Abstract
New cells are added in the brains of all adult vertebrates, but fishes have some of the greatest potential for neurogenesis and gliogenesis among all taxa, partly due to their indeterminate growth. Little is known, however, about how social interactions influence cell proliferation in the brain of these fishes that comprise the largest group of vertebrates. We used 5-bromo-2′-deoxy-uridine (BrdU) to identify and localize proliferation zones in the telencephalon, diencephalon, mesencephalon, and rhombencephalon that were primarily associated with ventricular surfaces in the brain of the African cichlid fish Astatotilapia burtoni. Cell migration was evident in some regions by 1 day post injection, and many newborn cells coexpressed the neuronal marker HuC/D at 30 days, suggesting they had differentiated into neurons. To test the hypothesis that social status and perception of an opportunity to rise in rank influenced cell proliferation, we compared numbers of BrdU-labeled cells in multiple brain nuclei among fish of different social status. Socially suppressed subordinate males had the lowest numbers of proliferating cells in all brain regions examined, but males that were given an opportunity to rise in status had higher cell proliferation rates within 1 day, suggesting rapid upregulation of brain mitotic activity associated with this social transition. Furthermore, socially isolated dominant males had similar numbers of BrdU-labeled cells compared with dominant males that were housed in a socially rich environment, suggesting that isolation has little effect on proliferation and that reduced proliferation in subordinates is a result of the social subordination. These results suggest that A. burtoni will be a useful model to analyze the mechanisms of socially induced neurogenesis in vertebrates.
Keywords: BrdU, dominance, HuC/D, neurogenesis, subordinate, teleost
Social interactions among conspecifics in both aggressive and reproductive contexts can have profound effects on the behavior and physiology of individuals. In particular, brain structure and function can be dramatically altered by an animal’s social environment, with important consequences for survival and reproductive fitness (Gheusi et al., 2009; Gonda et al., 2009; Lau et al., 2011). However, little is known about how position in a dominance hierarchy or the transition between low- and high-ranking social states might influence structural plasticity and neurogenesis in the vertebrate brain.
In many vertebrates, social stimuli can promote the production, migration, and differentiation of new cells into brain regions that control behaviors, where brain plasticity contributes to learning and memory formation (Castilla-Ortega et al., 2011; Nogues et al., 2011). For example, dominant male rats had higher cell proliferation in the hippocampus compared with subordinate animals (Hoshaw et al., 2006), dominant mountain chickadees (Poecile gambeli) that performed better on spatial memory tests had higher levels of cell proliferation in the hippocampus (Pravosudov and Omanska, 2005), repeated social defeat decreased cell proliferation in the dentate gyrus of mice (Yap et al., 2006), and social interactions including mating, exposure to the opposite sex, and maternal behavior alters cell proliferation rates in the brain across several taxa (Fowler et al., 2002; Mak et al., 2007; Ruscio et al., 2008; Gheusi et al., 2009; Hawken et al., 2009; Lau et al., 2011).
Social isolation and nonenriched environments can also have profound negative effects on brain cell proliferation in many taxa ( Greenough, 1975; Kempermann et al., 1997; Fowler et al., 2002; Dunlap et al., 2006; Rizzi et al., 2007; Barnea, 2009), and decreased neurogenesis is implicated in the pathogenesis of depressive illness in mammals (Snyder et al., 2011). In addition to effects on cell proliferation, social and environmental conditions can also influence cell fate and neuron survival in birds and mammals (Kempermann et al., 1997; Adar et al., 2008; Barnea, 2009; Gheusi et al., 2009; Ming and Song, 2011). In rats, for example, position in a dominance hierarchy had no effect on cell proliferation in the hippocampal dentate gyrus, but dominant males had more new neurons compared with subordinate animals. Thus, determining how social interactions can influence the production, differentiation, and migration of new brain cells is important for understanding the selective pressures that act on socially induced plastic changes in neural circuitry.
Adult neurogenesis occurs in the brains of all vertebrates, but there are distinct differences in cell proliferation capacity among taxa (Chapouton et al., 2007; Kaslin et al., 2008; Ming and Song, 2011). In mammals, for example, adult neurogenesis occurs primarily in two telencephalic areas, the subependymal/subventricular zone of the lateral ventricle, which generates olfactory bulb interneurons, and the subgranular zone of the dentate gyrus, which produces hippocampal granular neurons (Abrous et al., 2005; Kaslin et al., 2008; Ming and Song, 2011). In contrast, cell proliferation in teleost fishes, which continue to grow throughout life, is orders of magnitude greater than that observed in mammals and occurs throughout the brain and eye in dozens of well-defined areas called “proliferation zones” that are located primarily at or near the surfaces of ventricles (Fernald, 1991; Zupanc and Horschke, 1995; Zikopoulos et al., 2000; Ekstrom et al., 2001; Zupanc et al., 2005; Zupanc, 2008; Kuroyanagi et al., 2010). This widespread and abundant cell proliferation in teleosts makes them ideal models to study which factors influence neurogenesis in the adult vertebrate brain.
In fishes, differences in cell proliferation in the brain are associated with environmental complexity and rearing conditions (Lema et al., 2005; von Krogh et al., 2010; Dunlap et al., 2011), sex and season (Zikopoulos et al., 2000, 2001; Ampatzis and Dermon, 2007; Dunlap et al., 2011), and social interactions and stress (Dunlap et al., 2006, 2008; Sorensen et al., 2007, 2011). However, most of these studies have examined only one or a limited number of proliferation areas, and none have tested how the perception of a social opportunity (i.e., chance to rise in rank to become socially dominant) might rapidly influence cell proliferation in the brain.
The African cichlid fish Astatotilapia burtoni is an excellent model to test how the social environment influences cell proliferation in the brain because in males, their behavioral repertoire and physiology are tightly coupled to dominance status, which is ultimately controlled by the brain (Fernald, 2009). Moreover, retinal cell addition patterns, including the insertion of new rod photoreceptors (Johns and Fernald, 1981) and lens growth (Fernald and Wright, 1983), were first understood in this species (Fernald, 1991). Male A. burtoni have evolved two distinct, reversible phenotypes as an adaptation to their dynamic social environment: 1) dominant males that are brightly colored defend a spawning and feeding territory and display aggressive and courtship behaviors; and 2) subordinate males that are drably colored, similar to females, lack a spawning territory, display submissive behaviors, and do not typically court females (Fernald and Hirata, 1977).
In addition to these behavioral and coloration differences, dominant and subordinate males also differ in several key reproductive physiological traits. For example, dominant males have larger gonadotropin-releasing hormone (GnRH)1 somata in the brain (Davis and Fernald, 1990; Francis et al., 1993), higher levels of brain GnRH1 mRNA (White et al., 2002), higher luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in the pituitary and bloodstream (Maruska et al., 2011), higher circulating sex-steroid hormone levels (Parikh et al., 2006; Maruska and Fernald, 2010b), and larger testes (Fraley and Fernald, 1982; Maruska and Fernald, 2011a) compared with subordinate individuals. When a subordinate male perceives an opportunity to ascend in social rank and become dominant, he displays territorial and reproductive behaviors within minutes (Burmeister et al., 2005; Maruska and Fernald, 2010a) and shows rapid (minutes to hours) upregulation of the entire reproductive axis from the brain to the testes (Burmeister et al., 2005; Kustan et al., 2011; Maruska and Fernald, 2011a,b; Maruska et al., 2011). Whereas this social opportunity triggers dramatic physiological changes to prepare the animal for his new status as a reproductively active and dominant territory holder, it is not known whether this transition is also associated with changes in cell proliferation in the brain.
The goal of this study was first to identify the cell proliferation zones in the brain of a recently derived, and highly social, perciform fish species, and then to test the hypotheses that any of three distinct social situations, dominance status (subordinate or dominant), social isolation, or social opportunity, could influence cell proliferation in the fish brain. Our results show that in addition to the dramatic differences in reproductive physiology and behavior associated with social status in A. burtoni, the social environment also has profound effects on brain structure, as evidenced by differences in cell proliferation in behaviorally relevant brain regions depending on social group.
MATERIALS AND METHODS
Animals and social manipulation
Laboratory-bred Astatotilapia burtoni used in all experiments were derived from wild-caught stock from Lake Tanganyika, Africa, maintained in community aquaria under environmental conditions that mimic their natural equatorial habitat (24–26°C; pH 8.0; 12:12-hour light/dark with full-spectrum illumination; constant aeration), and fed cichlid flakes (AquaDine, Healdsburg, CA) each morning. All experimental procedures were approved by the Stanford Administrative Panel for Laboratory Animal Care.
To test how social status and the social environment influenced brain cell proliferation, we created four different groups of experimental males: 1) stable subordinate males (n = 9; standard length [SL], 55.2 ± 2.6 SD mm; BM, 4.0 ± 0.50 SD g); 2) stable dominant males (n = 9; SL, 55.0± 2.9 mm; body mass [BM], 4.9 ± 0.86 g); 3) socially ascending males (subordinate to dominant) (n = 7; SL, 61.0 ± 3.6 mm; BM, 6.3 ± 1.6 g); and 4) socially isolated dominant males (n = 8; SL, 63.0 ± 4.0 mm; BM, 7.0 ± 1.3 g) (Fig. 1A–C). It is not possible to obtain socially isolated subordinate males for comparison because dominance is the default status in this species, such that without aggressive interactions from other males to keep them suppressed, subordinate males placed in isolated tanks will become dominant (Fraley and Fernald, 1982; Fernald, 2009).
Figure 1.
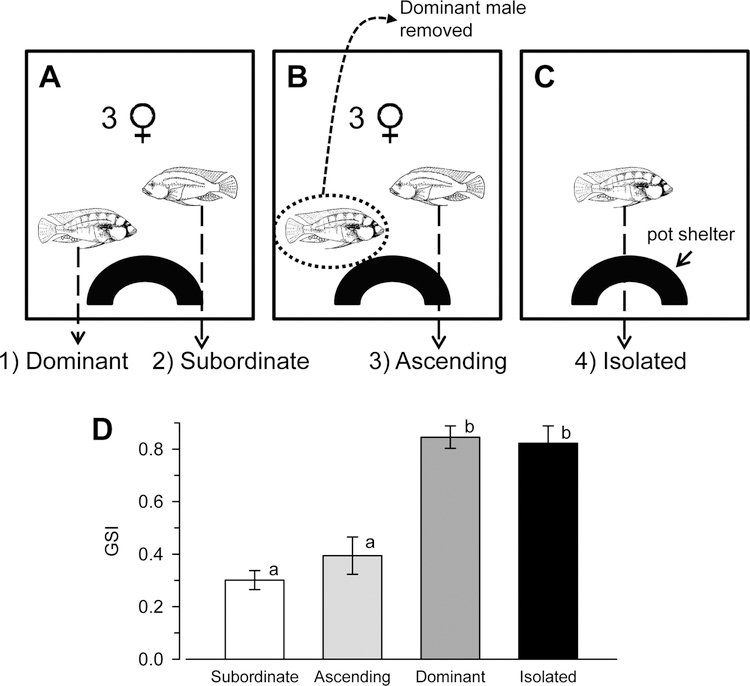
Experimental paradigm and social groups used to examine cell proliferation in the brain of the male African cichlid fish Astatotilapia burtoni. A: Stable dominant and subordinate males were housed together for 4–5 weeks along with three females and a single pot shelter to serve as a territory. B: Ascending males were created by establishing fish as in A, but after the 4–5-week period, the dominant male was removed prior to light onset, providing an opportunity for the subordinate male to rise in social rank. C: Isolated dominant males were housed alone in identical tanks for 4–5 weeks. Fish in all groups were sacrificed 24 hours after BrdU injection. D: Mean (±SEM) gonadosomatic index (GSI) was higher in dominant (n = 9) and isolated dominant (n = 8) males compared with both subordinate (n = 9) and ascending (n = 7) males. Different letters indicate statistical differences at P < 0.05.
Stable subordinates and dominants were created by removing two size-matched dominant males from different community tanks and putting them together with four females in a new experimental tank that contained a single terra cotta pot territory. Within about an hour, one male becomes dominant and the other subordinate. Fish were observed daily to verify that each male maintained his social status for 4–5 weeks, a time sufficient to suppress the reproductive axis (White et al., 2002; Maruska and Fernald, 2011a; Maruska et al., 2011). Ascending males were generated as described above for stable animals except that after 5-bromo-2′-deoxyuridine (BrdU) injection (see below), the stable subordinate was placed back into the experimental tank without the dominant male, creating an opportunity for social ascent. These ascending males begin to display reproductive and territorial behaviors characteristic of dominant males within minutes (Burmeister et al., 2005; Maruska and Fernald, 2010a). Socially isolated males were created by removing stable dominant males from community tanks and placing them alone into new individual experimental tanks, each with a single terra cotta pot, where they were physically and visually isolated from any other fish. As mentioned above, because dominance is the default state in A. burtoni, these socially isolated males are also dominant (Fraley and Fernald, 1982; Fernald, 2009). As expected, stable dominant and socially isolated dominant males had higher gonadosomatic indices than both stable subordinate and ascending males (ANOVA, F(3,29) = 29.05, P < 0.001, Student-Newman-Keuls test, P < 0.001), which provided further verification of social status (Fig. 1D).
BrdU injections and tissue preparation
Following the 4–5-week social exposure period, all males were briefly anesthetized in ice-cold tank water, weighed, given an intraperitoneal injection of BrdU (100 μg/g body mass; total injection volume ~ 16–30 μl), and immediately placed back into their tanks for recovery. BrdU is a thymine analog that incorporates into the DNA of actively dividing cells during the S-phase of the cell cycle, and is commonly used as a marker of brain and retinal cell proliferation from fishes through mammals (Chiu et al., 1995; Kwan et al., 1996; Kozorovitskiy and Gould, 2004; Zupanc, 2006, 2008; Almli and Wilczynski, 2009). Previous studies have also shown that the metabolic availability of BrdU is approximately 2–4 hours (Takahashi et al., 1993; Zupanc and Horschke, 1995), indicating that any observed cell proliferation from BrdU labeling occurs within the first few hours after injection.
Males from all experimental groups were sacrificed 24 hours post injection, and at the same time of day (10:00–12:00) because a previous study in this species showed circadian differences in cell proliferation in the retina (Chiu et al., 1995). Ascending males were sacrificed 24 hours after the first display of dominance behaviors as previously described (Maruska and Fernald, 2010a). Fish were anesthetized as described above, and SL and BM were measured; they were then sacrificed by rapid cervical transection. Testes were removed and weighed to calculate the gonadosomatic index [GSI = (gonad mass/body mass) × 100]. Brains were removed and fixed in 4% buffered-formalin overnight at 4°C, rinsed in phosphate-buffered saline (1X PBS, pH 7.4, Gibco, Invitrogen, Carlsbad, CA), and cryoprotected overnight in 30% sucrose in PBS prior to sectioning. Brains were then embedded (Neg50 media, Thermo Scientific, Waltham, MA) and sectioned in the coronal plane at 20 μm on a cryostat (HM550, Microm, Walldorf, Germany), alternately collected onto charged slides (Superfrost plus, VWR, Chicago, IL), and stored in the dark at 4°C until staining.
BrdU immunohistochemistry
To examine cell proliferation, one set of alternate slides that contained 20-μm-thick sections through the entire brain, from the olfactory nerve to the rostral spinal cord, was reacted with BrdU antiserum by using immunohistochemical techniques described previously (Kustan et al., 2011), whereas the second slide set was used for a separate study. Briefly, BrdU label was detected by using the following protocol: PBS incubation (30 minutes), quenching of endogenous peroxidases with 1.5% hydrogen peroxide (30 minutes), PBS wash (3X 5 minutes), incubation in DNAse I buffer (40 mM Tris-HCl, 10 mM NaCl, 6 mM MgCl2, 10 mM CaCl2, pH 7.9; 5 minutes), denaturing of DNA with 500 U/ml DNAse I in DNAse buffer (10 minutes), PBS wash (3X 5 minutes), blocking of nonspecific staining (5% normal goat serum, 0.2% bovine serum albumin [BSA], and 0.3%Triton X-100 in PBS; 1 hour), overnight incubation at 4°C with rat monoclonal anti-BrdU (#ab6326, clone number BU1/75 [ICR1], Abcam, Boston, MA; 1:1,000 final concentration in blocking solution), PBS wash (3X 10 minutes), 1 hour of incubation with biotinylated goat anti-rat secondary antibody (Vector, Burlingame, CA) in PBS with 10% normal goat serum, PBS wash (3X 10 minutes), quenching with 1.5% H202 (10 minutes), PBS wash (3X 10 minutes), incubation with Vectastain ABC kit (Vector; 2 hours), PBS wash (3X 10 minutes), and reaction with 3′3-diaminobenzidine (DAB) substrate kit (Vector; 5 minutes).
Slides were then rinsed in distilled water to stop the reaction, counterstained with 0.5% cresyl violet, dehydrated in an ethanol series (50–100%), cleared in xylene, and coverslipped with cytoseal 60 mounting media (Richard-Allan Scientific, Kalamazoo, MI). Slides were viewed and photographed with a camera (SPOT, Diagnostic Instruments, Sterling Heights, MI) attached to a Zeiss Axiophot microscope, and photos were adjusted for brightness, contrast, and levels with Photoshop CS3 software (Adobe Systems, San Jose, CA).
Localization of cell proliferation zones and quantification
To localize cell proliferation zones in the A. burtoni brain and to determine distribution, migration, and cell fate of BrdU-labeled cells after different survival times, several males were sacrificed at 3 hours (n = 4), 11 days (n = 2), and 30 days (n = 3) after injection. Double-label fluorescent immunohistochemistry was performed on the brains collected 30 days after injection to examine colocalization with the RNA-binding protein neuronal marker HuC/D or the radial glial marker glial fibrillary acidic protein (GFAP). These reactions were performed as described above with the following exceptions: the quenching step was eliminated; primary antibodies were applied simultaneously (rat anti-BrdU 1:300 and either mouse monoclonal anti-HuC/HuD [16A11] 1:200, Invitrogen #A21271 or mouse monoclonal anti-GFAP [GA5] 1:300, #3670, Cell Signaling Technology, Danvers, MA); fluorescent-conjugated secondary antibodies were applied simultaneously (goat anti-mouse Alexa Fluor 488, #A11029, Invitrogen; goat anti-rat Alexa Fluor 568, #A11077, Invitrogen); and slides were mounted and coverslipped with Fluoromount-G (#0100–01, Southern Biotechnology, Birmingham, AL).
To compare cell proliferation across experimental social groups, BrdU labeling was quantified without knowledge of fish experimental group. We quantified the number of BrdU-labeled cells in the following brain regions that were classified into proliferation zones: ventral nucleus of the ventral telencephalon (Vv); anterior preoptic area (POA); hypothalamic nucleus of the lateral recess (NRL); central posterior thalamic nucleus (CP); periventricular nucleus of the posterior tuberculum (TPp); and caudal molecular layer of the corpus cerebellum (CCeM). Within each zone, the total number of BrdU-positive cells was counted on one half of the brain in each of three adjacent sections and then summed across these three sections. To obtain more accurate cell counts, we also applied a correction factor to the counts by using the following formula developed by Abercrombie (1946): Ni = ni (t/t + d), where Ni is the number of BrdU-labeled cells in section i, ni is the number of labeled cells actually counted in section i, t is the section thickness (20 μm), and d is the mean diameter of the BrdU-labeled cell nuclei measured in sections orthogonal (i.e., sagittal) to the plane of sections used for quantification (i.e., coronal).
The brain areas analyzed were photographed from each section and then the area in μm2 was measured by tracing around the quantified region within the SPOT software (Diagnostic Instruments). The quantified region was in the center of the rostrocaudal extent of each nucleus, and only the proliferation zone itself was used in the analysis, defined as a 50-μm band surrounding the ventricle that contained BrdU-labeled cells. Because the sectioned brains were alternately collected onto slides, and we only used one set of these alternates for BrdU immunohistochemistry, the adjacent 20-μm-thick sections used for quantification were 20 μm apart. To verify that the size of BrdU-labeled cell nuclei were similar among social groups, and therefore could be compared, we also measured the diameters of 10 randomly selected cells in each brain region of each individual. The overall mean diameter of BrdU-labeled cells was 5.87 ± 0.02 μm (mean ± SE). This analysis showed there was no difference in mean cell diameters in any region among subordinate, ascending, dominant, and isolated dominant males (ANOVA, P > 0.10). Furthermore, the size of the analyzed proliferation zone within each quantified brain region did not differ among social groups (ANOVAs, P > 0.10). Regions chosen for analysis within each brain area were also standardized among individuals by using neuroanatomical landmarks based on atlases from both A. burtoni (Fernald and Shelton, 1985; Burmeister et al., 2009; Munchrath and Hofmann, 2010) and other teleost fishes (Maler et al., 1991; Wulliman et al., 1996; Anken and Bourrat, 1998; Munoz-Cueto et al., 2001).
Antibody details and specificity
BrdU label was detected with a rat monoclonal BrdU antibody (#ab6326, clone number BU1/75 [ICR1], Abcam). This antibody reacts with BrdU in single-stranded DNA, BrdU attached to a protein carrier, or free BrdU, and detects nucleated cells in S-phase that have had BrdU incorporated into their DNA. In A. burtoni, positive staining was detected only in cell nuclei, similar to that described for brain BrdU labeling in other fish studies (Ekstrom et al., 2001; Zupanc et al., 2005; Dunlap et al., 2011). Negative controls included omission of primary antibody, secondary antibody, ABC solution, or DAB, and application of BrdU antiserum to tissue from non-BrdU-injected fish, all of which resulted in no staining.
GFAP is an intermediate filament protein found in astrocytes and radial glia in vertebrates (Levitt and Rakic, 1980). GFAP staining was detected with mouse monoclonal anti-GFAP (GA5; #3670, Cell Signaling Technology), produced by immunizing animals with native GFAP purified from pig spinal cord. In A. burtoni, this antibody labeled the cytoplasm of cells with the characteristic morphology and distribution of radial glial cells seen in other teleosts (Forlano et al., 2001; Zupanc and Clint, 2003; Dunlap et al., 2006) and stained a single band of the appropriate molecular weight (~50 kDa) on western blots run on protein isolates of whole male A. burtoni brains (data not shown). Omission of primary antibody or secondary antibody during immunohistochemistry resulted in no staining.
The HuC/D antiserum was mouse monoclonal anti-HuC/HuD (16A11) (#A21271, Invitrogen), produced from a synthetic peptide representing amino acids 240–251 within the carboxy-terminal domain of human HuD, which labels the Elav family of neuronal proteins, HuC, HuD, and HeI-N1. This antibody was previously shown to specifically label neuronal cells in zebrafish, chick, canaries, and humans (Marusich et al., 1994; Barami et al., 1995; Wakamatsu and Weston, 1997; Ampatzis and Dermon, 2011). In A. burtoni, it similarly only stained the cytoplasm of cells with the characteristic morphology and distribution of neurons. Omission of primary antibody or secondary antibody during immunohistochemistry resulted in no staining.
Statistical analyses
We used analysis of variance (ANOVA) to test for differences in GSI, BrdU-labeled cell diameters, and proliferation zone volumes among the four social groups, followed by post hoc pairwise comparisons. To test for differences in cell proliferation among social groups, we used ANOVA to compare BrdU-labeled cell numbers (raw and Abercrombie-corrected; uncorrected comparison), as well as analysis of covariance (ANCOVA) with analyzed proliferation zone volume as a covariate to control for the structure volume (corrected comparison), followed by post hoc pairwise comparisons. Statistical comparisons were performed with SPSS 19.0 (IBM, Armonk, NY).
RESULTS
Distribution of BrdU-labeled cells in male A. burtoni brain
To describe the overall distribution of proliferating cells in the A. burtoni brain, we used males at 1 day post BrdU injection because preliminary experiments showed there were more labeled cells at this time compared with shorter survival times of 3–4 hours (n = 4 per time point; t-tests, P < 0.05), and thus a more complete representation of all actively dividing cells was more likely depicted.
Olfactory bulbs and telencephalon
BrdU-labeled cells were sparse in the olfactory bulbs, with the majority observed in the glomerular or external cellular layer (ECL) along the outer ventricular margin; only a few scattered cells were seen in the internal cellular layer (ICL; Fig. 2A).
Figure 2.
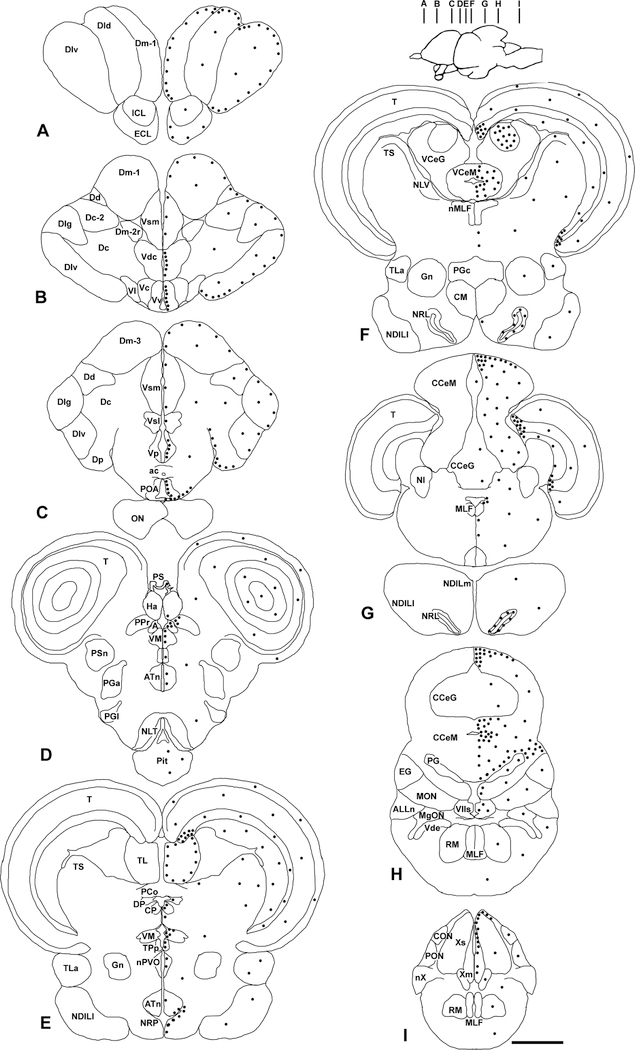
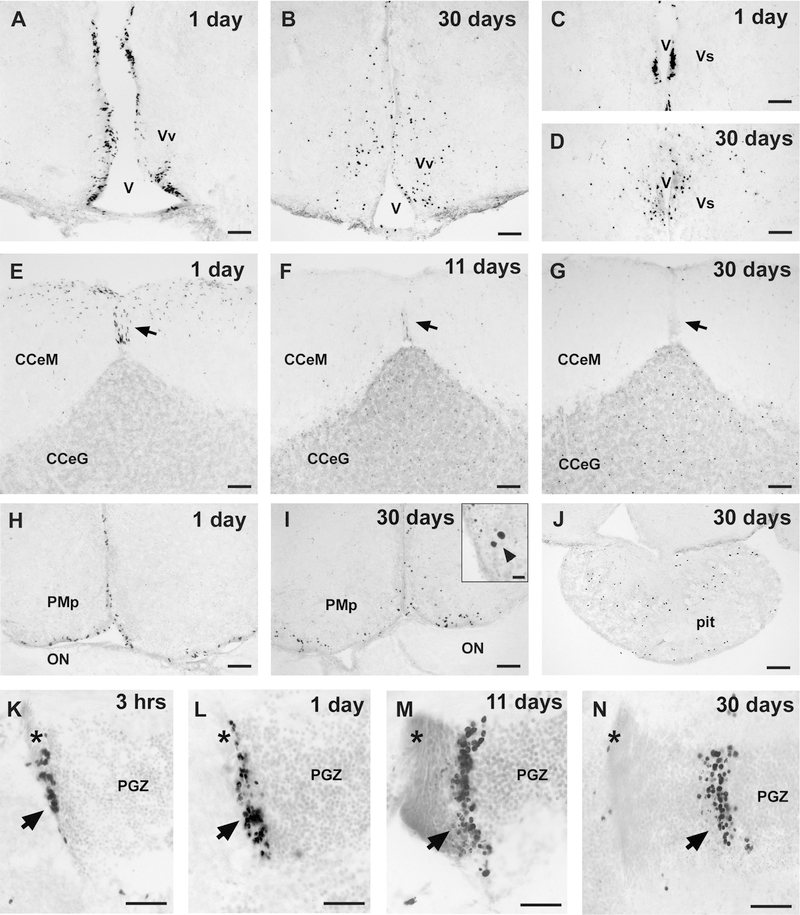
Distribution of cell proliferation zones in the brain of adult male Astatotilapia burtoni. A-I: Representative coronal sections show locations of BrdU-labeled cells (dots) after 1-day survival (on the right) and neuroanatomical labels (on the left). The number of dots is a semiquantitative representation of the relative density of BrdU-labeled cells in each region. Brain inset shows the approximate location of each section. For abbreviations, see list. Scale bar = 1 mm in A-I.
In the telencephalon, BrdU labeling was primarily associated with the ventricular surfaces of both the midline and outer brain margins (Figs. 2A–C, 3A–E). Proliferating cells were found along the ventricular surface at the lateral margin of the brain in all dorsal telencephalic nuclei (Dm, Dl, Dd, Dp), whereas only a few scattered labeled cells were observed in the deeper parenchyma of these nuclei and in the medially positioned Dc. In several locations and often at the borders of two adjacent nuclei, there were also aggregations of labeled cells (e.g., caudal pole of Dp; border of Dlv and Dld and Dlg and Dc-2; Fig. 3B).
Figure 3.
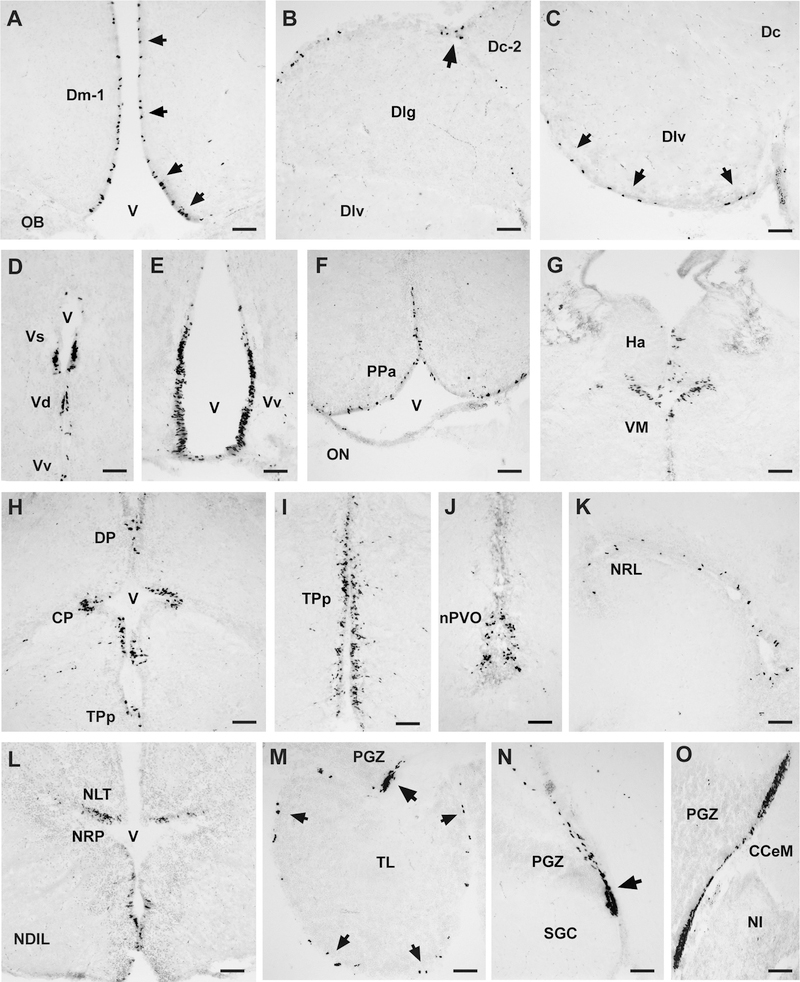
Representative photomicrographs of coronal sections show BrdU-labeled proliferating cells within the telencephalon, diencephalon, and mesencephalon of male A. burtoni. A: BrdU cells (arrows) lined the ventricular surface along the midline of the medial part of the dorsal telencephalon (Dm). B: Proliferating cells lined the marginal surface of the lateral part of the dorsal telencephalon and often clustered at the margin between Dlg and Dc-2 (arrow). C: BrdU cells along the ventral margin of the lateral part of the dorsal telencephalon (Dlv) (arrows). D: A distinct proliferation zone along the midline in the supracommissural (Vs) and dorsal (Vd) nucleus of the ventral telencephalon. E: Extensive proliferation zone along the ventricle within the ventral nucleus of the ventral telencephalon (Vv). F–J: In the diencephalon, proliferating cells were found in the anterior preoptic area (PPa) along the ventricular margin (F), in the habenula and ventromedial thalamic nucleus (G), within the dorsal (DP) and central (CP) posterior thalamic nuclei (H), posterior tuberculum (TPp; I), and paraventricular organ (nPVO; J). K,L: In the hypothalamus, proliferating cells were abundant along the nucleus of the lateral recess (K) and posterior recess (L). M: BrdU cells (arrows) lined the ventricular margin of the torus longitudinalis (TL). A distinct zone of clustered proliferating cells was also found in the dorsal periventricular granular zone (PGZ) of the tectum (arrow). Midline is to the right. N: The ventral portion of the proliferation zone associated with the caudal PGZ (arrow). O: Region in the caudal tectum where the dorsal and ventral portions of the tectal proliferation zones connect via a thin ependymal lining. Examples are from male brains at 1 day post BrdU injection and were counterstained with cresyl violet. For abbreviations, see list. Scale bar = 50 μm in A–O.
In the ventral telencephalon, BrdU-labeled cells were abundant along the midline ventricle in the ventral subdivision of the ventral telencephalon (Vv; Figs. 2B, 3E). More caudally, a distinct aggregation of proliferating cells was also observed in a furrow within Vd/Vs (Fig. 3D). Labeled cells were present in all other ventral telencephalic nuclei (Vs, Vp, Vc, Vl), but were scattered and appeared fewer in number as the distance from the ventricular surface increased.
Diencephalon
Similar to the telencephalon, BrdU-labeled cells in the diencephalon were primarily concentrated at the ventricular surfaces (Fig. 2C,D). In the preoptic area, proliferating cells were abundant along the floor of the preoptic recess and extended laterally in the region above the optic chiasm (Figs. 2C, 3F). Outside of this proliferation zone, low to modest numbers of BrdU-labeled cells were found scattered in all nuclei of the preoptic area including the anterior and posterior periventricular preoptic nuclei, parvocellular and magnocellular parts of the magnocellular preoptic nuclei, and suprachiasmatic nucleus.
The ventricular surface along the midline of the pretectal and thalamic regions contained several conspicuous proliferation zones with clustered BrdU-labeled cells that were both distinct and continuous at different points and often spanned multiple brain nuclei (Figs. 2D,E, 3G–J). In the dorsal pretectum and thalamus, numerous labeled cells were found in the rostral periventricular pretectal nucleus (PPr), dorsal posterior nucleus (DP), central posterior nucleus (CP), and anterior thalamic nucleus (A; Fig. 3G,H). Proliferating cells were also present in the ventral habenular nucleus and just beneath the habenula in the nucleus intermedius (I) and eminentia thalami (ET). Sparse numbers of BrdU cells were also observed in the dorsal habenular nucleus in some individuals, and scattered labeled cells occurred in the pineal stalk and body.
In the ventral thalamus, BrdU-labeled cells were concentrated along the ventricular midline within the ventromedial thalamic nucleus (VM), and some scattered cells were observed in the nucleus of the paraventricular organ (nPVO; Fig. 3J). A conspicuous proliferation zone in this region is also located along the ventricular midline in the periventricular nucleus of the posterior tuberculum (TPp; Fig. 3I). This proliferation zone extends throughout the TPp in both rostrocaudal and dorsoventral directions.
The hypothalamus contained an extensive and complex proliferation zone that essentially lined the paraventricular recesses throughout this brain region. Proliferating cells were moderate to abundant in the nucleus of the lateral recess (NRL) along its entire rostrocaudal length (Figs. 2F,G, 3K), and within all other dorsal and ventral hypothalamic nuclei associated with these recesses including the nucleus of the posterior recess (NRP), lateral tuberal nucleus (NLT), posterior tuberal nucleus (NPT), anterior tuberal nucleus (ATn), and ventral tuberal nucleus (VTn). Only a few scattered cells were located away from these ventricular regions in the hypothalamus including the various subdivisions of the inferior lobe (Fig. 2E–G). The pituitary gland also contained moderate numbers of BrdU-labeled cells throughout, which did not appear to be localized to any specific regional compartment (Fig. 2D).
Mesencephalon
One of the most conspicuous cell proliferation zones in the entire brain was located in the midbrain, and was composed of two distinct regions of densely clustered cells associated with the periventricular gray zone (PGZ) of the tectum. One region was located dorsally in the peripheral growth zone of the tectum and extended along the border between the torus longitudinalis and the PGZ of the tectum (Figs. 2E–G, 3M). The second region was located more ventral and caudal within the PGZ and was associated with the ependymal region that connects the ventrocaudal pole of the tectum with the torus semicircularis (Figs. 2F,G,; 3N,O). In the most caudal sections, these two proliferation zones were continuous and connected via the thin ependymal fold between the tectum and caudal TS, nucleus isthmi (NI), and tractus tectobulbaris (TTB). There were also scattered BrdU-labeled cells throughout all tectal layers including the superficial, periventricular, and deep layers. In the torus longitudinalis (TL), BrdU-labeled cells were located along the entire lateral ventricular border throughout its rostrocaudal extent (Figs. 2E, 3M). Only a few scattered labeled cells were found within the torus semicircularis, primarily at the ventricular surface, but some were also localized to deeper levels within both the dorsal and ventral TS subdivisions. Occasional scattered BrdU-labeled nuclei were also observed in other midbrain regions including the glomerular nucleus (Gn), torus lateralis (TLa), and tegmentum (Fig. 2E,F).
Rhombencephalon
In the medulla oblongata, proliferating cells were seen scattered throughout many nuclei, but the most prominent zone was located in the vagal lobe (VL) where labeled cells were concentrated along the dorsomedial ventricular surface throughout its rostrocaudal extent (Fig. 2I). Sparse to moderate label was also observed around the fourth ventricle and in the vagal motor nucleus, vagal sensory nucleus, and facial sensory nucleus. There were also a few BrdU-labeled cells observed within all octavolateralis nuclei in the hindbrain (AON, MON, MgON, TON, DON, CON, PON), subdivisions of the reticular formation (RF), cerebellar crest, and all cranial nerve nuclei, and within the cranial nerves themselves as they entered the brainstem (Fig. 2G–I).
The cerebellum contained the most extensive proliferation zones in the brain, and included large aggregations of labeled cells in the two main subdivisions, the valvula cerebelli and corpus cerebelli, and to a lesser extent in the third subdivision, the eminentia granularis (Figs. 2F–H, 4). In the valvula cerebelli, BrdU-labeled cells were concentrated in the molecular layer of both the pars lateralis (Fig. 4A) and pars medialis (Fig. 4B), especially along the midline of each subdivision where proliferating cells radiated outward from the center in both dorsoventral and mediolateral directions. Only a few scattered cells were observed in the granule cell layer of the valvula cerebelli at the 1-day time point.
Figure 4.
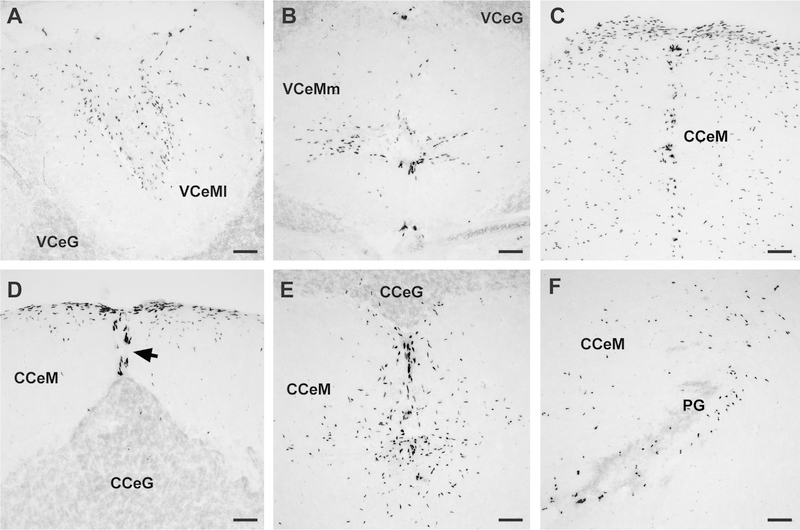
Representative photomicrographs of coronal sections show BrdU-labeled proliferating cells within the cerebellum of male A. burtoni. A,B: Abundant BrdU cells were found in both the lateral (A) (VCeMl) and medial (B) (VCeMm) regions of the valvula cerebelli. C: Large proliferation zone in the molecular layer of the rostral corpus cerebellum (CCeM). D: Elongated BrdU-labeled cells (arrow) along the midline of the CCeM from the tip of the granule cell layer (CCeG) to the dorsal brain margin in the caudal region of the corpus cerebellum. E: Large proliferation zone at the midline in the caudal CCeM below the CCeG. F: Proliferating cells in the lateral CCeM around the periventricular granular cell mass of the caudal cerebellar lobe (PG). Examples are from male brains at 1 day post BrdU injection and were counterstained with cresyl violet. For abbreviations, see list. Scale bar = 50 μm in A-F.
In the corpus cerebelli, numerous BrdU-labeled cells were distributed in a large proliferation zone primarily in the dorsal molecular layer (Figs. 2G,H, 4C–F). This zone was characterized by aggregations of labeled cells with elongated nuclei concentrated along the midline from the outer dorsal surface of the brain to the top of the granule cell layer (Fig. 4D). This zone extended bilaterally along the dorsal surface of the brain, and the number of labeled cells decreased with distance away from the midline. Within this corpus proliferation zone, labeled cells were abundant in both rostral and caudal regions of the molecular layer (Fig. 4C,E). The stretch of molecular layer in the caudal cerebellar lobe that is located above the ventricle and extends laterally from the periventricular granular cell mass of the caudal lobe (PG) out to the EG was also a significant proliferation zone (Fig. 4F). Only very few scattered cells were observed in the granule cell layer of the corpus cerebelli throughout its rostrocaudal extent. In the eminentia granularis (EG), BrdU-labeled cells were few in number at rostral locations, but appeared more abundant in caudal regions.
Distribution of BrdU-labeled cells at different survival times
To analyze the migration patterns of proliferating cells in the A. burtoni brain, we examined dominant male individuals at post-BrdU injection survival times of 3 hours, 1 day, 11 days, and 30 days (Fig. 5). In general, the number of BrdU-labeled cells was most abundant at the 1-day survival time, with fewer cells observed at 3 hours (n = 4 per time point in CCe, Vv, and TPp; t-tests, P < 0.05). Although they were not quantified, there appeared to be fewer labeled cells overall at 11 and 30 days, but many BrdU+ cells had migrated away from their original ventricular proliferation zones into the brain parenchyma at these later time points (Fig. 5). At the later survival times, there was also a combination of intensely labeled cells as well as more faintly labeled cells throughout the brain, which is likely due to dilution of the BrdU label with successive divisions. The rate at which newborn cells migrated away from proliferation zones also differed among brain regions, ranging from slow migration of distances equivalent to only a few cell diameters in many forebrain regions, to more rapid migration of tens to hundreds of microns in regions of the cerebellum. Many BrdU-labeled cell nuclei also remained in or near proliferation zones with little evidence of migration, which also varied among brain regions (see below).
Figure 5.
Representative photomicrographs of coronal sections from male A. burtoni brains at different time points after BrdU injection show the survival and migration of labeled cells from the ventricular proliferation zones. A: BrdU cells are abundant around the ventricle (V) in the ventral nucleus of the ventral telencephalon (Vv) at 1 day. B: At 30 days post BrdU injection, many labeled cells in Vv have migrated into the brain parenchyma. C: Small proliferation zone in the region of Vs and Vd at 1 day. D: Migration of BrdU-labeled cells away from the ventricle into Vs at 30 days. E–G: Proliferating cells in the corpus cerebellum are concentrated in the molecular layer (CCeM) at 1 day (E), with a distinct zone of elongated nuclei along the midline from the tip of the granule cell layer (CCeG) to the dorsal margin of the brain (arrow). By 11 days (F), the new cells have migrated into the CCeG, with only a few remaining at the midline zone (arrow), and by 30 days (G), BrdU cells are homogeneously distributed within the CCeG, with few to no cells remaining in the CCeM. H: Proliferating cells in the preoptic area line the ventral and midline ventricular surface at 1 day. I: By 30 days, many cells have migrated into the parenchyma of the preoptic area, and labeled nuclei of different diameters were common. Inset: Large BrdU-labeled nuclei (arrowhead) in the magnocellular region of the preoptic area. J: BrdU-labeled cells are scattered throughout the pituitary gland at 30 days post survival. K–N: BrdU-labeled cells in the dorsomedial mitotic region of the PGZ (arrow) after different survival times: 3 hours (K), 1 day (L), 11 days (M), and 30 days (N). Asterisk marks the dorsal portion of the PGZ mitotic zone at the same approximate location in each micrograph to illustrate the lateral migration of the proliferating cells (midline is to the left in K–N). Sections were counterstained with cresyl violet. For abbreviations, see list. Scale bar = 50 μm in A–N; 20 μm in inset in I.
In the telencephalon and diencephalon, BrdU-labeled cells at the early survival times (3 hours and 1 day) were primarily located along the ventricular surfaces, whereas after longer survival times (11 and 30 days) the cells were located in these same regions but many had also migrated deeper into the parenchyma of nearby nuclei (Fig. 5). Proliferating cells in the preoptic area also migrated away from the ventricular surface, and there were often cells with very large BrdU-labeled nuclei (~10–20 μm in diameter) seen in the PPa and magnocelluar region of the preoptic nucleus (PMn) after 11 and 30 days (Fig. 5I, inset). The pituitary had scattered BrdU cells distributed throughout that appeared similar in number between the short and long survival times (Fig. 5J), but this was not quantified. Interestingly, the thalamic and TPp ventricular regions of the diencephalon were some of the most conspicuous proliferation areas at 1 day, where lateral migration from the ventricle was already apparent (Fig. 6A), but this region contained only a few scattered BrdU-labeled cells at 11 and 30 days. In both the dorso-medial and ventrocaudal PGZ proliferation zones of the tectum, the BrdU cells migrated laterally within the PGZ as a distinct band (Fig. 5K–N), and there were often scattered cells that migrated to the more superficial tectal zones directly above the PGZ.
Figure 6.
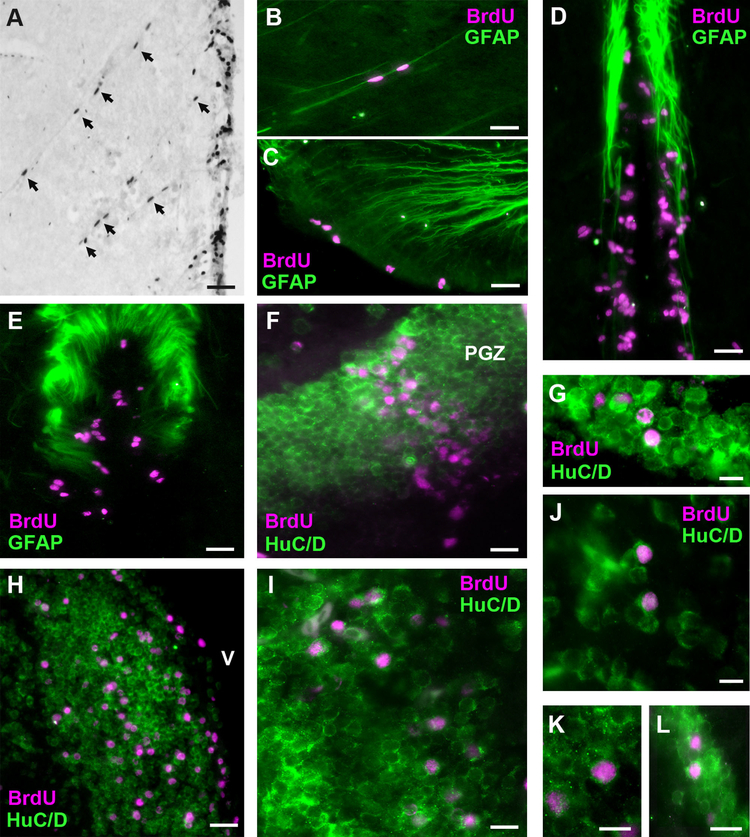
Migration and differentiation of BrdU-labeled cells in the brain of male A. burtoni. A: Elongated BrdU-labeled nuclei (arrows) migrating away from the midline ventricular proliferation zone (on right) in the periventricular nucleus of the posterior tuberculum (TPp) at 1 day post injection. B: GFAP-labeled radial glial fiber guiding BrdU cells in the TPp. C: GFAP radial glial fibers extending to the proliferation zone in the preoptic recess of the PPa. D: GFAP-labeled glial fibers along the midline near the diencephalic proliferation zone in the region of TPp. E: GFAP fibers near BrdU-labeled cells in the NRP of the hypothalamus. F: BrdU-labeled cells in the periventricular gray zone (PGZ) of the dorsal tectum are colabeled with the neuronal marker HuC/D. G: Proliferating cells that express HuC/D in the ventromedial thalamic nucleus. H: Double-label of BrdU-labeled cells and HuC/D in the ventral nucleus of the ventral telencephalon (Vv) along the midline ventricle (V). I: Example of HuC/D-expressing BrdU-labeled cells in the preoptic area. J–K: Higher magnifications of BrdU-labeled cells colabeled with HuC/D in the lateral part of the dorsal telencephalon (Dl) (J), preoptic area (K), and vagal lobe of the rhombencephalon (L). Representative photomicrographs in B–L are of coronal sections at 30 days post BrdU injection to show expression of BrdU-labeled cells (magenta) with the radial glial marker GFAP (green; B–E) or the neuronal marker HuC/D (green; F–L). For abbreviations, see list. Scale bar = 50 μm in A,C–F,H,I; 20 μm in B,G,J–L.
In all subdivisions of the cerebellum (valvula cerebelli, corpus cerebelli, eminentia granularis), proliferating cells were abundant in the molecular layer at 3 hours and 1 day, but by 11 days, the majority of BrdU cells had migrated into the granule cell layer and by 30 days were uniformly distributed throughout the granule layer with few cells seen in the molecular layer (Fig. 5E–G). At the earlier survival times (3 hours and 1 day), the BrdU-labeled cell nuclei in the molecular layer were primarily elongated (long axis ~7–10 μm in diameter), whereas cell nuclei at the later times of 11 and 30 days were small (~2–3 μm in diameter) and spherical once they migrated into the granule cell layer (Fig. 5E–G).
Fate of proliferating cells
To examine the fate of the new cells, we performed double-label immunohistochemistry to colocalize BrdU with either HuC/D (RNA-binding proteins primarily expressed in neurons) or GFAP (an intermediate filament protein expressed in radial glial cells and astrocytes) at the 30-day post-survival time (Fig. 6). We saw no evidence for colocalization of BrdU within GFAP-labeled radial glial cells in any individual. However, GFAP-stained radial glial processes were located near BrdU-labeled cells and proliferation zones (Fig. 6B–E). Furthermore, there were often BrdU cells with elongated nuclei that appeared to be guided by radial glial fibers in as little as 1 day post injection, especially in the TPp and thalamic proliferation regions (Fig. 6A,B).
Many cells were colabeled with BrdU and the neuronal marker HuC/D, and examples of this colocalization were evident throughout the brain in many different nuclei from the telencephalon to the rhombencephalon (Fig. 6F–L). Specifically, double-labeled cells were observed in nuclei of the dorsal and ventral telencephalon (Fig. 6H,J), preoptic area (Fig. 6I; 6K), thalamic and habenular nuclei (Fig. 6G), posterior tuberculum, hypothalamic nuclei, tectum, and hindbrain vagal lobe (Fig. 6L). The HuC/D antibody did not label granule cells in the corpus or valvula cerebelli, similar to that observed in the zebrafish (Zupanc et al., 2005), so we are unable to comment on whether the BrdU-labeled cells distributed throughout these layers were in fact granule neurons, although they did show the morphology and localization characteristic of granule neurons. Similarly, anti-HuC/D did not label cells in the ICL of the olfactory bulbs, so the fate of BrdU-labeled cells in this brain region is also unknown. There were also many BrdU-labeled cells throughout the brain that were not colabeled with either GFAP or HuC/D at the 30-day post-injection time, and these even occurred within regions of abundant HuC/D-stained neurons.
Effects of social status and environment on cell proliferation
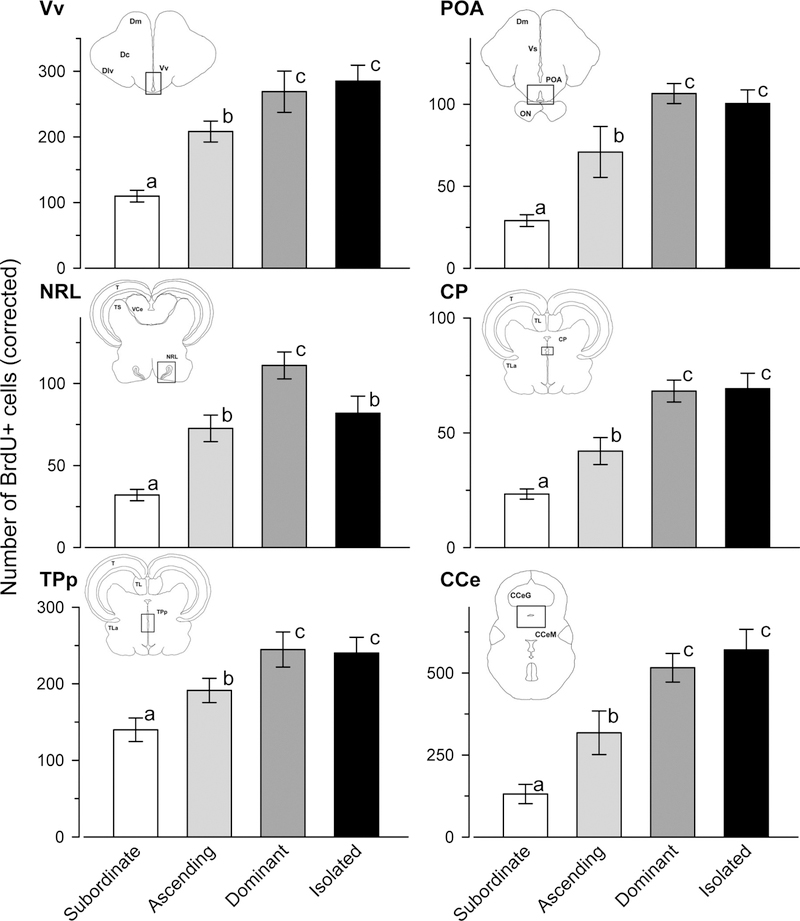
The main proliferation zones described above were present in the brain of every individual from all social groups, but subordinate males had the lowest numbers of proliferating cells in all brain regions examined compared with the other social groups when raw or Abercrombie-corrected counts were analyzed (ANOVA, uncorrected), as well as when the effects of structure volume were accounted for with ANCOVA (corrected; Figs. 7, 8; Table 1). Mean cell numbers and the volume of each proliferation zone analyzed across social groups are presented in Table 1, whereas Abercrombie-corrected cell counts are plotted in Figure 7. In the Vv, ascending males had more proliferating cells compared with subordinate males, but dominant and isolated dominant males had more cells than both subordinate and ascending animals (ANCOVA, F(3,28) = 19.56, P < 0.001; ANOVA, F(3,28) = 9.73, P < 0.001) (Figs. 7, 8). In the anterior preoptic area, ascending males had more proliferating cells compared with subordinate males, and both had fewer proliferating cells than dominant and isolated dominant males (ANCOVA, F(3,28) = 12.35, P < 0.001; ANOVA, F(3,28) = 13.67, P < 0.001).
Figure 7.
Social status influences cell proliferation in the brain of male A. burtoni. Males ascending in social status had higher cell proliferation compared with socially subordinate males. Furthermore, dominant males in a social environment had BrdU-labeled cell numbers similar to those that were socially isolated. Data are plotted as the number of BrdU-labeled cells (with Abercrombie correction factor; mean ± SEM) based on quantification from cell proliferation zones within each brain region (see Materials and Methods). Insets show brain coronal sections to indicate the location of quantified regions (boxed). CCe, corpus cerebellum; CP, central posterior thalamic nucleus; NRL, nucleus of the lateral recess; POA, preoptic area; TPp, periventricular nucleus of the posterior tuberculum; Vv, ventral nucleus of the ventral telencephalon. For other abbreviations, see list. Sample sizes are: subordinate males (n = 9), ascending males (n = 7), socially dominant males (n = 9), isolated dominant males (n = 8). Different letters indicate statistical differences among groups at P < 0.05 from the ANCOVA analysis of cell numbers with analyzed proliferation zone volume as a covariate followed by pairwise comparisons.
Figure 8.
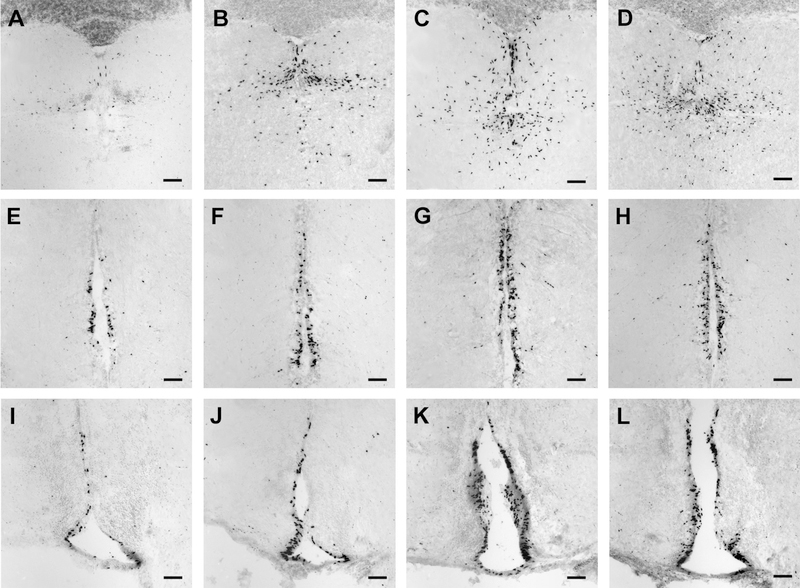
Relative number of BrdU-labeled cells varies with social status in the brain of male A. burtoni. Representative photomicrographs of coronal sections in the cerebellum (A–D), periventricular nucleus of the posterior tuberculum (E–H), and ventral nucleus of the ventral telencephalon (I–L) illustrate the relative differences in the total number of BrdU-labeled cell nuclei (black label) among subordinate (A,E,I), ascending (B,F,J), dominant (C,G,K), and isolated dominant males (D,H,L). Sections were counterstained with cresyl violet. Scale bar = 50 μm in A–L.
TABLE 1.
Summary of the Mean Number of BrdU-Labeled Cells and Total Volume Analyzed for Proliferation Zones in Each Brain Region Across Social Groups in Male Astatotilapia burtoni1
| Vv |
POA |
NRL |
Cp |
TPp |
CCe |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cells | Volume (mm3) | No. of cells | Volume (mm3) | No. of cells | Volume (mm3) | No. of cells | Volume (mm3) | No. of cells | Volume (mm3) | No. of cells | Volume (mm3) | |
| Subordinate (n = 9) | 120.9 (±9.8) | 0.0024 (±0.0001) | 31.8 (±3.9) | 0.0018 (±0.0001) | 35.1 (±3.8) | 0.0228 (±0.003) | 25.5 (±2.4) | 0.0007 (±0.0001) | 153.2 (±16.9) | 0.0024 (±0.0005) | 145.6 (±32.5) | 0.0165 (±0.002) |
| Ascending (n = 7) | 229.1 (±39.4) | 0.0023 (±0.0002) | 77.4 (±16.9) | 0.0021 (±0.0001) | 79.7 (±24.2) | 0.0180 (±0.003) | 46.0 (±6.4) | 0.0007 (±0.0001) | 209.4 (±17.3) | 0.0017 (±0.0001) | 352.4 (±73.7) | 0.0134 (±0.003) |
| Dominant (n = 9) | 296.0 (±34.6) | 0.0023 (±0.0002) | 116.3 (±6.6) | 0.0020 (±0.0001) | 110.8 (±9.0) | 0.0214 (±0.003) | 74.5 (±5.2) | 0.0007 (±0.00005) | 268.0 (±30.5) | 0.0017 (±0.0001) | 572.5 (±48.5) | 0.0147 (±0.001) |
| Isolated (n = 8) | 313.6 (±25.2) | 0.0025 (±0.0002) | 109.8 (±13.6) | 0.0021 (±0.0001) | 94.3 (±10.6) | 0.0223 (±0.002) | 75.8 (±6.9) | 0.0007 (±0.00007) | 263.1 (±21.1) | 0.0019 (±0.0001) | 633.1 (±65.6) | 0.0151 (±0.001) |
Data are expressed as mean (±SE). For abbreviations, see list.
In the hypothalamic NRL, the number of BrdU-labeled cells was higher in ascending compared with subordinate males, and these ascended males had counts that did not differ from isolated dominant males but were lower than those of socially exposed dominant males (ANCOVA, F(3,28) = 8.39, P < 0.001; ANOVA, F(3,28) = 7.06, P = 0.001). In the thalamic CP, ascending males had more proliferating cells than subordinate animals, whereas dominant and isolated males had the highest levels (ANCOVA, F(3,28) = 22.02, P < 0.001; ANOVA, F(3,28) = 20.75, P < 0.001). In the TPp, ascending males also had higher BrdU+ cell numbers compared with subordinates, but thesewere lower than dominant and isolated male levels (ANCOVA, F(3,28) = 10.86, P < 0.001; ANOVA, F(3,28) = 5.73, P = 0.003). In the caudal CCe, ascending males had higher BrdU+ cell numbers compared with subordinates, but the highest values were found in both dominant and isolated dominant animals (ANCOVA, F(3,28) = 22.58, P < 0.001; ANOVA, F(3,28) = 16.62, P < 0.001; Figs. 7, 8).
DISCUSSION
The African cichlid fish Astatotilapia burtoni has extensive cell proliferation zones throughout the brain similar to those found in other teleost fishes, and many of the new cells that survive after 4 weeks have differentiated into neurons. We also show that the social environment influences cell proliferation in multiple brain regions, and that cell proliferation is rapidly (within hours) increased in males that rise in social rank. To our knowledge, this is the first study to show such rapid changes in brain cell proliferation due to a shift in social status, and provides important insights about how an animal’s social environment can influence structural plasticity in the brain.
Distribution of proliferation zones
The overall distribution of BrdU-labeled cells in the brain of A. burtoni was similar to that described in other fishes including the zebrafish (Grandel et al., 2006; Zupanc et al., 2005), medaka (Kuroyanagi et al., 2010), brown ghost knifefish (Zupanc and Horschke, 1995), three-spined stickleback (Ekstrom et al., 2001), rivulus (Fernandez et al., 2011), and gilthead seabream (Zikopoulos et al., 2000). Although this is the first study to describe proliferation zones in a cichlid fish, the similar distribution suggests strong evolutionary conservation of proliferation zones near ventricular surfaces in vertebrates generally, and specifically among these fish species, despite their dramatic differences in behavior, ecology, and phylogenetic relationships. The presence of abundant proliferating cells in the teleost homologs of the main mammalian constitutive proliferation zones, ventricular and subventricular telencephalic zones (homologous in part to ventricular regions of the teleost pallial and sub-pallial telencephalon) and dentate gyrus of hippocampus (homologous in part to the teleost lateral part of the dorsal telencephalon, Dl) in A. burtoni and other fishes further supports the proposed hypothesis that production of new neurons in these regions is a conserved trait across all vertebrates (Chapouton et al., 2007; Kaslin et al., 2008; Zupanc, 2008).
Although the neuroanatomical position of proliferation zones in A. burtoni was similar to that of other described fishes, most notably the stickleback (Ekstrom et al., 2001), medaka (Kuroyanagi et al., 2010), sea bream (Zikopoulos et al., 2000), and zebrafish (Grandel et al., 2006), some small differences were also apparent. For example, similar to medaka and stickleback (Ekstrom et al., 2001; Kuroyanagi et al., 2010), two marginal well-defined proliferation zones were found in the tectum of A. burtoni, but this differs from zebrafish and the brown ghost, in which new cells are primarily generated at the caudal PGZ (Zupanc and Horschke, 1995; Zupanc et al., 2005). Furthermore, we observed scattered BrdU-labeled cells throughout most regions of the brain that were not located in proliferation zones, a phenomenon also described for the brown ghost (Zupanc and Horschke, 1995), but not the stickleback (Ekstrom et al., 2001). A. burtoni also showed little evidence of cell proliferation in the eminentia granularis at the 1-day survival time, similar to zebrafish and stickleback (Ekstrom et al., 2001; Grandel et al., 2006), but different from the brown ghost, which showed abundant proliferation in this cerebellar subdivision (Zupanc and Horschke, 1995). The significance of any of these relatively small variations is unknown, but may result from species-specific differences in behavior, ecology, habitat, sensory inputs, or phylogeny, and further study is needed to test whether they contribute to adaptations in neuronal function.
Distribution, migration, and fate of BrdU-labeled cells
In A. burtoni, we saw no evidence of BrdU labeling in GFAP-positive radial glial cells, but most proliferation zones were close to regions of dense radial glial processes and there was evidence of these glial fibers presumably guiding BrdU-labeled cells away from proliferation zones toward their target locations. BrdU-labeled cells also did not coexpress GFAP in the zebrafish (Zupanc et al., 2005), and BrdU/GFAP cells were infrequent in the brain of rivulus fish (Fernandez et al., 2011). Guidance of newborn cells by radial glial fibers, however, is more common across vertebrates and was also described in the brain of the brown ghost knifefish (Apteronotus leptorhynchus) (Zupanc and Clint, 2003; Zupanc, 2008) and zebrafish (Ampatzis and Dermon, 2007). In A. leptorhynchus, social interactions also increased the density of radial glia fibers in the periventricular nucleus of the diencephalon, which may facilitate migration of newborn cells into the nearby electro-communication circuitry used for reproduction (Dunlap et al., 2006). The high density of GFAP-containing radial glial fibers near most proliferation zones in A. burtoni suggests an important role in guiding these newborn cells to their designated locations. Future studies are needed, however, to test whether glial fiber density may also be influenced by the social environment in A. burtoni.
In contrast to the lack of BrdU/GFAP cells in A. burtoni, there were many examples of BrdU+ cells colabeled with the neuronal marker HuC/D throughout the brain at 30 days post injection, suggesting they had differentiated into neurons. Similarly, some newborn cells also expressed HuC/D protein in zebrafish after long survival times (weeks to months) (Zupanc et al., 2005; Grandel et al., 2006; Ampatzis and Dermon, 2007). In the rivulus fishes (Austrolebias spp.), the neuronal marker HuC/D was colocalized to BrdU-labeled cells in several brain areas as early as 24 hours after BrdU injection, demonstrating rapid neurogenesis in these species that have a short annual lifespan (Fernandez et al., 2011). There is also increasing evidence that neurons develop from progenitor radial glial cells in several regions of the teleost brain (Pellegrini et al., 2007; Ito et al., 2010; Strobl-Mazzulla et al., 2010). In the zebrafish Danio rerio, for example, new cells in the PGZ mitotic zone of the tectum possess characteristics of neural stem/progenitor cells and contribute to both neuronal (glutamatergic and γ-aminobutyric acid [GABA]ergic neurons) and glial (oligodendrocytes and radial glia) cell lineages to maintain tectal structure (Ito et al., 2010), and aromatase-positive radial glial cells were shown to be neuron progenitors in the forebrain ventricular zone (Pellegrini et al., 2007). The cellular phenotype of BrdU-labeled cells throughout the A. burtoni brain that did not coexpress GFAP or HuC/D at 30 days is unknown, but longer survival times and additional cell markers may help resolve this issue. Future studies should also test how the social environment might influence the regulation of cell numbers by apoptosis or other cell death/survival mechanisms, because high cell proliferation in the brain is often also correlated with regions of high apoptosis (Soutschek and Zupanc, 1996; Ampatzis and Dermon, 2007).
Effects of social status and environment on cell proliferation
Our results comparing brain cell proliferation among fish in different social conditions demonstrate that 1) subordinate socially suppressed males had low numbers of proliferating cells compared with all other social groups; 2) males given an opportunity to ascend in social rank rapidly showed higher BrdU+ cell numbers compared with subordinate males; and 3) dominant males that were socially isolated had similar BrdU+ cell numbers to dominant males that were housed in socially interactive groups. These results are discussed below in relation to what we know about the behavior, physiology, and natural history of A. burtoni.
Subordinate male A. burtoni had fewer proliferating cells in the brain compared with all other groups, suggesting that chronic social stress inhibits cell proliferation similar to that seen across vertebrates (Kozorovitskiy and Gould, 2004; Yap et al., 2006; Barnea, 2009; Sorensen et al., 2011). Reduction of neurogenesis in response to social stress is thought to have evolved as an adaptive response, or coping strategy, that is part of the behavioral and physiological response to subordination. This response serves to signal to the dominant animal that the subordinate is no longer a threat, ultimately increasing survival and future reproductive opportunities for the lower ranking individual (Matsumura and Hayden, 2006). Because the subordinate males in our study all started out as dominant individuals prior to the 4–5-week suppression period, the low levels of cell proliferation were more likely caused by the chronic social stress rather than by some pre-existing or genetic differences among individuals. Also, the observation that socially isolated dominant males have similar cell proliferation to those in socially interactive environments suggests that the lower proliferation seen in subordinate males is specifically due to the social subordination, and not merely a result of a socially stressful situation.
Subordinate male A. burtoni are physically prevented from holding a territory by aggressive attacks from dominant males, and have a suppressed reproductive axis that includes small GnRH1 neurons in the brain (Davis and Fernald, 1990), low levels of circulating gonadal steroids (Parikh et al., 2006; Maruska and Fernald, 2010b), and reduced testes size (Fraley and Fernald, 1982; Maruska and Fernald, 2011a). Despite this reproductive suppression, however, subordinate males often have higher somatic growth rates that may allow them to quickly attain a larger size so they are more likely to gain a territory and reproductive opportunities in the future (Hofmann et al., 1999). In contrast to somatic growth, our results here indicate that neuronal growth in the metabolically demanding brain remains suppressed during social subordination, but can be quickly stimulated upon social opportunity (see below).
Male A. burtoni that were given an opportunity to acquire a territory and become dominant had higher cell proliferation throughout the brain compared with suppressed subordinate males. Previous studies have shown that the metabolic availability of BrdU is approximately 2–4 hours (Takahashi et al., 1993; Zupanc and Horschke, 1995), suggesting that cell proliferation in ascending males (injected immediately before given a social opportunity) increases within this short time period. This rapid increase in brain cell proliferation is also coupled with rapid changes in behavior and physiology at every level of the hypothalamic-pituitary-gonadal (HPG) axis (Burmeister et al., 2005; Maruska and Fernald, 2010a; 2011a,b; Kustan et al., 2011; Maruska et al., 2011), which may serve to accommodate the increased neural and cognitive demands associated with the transition to dominance. For example, subordinate males spend most of their time fleeing the attacks of dominant males, whereas a socially transitioned male must now defend and maintain a territory from intruders and court and spawn with females, a switch that includes the addition of over a dozen different behaviors to his repertoire (Fernald, 1977). In a study in ring doves, neurogenesis in the ventromedial hypothalamic nucleus was not necessary for expression of normal courtship behaviors, but was required for the recovery of courtship behaviors after lesions of this nucleus, suggesting that newborn cells are important for behavioral recovery (Chen et al., 2006; Chen and Cheng, 2007). This behavioral recovery may be similar to reacquisition of the complex dominance behavioral repertoire that occurs in ascending male A. burtoni, necessitating the quick production of new brain cells.
In addition to the dramatic behavioral transformation, the most significant physiological changes for these newly ascended A. burtoni males is the rapid upregulation of the HPG reproductive axis (Burmeister et al., 2005; Maruska and Fernald, 2010a, 2011a,b; Kustan et al., 2011; Maruska et al., 2011). Neurogenesis is extremely important for reproductive function in mammals, as evidenced by stimulation of cell proliferation by reproductive-related signals involved in courtship and mating (e.g., pheromones; Hawken et al., 2009; Lau et al., 2011; Mak et al., 2007), and our results now suggest that a similar phenomenon may also occur in fishes. Interestingly, estrogens are well known for influencing cell proliferation, migration, and differentiation of newborn brain cells in mammals (Fowler et al., 2008), and we know that estrogen receptors in A. burtoni are concentrated in the same regions described here as proliferation zones (Munchrath and Hofmann, 2010), and that ascending and dominant males have higher circulating levels of 17β-estradiol compared with subordinate animals (Maruska and Fernald, 2010b; K.P. Maruska, unpublished observations). Thus, it is possible that estrogenic pathways may contribute to the socially induced cell proliferation patterns observed here, but this hypothesis requires further study.
Cell proliferation and neurogenesis have also recently been described as a necessary component of learning and memory in several taxa (Castilla-Ortega et al., 2011; Nogues et al., 2011). Because the males used in our study had all previously been dominant, and this species can reversibly switch between social states depending on the composition of the social environment, the ascension to dominance also likely involves neuronal circuitry involved in learning and memory, and reward pathways. In fact, it has been suggested that newly generated neurons in the hippocampus of mammals possess unique characteristics that facilitate synaptic plasticity and associative long-term potentiation to promote memory formation and retrieval (Schmidt-Hieber et al., 2004; Mongiat et al., 2009). Although we did not quantify cell proliferation in the teleost homolog of the hippocampus for technical reasons, other regions of the brain that we did examine, such as the cerebellum and ventral nucleus of the ventral telencephalon (lateral septum homolog), are also implicated in social and emotional learning and memory, which is relevant to ascending males (Rodriguez et al., 2005; Salas et al., 2006; Engelmann, 2008). Future analyses of the cell fate, migration patterns, and neuronal survival of new cells in ascending male A. burtoni should provide further insight into the significance of this higher cell proliferation and which brain regions and neural circuits might receive the new cells during social transition. It is also important to note that the higher cell proliferation at social opportunity could be controlled by stimulation of proliferation, removal of inhibition, or some combination of both, potentially regulated by epigenetic or microRNA-mediated mechanisms (Kim and Rosenfeld, 2010; Shi et al., 2010). Nevertheless, the higher level of cell proliferation in ascending males further highlights the dramatic and rapid neural plasticity that can be induced solely by the perception of a social opportunity.
Another intriguing result of our study was that socially isolated dominant male A. burtoni had high levels of cell proliferation throughout the brain that did not differ from dominant males housed in a typical socially rich environment. This differs from many other studies in mammals (Fowler et al., 2002; Rizzi et al., 2007), birds (Barnea, 2009), and fishes (Dunlap et al., 2006, 2011), in which social isolation is often associated with reduced cell proliferation in the brain. There are, however, some examples in which socially isolated individuals did not have fewer numbers of proliferating cells compared with socially exposed individuals (Fowler et al., 2002; Dunlap et al., 2006; Sorensen et al., 2007), but these effects are often brain region specific rather than more global as observed in A. burtoni. The default social status in A. burtoni is dominance, and therefore, these two groups were of the same status and reproductive condition (as measured by GSI), but differed in the amount of social interaction. The recent highlighted importance of cell proliferation in mammalian reproduction (Lau et al., 2011) raises the possibility that the maintenance of reproductive physiology in these socially isolated, but dominant, male A. burtoni may require an addition or turnover of new cells that is more important than any stress-related physiological response induced by the isolated social environment. Furthermore, these data also support the idea that lower cell proliferation in subordinate males is a result of the social subordination, rather than stress per se.
In summary, we demonstrate that the African cichlid fish A. burtoni has significant proliferation zones throughout the brain that are similar to those described in other teleosts, that many of the newborn cells differentiate into neurons within a few weeks, and that social status influences the number of proliferating cells in multiple brain nuclei. With the recent availability of genomic resources for A. burtoni, and the unique ability to manipulate its social environment and reproductive capacity, we also propose that this species will serve as an ideal vertebrate model to test specific hypotheses on the cellular and molecular regulation of neurogenesis and gliogenesis, and how different environmental and physiological factors might influence these processes that will be applicable to other vertebrate taxa.
ACKNOWLEDGMENTS
We thank Jackie Kustan and Geet Chakraborty for experimental assistance, and the reviewers and editors for insightful comments that improved the manuscript.
Grant sponsor: National Institutes of Health; Grant numbers: F32NS061431 (to K.P.M.), F32HD063234 (to R.E.C.) and NS034950 (to R.D.F); Grant sponsor: National Science Foundation; Grant number: IOS-0923588 (to R.D.F.).
Abbreviations
- A
Anterior thalamic nucleus
- ac
Anterior commissure
- ALLn
Anterior lateral line nerve
- ATn
Anterior tuberal nucleus
- BrdU
5-Bromo-2′-deoxyuridine
- CCe
Corpus cerebelli
- CCeG
Granular layer of the corpus cerebelli
- CCeM
Molecular layer of the corpus cerebelli
- CON
Caudal octaval nucleus
- CP
Central posterior thalamic nucleus
- CM
Corpus mammillare
- Dc
Central part of the dorsal telencephalon
- Dc-2
Subdivision 2 of Dc
- Dd
Dorsal nucleus of the dorsal telencephalon
- Dl
Lateral part of the dorsal telencephalon
- Dld
Dorsal division of the lateral part of the dorsal telencephalon
- Dlg
Granular division of the lateral part of the dorsal telencephalon
- Dlv
Ventral division of the lateral part of the dorsal telencephalon
- Dm
Medial part of the dorsal telencephalon
- Dm-1,2,3
Subdivisions 1, 2, and 3 of Dm
- Dm-2r
Rostral part of Dm-2
- Dp
Posterior part of the dorsal telencephalon
- DP
Dorsal posterior thalamic nucleus
- ECL
External cellular layer of OB
- EG
Granular eminence
- ET
Eminentia thalami
- GFAP
Glial fibrillary acidic protein
- Gn
Glomerular nucleus
- Ha
Habenula
- I
Intermediate thalamic nucleus
- ICL
Internal cellular layer of OB
- MgON
Magnocellular octaval nucleus
- MON
Medial octavolateralis nucleus
- MLF
Medial longitudinal fasciculus
- NDIL
Diffuse nucleus of the inferior lobe
- NDILl
Lateral part of NDIL
- NDILm
Medial part of NDIL
- NI
Nucleus isthmi
- NLT
Lateral tuberal nucleus
- NLV
Nucleus of the lateral valvulae
- nMLF
Nucleus of the medial longitudinal fasciculus
- NPT
Posterior tuberal nucleus
- NRL
Nucleus of the lateral recess
- NRP
Nucleus of the posterior recess
- nPVO
Nucleus of the paraventricular organ
- nX
Vagal nerve
- OB
Olfactory bulb
- ON
Optic nerve
- PCo
Posterior commissure
- PG
Periventricular granular cell mass of caudal cerebellar lobe
- PGa
Anterior preglomerular nucleus
- PGc
Commissural preglomerular nucleus
- PGl
Lateral preglomerular nucleus
- PGZ
Periventricular gray zone of tectum
- Pit
Pituitary
- PMp
Magnocellular preoptic nucleus
- POA
Preoptic area
- PPa
Anterior parvocellular preoptic nucleus
- PPr
Rostral periventricular pretectal nucleus
- PS
Pineal stalk
- PSn
Superficial pretectal nucleus
- PON
Posterior octaval nucleus
- RM
Medial reticular nucleus
- SGC
Stratum griseum centrale
- T
Tectum
- TL
Torus longitudinalis
- TLa
Nucleus of the torus lateralis
- TPp
Periventricular nucleus of the posterior tuberculum
- TS
Torus semicircularis
- TTB
Tractus tectobulbaris
- V
Ventricle
- Vc
Central nucleus of the ventral telencephalon
- VCe
Valvula cerebelli
- VCeG
Granular layer of the valvula cerebelli
- VCeM
Molecular layer of the valvula cerebelli
- VCeMl
Molecular layer of the valvula cerebelli, pars lateralis
- VCeMm
Molecular layer of the valvula cerebelli, pars medialis
- Vd
Dorsal nucleus of the ventral telencephalon
- Vdc
Caudal part of Vd
- Vde
Descending tract of the trigeminal nerve
- VIIs
Facial sensory nucleus
- Vl
Lateral nucleus of the ventral telencephalon
- VM
Ventromedial thalamic nucleus
- Vp
Postcommissural nucleus of the ventral telencephalon
- Vs
Supracommissural nucleus of the ventral telencephalon
- Vsm
Medial part of Vs
- Vsl
Lateral part of Vs
- VTn
Ventral tuberal nucleus
- Vv
Ventral nucleus of the ventral telencephalon
- Xm
Vagal motor nucleus
- Xs
Vagal sensory nucleus
LITERATURE CITED
- Abercrombie M 1946. Estimation of nuclear population from microtome sections. Anat Rec 94:239–247. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. 2005. Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569. [DOI] [PubMed] [Google Scholar]
- Adar E, Nottebohm F, Barnea A. 2008. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J Neurosci 28:5394–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Wilczynski W. 2009. Sex-specific modulation of cell proliferation by socially relevant stimuli in the adult green treefrog brain (Hyla cinerea). Brain Behav Evol 74: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampatzis K, Dermon CR. 2007. Sex differences in adult cell proliferation within the zebrafish (Danio rerio) cerebellum. Eur J Neurosci 25:1030–1040. [DOI] [PubMed] [Google Scholar]
- Ampatzis K, Dermon CR. 2011. Regional distribution and cellular localization of b2-adrenoceptors in the adult zebrafish brain (Danio rerio). J Comp Neurol 518:1418–1441. [DOI] [PubMed] [Google Scholar]
- Anken RH, Bourrat F. 1998. Brain atlas of the medakafish Oryzias latipes. Paris: Institut National de la Recherche Agronomique. [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. 1995. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol 28:82–101. [DOI] [PubMed] [Google Scholar]
- Barnea A 2009. Interactions between environmental changes and brain plasticity in birds. Gen Comp Endocrinol 163: 128–134. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. 2005. Rapid behavioral and genomic responses to social opportunity. PLoS Biol 3: e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Munshi RG, Fernald RD. 2009. Cytoarchitecture of a cichlid fish telencephalon. Brain Behav Evol 74: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-Ortega E, Pedraza C, Estivill-Torrus G, Santin LJ. 2011. When is adult hippocampal neurogenesis necessary for learning? Evidence from animal research. Rev Neurosci 22: 267–283. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R, Bally-Cuif L. 2007. Adult neurogenesis in non-mammalian vertebrates. Bioessays 29:745–757. [DOI] [PubMed] [Google Scholar]
- Chen G, Cheng MF. 2007. Inhibition of lesion-induced neurogenesis impaired behavioral recovery in adult ring doves. Behav Brain Res 177:358–363. [DOI] [PubMed] [Google Scholar]
- Chen G, Bonder EM, Cheng MF. 2006. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol 66:537–551. [DOI] [PubMed] [Google Scholar]
- Chiu JF, Mack AF, Fernald RD. 1995. Daily rhythm of cell proliferation in the teleost retina. Brain Res 673:119–125. [DOI] [PubMed] [Google Scholar]
- Davis MR, Fernald RD. 1990. Social control of neuronal soma size. J Neurobiol 21:1180–1188. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Castellano JF, Prendaj E. 2006. Social interaction and cortisol treatment increase cell addition and radial glia fiber density in the diencephalic periventricular zone of adult electric fish, Apteronotus leptorhynchus. Horm Behav 50:10–17. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, McCarthy EA, Jashari D. 2008. Electrocommunication signals alone are sufficient to increase neurogenesis in the brain of adult electric fish, Apteronotus leptorhynchus. Dev Neurobiol 68:1420–1428. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Silva AC, Chung M. 2011. Environmental complexity, seasonality and brain cell proliferation in a weakly electric fish, Brachyhypopomus gauderio. J Exp Biol 214: 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom P, Johnsson CM, Ohlin LM. 2001. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J Comp Neurol 436:92–110. [PubMed] [Google Scholar]
- Engelmann M 2008. Vasopressin in the septum: not important versus causally involved in learning and memory-two faces of the same coin? Prog Brain Res 170:389–395. [DOI] [PubMed] [Google Scholar]
- Fernald RD. 1977. Quantitative behavioral observations of Haplochromis burtoni under semi-natural conditions. Anim Behav 25:643–653. [Google Scholar]
- Fernald RD. 1991. Teleost vision: seeing while growing. J Exp Zool Suppl 5:167–180. [DOI] [PubMed] [Google Scholar]
- Fernald RD. 2009. Social regulation of reproduction: what changes and why? Horm Brain Behav 1:683–691. [Google Scholar]
- Fernald RD, Hirata NR. 1977. Field study of Haplochromis burtoni: quantitative behavioral observations. Anim Behav 25: 964–975. [Google Scholar]
- Fernald RD, Shelton LC. 1985. The organization of the diencephalon and the pretectum in the cichlid fish, Haplochromis burtoni. J Comp Neurol 238:202–217. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Wright SE. 1983. Maintenance of optical quality during crystalline lens growth. Nature 301:618–620. [DOI] [PubMed] [Google Scholar]
- Fernandez AS, Rosillo JC, Casanova G, Olivera-Bravo S. 2011. Proliferation zones in the brain of adult fish Austrolebias (Cyprinodontiform: Rivulidae): a comparative study. Neuroscience 189:12–24. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. 2001. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci 21:8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. 2002. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol 51:115–128. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. 2008. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev 57:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley NB, Fernald RD. 1982. Social control of developmental rate in the African cichlid fish, Haplochromis burtoni. Z Tierpsychol 60:66–82. [Google Scholar]
- Francis RC, Soma K, Fernald RD. 1993. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci U S A 90:7794–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. 2009. A niche for adult neurogenesis in social behavior. Behav Brain Res 200:315–322. [DOI] [PubMed] [Google Scholar]
- Gonda A, Herczeg G, Merila J. 2009. Habitat-dependent and -independent plastic responses to social environment in the nine-spined stickleback (Pungitius pungitius) brain. Proc Biol Sci 276:2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. 2006. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol 295:263–277. [DOI] [PubMed] [Google Scholar]
- Greenough WT. 1975. Experiential modification of the developing brain. Am Sci 63:37–46. [PubMed] [Google Scholar]
- Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. 2009. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod 80:1146–1151. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. 1999. Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci U S A 96:14171–14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Evans JC, Mueller B, Valentino RJ, Lucki I. 2006. Social competition in rats: cell proliferation and behavior. Behav Brain Res 175:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Tanaka H, Okamoto H, Ohshima T. 2010. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev Biol 342:26–38. [DOI] [PubMed] [Google Scholar]
- Johns PR, Fernald RD. 1981. Genesis of rods in teleost fish retina. Nature 293:141–142. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Brand M. 2008. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci 363:101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rosenfeld MG. 2010. Epigenetic control of stem cell fate to neurons and glia. Arch Pharm Res 33:1467–1473. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. 2004. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci 24:6755–6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyanagi Y, Okuyama T, Suehiro Y, Imada H, Shimada A, Naruse K, Takeda H, Kubo T, Takeuchi H. 2010. Proliferation zones in adult medaka (Oryzias latipes) brain. Brain Res 1323:33–40. [DOI] [PubMed] [Google Scholar]
- Kustan JM, Maruska KP, Fernald RD. 2011. Subordinate male cichlids retain reproductive competence during social suppression. Proc Biol Sci 279:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JW, Lee MJ, Mack AF, Chiu JF, Fernald RD. 1996. Nonuniform distribution of cell proliferation in the adult teleost retina. Brain Res 712:40–44. [DOI] [PubMed] [Google Scholar]
- Lau BW, Yau SY, So KF. 2011. Reproduction: a new venue for studying function of adult neurogenesis? Cell Transplant 20:21–35. [DOI] [PubMed] [Google Scholar]
- Lema SC, Hodges MJ, Marchetti MP, Nevitt GA. 2005. Proliferation zones in the salmon telencephalon and evidence for environmental influence on proliferation rate. Comp Biochem Physiol A Mol Integr Physiol 141:327–335. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. 1980. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol 193: 815–840. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. 2007. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci 10:1003–1011. [DOI] [PubMed] [Google Scholar]
- Maler L, Sas E, Johnston S, Ellis W. 1991. An atlas of the brain of the electric fish Apteronotus leptorhynchus. J Chem Neuroanat 4:1–38. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. 1994. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol 25:143–155. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. 2010a. Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm Behav 58:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. 2010b. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. 2011a. Plasticity of the reproductive axis caused by social status change in an African cichlid fish: II. Testicular gene expression and spermatogenesis. Endocrinology 152:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. 2011b. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 26:412–423. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Levavi-Sivan B, Biran J, Fernald RD. 2011. Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. Pituitary gonadotropins. Endocrinology 152:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Hayden TJ. 2006. When should signals of submission be given? A game theory model. J Theor Biol 240:425–433. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. 2011. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. 2009. Reliable activation of immature neurons in the adult hippocampus. PLoS One 4:e5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munchrath LA, Hofmann HA. 2010. Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. J Comp Neurol 518:3302–3326. [DOI] [PubMed] [Google Scholar]
- Munoz-Cueto JA, Sarasquete C, Zohar AH, Kah O. 2001. An atlas of the brain of the gilthead seabream (Sparus aurata). College Park, MD: Sea Grant Press. [Google Scholar]
- Nogues X, Corsini MM, Marighetto A, Abrous DN. 2011. Functions for adult neurogenesis in memory: an introduction to the neurocomputational approach and to its contribution. Behav Brain Res 227:418–425. [DOI] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. 2006. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav Brain Res 166:291–295. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y, Gueguen MM, Marmignon MH, Brion F, Pakdel F, Kah O. 2007. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol 501:150–167. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Omanska A. 2005. Dominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. J Neurobiol 62:31–41. [DOI] [PubMed] [Google Scholar]
- Rizzi S, Bianchi P, Guidi S, Ciani E, Bartesaghi R. 2007. Neonatal isolation impairs neurogenesis in the dentate gyrus of the guinea pig. Hippocampus 17:78–91. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Duran E, Gomez A, Ocana FM, Alvarez E, Jimenez-Moya F, Broglio C, Salas C. 2005. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res Bull 66:365–370. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. 2008. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res 187:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas C, Broglio C, Duran E, Gomez A, Ocana FM, Jimenez-Moya F, Rodriguez F. 2006. Neuropsychology of learning and memory in teleost fish. Zebrafish 3:157–171. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS. 2010. MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci 30:14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen C, Overli O, Summers CH, Nilsson GE. 2007. Social regulation of neurogenesis in teleosts. Brain Behav Evol 70:239–246. [DOI] [PubMed] [Google Scholar]
- Sorensen C, Nilsson GE, Summers CH, Overli O. 2011. Social stress reduces forebrain cell proliferation in rainbow trout (Oncorhynchus mykiss). Behav Brain Res 227:311–318. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Zupanc GK. 1996. Apoptosis in the cerebellum of adult teleost fish, Apteronotus leptorhynchus. Brain Res Dev Brain Res 97:279–286. [DOI] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Nunez A, Pellegrini E, Gueguen MM, Kah O, Somoza GM. 2010. Progenitor radial cells and neurogenesis in pejerrey fish forebrain. Brain Behav Evol 76:20–31. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS Jr. 1993. Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci 13:820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krogh K, Sorensen C, Nilsson GE, Overli O. 2010. Forebrain cell proliferation, behavior, and physiology of zebra-fish, Danio rerio, kept in enriched or barren environments. Physiol Behav 101:32–39. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Weston JA. 1997. Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development 124:3449–3460. [DOI] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. 2002. Social regulation of gonadotropin-releasing hormone. J Exp Biol 205: 2567–2581. [DOI] [PubMed] [Google Scholar]
- Wulliman MF, Rupp B, Reichert H. 1996. Neuroanatomy of the zebrafish brain: a topological atlas. Basel, Switzerland: Birkhauser Verlag. [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. 2006. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res 172:344–350. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Kentouri M, Dermon CR. 2000. Proliferation zones in the adult brain of a sequential hermaphrodite teleost species (Sparus aurata). Brain Behav Evol 56: 310–322. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Kentouri M, Dermon CR. 2001. Cell genesis in the hypothalamus is associated to the sexual phase of a hermaphrodite teleost. Neuroreport 12:2477–2481. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. 2006. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192:649–670. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. 2008. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol Paris 102: 357–373. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Clint SC. 2003. Potential role of radial glia in adult neurogenesis of teleost fish. Glia 43:77–86. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Horschke I. 1995. Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J Comp Neurol 353:213–233. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Hinsch K, Gage FH. 2005. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol 488:290–319. [DOI] [PubMed] [Google Scholar]