascular function changes in breast cancer cases on AIs compared to postmenopausal women. Further work is needed to evaluate vascular changes over time.
Keywords: Cardiotoxicity, Breast cancer, Aromatase inhibitors, Endothelial function, Vascular function
Introduction
With over 230,000 new cases of breast cancer diagnosed yearly, of which two-thirds are estrogen receptor (ER) positive, adjuvant endocrine therapy using an aromatase inhibitor (AI) is the standard of care to help reduce disease recurrence and breast cancer-related mortality [1]. The selective ER modulator tamoxifen and the third generation AIs have been shown to reduce both disease recurrence and breast cancer-related mortality in women with ER-positive early disease [2, 3]. With superior outcomes, third generation AIs have replaced tamoxifen as first-choice adjuvant endocrine therapy for postmenopausal women with early breast cancer [4].
Aromatase inhibitors, however, may increase the risk of hypertension, hyperlipidemia, and cardiovascular (CV) disease [5]. The major clinical trials that examined the safety of AIs include the Arimidex Tamoxifen Alone or in Combination (ATAC) study, the Breast International Group (BIG 1–98), and the Intergroup Exemestane Study (IES). In the ATAC trial, anastrozole was associated with higher rates of hypercholesterolemia and hypertension [4]. At 68 months, there was a trend toward a higher incidence of ischemic cardiovascular events (n = 127 vs. n = 104). In more recent data, the concern continues as there were more grade 3 and 4 cardiovascular events (angina pectoris and MI) reported in those on anastrozole as compared with tamoxifen. In the BIG 1–98 trial, there was an increase in the incidence of grade 3 through 5 cardiac events with the AI letrozole [4, 6]. There have also been trends towards increases in hypertension and ischemic cardiovascular disease with the AI exemestane as well [7]. A recent systemic review of 19 randomized controlled trials (n = 62,345 women) demonstrated a 19% increased risk of cardiovascular events in those on AIs as compared with tamoxifen (RR 1.19, 95% CI 1.07–1.34). The relative risk of ischemic heart disease increased by 30% in those on AIs as compared to those on tamoxifen (RR 1.30, 95% CI 1.11–1.53) [8]. In a subsequent population-based cohort study of women >55 years in the Ontario Health Insurance Plan with stage 1–3 breast cancer, women on AIs were much more likely to have a myocardial infarction (HR 2.02, 95% CO 1.15–3.53) as compared to those women treated with tamoxifen [9]. Other studies suggest there may not be an increased risk of cardiovascular complications in women on AIs [10]. Having a better understanding of the exact mechanism of AIs on the cardiovascular system is vital given both the conflicting data and the inconsistency with which these data have been collected.
Injury to the cardiovascular system results from disruptions in inflammation, hemostasis, endothelial damage, and vascular function, all leading to the development of atherosclerosis. While traditional risk factors such as advancing age, hypertension, hyperlipidemia, and tobacco use can identify risk factors for cardiovascular disease, endothelial dysfunction identified by reactive hyperemia using EndoPAT has been associated with an increased risk of CV events, independent of these traditional risk factors in the Framingham risk score [11]. As a result, we hypothesized that use of AIs, and the associated reduction in estrogen, would result in a decrease in endothelial function, a predictor of early CV disease in women.
We report the results of a cross-sectional study examining the reactive hyperemia by EndoPAT in post-menopausal women with breast cancer on an AI in comparison to healthy, postmenopausal women.
Methods
Subjects
We conducted a cross-sectional study of 36 post-menopausal women with ER+ breast cancer prescribed an AI (cases) and 25 healthy, postmenopausal women (controls) at the University of Minnesota (UMN) Masonic Cancer Center between 2014 and 2015. Eligible cases had a diagnosis of locally advanced, curative intent breast cancer. All cases had completed breast cancer treatment and were taking an AI. Subjects with a history of tobacco use, myocardial infarction, congestive heart failure, or cardiac catheterization requiring intervention were excluded. Medical record abstraction for cases confirmed diagnosis, stage at diagnosis, use of chemotherapy, use of radiation, and personal medical history including history of cardiovascular disease and medications. Type of prescribed aromatase inhibitor was also abstracted.
Twenty-five healthy, postmenopausal women without a history of breast cancer, myocardial infarction, congestive heart failure, or cardiac catheterization were enrolled. Five of the twenty-five controls were subsequently found to be taking exogenous estrogen; they were excluded from the final analysis.
The protocol was approved by the University of Minnesota Institutional Review Board and Cancer Center Review Committee. All patients provided written informed consent according to the Declaration of Helsinki.
Study recruitment
Potential cases were mailed a letter of recruitment inviting them to participate in the study. A postcard was provided to indicate whether they were interested in the study or whether they wanted to actively decline participation. For those who expressed interest, a screening phone call was placed to discuss the study and identify participants. For those who did not return the postcard, a follow-up phone call was placed to the subjects. Failure to answer the phone call or return the postcard was identified as passive refusal to participate in the study. Consented subjects were invited to undergo vascular assessment and biomarker assessment. Those consented subjects who completed the protocol received one $50 gift card.
Healthy postmenopausal women in the control group were recruited from fliers in the Minneapolis St Paul area.
Vascular assessment
Vascular assessment was performed using the EndoPAT 2000 and HDI/PulseWave CR-2000 Cardiovascular Profiling System (Hypertension Diagnostic Inc., Eagan, MN) and pulse contour analysis on one occasion as previously described [11–13]. HDI/Pulse Wave CR-2000 Cardiovascular Profiling System uses a pencil-shaped applanation tonometer to measure radial arterial pulse wave forms which are then digitized, analyzed, and stored by a computer. Large artery elasticity (LAE) and small artery elasticity (SAE) are derived from the diastolic pulse contour analysis based on a modified Windkessel model of circulation (model HDI/PulseWave CR-2000 Cardiovascular Profiling System). In performing the procedure, a tonometer was placed at the dominant wrist overlying the radial artery of resting participants. A stable 30 s measure of the radial pulse waveform was achieved, excluding the dicrotic notch, and digitized at 200 samples per second. Before and during the waveform assessment, an automated oscillatory blood pressure measurement was taken on the contralateral arm. A typical waveform has 2 maxima present within the diastolic decay curve. The first occurs at the beginning of diastole and represents the capacitance of the proximal aorta and major branches after cardiac ejection. The second maximum results from a reflective, or oscillatory, wave, corresponding to elasticity in the smaller arteries. This system has been standardized and correlated with cardiovascular risk factors. It has been shown by Grey et al. [14] that SAE is predictive for coronary heart disease events and that LAE and SAE are predictive for heart failure events above and beyond arterial blood pressure [13]. A reduction in reactive hyperemia assessed by the EndoPAT ratio (<1.67) has also been correlated with an increase in cardiac events independent of other traditional cardiac risk factors [11].
The flow mediated dilation examination required the patient to be supine, at rest, in a quiet, air-conditioned room. Subjects were fasting at least 8 h before the study. All vaso-active medications were withheld for at least four half-lives, when possible. In addition subjects were asked not to exercise, use caffeine, high-fat foods, or vitamin C for at least 4–6 h before the test. SonoSite Ultrasound system was equipped with vascular software for two-dimensional (2D) imaging, color and spectral Doppler, an internal electrocardiogram (ECG) monitor, and a high-frequency vascular transducer. Timing of each image with respect to the cardiac cycle was determined with simultaneous ECG recording on the ultrasound system monitor.
All study assessments were conducted in a specifically designed research space for clinical vascular assessments. Blood collection and vascular functional measurements were conducted in the morning by a trained vascular technician (NF). All testing was completed by the same technician to reduce inter-technician variability. The pulse waveform assessments were performed in triplicate, and the mean (average of 3 values for each participant) LAE and SAE indices were used in statistical analyses.
Biomarker assessment
Biomarkers were obtained using a fasting blood draw to evaluate the following lipids and inflammatory markers: serum ultrasensitive estradiol, serum glucose, total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides (TG), C-reactive protein (CRP), plasminogen activator 1 (PA1), tissue-type plasminogen activator (t-PA), soluble vascular cell adhesion molecule (p-VCAM), and circulating endothelial cells. Blood samples were collected first, prior to vascular functional assessments. All blood samples were appropriately processed and aliquoted for measurement of respective biomarkers.
Statistics
Demographic and clinical characteristics of participants by group were obtained. For measurements taken in triplicate, the average value of those measurements was used in analysis. Means ± standard deviations or medians and ranges of the biomarkers and functional test measurements are presented as appropriate. Differences between cases and controls were carried out using χ2 and Fisher’s Exact tests for categorical variables and t tests for continuous variables. Wilcoxon Rank Sum tests were used for vascular function test comparisons. Data were analyzed using SAS Version 9.3.
Results
Baseline characteristics are presented in Table 1. Most subjects were Caucasian (>90%) and overweight. Cases had a higher mean systolic blood pressure (SBP) of ± 16.9 mmHg compared to controls (116.2 ± 13.3 mmHg; p = 0.004). Most cases had Stage 1 breast cancer (54.0%), about half received chemotherapy (48.6%), and two-thirds received radiation (67.7%) with 37.1% having received left sided breast radiation. Median time on AIs was 35 (range 1–85) months. There were no differences in cholesterol or glucose between cases and controls. Cases had a reduced estradiol level (median 2 vs. 15 pg/ml, p < 0.0001).
Table 1.
Demographic and Clinical Characteristics compared between women with breast cancer and controls
| Outcome | BRCA (N = 36) |
Controls (N = 20) |
|||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | p value | |
| Baseline characteristics | |||||
| Age | 36 | 61.7 (7.1) | 20 | 59.6 (4.7) | 0.18 |
| Body mass index (BMI) | 36 | 27.6 (7.8) | 20 | 26.5 (5.8) | 0.66 |
| Heart rate (bpm) | 36 | 63.1 (8.5) | 20 | 64.1 (8.1) | 0.66 |
| Systolic blood pressure | 36 | 128.6 (16.9) | 20 | 116.2 (13.3) | 0.004 |
| Diastolic blood pressure | 36 | 70.7 (9.2) | 20 | 70.1 (9.3) | 0.81 |
| Outcome | BRCA (N = 36) |
||||
| N | Percent (%) | ||||
| Cancer characteristics | |||||
| Stage breast cancer | |||||
| Stage 1 | 20 | 54.0 | |||
| Stage 2 | 13 | 35.2 | |||
| Stage 3 | 4 | 10.8 | |||
| Cancer | |||||
| Left | 13 | 37.1 | |||
| Right | 22 | 62.9 | |||
| Chemotherapy (y) | 17 | 48.6 | |||
| Radiation (y) | 23 | 67.7 | |||
| Aromatase inhibitor | |||||
| Anastrazole | 14 | 41.2 | |||
| Exemestane | 5 | 14.7 | |||
| Letrozole | 15 | 44.1 | |||
Cases had a statistically significant reduced EndoPAT ratio of 0.8 (0.2–2.6), while controls measured 2.7 (1.3–3.8) (Fig. 1a; p < 0.0001); this remained statistically significant after adjusting for SBP. Differences in the same direction were observed for large artery elasticity (LAE) and small artery elasticity (SAE); however, they were not statistically significant. LAE was 12.9 ml/mmHg × 10 (range 5.4–22.2) in cases and 14.6 (9.4–24.6) in controls (Fig. 1b; p = 0.12). SAE was 5.2 (1.8–15.6)ml/mmHg × 100 in cases and 7.0 (2.4–15.7) mmHg × 100 in controls (Fig. 1c; p = 0.07). There were no differences in LAE, SAE, and endoPAT ratio when examining laterality of breast cancer (left/right), use of chemotherapy, or use of radiation. There was a decrease in LAE in those receiving anastrazole (median 11.7 ml/mmHg) as compared to exemestane (median 14.6 ml/mmHg) and letrozole (median 13.9 ml/mmHg; p = 0.03).
Fig. 1.
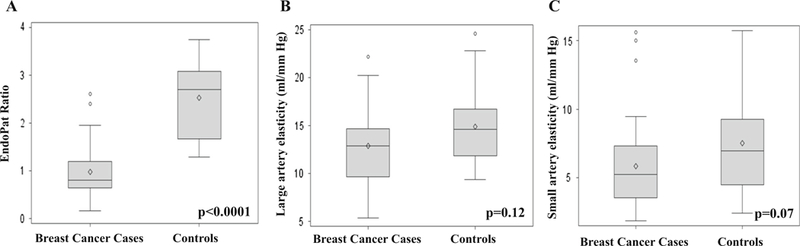
a–c Vascular function measurements in breast cancer cases on aromatase inhibitors and controls (a). EndoPat ratio (b). Large artery elasticity (c). Small artery elasticity
There was no association between duration of AI use and vascular function. The majority of cases, however, had an EndoPAT ratio associated with an increased risk of CV events based on prior studies (<1.67) [11].
Women on Als had a significant reduction in estradiol levels as compared to healthy postmenopausal women (2 vs. 15, p < 0.0001). There was a correlation between estradiol levels and EndoPAT ratio (Spearman coefficient, r = 0.68, p < 0.0001).
Women on AIs had a trend towards increases in various biomarkers of hemostasis and endothelial damage (Table 2). No changes in biomarkers of inflammation were detected. There were no differences in circulating endothelial cells between cases and controls.
Table 2.
Biomarkers in breast cancer subjects on AIs as compared to healthy postmenopausal women
| Measure | BRCA* |
Controls |
p value | ||
|---|---|---|---|---|---|
| N | Median (Min, Max) | N | Median (Min, Max) | ||
| Cardiac Biomarkers | |||||
| Cholesterol (mg/dl) | 34 | 220 (155, 298) | 20 | 206 (171, 262) | 0.22 |
| Triglycerides (mg/dl) | 34 | 76 (44, 224) | 20 | 68 (40, 245) | 0.19 |
| HDL cholesterol (mg/dl) | 34 | 72 (36, 102) | 20 | 69 (45, 106) | 0.72 |
| LDL cholesterol (mg/dl) | 34 | 132 (93, 195) | 20 | 125 (74, 199) | 0.29 |
| Blood glucose (mmol/l) | 34 | 87 (67, 106) | 20 | 88 (72, 114) | 0.53 |
| Estradiol (pg/ml) | 30 | 2 (2, 20) | 20 | 15 (4, 21) | <0.0001 |
| Inflammation | |||||
| High sensitive C-reactive protein (hsCRP) (mg/l) | 33 | 1.1 (2.0, 12.7) | 20 | 1.2 (3.0, 5.3) | 0.91 |
| White Blood Cells (×109 l−1) | 34 | 5.0 (2.6, 7.4) | 20 | 4.6 (3.3, 8.1) | 0.29 |
| Hemostasis | |||||
| Plasminogen activator inhibitor-1 (pg/ml) | 33 | 1131 (175, 4874) | 20 | 757 (146, 4082) | 0.06 |
| Tissue-type plasminogen activator (t-PA) (pg/ml) | 33 | 989.5 (186.1, 6183.4) | 20 | 721.3 (271.5, 4081.5) | 0.05 |
| Thrombomodulin (ng/ml) | 31 | 2.9 (1.6, 6.5) | 20 | 2.7 (1.2, 5.0) | 0.11 |
| Ddimer (pg/ml) | 31 | 21,135 (78, 189,375) | 20 | 6365 (164, 73,507) | 0.06 |
| Endothelial Damage | |||||
| Surface VCAM-1 (ng/ml) | 31 | 67 (0,100) | 20 | 55 (14, 82) | 0.07 |
BRCA breast cancer cases
Discussion
In this study, postmenopausal breast cancer survivors on AIs had reduced endothelial function compared to healthy postmenopausal women. Reduced endothelial function was appreciable, independent of prior chemotherapy use, radiation use, or type of AI. These results provide additional evidence that AI use, and the lack of associated estrogen, results in endothelial dysfunction.
Endothelial dysfunction and impairments in vascular relaxation reflect a vascular phenotype prone to atherosclerosis. In prior cardiac studies, individuals with impaired vascular function are at risk for cardiac adverse events [11, 13, 15]. In one cohort of 329 asymptomatic outpatients, those with reduced endoPat at baseline (<1.67) were at significantly higher risk of adverse cardiac events in a seven-year follow-up period (48 vs. 28%). A reduction in EndoPAT has also shown to be a predictor of adverse clinical events in individuals seen in emergency departments with chest pain [16]. Finally, additional studies have demonstrated that reduced small artery elasticity is predictive of increasing rates of CV events and stroke [15, 17, 18]. Endothelial dysfunction is an independent predictor of cardiovascular events, independent of traditional cardiac risk factors in the Framingham risk score. In this study, the median endoPAT ratio was 0.8, significantly lower than that seen in cardiac studies of individuals with an increased risk of future cardiac events. This non-invasive testing may be easily adaptable in identifying breast cancer patients at highest risk for future cardiac events.
While longitudinal clinical trials of AIs demonstrated mixed results as to whether AIs resulted in an increased risk of cardiac events, these trials were predominantly done on healthy individuals, and reported cardiac events inconsistently [6, 7, 10]. Additionally, some of these trials included women who were treated with tamoxifen prior to using an AI. Tamoxifen has been demonstrated to improve lipid profiles [19]. As a result, it is possible that our study demonstrates significant changes in endothelial dysfunction, given that endothelial dysfunction is typically seen as an early change in the development of atherosclerotic disease, as opposed to an end event such as myocardial infarction that is captured in a clinical trial.
It has been hypothesized that estrogen has both genomic and non-genomic effects resulting in a protective effect on the development of atherosclerosis [19]. Rapidly, estrogen can result in vasodilation, increases in nitric oxide, helping to prevent changes in the endothelium. However, estrogen can also cause longer, genomic like effects resulting in decreases in smooth muscle cell growth, increases in endothelial-cell growth, and a decrease in vascular injury. The effects of estrogen on endothelial function have been confirmed in animal models and more recently in a small study of healthy, premenopausal women in which there was significant inverse relationship found between estradiol concentration and vascular elasticity [20].
Changes in endothelial function would be expected soon after starting an AI. It remains unclear whether genomic changes occur the longer a woman is on AI, impacting long-term endothelial function as well as the reversibility of its impact. The current prescription for women with ER + breast cancer is an AI for five years, and possibly ten years [21]. Given these results, it is important to question whether choosing an AI for ten years warrants prescription, when it may not improve overall survival. The Long Island Breast Cancer study looked at long-term mortality in breast cancer survivors as compared to 1411 age-matched women without breast cancer. The risk of CV death in breast cancer survivors almost doubled that of the control group at 7 years after diagnosis (HR 1.9) [22]. This Long Island Breast Cancer study did not separate hormonal therapy by tamoxifen or AI.
This study is limited. It is a cross-sectional, single institution study of non-smokers. It does not answer how AIs impact endothelial function over time or the impact of AIs in premenopausal women or in those with preexisting atherosclerosis. Studies are ongoing to look at the longitudinal impact of AIs on vascular function.
In conclusion, we observed breast cancer survivors on AIs demonstrate reduced endothelial function, a measure of early CV disease, compared to controls, likely the result of reduced estradiol levels. Further work is needed to understand the long-term impact of AIs over time in breast cancer survivors as well as the mechanism associated with AI endothelial dysfunction.
Acknowledgments
Funding The work in this paper was supposed by the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) NIH# K12-HD055887 Grant.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A et al. (2017) NCCN guidelines insights: breast cancer, Version 1.2017. J Natl Compr Canc Netw 15(4):433–451 [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J et al. (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooning MJ, Dorresteijn LD, Aleman BM, Kappelle AC, Klijn JG, Boogerd W et al. (2006) Decreased risk of stroke among 10-year survivors of breast cancer. J Clin Oncol 24(34):5388–5394. doi: 10.1200/JC0.2006.06.5516 [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M et al. (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 11(12):1135–1141. doi: 10.1016/S1470-2045(10)70257-6 [DOI] [PubMed] [Google Scholar]
- 5.Chapman JA, Shepherd LE, Ingle JN, Muss HB, Pritchard KI, Gelmon KA et al. (2016) Competing risks of death in women treated with adjuvant aromatase inhibitors for early breast cancer on NCIC CTG MA.27. Breast Cancer Res Treat 156(2):343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L et al. (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 12(12):1101–1108. doi: 10.1016/S1470-2045(11)70270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE et al. (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Inter-group Exemestane Study): a randomised controlled trial. Lancet 369(9561):559–570. doi: 10.1016/S0140-6736(07)60200-1 [DOI] [PubMed] [Google Scholar]
- 8.Khosrow-Khavar F, Filion KB, Al-Qurashi S, Torabi N, Bouganim N, Suissa S et al. (2017) Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 28(3):487–496. doi: 10.1093/annonc/mdw673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Qadir H, Amir E, Fischer HD, Fu L, Austin PC, Harvey PJ et al. (2016) The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer 68:11–21 [DOI] [PubMed] [Google Scholar]
- 10.Ligibel JA, James O’Malley A, Fisher M, Daniel GW, Winer EP, Keating NL (2012) Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat 131(2):589–597 [DOI] [PubMed] [Google Scholar]
- 11.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE et al. (2010) Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 31(9):1142–1148. doi: 10.1093/eurheartj/ehq010 [DOI] [PubMed] [Google Scholar]
- 12.Blaes AH, Mulrooney DA, Vogel RI, Peterson BA, Yee D, Neglia JP et al. (2014) Arterial elasticity in testicular cancer survivors. J Clin Oncol 32:524190110 [Google Scholar]
- 13.Duprez DA, Jacobs DR Jr, Lutsey PL, Bluemke DA, Brumback LC, Polak JF et al. (2011) Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol 174(5):528–536. doi: 10.1093/aje/kwr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grey E, Bratteli C, Glasser SP, Alinder C, Finkelstein SM, Lindgren BR et al. (2003) Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am J Hypertens 16(4):265–269 [DOI] [PubMed] [Google Scholar]
- 15.Hom EK, Duprez DA, Jacobs DR Jr, Bluemke DA, Brumback LC, Polak JF et al. (2016) Comparing arterial function parameters for the prediction of coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Am J Epidemiol 184(12):894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shechter M, Matetzky S, Prasad M, Goitein O, Goldkorn R, Naroditsky M et al. (2017) Endothelial function predicts 1-year adverse clinical outcome in patients hospitalized in the emergency department chest pain unit. Int J Cardiol 240:14–19 [DOI] [PubMed] [Google Scholar]
- 17.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y et al. (2009) Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134(1):52–58. doi: 10.1016/j.ijcard.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 18.Hirata Y, Sugiyama S, Yamamoto E, Matsuzawa Y, Akiyama E, Kusaka H et al. (2014) Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol 173(3):481–486. doi: 10.1016/j.ijcard.2014.03.085 [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn ME, Karas RH (1999) The protective effects of estrogen on the cardiovascular system. N Engl J Med 340(23):1801–1811. doi: 10.1056/NEJM199906103402306 [DOI] [PubMed] [Google Scholar]
- 20.Luca MC, Liuni A, Harvey P, Mak S, Parker JD (2016) Effects of estradiol on measurements of conduit artery endothelial function after ischemia and reperfusion in premenopausal women. Can J Physiol Pharmacol 94(12):1304–1308. doi: 10.1139/cjpp-2015-0589 [DOI] [PubMed] [Google Scholar]
- 21.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J et al. (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375(3):209–219. doi: 10.1056/NEJMoa1604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD (2016) Cardiovascular disease mortality among breast cancer survivors. Epidemiology 27(1):6–13. doi: 10.1097/EDE.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]


