Abstract
The discovery of optogenetics has revolutionized research in neuroscience by providing the tools for noninvasive, cell-type selective modulation of membrane potential and cellular function in vitro and in vivo. Rhodopsin-based optogenetics has later been introduced in experimental cardiology studies and used as a tool to photoactivate cardiac contractions or to identify the sites, timing, and location most effective for defibrillating impulses to interrupt cardiac arrhythmias. The exploitation of cell-selectivity of optogenetics, and the generation of model organisms with myocardial cell type targeted expression of opsins has started to yield novel and sometimes unexpected notions on myocardial biology. This review summarizes the main results, the different uses, and the prospective developments of cardiac optogenetics.
Keywords: heart, optogenetics, Channelrhodopsin-2, arrhythmias, heart network
1. Introduction of Optogenetics
If you were seeking information on “optogenetics” in a biology, physiology, or even neuroscience textbook published roughly ten years ago, you would be left wanting. Yet, a pubmed search using the same keyword in early 2019 returns more than 5000 hits, attesting to the tremendous success of this technique in biological and biomedical sciences. The recently coined word “optogenetics” summarizes the peculiar aspects of the methodology, which relies on the expression of microbial derived genes (-genetics) encoding one or more light-controlled ion channels or pumps (opsins) in the plasma membrane of a specific cell type. Although the word optogenetics now refers more comprehensively to both such actuators and any genetically encoded sensor emitting light, in response to variations of the intracellular environment (e.g., pH, voltage, Ca2+, cAMP to name a few), here, we will focus on optogenetics based on the use of channel-forming opsins.
Most opsins are light-sensitive ion channel proteins which open rapidly upon illumination with visible light at a specific wavelength, causing redistribution of either cations (e.g., sodium, protons) or anions (e.g., chloride) across the membrane which, depending on the experimental conditions and cell types, results in cell depolarization or hyperpolarization [1,2]. The capacity of opsins to modify the cellular membrane potential has thus offered investigators unique tools to control, with minimal invasiveness on cellular homeostasis and cell-type precision, the quintessential property of excitable cells.
The immediate success of optogenetics has prompted research of additional light-gated proteins present in nature, as well as the molecular modification of the ones already known, to expand the opsin toolkit with variants endowed with different spectral sensitivity (i.e., photoactivating light color), ion selectivity [3], activation/inactivation kinetics [4] or light-dependent conversion between on and off states [5]. As a result, more than 200 opsin variants are now available, as described in (for reference, see www.optogenetics.org) [1,6,7].
Optogenetics has primarily and most often been exploited in neuroscience to isolate (or interrogate) the function of specific neuronal types forming brain networks too densely juxtaposed to allow functional investigation with conventional methodologies. The method has revolutionized the study of brain connectivity in both normal and diseased conditions [8] and allowed association of the function of specific cell types to behavior [9,10,11,12], as well as the identification of pathogenetic mechanisms and potential therapeutic approaches in neuro-pathologies including, e.g., Parkinson’s disease [13,14,15] and retinal degeneration [16].
Unsurprisingly, “neuron+optogenetics” returns more than 3000 out of the total number of pubmed hits of optogenetics. While such abundant research has focused on the complex neuronal systems at the base of brain function, using various molecular tools, delivery strategies, and model systems [17], very little has comparatively been done to disentangle another intricated and equally vital cellular network, such as the heart.
2. The Myocardium: A Complex Network of Excitable and Non-excitable Cells
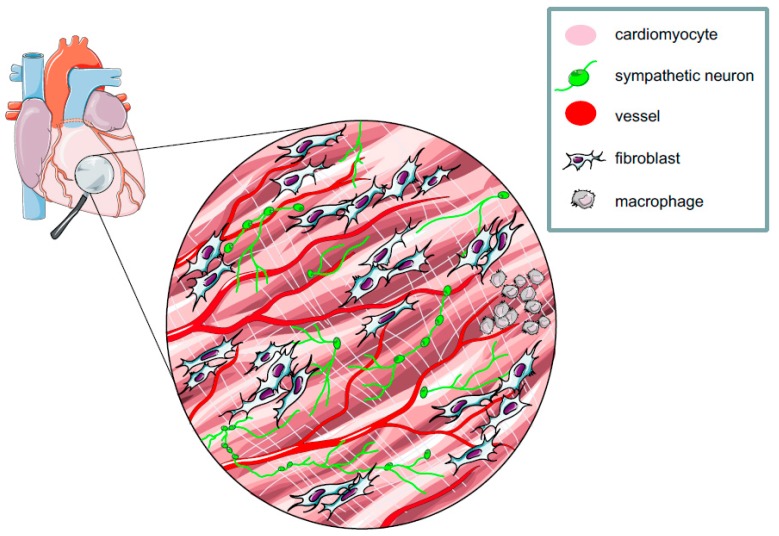
The heart is a strenuous worker contracting unceasingly through the entire life to provide oxygen and nutrients to all cells of the organism. The ability to sustain such crucial activity results from the cooperation of multiple specialized cell types, which are finely interconnected to one another in a well-defined arrangement [18,19]. The concept of the heart as a multicellular structure has been overlooked for a long time, as research focused on cardiomyocytes (CMs) (30–40% of all cardiac cells), responsible for both heart contraction (working CMs) and the conduction of electrical impulses (conducting CMs). This biased view caused the larger non-CM cell populations (60–70% of total cells in the heart), including excitable cells, such as neurons and vascular smooth muscle cells, and non-excitable cells (i.e., endothelial cells, fibroblasts, and immune cells) to sit in the background (Figure 1) [20,21,22,23]. However, these latter, seemingly silent cells have a key role in maintaining the electromechanical integrity of the heart and contribute to its adaptation to the intrinsic and extrinsic stimuli of daily activities, as well as in mediating myocardial remodeling upon tissue damage [24,25,26,27,28,29]. Interestingly, these functions are based upon various means of intercellular communication, including direct cell-to-cell interaction, secreted neurotransmitters or paracrine factors, and heterocellular electrical coupling, altogether orchestrating a well-regulated cross-talk between different CMs and non-CM cell types [30,31,32,33,34,35]. Thus far, such unique myocardial complexity has mostly been disarranged in in vitro studies on isolated cells, or analyzed upon pharmacologic or genetic perturbation of cell specific gene or protein functions [36,37,38,39,40,41,42,43], while strategies to interrogate specific cells in their native environment, the intact heart, have poorly been exploited. The view on the multicellular complexity of the myocardium is similar to the framework of the functional microcircuits of the brain, suggesting that optogenetics may represent, in molecular cardiology, as it does in neurobiology, an appropriate tool to disentangle heart circuitries.
Figure 1.
The heart is a multicellular network of excitable and non-excitable cells. Schematic representation of the myocardial network. As described in paragraphs 2 and 5, all cell types represented here have been targeted with opsins.
3. The Heart Goes to Optogenetics
After the initial run-in, while several groups provided evidence to support the use of Channelrhodopsin-2 (ChR2) as a tool to modulate the activity of mammalian neurons [8,44,45,46,47], optogenetics exploded in 2010 when it laureated Nature’s “Method of the Year” [48], and soon after touched the heart. The charme of this new technology enlightened molecular cardiologists, who first aimed at optically pacing the heart in vivo. On this trail, Bruegmann et al. [49] expressed ChR2 in mouse embryonic stem cell-derived CMs to test the method in vitro and generated transgenic mice with cardiac-specific expression of ChR2, which replicated, with light flashes instead of electrodes, epicardial pacing conventionally achieved in the electrophysiology (EP) lab. Simultaneously, ChR2 was expressed in the zebrafish heart and used to map pacemaker regions, while the yellow-light-activated chloride channel HaloRhodopsin from N. pharaonis (NpHR) was employed to inhibit cardiac function [50]. In addition, Jia et al. were able to control cardiac excitation and contraction with light by generating cx-43-coupled HEK cells stably expressing ChR2, using a so-called tandem-cell-unit (TCU) strategy [51], and demonstrated the feasibility of biological optical pacemakers. Taken altogether, these initial seminal works demonstrated that opsin targeting to the heart enables optical pacing of heartbeats in different regions of atria and ventricles, with minimal interference on the endogenous CM activity. These experiments were fundamental ‘proof-of concept’, which marked the beginning of the era of cardiac optogenetics, which was used later as a tool to delve into other aspects of cardiac physiology and pathology [52,53]. Cardiac optogenetic control required further research into the optical properties of the myocardium. By characterizing the attenuation function of ChR2-activating blue light across the heart wall, it was possible to photoactivate different tissue volumes. This was used to experimentally determine the liminal cell number required to generate extra-systolic foci, which directly assessed the threshold to overcome the protective effect of electrotonic coupling between CMs. Before optogenetics, such a physiological concept was only theoretical and inferred through numerical modeling. By applying the photoactivation assay to different regions of normal and ischemic hearts, optogenetics revealed the high arrhythmogenic potential of ectopies occurring during acute myocardial ischemia in the right ventricular outflow tract [52].
Preclinical research using cardiac optogenetics has soon aimed at developing therapeutic concepts, ranging from light-operated termination of arrhythmias to the development of biological pacemakers for cell therapy, to cardiac resynchronization [54]. In 2014, Entcheva and, simultaneously, Trayanova asked cautiously whether optogenetics would, in the following years, maintain the promise of restoring healthy heart rhythm in patients, and provided the groundwork for computational modelling of light-assisted modulation of normal and arrhythmic heartbeats [55,56]. In the following five years, modelling of cardiac optogenetics evolved to address opsin spectral sensitivity and illumination protocols, and was computed on realistic imaging data from patient’s hearts [56,57]. In parallel, the path towards clinical translation of optogenetics was signed by several reports testing suitable opsin delivery strategies [58], and showing in preclinical models the feasibility of optical antiarrhythmic therapy [53,59,60,61].
4. All-Optical: Optogenetics, Optical Mapping, and Optoelectronics
The possibilities offered by optogenetics in the understanding of physiologic and pathologic mechanisms of heart diseases have prompted the combination of light-activation with optical mapping of voltage or Ca2+ dynamics, on one hand, and methods to achieve spatially tunable light delivery on the other. All-optical investigation of cardiac electrical activity in ChR2-expressing hearts is possible by using voltage sensitive dyes, with excitation and emission spectra separated from the opsin activating light [52]. Combination of a temporally accurate actuator with high speed optical voltage mapping has allowed interruption of sustained arrhythmic waves by illuminating spatially defined areas based on the wave dynamics (Figure 2) [54,59,61]. In addition to voltage, given the central role of Ca2+ in cardiac function, optogenetics has been combined with Ca2+ sensitive dyes to monitor the second messenger dynamics upon photoactivation of specific CM subpopulations in isolated hearts [62]. Since alterations in Ca2+ dynamics are associated with arrhythmogenesis, all-optical depolarization/Ca2+ detection has been employed in vitro to develop a semiautomated antiarrhythmic drug screening platform [63,64,65,66].
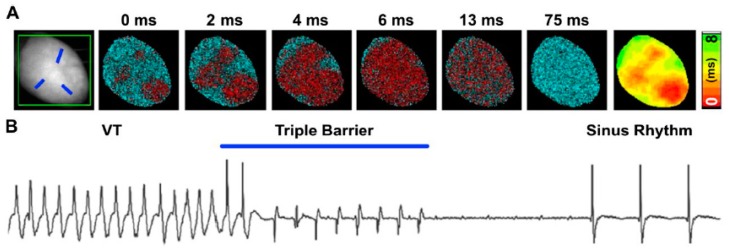
Figure 2.
Optogenetic defibrillation of mouse ventricular tachycardia via patterned illumination. (A) Pattern of optogenetic stimulation designed with three discrete sites simultaneously illuminated (referred to as Triple-barrier illumination strategy), as indicated by the blue traits on the fluorescence image shown in the left panel. The remainder pseudocolored maps show propagation of light-induced excitation in ChR2-expressing transgenic mouse heart, obtained with the red-shifted voltage sensitive dye (Di-4-ANBDQPQ). (B) ECG signal demonstrating interruption of ventricular arrhythmia and restoration of the normal sinusal rhythm upon triple-barrier pattern optogenetic stimulation (modified with permission from Crocini et al.).
The development of light crafting tools to define dynamically and at high resolution the localization of the incident photostimuli is rapidly endowing cardiac optogenetics with ways to both generate and interrupt arrhythmic waves [67,68]. The combination of techniques used in these studies inform on arrhythmia mechanisms and help refining the therapeutic electrophysiologic protocols routinely used in the clinic to interfere with arrhythmic circuits. In parallel, the evolution of light emitting device technologies has miniaturized light sources, allowing in vivo optogenetics in freely moving animals [60,69]. Further technologic improvement has allowed to couple arrhythmia-detecting telemetry ECG with the activation of implanted light sources and develop hybrid bioelectronic photo-defibrillating devices [70]. Finally, all-optical approaches have used opsins in vitro to implement automated platforms tailored for multiplexed, high-yield drug screening or cardiotoxicity studies [62,64]. These examples underline the bond between optogenetics and progress in optoelectronics, which will likely expand the applicability of the tool for cardiac research and therapy.
5. Optogenetics Targets Specific Heart Cells
As discussed above, the distinctive advantage of optogenetics over conventional methods to control cellular activity is cell type specificity. To achieve this goal, either of two main strategies have commonly been used: (i) expression of opsins under control of a cell-specific genetic driver (e.g., cell type specific promoters such as α-MyHC) or (ii) cell-selective tropism of viral vectors incorporating the opsin transgene [54,71]. While each of the two strategies has its own advantages relative to the experimental needs, which include on one hand the generation of stably expressing mouse strains, and on the other the use of different model systems (including larger mammals, or human-derived cells), they increase the versatility of optogenetics. These strategies have allowed investigations of selected cell types forming the myocardial network, some of which are summarized below.
Conduction system cells. With respect to the excitable cell component of the myocardium, the working and conducting CMs coexist, with the former making up most of the muscular mass of the heart, and the latter organized in a tree-like network which is anatomically defined at the septal root (His bundle) and infiltrates, with progressively narrower branches, the muscular matrix of the cardiac walls [72]. The distal sector of conduction system, the so-called Purkinje fiber (PF) network, has a fundamental role in physiological heart activation, and despite its dysfunction has been centrally implicated in arrhythmogenesis [73,74], its distribution in the heart sub-endocardium has limited specific investigation to destructive approaches (e.g., chemical ablation) [75]. PF-targeting of ChR2 was achieved by Zaglia et al. using the cx-40 promoter and allowed to optically pace the heart through distal PF activation [52]. The cell-specific approach was used to assess in vivo electrophysiological properties of the intact conduction system, i.e., refractoriness (Figure 3), a parameter hardly possible to determine with other methods. In addition, an estimate of the minimal number of cells needed to activate conducted beats from the PF network was obtained, proving in intact hearts the previously simulated result [76,77] that, due to their cable-like arrangement, reduced dispersion of depolarization increases the susceptibility of PFs to originate ventricular beats [52].
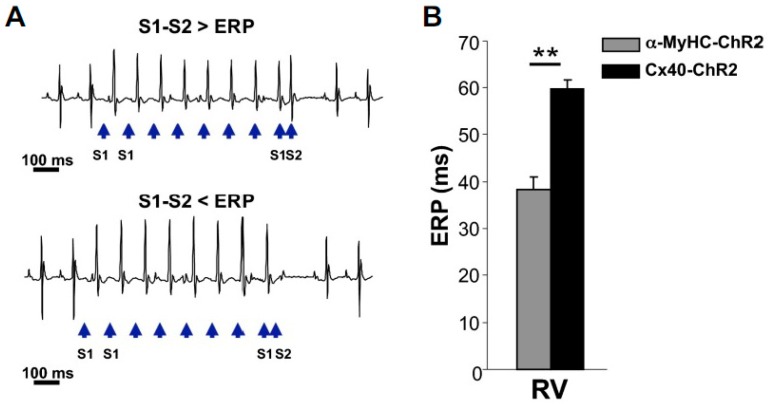
Figure 3.
Optogenetics allows selective interrogation of Purkinje fibers electrophysiology in vivo. (A) Optical programmed stimulation using the extrastimulus (S1–S2) protocol to measure the PF effective refractory period (ERP). Blue arrows indicate the light pulses. (B) Comparison of the ERP of ventricular myocytes and PF as obtained with photostimulation of the RV surface in α-MyHC-ChR2 (gray bar) and Cx40-ChR2 (black bar) mice, respectively. Bars represent s.e.m. (**, p < 0.01; n = 10 α-MyHC-ChR2 mice and n = 5 Cx40-ChR2 mice) (modified with permission from Zaglia et al.).
Cardiac neurons. Regarding cardiac excitable cells, the neuronal component of the myocardium has been neglected for a long time. However, heart function is continuously tuned by the balance between the activity of parasympathetic and sympathetic autonomic nervous inputs, which match heart rate and contractility to the instantaneous requirements of the organism. While acetylcholine, released by parasympathetic fibers, at the SinoAtrial (SAN) and AtrioVentricular (AVN) Nodes, reduces heart rate, noradrenaline (NE), discharged by the sympathetic neurons (SNs) innervating both the atria and the ventricles, is responsible for positive chronotropic and inotropic responses via β-adrenoceptors (β-AR) [78]. Alterations in the pattern of cardiac innervation and β-AR signaling have been linked to cardiac arrhythmias and, unsurprisingly, β-AR are targets of key cardiovascular therapeutics [79,80]. Despite this evidence, the mechanisms underlying autonomic control of heart function, including the biophysics of intercellular neurocardiac communication, have been somewhat poorly addressed. Recently, optogenetics started to light up the aspects of neurocardiology, and in 2015 Wengrowski et al. generated a novel mouse model expressing ChR2 under control of the SN promoter Tyrosine Hydroxylase (TH). Consistent with the effects of NE released by SNs, epicardial photostimulation of isolated hearts resulted in a positive chronotropic response, increased inotropism, and reduced Action Potential Duration (APD). In addition, fast pacing elicited sustained arrhythmic events [81]. By using the same murine model in an open-chest configuration, Prando et al. developed a optogenetic neurocardiac coupling assay which demonstrated in vivo that SNs communicate to target CMs in a ‘quasi-synaptic’ fashion, thanks to the establishment of a specific interaction site, in which NE is released in a restricted intercellular cleft [82]. More recently, the Kay group has targeted ChR2 to cholinergic neurons, contributing the specular concept that dense innervation of the SAN allows parasympathetic acetylcholine to directly control heart rhythm with a high temporal resolution [69]. That optogenetics can be used to study neuronal influence on heart physiology has also been demonstrated in different model systems, e.g., Drosophila melanogaster, upon expression of neuronally-targeted ChR2 in SNs, showing the photoactivated increase in heart rate [83].
The increasing attention to autonomic neuromodulation, as a strategy to target the arrhythmogenic effect of SNs, especially in conditions favoring electrophysiologic vulnerability, such as myocardial ischemia or heart failure [84,85], prompted the use of optogenetics to achieve optical SN inhibition. This concept was successfully proved by Yu et al. who delivered viral vectors transducing the inhibitory light-sensitive opsin, archeorhodopsin (ArchT), to the left stellate ganglia neurons of dogs. Optogenetic modulation could reversibly inhibit SN activity and was effective in preventing ventricular arrhythmias upon myocardial ischemia [86]. The use of autonomic neuron-targeted opsins, combined with implantable devices to achieve local illumination of cardiac sympathetic and parasympathetic neurons (e.g., [69]) is, in prospect, transferable to clinical applications as a flexible tool for heart neuromodulation.
Cardiac non-excitable cells. A large part of the myocardium is constituted by non-CM cell populations, mostly interstitial cells or components of blood vessels, all of which fall into the general classification of non-excitable cell types, for the lack of activation mechanisms (e.g., voltage dependent ion channels) responding to the variation of membrane potential. As such, the contribution of these cells to myocardial electrophysiology has, for a long time, not been supposed. Recent research, mostly conducted in vitro or in silico [51,57,87,88], and less frequently using electrophysiological mapping of the intact heart [89] has, however, taken into account the electrophysiological role of the cell types which, although not native parts of the CM syncytium, may electrically couple to it. These instances include the electrotonic effect of fibroblasts intertwined to CMs in the intact myocardium, or, e.g., the conditional coupling of inflammatory cells, like macrophages (MΦ), to CMs. The use of opsins, by allowing cell specific modulation of the membrane potential, has enabled, as exemplified in more detail below, the uncovering of emerging properties of nonexcitable cells in the conduction of cardiac activation waves.
Cardiac fibroblasts. Fibroblasts are the most abundant cardiac interstitial cells which are commonly recognized as having a structural role of generating the scaffold sustaining the complex myocardial architecture. While it is difficult to define the signature of cardiac fibroblasts (CFs) in terms of cell origin and surface markers [90,91], researchers agree that correct CF function is key for the maintenance of the healthy extracellular matrix, and in its remodeling after tissue damage [92]. In addition, CFs modulate the activity of CMs, both through the release of extracellular factors [23,34,35], and via direct mechanical or, interestingly, electrical interaction. This latter concept collides with the traditional notion of CFs as electrically inert "insulators" with regards to the cardiac activation wave. However, evidence is recently accruing which demonstrates that: (i) CFs express, although at low levels, cx-43, cx-45 and cx-40 [37,38], which are the CM connexin isoforms, and consistently mediate both ‘CF-CF’ homo- and ‘CF-CM’ hetero-cellular coupling; (ii) in certain sectors of the conduction system, i.e., the SAN [31], CFs are functionally connected to CMs; (iii) CFs may change their membrane potential during mechanical perturbation [93], and (iv) due to their low membrane capacitance and a high coupling resistance, CFs may function as slow conductors of electrical signals for long intramyocardial distances.
Although they are “non-excitable cells”, the aforementioned properties may change upon damage inducing CFs activation, resulting in membrane hyperpolarization, increased outward current density and membrane resistance [94,95] and enhanced cx-43 expression [96,97]. Excitingly, activated CFs not only contribute to the structural, but also to the electrophysiological myocardial remodeling, synergizing to explain the increased arrhythmogenic vulnerability [98]. Altogether, this justifies the appeal of CF optogenetics as a tool to illuminate the obscure aspects of “CF–CM” communication.
When using CF targeted optogenetics, the main problem research had to overcome was the lack of a fibroblast-specific marker. At the time, expression of opsins was achieved in isolated cell lines (e.g., HEK293, 3T3) by stable transfection or infection with specific adenoviral vectors, which allowed study of “CF–CM” coupling in vitro. As proof of principle, expression of ChR2 or ArchT was used to demonstrate that light-assisted modulation of CF membrane potential may affect the coupled CM electrical properties. Although the in vitro context is far from the complexity of the intact myocardial network, and stable cell lines may not faithfully replicate the peculiar properties of CF, in a recent report, hetero-cellular coupling between myocytes and non-myocytes has been assessed in the intact damaged myocardium using genetically encoded voltage sensitive fluorescent proteins (i.e., falling into “optogenetics” in its broader sense) [99]. The results of these studies have opened a novel view on interstitial myocardial cells, suggesting optogenetics may represent a tool to investigate it further.,The results of these studies open a novel view on interstitial myocardial cells. In a somewhat counterintuitive approach, the optical modulation of CF membrane potential may be exploited in future studies for antiarrhythmic purposes or resynchronization therapy, tasks currently reserved for the noble excitable components of the heart [71,87,100].
Vascular cells. Several cardiac pathologies are caused or accompanied by defects in the myocardial vascular system, which therefore represents a fundamental point of intervention for the therapy of heart diseases. The direct investigation of the cellular components of coronary arteries and capillaries in the intact heart in vivo has been hampered by their anatomy, which makes them difficult to reach, except for large epicardial vessels. Recently, Wu et al. generated a mouse model expressing ChR2 selectively in the excitable, contractile component of blood vessels, the vascular smooth muscle cells (SMCs), and demonstrated that spatially restricted photoactivation could be used to trigger localized vasoconstriction [101]. Given that the control over vasomotor tone is grounded on the coordinated function of both the contractile smooth muscle and the non-excitable endothelial cells (ECs), in analogy to the “CF–CM” hetero-cellular coupling in the working myocardium, the study of intercellular communication within the vessel wall in vivo is of paramount physiologic importance. Remarkably, ECs may modulate SMCs function both through the release of paracrine factors (either vasoconstrictors, i.e., ET-1 TxA2, or vasodilators, i.e., NO) and through direct electrical coupling mediated by different connexin isoforms [102]. Furthering the study of vascular physiology in vivo, Zhang et al. generated mice expressing ChR2 in ECs, and assessed the role of selectively modulating EC membrane potential on SMC contractile tone, demonstrating that photostimulation of ECs elicited in the intact excised heart “fast, robust, reproducible and long lasting vasoconstriction” [103]. Although currently vascular cell optogenetics has had very little use, it is foreseeable that the method will shortly yield important advancements in vascular cell physiology.
Cardiac inflammatory cells. Inflammation plays a central role in heart pathology and the study of cardiac inflammatory cells represents an expanding branch of cardiovascular research. Although in a simplistic view, inflammatory cells are recruited from the bloodstream to the site of tissue damage, resident cell populations have commonly been identified in all organs, including the heart. Resident cardiac macrophages (MΦ) have a key role in both the early response and subsequent phase of tissue repair, e.g., following myocardial ischemia [104]. Detailed histopathological analysis of the myocardium identified MΦ interspersed within the ventricles and in proximity of the AVN and conduction system [105]. Such peculiar topology prompted investigation of whether MΦ could have, in addition to its best-known role in the inflammatory response, effects more directly related to the conduction of electrical impulses in the heart. That MΦ could electrically couple to CMs was initially predicted based on computational modelling, and subsequently demonstrated to occur through gap junctions, allowing synchronous depolarization of the coupled cells [106]. Interestingly, expression of ChR2 in MΦ allowed demonstration that modulation of MΦ membrane potential had, conversely, the effect of facilitating AV conduction. Consistently, conditional deletion of cx-43 in MΦ or the congenital absence of these cells delayed AV conduction [107]. Thus, optogenetics allowed identification of a previously unrecognized and unpredictable function of MΦ in the heart and indicated that these cells participate directly in normal and altered conduction of cardiac electrical signals.
6. Conclusions
Here, we have briefly covered the applications of optogenetics in the study of cardiac physiology and pathology. This summary is by no means comprehensive and we apologize to the many colleagues who contributed to the field but have not been cited. In our opinion, cardiac optogenetics is still at a rather naïve stage, but is rapidly progressing beyond being a stylish tool for heart pacing. The opsin toolkit includes a large number of variants with specific features, only a few of which have been used in cardiac optogenetics. For instance, light control of intracellular signaling may be exploited in the study of myocardial development and pathologic remodeling (e.g., hypertrophy, failure). An open field of study which will benefit from the use of optogenetics is the understanding of the roles of the many different cell populations forming the heart, explored in their intact environment. With the pace of current progress in molecular biology and light crafting technology, a multicolored heart with every cell type activatable with its appropriate light beam to interrogate the myocardial network in healthy and diseased hearts can be pictured in the near future.
Abbreviation
| α-MyHC | alpha-myosin heavy chain |
| ArchT | archeorhodopsin |
| AVN | atrioventricular node |
| APD | action potential duration |
| β-AR | β-adrenoceptors |
| ChR2 | Channelrhodopsin-2 |
| CF | cardiac fibroblast |
| CM | cardiomyocyte |
| cx | connexin |
| EC | endothetial cell |
| ECG | electrocardiography |
| EP | electrophysiology |
| ERP | effective refractory period |
| ET-1 | endothelin-1 |
| MΦ | macrophage |
| NE | noradrenaline |
| NO | nitric oxide |
| NpHR | N. pharaonis HaloRhodopsin |
| PF | Purkinje fiber |
| RVSAN | right ventriclesino atrial node |
| SMC | smooth muscle cell |
| SNs | sympathetic neurons |
| TCU | tandem cell unit |
| TH | tyrosine hydroxylase |
| TxA2 | thromboxane-A2. |
Author Contributions
Conceptualization, T.Z. and M.M; Writing-Original Draft Preparation, T.Z. and M.M.; Writing-Review & Editing, T.Z., A.D.B., M.M.; Funding Acquisition, T.Z. and M.M.
Funding
This work was supported by the University of Padova (StarsWiC2017 “miniheartwork” to MM) and ARISLA (SNop) to TZ.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mattis J., Tye K.M., Ferenczi E.A., Ramakrishnan C., O’Shea D.J., Prakash R., Gunaydin L.A., Hyun M., Fenno L.E., Gradinaru V., et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods. 2011;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopton R.A., Baillie J.S., Rafferty S.A., Moss R., Zgierski-Johnston C.M., Prykhozhij S.V., Stoyek M.R., Smith F.M., Kohl P., Quinn T.A., et al. Cardiac Electrophysiological Effects of Light-Activated Chloride Channels. Front. Physiol. 2018;9:1806. doi: 10.3389/fphys.2018.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinlogel S., Feldbauer K., Dempski R.E., Fotis H., Wood P.G., Bamann C., Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca(2)+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011;14:513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 4.Gunaydin L.A., Yizhar O., Berndt A., Sohal V.S., Deisseroth K., Hegemann P. Ultrafast optogenetic control. Nat. Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 5.Berndt A., Yizhar O., Gunaydin L.A., Hegemann P., Deisseroth K. Bi-stable neural state switches. Nat. Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F., Vierock J., Yizhar O., Fenno L.E., Tsunoda S., Kianianmomeni A., Prigge M., Berndt A., Cushman J., Polle J., et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deisseroth K., Hegemann P. The form and function of channelrhodopsin. Science. 2017;357 doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein J.G., Boyden E.S. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K., Feng G., Majewska A.K., Miesenbock G., Ting A., Schnitzer M.J. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F., Aravanis A.M., Adamantidis A., de Lecea L., Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 13.Aravanis A.M., Wang L.P., Zhang F., Meltzer L.A., Mogri M.Z., Schneider M.B., Deisseroth K. An optical neural interface: In vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 14.Kravitz A.V., Freeze B.S., Parker P.R., Kay K., Thwin M.T., Deisseroth K., Kreitzer A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonnesen J., Parish C.L., Sorensen A.T., Andersson A., Lundberg C., Deisseroth K., Arenas E., Lindvall O., Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS ONE. 2011;6:e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.G N., Tan A., Farhatnia Y., Rajadas J., Hamblin M.R., Khaw P.T., Seifalian A.M. Channelrhodopsins: Visual regeneration and neural activation by a light switch. New Biotechnol. 2013;30:461–474. doi: 10.1016/j.nbt.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yizhar O., Fenno L.E., Davidson T.J., Mogri M., Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Wanjare M., Huang N.F. Regulation of the microenvironment for cardiac tissue engineering. Regen. Med. 2017;12:187–201. doi: 10.2217/rme-2016-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee I., Fuseler J.W., Price R.L., Borg T.K., Baudino T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 20.LeGrice I., Pope A., Smaill B. The Architecture of the Heart: Myocyte Organization and the Cardiac Extracellular Matrix. Dev. Cardiovasc. Med. 2005;253:3–22. [Google Scholar]
- 21.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ. Res. 1974;35:17–26. [PubMed] [Google Scholar]
- 22.Nag A.C. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 23.Tian Y., Morrisey E.E. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ. Res. 2012;110:1023–1034. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto A.R., Ilinykh A., Ivey M.J., Kuwabara J.T., D’Antoni M.L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N.A., et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ongstad E., Kohl P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell. Cardiol. 2016;91:238–246. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rog-Zielinska E.A., Norris R.A., Kohl P., Markwald R. The Living Scar—Cardiac Fibroblasts and the Injured Heart. Trends Mol. Med. 2016;22:99–114. doi: 10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mollmann H., Nef H.M., Kostin S., von Kalle C., Pilz I., Weber M., Schaper J., Hamm C.W., Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Endo J., Sano M., Fujita J., Hayashida K., Yuasa S., Aoyama N., Takehara Y., Kato O., Makino S., Ogawa S., et al. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116:1176–1184. doi: 10.1161/CIRCULATIONAHA.106.650903. [DOI] [PubMed] [Google Scholar]
- 29.Tillmanns J., Hoffmann D., Habbaba Y., Schmitto J.D., Sedding D., Fraccarollo D., Galuppo P., Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell. Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Chilton L., Giles W.R., Smith G.L. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J. Physiol. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camelliti P., Green C.R., LeGrice I., Kohl P. Fibroblast network in rabbit sinoatrial node: Structural and functional identification of homogeneous and heterogeneous cell coupling. Circ. Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 32.Kohl P., Camelliti P. Fibroblast-myocyte connections in the heart. Heart Rhythm. 2012;9:461–464. doi: 10.1016/j.hrthm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 33.De Maziere A.M., van Ginneken A.C., Wilders R., Jongsma H.J., Bouman L.N. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J. Mol. Cell. Cardiol. 1992;24:567–578. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 34.Kakkar R., Lee R.T. Intramyocardial fibroblast myocyte communication. Circ. Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottaviano F.G., Yee K.O. Communication signals between cardiac fibroblasts and cardiac myocytes. J. Cardiovasc. Pharmacol. 2011;57:513–521. doi: 10.1097/FJC.0b013e31821209ee. [DOI] [PubMed] [Google Scholar]
- 36.Kofron C.M., Mende U. In vitro models of the cardiac microenvironment to study myocyte and non-myocyte crosstalk: Bioinspired approaches beyond the polystyrene dish. J. Physiol. 2017;595:3891–3905. doi: 10.1113/JP273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Kanter E.M., Laing J.G., Aprhys C., Johns D.C., Kardami E., Yamada K.A. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun. Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louault C., Benamer N., Faivre J.F., Potreau D., Bescond J. Implication of connexins 40 and 43 in functional coupling between mouse cardiac fibroblasts in primary culture. Biochim. Biophys. Acta. 2008;1778:2097–2104. doi: 10.1016/j.bbamem.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Rook M.B., van Ginneken A.C., de Jonge B., el Aoumari A., Gros D., Jongsma H.J. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am. J. Physiol. 1992;263:C959–C977. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- 40.He K., Shi X., Zhang X., Dang S., Ma X., Liu F., Xu M., Lv Z., Han D., Fang X., et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 2011;92:39–47. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Gerdes H.H. Long-distance electrical coupling via tunneling nanotubes. Biochim. Biophys. Acta. 2012;1818:2082–2086. doi: 10.1016/j.bbamem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.P., Xie Y., Garfinkel A., Qu Z., Weiss J.N. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc. Res. 2012;93:242–251. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goshima K. Formation of nexuses and electrotonic transmission between myocardial and FL cells in monolayer culture. Exp. Cell Res. 1970;63:124–130. doi: 10.1016/0014-4827(70)90339-3. [DOI] [PubMed] [Google Scholar]
- 44.Nagel G., Ollig D., Fuhrmann M., Kateriya S., Musti A.M., Bamberg E., Hegemann P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 45.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Gutierrez D.V., Hanson M.G., Han J., Mark M.D., Chiel H., Hegemann P., Landmesser L.T., Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagel G., Szellas T., Kateriya S., Adeishvili N., Hegemann P., Bamberg E. Channelrhodopsins: Directly light-gated cation channels. Biochem. Soc. Trans. 2005;33:863–866. doi: 10.1042/BST0330863. [DOI] [PubMed] [Google Scholar]
- 48.Method of the Year 2010. Nat. Method. 2011;8:1. doi: 10.1038/nmeth.f.321. [DOI] [PubMed] [Google Scholar]
- 49.Bruegmann T., Malan D., Hesse M., Beiert T., Fuegemann C.J., Fleischmann B.K., Sasse P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 50.Arrenberg A.B., Stainier D.Y., Baier H., Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–974. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- 51.Jia Z., Valiunas V., Lu Z., Bien H., Liu H., Wang H.Z., Rosati B., Brink P.R., Cohen I.S., Entcheva E. Stimulating cardiac muscle by light: Cardiac optogenetics by cell delivery. Circ. Arrhythm. Electrophysiol. 2011;4:753–760. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaglia T., Pianca N., Borile G., Da Broi F., Richter C., Campione M., Lehnart S.E., Luther S., Corrado D., Miquerol L., et al. Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of ChannelRhodopsin-2. Proc. Natl. Acad. Sci. USA. 2015;112:E4495–E4504. doi: 10.1073/pnas.1509380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruegmann T., Boyle P.M., Vogt C.C., Karathanos T.V., Arevalo H.J., Fleischmann B.K., Trayanova N.A., Sasse P. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J. Clin. Investig. 2016;126:3894–3904. doi: 10.1172/JCI88950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyle P.M., Karathanos T.V., Trayanova N.A. Cardiac Optogenetics: 2018. JACC Clin. Electrophysiol. 2018;4:155–167. doi: 10.1016/j.jacep.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams J.C., Entcheva E. Optogenetic versus Electrical Stimulation of Human Cardiomyocytes: Modeling Insights. Biophys. J. 2015;108:1934–1945. doi: 10.1016/j.bpj.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karathanos T.V., Bayer J.D., Wang D., Boyle P.M., Trayanova N.A. Opsin spectral sensitivity determines the effectiveness of optogenetic termination of ventricular fibrillation in the human heart: A simulation study. J. Physiol. 2016;594:6879–6891. doi: 10.1113/JP271739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Entcheva E., Bub G. All-optical control of cardiac excitation: Combined high-resolution optogenetic actuation and optical mapping. J. Physiol. 2016;594:2503–2510. doi: 10.1113/JP271559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogt C.C., Bruegmann T., Malan D., Ottersbach A., Roell W., Fleischmann B.K., Sasse P. Systemic gene transfer enables optogenetic pacing of mouse hearts. Cardiovasc. Res. 2015;106:338–343. doi: 10.1093/cvr/cvv004. [DOI] [PubMed] [Google Scholar]
- 59.Crocini C., Ferrantini C., Coppini R., Scardigli M., Yan P., Loew L.M., Smith G., Cerbai E., Poggesi C., Pavone F.S., et al. Optogenetics design of mechanistically-based stimulation patterns for cardiac defibrillation. Sci. Rep. 2016;6:35628. doi: 10.1038/srep35628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe M., Feola I., Majumder R., Jangsangthong W., Teplenin A.S., Ypey D.L., Schalij M.J., Zeppenfeld K., de Vries A.A., Pijnappels D.A. Optogenetic manipulation of anatomical re-entry by light-guided generation of a reversible local conduction block. Cardiovasc. Res. 2017;113:354–366. doi: 10.1093/cvr/cvx003. [DOI] [PubMed] [Google Scholar]
- 61.Nyns E.C.A., Kip A., Bart C.I., Plomp J.J., Zeppenfeld K., Schalij M.J., de Vries A.A.F., Pijnappels D.A. Optogenetic termination of ventricular arrhythmias in the whole heart: Towards biological cardiac rhythm management. Eur. Heart J. 2017;38:2132–2136. doi: 10.1093/eurheartj/ehw574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S., Kugelman T., Buch A., Herman M., Han Y., Karakatsani M.E., Hussaini S.A., Duff K., Konofagou E.E. Non-invasive, Focused Ultrasound-Facilitated Gene Delivery for Optogenetics. Sci. Rep. 2017;7:39955. doi: 10.1038/srep39955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klimas A., Ambrosi C.M., Yu J., Williams J.C., Bien H., Entcheva E. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat. Commun. 2016;7:11542. doi: 10.1038/ncomms11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colatsky T., Fermini B., Gintant G., Pierson J.B., Sager P., Sekino Y., Strauss D.G., Stockbridge N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods. 2016;81:15–20. doi: 10.1016/j.vascn.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Dempsey G.T., Chaudhary K.W., Atwater N., Nguyen C., Brown B.S., McNeish J.D., Cohen A.E., Kralj J.M. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Lapp H., Bruegmann T., Malan D., Friedrichs S., Kilgus C., Heidsieck A., Sasse P. Frequency-dependent drug screening using optogenetic stimulation of human iPSC-derived cardiomyocytes. Sci. Rep. 2017;7:9629. doi: 10.1038/s41598-017-09760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter C., Christoph J., Lehnart S.E., Luther S. Optogenetic Light Crafting Tools for the Control of Cardiac Arrhythmias. Methods Mol. Biol. 2016;1408:293–302. doi: 10.1007/978-1-4939-3512-3_20. [DOI] [PubMed] [Google Scholar]
- 68.Majumder R., Feola I., Teplenin A.S., de Vries A.A., Panfilov A.V., Pijnappels D.A. Optogenetics enables real-time spatiotemporal control over spiral wave dynamics in an excitable cardiac system. eLife. 2018;7 doi: 10.7554/eLife.41076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno A., Endicott K., Skancke M., Dwyer M.K., Brennan J., Efimov I.R., Trachiotis G., Mendelowitz D., Kay M.W. Sudden Heart Rate Reduction Upon Optogenetic Release of Acetylcholine From Cardiac Parasympathetic Neurons in Perfused Hearts. Front. Physiol. 2019;10:16. doi: 10.3389/fphys.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nyns E.C.A., Poelma R.H., Volkers L., Plomp J.J., Bart C.I., Kip A.M., van Brakel T.J., Zeppenfeld K., Schalij M.J., Zhang G.Q., et al. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau6447. [DOI] [PubMed] [Google Scholar]
- 71.Ambrosi C.M., Klimas A., Yu J., Entcheva E. Cardiac applications of optogenetics. Prog. Biophys. Mol. Biol. 2014;115:294–304. doi: 10.1016/j.pbiomolbio.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham J.G., Klein B.A. Textbook of Veterinary Physiology. Elsevier Saunders; St. Louis, MO, USA: 2013. [Google Scholar]
- 73.Boyden P.A., Dun W., Robinson R.B. Cardiac Purkinje fibers and arrhythmias; The GK Moe Award Lecture 2015. Heart Rhythm. 2016;13:1172–1181. doi: 10.1016/j.hrthm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheinman M.M. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm. 2009;6:1050–1058. doi: 10.1016/j.hrthm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Cerrone M., Noujaim S.F., Tolkacheva E.G., Talkachou A., O’Connell R., Berenfeld O., Anumonwo J., Pandit S.V., Vikstrom K., Napolitano C., et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss J.N., Nivala M., Garfinkel A., Qu Z. Alternans and arrhythmias: From cell to heart. Circ. Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie Y., Sato D., Garfinkel A., Qu Z., Weiss J.N. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys. J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franzoso M., Zaglia T., Mongillo M. Putting together the clues of the everlasting neuro-cardiac liaison. Biochim. Biophys. Acta. 2016;1863:1904–1915. doi: 10.1016/j.bbamcr.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Yang J., Liu Y., Fan X., Li Z., Cheng Y. A pathway and network review on beta-adrenoceptor signaling and beta blockers in cardiac remodeling. Heart Fail. Rev. 2014;19:799–814. doi: 10.1007/s10741-013-9417-4. [DOI] [PubMed] [Google Scholar]
- 80.Vilar S., Sobarzo-Sanchez E., Santana L., Uriarte E. Molecular Docking and Drug Discovery in beta-Adrenergic Receptors. Curr. Med. Chem. 2017;24:4340–4359. doi: 10.2174/0929867324666170724101448. [DOI] [PubMed] [Google Scholar]
- 81.Wengrowski A.M., Wang X., Tapa S., Posnack N.G., Mendelowitz D., Kay M.W. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc. Res. 2015;105:143–150. doi: 10.1093/cvr/cvu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prando V., Da Broi F., Franzoso M., Plazzo A.P., Pianca N., Francolini M., Basso C., Kay M.W., Zaglia T., Mongillo M. Dynamics of neuroeffector coupling at cardiac sympathetic synapses. J. Physiol. 2018;596:2055–2075. doi: 10.1113/JP275693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malloy C., Sifers J., Mikos A., Samadi A., Omar A., Hermanns C., Cooper R.L. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2017;203:791–806. doi: 10.1007/s00359-017-1191-7. [DOI] [PubMed] [Google Scholar]
- 84.Buckley U., Shivkumar K., Ardell J.L. Autonomic Regulation Therapy in Heart Failure. Curr. Heart Fail. Rep. 2015;12:284–293. doi: 10.1007/s11897-015-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen M.J., Zipes D.P. Interventional and device-based autonomic modulation in heart failure. Heart Fail. Clin. 2015;11:337–348. doi: 10.1016/j.hfc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Yu L., Zhou L., Cao G., Po S.S., Huang B., Zhou X., Wang M., Yuan S., Wang Z., Wang S., et al. Optogenetic Modulation of Cardiac Sympathetic Nerve Activity to Prevent Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2017;70:2778–2790. doi: 10.1016/j.jacc.2017.09.1107. [DOI] [PubMed] [Google Scholar]
- 87.Nussinovitch U., Shinnawi R., Gepstein L. Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc. Res. 2014;102:176–187. doi: 10.1093/cvr/cvu037. [DOI] [PubMed] [Google Scholar]
- 88.Nussinovitch U., Gepstein L. Optogenetics for suppression of cardiac electrical activity in human and rat cardiomyocyte cultures. Neurophotonics. 2015;2:031204. doi: 10.1117/1.NPh.2.3.031204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahoney V.M., Mezzano V., Mirams G.R., Maass K., Li Z., Cerrone M., Vasquez C., Bapat A., Delmar M., Morley G.E. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci. Rep. 2016;6:26744. doi: 10.1038/srep26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moore-Morris T., Cattaneo P., Puceat M., Evans S.M. Origins of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mikawa T., Gourdie R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 92.Vasquez C., Morley G.E. The origin and arrhythmogenic potential of fibroblasts in cardiac disease. J. Cardiovasc. Transl. Res. 2012;5:760–767. doi: 10.1007/s12265-012-9408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohl P., Kamkin A.G., Kiseleva I.S., Streubel T. Mechanosensitive cells in the atrium of frog heart. Exp. Physiol. 1992;77:213–216. doi: 10.1113/expphysiol.1992.sp003576. [DOI] [PubMed] [Google Scholar]
- 94.Vasquez C., Mohandas P., Louie K.L., Benamer N., Bapat A.C., Morley G.E. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ. Res. 2010;107:1011–1020. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiseleva I., Kamkin A., Pylaev A., Kondratjev D., Leiterer K.P., Theres H., Wagner K.D., Persson P.B., Gunther J. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J. Mol. Cell. Cardiol. 1998;30:1083–1093. doi: 10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 96.Vasquez C., Benamer N., Morley G.E. The cardiac fibroblast: Functional and electrophysiological considerations in healthy and diseased hearts. J. Cardiovasc. Pharmacol. 2011;57:380–388. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y., Kanter E.M., Yamada K.A. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc. Pathol. 2010;19:e233–e240. doi: 10.1016/j.carpath.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roell W., Lewalter T., Sasse P., Tallini Y.N., Choi B.R., Breitbach M., Doran R., Becher U.M., Hwang S.M., Bostani T., et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 99.Quinn T.A., Camelliti P., Rog-Zielinska E.A., Siedlecka U., Poggioli T., O’Toole E.T., Knopfel T., Kohl P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl. Acad. Sci. USA. 2016;113:14852–14857. doi: 10.1073/pnas.1611184114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu J., Entcheva E. Inscribing Optical Excitability to Non-Excitable Cardiac Cells: Viral Delivery of Optogenetic Tools in Primary Cardiac Fibroblasts. Methods Mol. Biol. 2016;1408:303–317. doi: 10.1007/978-1-4939-3512-3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y., Li S.S., Jin X., Cui N., Zhang S., Jiang C. Optogenetic approach for functional assays of the cardiovascular system by light activation of the vascular smooth muscle. Vascul. Pharmacol. 2015;71:192–200. doi: 10.1016/j.vph.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Figueroa X.F., Duling B.R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S., Cui N., Wu Y., Zhong W., Johnson C.M., Jiang C. Optogenetic intervention to the vascular endothelium. Vascul. Pharmacol. 2015;74:122–129. doi: 10.1016/j.vph.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hulsmans M., Sam F., Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J. Mol. Cell. Cardiol. 2016;93:149–155. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hulsmans M., Clauss S., Xiao L., Aguirre A.D., King K.R., Hanley A., Hucker W.J., Wulfers E.M., Seemann G., Courties G., et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169:510–522. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munshi N.V. Resident Macrophages: Near and Dear to Your Heart. Cell. 2017;169:376–377. doi: 10.1016/j.cell.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hulsmans M., Sager H.B., Roh J.D., Valero-Munoz M., Houstis N.E., Iwamoto Y., Sun Y., Wilson R.M., Wojtkiewicz G., Tricot B., et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018;215:423–440. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]