Abstract
Pediatric patients are unique both in their diagnosis and clinical presentation before implantation of a ventricular assist device (VAD) and in their driveline site characteristics post-implant. There is limited evidence in scholarly literature that describes complications of pediatric VAD driveline sites or approaches by which to manage them. The Cardiac Center at The Children’s Hospital of Philadelphia (CHOP) follows a standard of care for HeartWare VAD (HVAD) dressing changes in the inpatient setting with the goal of transitioning patients to weekly dressing changes by the time they are discharged to home. As a patient with an HVAD nears discharge, members of an interprofessional team collaborate with insurance providers and home care agencies to procure the appropriate supplies needed at home. Individualized plans of care are necessary for patients who are unable to transition to weekly dressings; however, customized products (such as silicone foam border dressings and antimicrobial agents) may be challenging to supply as single items from home care agencies. Between March 2014 and June 2017, 15 patients underwent HVAD implantation, and eight (53%) were discharged home. Ten patients (67%) were able to transition to weekly dressing changes. Individualized plans of care for driveline site management were required for six (40%) patients with persistent drainage. Three patients (20%) experienced a driveline site infection. This article describes how a quality improvement (QI) initiative using rapid-cycle improvement methodology was executed to standardize HVAD dressing changes in our pediatric population.
Keywords: pediatric, ventricular assist device, HeartWare, driveline site, dressing change
The first HeartWare ventricular assist device (HVAD) implant at The Children’s Hospital of Philadelphia (CHOP) took place in March 2014, and the patient was discharged to home 28 days later. As of June 2017, 15 patients have undergone surgery for HVAD implantation. Nurses in the Cardiac Center follow a standard of care for VAD dressing changes in the inpatient setting that allows for individualization when persistent drainage, skin breakdown at the driveline site, dressing integrity issues, or sensitivities to selected products for wound care occur.
Historically, a sturdy, minimally absorptive, adhesive dressing was chosen to dress VAD driveline exit sites. With continued use, the nursing staff noticed increased erosion at the drive-line exit site and challenges with managing drainage, which increased the potential for more frequent dressing changes and/or driveline site infections. Silicone foam border dressings promote a healthy wound microclimate and were first evaluated on patients with pulsatile, extracorporeal VADs in 2012. They provide additional protection from device-related pressure injury resulting from direct pressure of the driveline on the skin and are less painful when removed. These types of dressings are typically used for patients who require more than one VAD dressing change per week.
The addition of the HeartWare (HVAD) to our existing repertoire of VADs presented us with an opportunity to consider a different approach for dressing and managing driveline sites. The HVAD, although not Food and Drug Administration (FDA) approved for use in children, has gained popularity in pediatrics because of lower profiles of side effects and adverse events.1,2 In addition, providers can consider discharging these patients to home in order to return to activities of daily living in the hopes of maximizing their post-transplant outcomes and quality of life.3,4
In order to meet the demands of active lifestyles in the outpatient environment, HVAD dressings need to remain sturdy and occlusive. A two-piece occlusive dressing with semipermeable window is used for HVAD patients who have transitioned to once-weekly dressing changes because of its stronger adhesive properties and ability to provide an occlusive seal for longer periods of time. We also recommended that pediatric HVAD patients utilize two types of stabilization devices in order to minimize movement of the driveline and provide added security during activity. All HVAD patients use an adhesive anchor as a primary securement device. Abdominal binders are recommended as a secondary securement for added stabilization once patients resume activities of daily living.
Every HVAD patient requires routine, sterile dressing changes as part of their nursing plan of care. This baseline evidence provided a starting point for standardizing dressing change procedures in the HVAD population. A team of nurses conducted a literature review and subsequently carried out a quality improvement (QI) project using rapid-cycle improvement methodology to investigate which types of dressing products best meet the clinical needs of pediatric HVAD patients.
Literature Review
There is limited evidence in scholarly literature that describes complications of pediatric VAD driveline sites or approaches by which to manage them. In adult populations, the presence of a percutaneous driveline that powers a VAD pump causes high risk for infection throughout the duration of therapy, making driveline stabilization and exit site management a priority in the patient’s plan of care.5 Persistent driveline drainage (PDD) may occur as a result of fluid collections around a device, ascites, or repetitive trauma secondary to poor fixation of the driveline at the exit site and is further exacerbated by poor immunologic function, poor nutritional status, and anasarca. Although this type of drainage may not be infectious in origin, it can cause infection if prolonged.6
Clinical teams are often empowered to offer individualized techniques for driveline site management rather than relying on a single, exclusive method.7 Selection of products is based on prior experience or clinical preference with minimal guidance in the literature in terms of standardization.8 Individualization results in variability in care in both the inpatient and outpatient setting, which can have a negative impact on patient compliance and increase the chances for infection.5
Despite efforts to provide education regarding dressing change procedures and supplies as part of the discharge process,9 standardized techniques for dressing changes among VAD centers do not exist in the United States.8 Survey results on outpatient management of adult patients with a continuous-flow VAD report a frequency in dressing changes ranging from once daily to once weekly.10 Dressing change kits help to standardize and simplify driveline site management while providing added stability, promoting comfort, and decreasing the risk of infection.5
Rapid-Cycle Improvement Methodology
We utilized a framework grounded in rapid-cycle improvement methodology in order to provide a standardized and structured approach to clinical QI. Rapid-cycle improvement methodology is recommended for small, focused projects such as ours and includes phases that define the clinical problem and diagnose areas where an opportunity for improvement exists.11 Any recommended changes are tested and implemented to evaluate their effectiveness. Sustained changes are consistently monitored and supported by key stakeholders to assure that the interventions consistently demonstrate improved patient care.
Definition of the Problem
Our initial goal was to transition HVAD patients to once-weekly dressing changes in an attempt to streamline home routines for the patient and family, as the procedure for sterile dressing changes can be cumbersome and time-consuming. Discharge goals for driveline site management included identifying supplies and techniques for site care and transitioning to once-weekly dressing changes. We believed that this could be accomplished after the first postoperative week. As our experience in caring for HVAD patients evolved, nurses identified trends in their clinical condition that delayed transition to once-weekly dressings.
Diagnosis of the Problem
Our length of inpatient admission for initial HVAD implant ranged between 21 and 423 days, with a median of 40 days. Many patients still had chest tubes and wound drains in place after the first postoperative week because of delayed wound healing. It was difficult to maintain the integrity of a long-term VAD dressing in the presence of other medical devices or healing insertion sites, as the residual wounds and chest tube insertion sites tended to ooze and were in close proximity to the VAD dressing edge. Additionally, nine (60%) patients experienced postoperative drainage from the driveline site that required more frequent VAD dressing changes in order to keep the driveline exit site clean and dry until it resolved. PDD was a concern for six (40%) patients.
Change Intervention
We aimed to standardize the dressing products where appropriate and also liberate the dressing change procedure based on the clinical needs of patients who experienced delayed wound healing and PDD. To evaluate impacting variables, we completed a series of Plan-Do-Study-Act (PDSA) cycles to test different types of dressings for patients with delayed wound healing and PDD. We examined length of time between dressing changes and driveline exit site appearance and agreed to implement changes to the dressing change procedure only when there was a decrease in time and/or an improvement in appearance.
Dressing Selection
Dressing type was individualized to support delayed wound healing, ameliorate PDD, and address new findings of erosion and altered dressing integrity resulting from profuse diaphoresis. Six patients (40%) experienced PDD at their driveline exit site, two (33%) of whom required hydro-conductive gauze in place of silicone foam. One patient (7%) was unable to maintain dressing integrity because of profuse diaphoresis, and one patient (7%) experienced erosion at the driveline exit site. Each required individualized plans of care for driveline site management. Table 1 describes each test of change in greater detail.
Table 1.
Tests of Change for Driveline Site Management
| Clinical Condition | Number of Patients Eligible for Test of Change (% Frequency) n = 15 | Test of Change | Application to Clinical Practice | Result | Number of Eligible Patients With Clinical Improvement as a Result of Test of Change (% Frequency) |
|---|---|---|---|---|---|
| PDD | 6 (40%) | Hydroconductive gauze | Used in place of silicone foam | Absorb moisture away from the skin | 2 (33%); n = 6 |
| Erosion | 1 (7%) | Silver impregnated dressings | Used in place of silicone foam or hydroconductive gauze | Debridement of unhealthy tissue. Promotion of granulation tissue | 1 (100%); n = 1 |
| Altered dressing integrity | 1 (7%) | Hydroconductive gauze multiple layers. Silicone foam multiple layers | Transparent dressing with strong holding power used in place of silicone foam border dressings or occlusive window dressings | Absorb moisture away from the skin. Promote occlusive seal against epidermis | 1 (100%); n =1 |
PDD, persistent driveline drainage.
Patients who experienced delayed wound healing were able to transition to once-weekly dressing changes once chest tubes and drains were removed. Patients who experienced PDD, erosion, or profuse diaphoresis were unable to transition to weekly dressing changes unless their condition resolved. As a result of these tests of change, we concluded that it was more feasible to wait until chest tubes and drains had been discontinued before transitioning to weekly VAD dressing changes.
Selection of Antimicrobial Cleanser
A 50/50 dilution of 4% Chlorhexidine Gluconate (CHG) soap and sterile water is used to cleanse driveline sites and surrounding skin during the immediate postoperative period. We attempted to transition patients to a 2% CHG and alcohol-based skin prep at the time of transition to once-weekly dressing changes; however, several challenges specific to skin integrity were identified as a result. Two patients (13%) suffered from intense burning and stinging at the exit site. Two patients (13%) who had successfully transitioned to once-weekly dressing changes using a two-piece occlusive dressing with semipermeable window developed a rash at their exit site after prolonged use of the alcohol-based skin prep that required treatment with a topical steroid cream and short-term transition back to silicone foam dressings. These cases resolved completely when cleanser was switched back to the 50/50 dilution of 4% CHG soap and sterile water. After conferring with the cardiothoracic surgeons, use of 2% CHG and alcohol-based skin prep was discontinued and replaced it with the 4% CHG soap and water dilution in both the inpatient and outpatient setting. Since this change in practice, no patients have reported stinging, burning, or rash under their dressing.
Evaluating Tests of Change
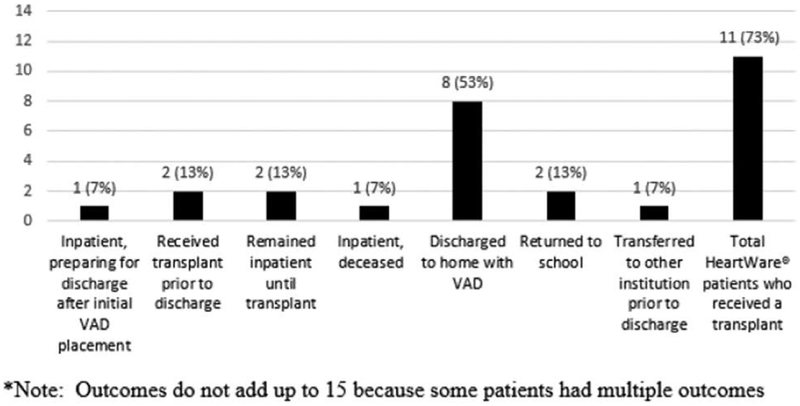
Between March 2014 and June 2017, 15 pediatric patients underwent surgery for implantation of an HVAD. Of these patients, four (26%) presented with single ventricle physiology and two (13%) required biventricular support. Ten patients (67%) met criteria for discharge, and eight (53%) were discharged to home with an HVAD in place. Discharge to home was not appropriate for four (27%) patients (Figure 1).
Figure 1.
Patient post-VAD implant outcomes (March 2014 to June 2017). VAD, ventricular assist device.
Ten (67%) patients were able to transition to weekly dressing changes using a two-piece occlusive window dressing. Six (40%) patients were classified as having PDD. The most common concerns for altered skin integrity at the driveline exit site resulted from infection (20%) and irritation from skin cleanser (13%). Two patients (25%) were readmitted from home for driveline site infection that was treated with antibiotics. Both required temporary modifications in the frequency and type of dressings used until the infection resolved, and one required modified supplies in the home setting for residual PDD post-infection that ultimately resolved. Table 2 describes patient characteristics specific to driveline site management in further detail.
Table 2.
Patient Characteristics (n = 15) Specific to HVAD Driveline Site Management Between March 2014 and June 2017
| Median (Range) Frequency (%) | |
|---|---|
| Age (years) | 11 (3–17) |
| Gender | |
| Male | 9 (60%) |
| Female | 6 (40%) |
| Weight (kg) | 45.8 (12.0–85.6) |
| BSA (m2) | 1.41 (0.55–2.07) |
| Diagnosis | |
| Cardiomyopathy | 7 (47%) |
| Myocarditis | 2 (13%) |
| Single ventricle physiology | 4 (26%) |
| Congenital heart disease | 1 (7%) |
| Kawasaki disease | 1 (7%) |
| Cannula site | |
| Left ventricular | 8 (53%) |
| Right ventricular | 2 (13%) |
| Biventricular | 2 (13%) |
| Atrial | 3 (10%) |
| Skin condition at driveline exit site | |
| PDD | 6 (40%) |
| Profuse diaphoresis | 1 (7%) |
| Irritation from skin prep or cleanser | 2 (13%) |
| Erosion | 1 (7%) |
| Risk for device-related pressure injury from driveline | 1 (7%) |
| Driveline site infection | 3 (20%) |
| Postoperative drainage characteristics | |
| Green serous | 8 (53%) |
| Serosanguinous | 8 (53%) |
| Bloody | 2 (13%) |
| Type of dressing at discharge * or transplant | |
| Weekly | 10 (67%) |
| Silicone foam | 7 (47%) |
| Two-piece occlusive window | 10 (67%) |
| Twice weekly | 5 (33%) |
| Silicone foam | 5 (33%) |
| Silicone foam with adhesive border | 4 (27%) |
| Silicone tape | 5 (33%) |
| Nonwoven, nonabsorptive, adhesive dressing | 1 (7%) |
| Hydrocolloid dressing | 1 (7%) |
| Patients discharged to home with an HVAD | 8 (53%) |
| Patient readmissions for driveline site infection | 2 (25%)† |
Actual discharge and preparing for initial discharge post-implant.
Frequency calculation based on eight HVAD patients who were discharged to home.
HVAD, HeartWare ventricular assist device; PDD, persistent drive-line drainage.
Sustaining Tests of Change
After several PDSA cycles, a silicone foam dressing was routinely placed under the driveline and covered with a silicone foam dressing with adhesive border. This dressing was adopted as the standard postoperative dressing for our HVAD population as well as the recommended dressing for patients with PDD or who developed drainage after transition to a once-weekly dressing. When the amount or quantity of drainage surpassed the absorbability of this dressing combination, the silicone foam dressing would be replaced with the hydroconductive dressing. Our standard long-term (once weekly) dressing consists of a silicone foam dressing covered with a two-piece occlusive window dressing. The timeline for transition to once-weekly dressing changes and a description of products for site care is detailed in Figure 2. Methods to support sustainability included revision of nursing procedures and standards of care to reflect the new approach, monitoring clinical practice, and initiating a skin integrity care plan for new and readmitted HVAD patient.
Figure 2.
Standard management of HeartWare ventricular assist device (HVAD) dressing changes. A: Standard postoperative dressing/standard dressing when drainage is present. B: Standard weekly dressing. For dressing shown in both panels, all sites are cleaned with a 50/50 solution of 4% Chlorhexidine Gluconate (CHG) soap and sterile water and dressed in the following fashion: (1) silicone foam dressing; (2A) silicone foam dressing with adhesive border (changed daily through postoperative day 7 and a minimum of twice weekly when chest tubes or active drainage are present), (2B) Two-piece semipermeable dressing with transparent window (changed once every 7 days when there is no active drainage from the driveline site); (3) securement device (changed a minimum of once weekly); and (4) silicone tape.
Discussion
Individualized plans of care for driveline site management were independent of patient age, diagnosis, or device placement. Drivelines were typically tunneled out of the left lower quadrant, but their exact location varied based on the origin of the HVAD device and whether biventricular support was needed. The velour cuff was internalized at the time of surgical implant. Drivelines did not fully incorporate into the patient’s skin for several weeks post-implant. During that time, the cuff could transverse the skin intermittently with changes in the patient’s weight or abdominal girth. When the cuff was visible at the driveline exit site, the team monitored the patient’s condition closely and continued with the prescribed dressing regimen unless signs or symptoms of skin injury, irritation, or infection were present. If the cuff was visible in the presence of these symptoms, then the frequency and type of products for dressing change were modified until the condition resolved and/or the felt reinternalized itself. Dean and colleagues12 report reduced driveline infection rates when the velour cuff of a VAD was internalized, and we had no cases where the cuff was externalized for prolonged periods of time.
The nature of PDD in our patients varied in quantity, consistency, onset, and duration. In each case, the frequency of dressing changes was increased in order to maintain skin integrity and prevent pooling of drainage at or around the exit site. While drainage by itself may not be infectious in nature, prolonged drainage—particularly in the setting of irritation and erosion at the driveline exit site—can increase the risk of infection.6,13 Schweiger and colleagues4 hypothesize that increased activity levels can cause movement of the driveline at the exit site. Added movement, with or without trauma to the exit site, can result in increased drainage and possible infection.5 We encouraged additional stabilization of the driveline using abdominal binders or elastic bandages in order to minimize movement of the driveline.
Rosenthal and colleagues14 were the first to describe adverse events in 109 children with long-term continuous-flow devices in a Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS) report spanning October 2012 to June 2015. Pump or driveline-related infection was reported in six (5%) children with continuous-flow VADs. We treated three (20%) patients for presumed driveline site infections between December 2015 and April 2017. Our experience was similar to that of Schweiger and colleagues,4 who reported four (33%) instances of driveline site infections in 12 patients between 2011 and 2013.
All of our patients who presented with a driveline site infection experienced new or increased drainage from the driveline exit site often described as purulent. The cause of infection was variable in nature: mediastinal abscess, methicillin susceptible Staphylococcus aureus (MSSA), and Staphylococcus epidermidis. Antibiotics were prescribed for each patient after consultation with colleagues from infectious disease. While all cases of infection ultimately resolved, two (66%) patients were maintained on long-term antibiotics for suppression of infection. Two patients (66%) were able to transition back to once-weekly dressings, while one (33%) remained on a twice-weekly schedule because of PDD.
Brandrud and colleagues15 studied 132 quality improvement projects in Norway in order to assess the perspectives of healthcare professionals specific to conditions for improvement and then evaluate the effectiveness of quality improvement methods. Their findings suggest that QI projects have the highest likelihood of success when current practices could be simply measured, best practices were assessed by subject matter experts, and the quality improvement effort was conducted in an organized fashion. PDSA cycles, which are rooted in the scientific method, are integral to improvement work that is grounded in rapid-cycle improvement methodology.11,16 PDSA cycles helped us learn about the challenges associated with driveline site management in order to develop and refine interventions aimed at reducing the risk of pressure injury, skin breakdown, and managing drainage, all of which have been associated with increased risk for driveline site infection.4,6,13 We were able to design two types of dressing regimes that met the immediate post-operative and long-term needs for 14 (94%) of our HVAD patients and are confident that these dressings will continue to meet driveline site management needs for the majority of our HVAD patients to come.
Interprofessional Management of HVAD Driveline Sites
The traditional responsibilities of a VAD coordinator are shared by an interprofessional team comprised a transplant coordinator, nurse practitioners (NPs), clinical nurse specialists (CNSs), physician assistants (PAs), and perfusionists. The coordinator team develops comprehensive plans of care that are supported and implemented by nursing leaders and bedside nurses. Every stakeholder is committed to delivering evidence-based care to achieve optimal patient outcomes for this population.
The CNSs in the Cardiac Intensive Care Unit (CICU) and Cardiac Care Unit (CCU) collaborate to address the unique needs of the pediatric patient with an HVAD and provide extensive clinical consult specific to driveline site management and dressing selection to support care across the continuum. The CNS team manages education and training for patients and caregivers specific to driveline site management and dressing changes and is responsible for assessing and verifying care-giver competence before discharge. They collaborate with the NPs when outpatient consultation or follow-up is needed specific to driveline site or dressing concerns.
The CNSs are also responsible for collaborating with case managers to procure the appropriate dressing supplies for home management. VAD dressing change kits are not currently used in the Cardiac Center, and supplies are ordered individually based on a projected number of dressing changes per month. The first two (25%) HVAD patients that were discharged received homecare services that were affiliated with the hospital, and experienced no difficulty in obtaining the same supplies that were used in the inpatient environment.
As VAD patients with varying types of insurance coverage and homecare service providers were identified, discharge planning specific to VAD dressings was initiated closer to the date of implant to assure that patients could receive the appropriate supplies immediately post-discharge. Two patients (25%) required product substitutions because of unavailability of preferred products through their homecare supplier. The CNSs and NPs drafted a standard letter of medical necessity that details the rationale behind product selection when faced with challenges in procuring appropriate supplies from homecare suppliers who may be unfamiliar with this patient population.
Several patients have reported instances where they have received incomplete orders or incorrect supplies, requiring follow-up with individual homecare companies and collaboration with outpatient providers in order to procure supplies to maintain the driveline and dressing until the situation was rectified. For these reasons, patients are sent home with enough supplies for three complete dressing changes. Many homecare supply companies are better equipped to collaborate with hospitals in order to supply customized dressing change kits as opposed to procuring individual items. A standardized dressing change kit is in development for the HVAD population at CHOP. Once available, it will be tested using rapid-cycle improvement methodology.
Conclusions
Pediatric patients are unique both in their diagnosis and clinical presentation before implantation of a VAD and in their driveline site characteristics post-implant. A QI framework grounded in rapid-cycle improvement methodology supported our efforts to test and implement different types of cleansing agents and dressings in order to identify a configuration that proved effective for 14 (94%) of our patients. Although a standard of care exists for driveline site management in the Cardiac Center at CHOP, patients may require modifications to the standard order to manage postoperative drainage or prevent alterations in skin integrity and decrease the risk of drive-line site infection. Customizing care is appropriate in these cases, and with thorough documentation and closed-loop communication, can be consistently carried out during each hospitalization.
Interprofessional collaboration is essential when managing pediatric HVAD driveline sites in the outpatient environment, as team members can partner with insurance providers and homecare agencies in order to procure the appropriate supplies for each patient. As our fast-paced healthcare environment continues to evolve, interprofessional teams will be challenged to identify and implement solutions to clinical problems in a timely fashion. There is opportunity to promote further standardization of HVAD dressings by implementing custom dressing change kits in both the inpatient and outpatient environment. Applying rapid-cycle improvement methodology can improve the quality of care delivered to pediatric VAD patients.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Crews KA, Kaiser SL, Walczak RJ, Jaquiss RD, Lodge AJ: Bridge to transplant with extracorporeal membrane oxygenation followed by HeartWare ventricular assist device in a child. Ann Thorac Surg 95: 1780–1782, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Miera O, Potapov EV, Redlin M, et al. : First experiences with the HeartWare ventricular assist system in children. Ann Thorac Surg 91: 1256–1260, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins B, Fynn-Thompson F, Daly KP, et al. : The Evolution of a Pediatric Ventricular Assist Device Program: The Boston Children’s Hospital Experience. Pediatr Cardiol 38: 1032–1041, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Schweiger M, Vanderpluym C, Jeewa A, et al. : Outpatient management of intra-corporeal left ventricular assist device system in children: A multi-center experience. Am J Transplant 15: 453–460, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Stahovich M, Sundareswaran KS, Fox S, et al. : Reduce driveline trauma through stabilization and exit site management: 30 days feasibility results from the multicenter RESIST study. ASAIO J 62: 240–245, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Baradarian S, Stahovich M, Krause S, Adamson R, Dembitsky W: Case series: clinical management of persistent mechanical assist device driveline drainage using vacuum-assisted closure therapy. ASAIO J 52: 354–356, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Hozayen SM, Soliman AM, Eckman PM: Comparison of two ventricular assist device dressing change protocols. J Heart Lung Transplant 31: 108–109, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Cannon A, Elliott T, Ballew C, et al. : Variability in infection control measures for the percutaneous lead among programs implanting long-term ventricular assist devices in the United States. Prog Transplant 22: 351–359, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Conway J, VanderPluym C, Jeewa A, Sinnadurai S, Schubert A, Lorts A: Now how do we get them home? Outpatient care of pediatric patients on mechanical circulatory support. Pediatr Transplant 20: 194–202, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Schlöglhofer T, Robson D, Bancroft J, et al. : International coordinator survey results on the outpatient management of patients with the HeartWare® ventricular assist system. Int J Artif Organs 39: 553–557, 2017. [DOI] [PubMed] [Google Scholar]
- 11.West B: Rapid cycle improvement: Avoid the pitfalls. Nurs Manage 43: 50–53, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Dean D, Kallel F, Ewald GA, et al. ; SSI Registry Investigators: Reduction in driveline infection rates: Results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J Heart Lung Transplant 34: 781–789, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Bentz B, Hupcey JE, Polomano RC, Boehmer JP: A retrospective study of left ventricular assist device-related infections. J Cardiovasc Manag 15: 9–16, 2004. [PubMed] [Google Scholar]
- 14.Rosenthal DN, Almond CS, Jaquiss RD, et al. : Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant 35: 569–577, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandrud AS, Nyen B, Hjortdahl P, et al. : Domains associated with successful quality improvement in healthcare: A nationwide case study. BMC Health Serv Res 17: 648, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leis JA, Shojania KG: A primer on PDSA: Executing plan-do-study-act cycles in practice, not just in name. BMJ Qual Saf 26: 572–577, 2017. [DOI] [PubMed] [Google Scholar]