Abstract
Background
Acute kidney injury (AKI) after out-of-hospital cardiac arrest (OHCA) is a well-known predictor for mortality. However, the natural course of AKI including recovery rate after OHCA is uncertain. This study investigated the clinical course of AKI after OHCA and determined whether recovery from AKI impacted the outcomes of OHCA.
Methods
This retrospective multicentre cohort study included adult OHCA patients treated with targeted temperature management (TTM) between January 2016 and December 2017. AKI was diagnosed using the Kidney Disease: Improving Global Outcomes criteria. The primary outcome was the recovery rate after AKI and its association with survival and good neurological outcome at discharge.
Results
A total of 3697 OHCA patients from six hospitals were screened and 275 were finally included. AKI developed in 175/275 (64%) patients and 69/175 (39%) patients recovered from AKI. In most cases, AKI developed within three days of return of spontaneous circulation [155/175 (89%), median time to AKI development 1 (1–2) day] and patients recovered within seven days of return of spontaneous circulation [59/69 (86%), median time to AKI recovery 3 (2–7) days]. Duration of AKI was significantly longer in the AKI non-recovery group than in the AKI recovery group [5 (2–9) vs. 1 (1–5) days; P < 0.001]. Most patients were diagnosed with AKI stage 1 initially [120/175 (69%)]. However, the number of stage 3 AKI patients increased from 30/175 (17%) to 77/175 (44%) after the initial diagnosis of AKI. The rate of survival discharge was significantly higher in the AKI recovery group than in the AKI non-recovery group [45/69 (65%) vs. 17/106 (16%); P < 0.001]. Recovery from AKI was a potent predictor of survival and good neurological outcome at discharge in the multivariate analysis (adjusted odds ratio, 8.308; 95% confidence interval, 3.120–22.123; P < 0.001 and adjusted odds ratio, 36.822; 95% confidence interval, 4.097–330.926; P = 0.001).
Conclusions
In our cohort of adult OHCA patients treated with TTM (n = 275), the recovery rate from AKI after OHCA was 39%, and recovery from AKI was a potent predictor of survival and good neurological outcome at discharge.
Electronic supplementary material
The online version of this article (10.1186/s13054-019-2535-1) contains supplementary material, which is available to authorized users.
Keywords: Acute kidney injury, Out-of-hospital cardiac arrest, Targeted temperature management, Therapeutic hypothermia, Survival rate
Background
Development of acute kidney injury (AKI) after out-of-hospital cardiac arrest (OHCA) is a well-known predictor for mortality and poor neurological outcomes in both adult and paediatric populations [1–6]. However, the natural course of AKI in terms of its recovery rate after OHCA is uncertain. Although the incidence and onset of AKI development have been reported several times, recovery rate after AKI has not been reported to date [3]. If we know the natural course of AKI after OHCA, it will be helpful in making appropriate clinical decisions such as determining the initiation time for renal replacement therapy (RRT) and making a prognosis in OHCA patients with AKI development. We hypothesised that recovery from AKI may affect the survival discharge and neurological status at discharge in OHCA patients treated with targeted temperature management (TTM). Therefore, this study investigated the clinical course of AKI (from development to recovery) after OHCA treated with TTM and determined whether recovery from AKI has an impact on the outcomes of OHCA after treatment with TTM.
Methods
Study design and setting
This was a retrospective, multicentre, cohort study. Data were collected by the review of medical record from the emergency departments of six academic hospitals located in South Korea. The study design and plan were approved by the institutional review boards of each hospital. Written informed consent was waived by the institutional review boards. We searched the medical records using the following diagnostic terminology: ‘death on arrival’, ‘cardiac arrest with successful resuscitation’, ‘cardiac arrest, unspecified’, ‘respiratory arrest’ and ‘post-resuscitation state’.
Study population
All adult patients who visited the emergency departments of the six hospitals for OHCA between January 2016 and December 2017 and were treated with TTM were screened. The following patients were excluded: patients aged < 19 years; patients reported dead on arrival to the hospital and did not receive cardiopulmonary resuscitation (CPR); patients who did not achieve return of spontaneous circulation (ROSC) despite performing CPR; patients who did not receive TTM despite achieving ROSC; patients who were diagnosed with end-stage renal disease (ESRD) with dialysis (peritoneal dialysis or haemodialysis) before developing cardiac arrest; patients who had a do-not-attempt resuscitation (DNAR) order prior to the development of cardiac arrest; patients who had acute intracranial haemorrhage or acute ischemic stroke; and patients who had active bleeding.
Definition of the development of and recovery of AKI
The definition and staging of AKI were based on the diagnostic criteria stipulated in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (Additional file 1) [7]. Development of AKI was diagnosed if one of the criteria was fulfilled (serum creatinine or hourly urine output). If both criteria, the one for serum creatinine and hourly urine output, were fulfilled to diagnose different stages of AKI development, higher stage of AKI was diagnosed. If the data for the serum creatinine level within the last 3 months before the development of cardiac arrest were available, it was regarded as baseline serum creatinine. If this data were unavailable, the lower value between the estimated serum creatinine level and the first measured serum creatinine after achieving ROSC was regarded as baseline serum creatinine [7, 8]. Recovery from AKI was characterised by the fulfilment of all of the following conditions (absence of AKI criteria): serum creatinine decreased below the level determined in the definition of stage 1 AKI and hourly urine output was maintained over 0.5 mL/kg/h for ≥ 12 h [9].
Variables
Primary outcome variables included the dates of AKI development and recovery, the duration of AKI, recovery rate, and survival discharge. If the AKI stage changed after the initial diagnosis, the highest stage of AKI was recorded. Secondary outcome variables included the following variables recommended by the Utstein resuscitation registry templates for OHCA: age (years), sex (male or female), weight (kg), past medical history (hypertension, diabetes mellitus, heart failure, or chronic kidney disease), witnessed arrest (witnessed or not witnessed), arrest location (home, workplace, sports or recreation, street or highway, public building, nursing home, and educational institution), bystander CPR (yes or no), first monitored rhythm (ventricular fibrillation, pulseless ventricular tachycardia, pulseless electrical activity, and asystole), cause of arrest (medical, trauma, drug overdose, drowning, electrocution and asphyxia), response time (time interval from call to visit), defibrillation time (time interval from call to the time the first shock), CPR duration (the sum of the prehospital and emergency room CPR times, min), epinephrine dose (mg), coronary angiography (yes or no), extracorporeal membrane oxygenation (yes or no), and targeted temperature of TTM (33 °C vs. 36 °C). The target temperature was determined by the physicians in charge of managing the patients at each institution because there is no difference in the outcomes between 33 °C and 36 °C. Therefore, a wide range of target temperatures [from 32 °C to 36 °C] are recommended in institutional standard operating procedures since the publication of the 2015 guidelines) [10, 11], maintenance period of TTM (24 h or 48 h), urine output (mL/kg/h) in the intensive care unit, event of shock (mean arterial pressure < 70 mmHg) after ROSC, daily serum creatinine level (mg/dL), RRT (yes or no), the type of RRT (continuous RRT or haemodialysis) and date of RRT (initiation, termination and duration), discharge date, survival status at discharge (yes or no) and Modified Rankin Scale (MRS) score at discharge. If the serum creatinine level was checked two or more times in a day, the highest value was recorded. All dates were calculated from the day of ROSC. The MRS score was used to assess neurological outcomes, with an MRS score of 0–3 being regarded as a good outcome and that of 4–6 as a poor outcome [12, 13].
Statistical methods
Descriptive statistics are reported as medians (interquartile range) or means with standard deviation for continuous variables according to the normality of distribution. The normal distribution of data was analysed using the Shapiro–Wilk test or Kolmogorov–Smirnov test. Categorical variables are reported as frequency (percentage). Demographics and clinical differences between groups were assessed using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. Comparisons of variables between groups were conducted using the two-sided Student t test or Mann–Whitney U test, as appropriate.
The association between predictors and outcome was quantified using odds ratio (OR) with 95% confidence interval (CI). To determine the independent factors associated with AKI development, recovery, and outcomes, we performed multivariate logistic regression analysis initially including all variables with P value < 0.2; we then applied a stepwise backward selection of the variables which remained significant. The Hosmer–Lemeshow test was used to evaluate the goodness of fit of the logistic regression model. The Cox regression analysis with time-varying covariate was used in the survival analysis to calculate the hazard ratio of AKI with respect to mortality. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Study population
Of the 3697 OHCA patients screened during the study period, 275 patients treated with TTM at six academic hospitals in South Korea were enrolled after excluding 3422 patients (Fig. 1). The baseline characteristics of the study population according to the development of and recovery from AKI are summarised in Tables 1 and 2.
Fig. 1.
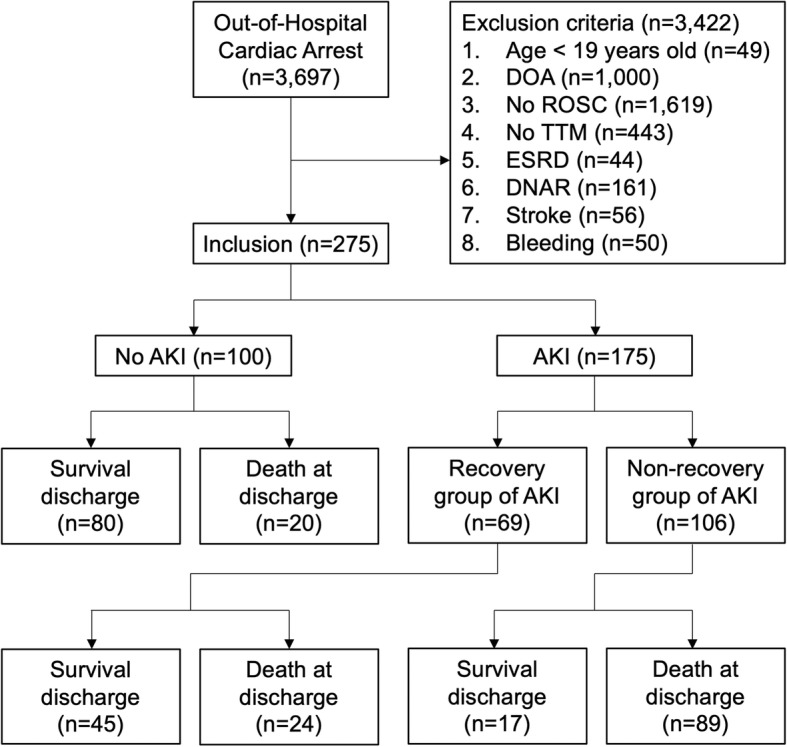
Flow chart of the study population. AKI, acute kidney injury; DNAR, do-not-attempt-resuscitation; DOA, death on arrival; ESRD, end-stage renal disease; ROSC, return of spontaneous circulation; TTM, targeted temperature management
Table 1.
Baseline characteristics of the study population according to acute kidney injury development
| Variable | All patients (n = 275) | No AKI (n = 100) | AKI (n = 175) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Male sex | 187/275 (68) | 70/100 (70) | 117/175 (67) | 0.591 |
| Age ≥ 60 years | 139/275 (51) | 40/100 (40) | 99/175 (57) | 0.008 |
| Weight ≥ 60 kg | 178/272 (65) | 63/98 (64) | 115/174 (66) | 0.764 |
| Medical history | ||||
| Heart failure | 7/272 (3) | 0/100 (0) | 7/172 (4) | 0.050 |
| Hypertension | 114/273 (42) | 30/100 (30) | 84/173 (49) | 0.003 |
| Diabetes mellitus | 67/273 (25) | 17/100 (17) | 50/173 (29) | 0.028 |
| Chronic kidney disease | 10/272 (4) | 0/100 (0) | 10/172 (6) | 0.015 |
| Resuscitation | ||||
| Arrest cause | 0.841 | |||
| Medical or uncertain | 224/275 (82) | 83/100 (83) | 141/175 (81) | |
| Trauma | 4/275 (2) | 0/100 (0) | 4/175 (2) | |
| Poisoning | 3/275 (1) | 1/100 (1) | 2/175 (1) | |
| Drowning | 1/275 (0) | 1/100 (1) | 0/175 (0) | |
| Asphyxia | 43/275 (16) | 15/100 (15) | 28/175 (16) | |
| Arrest location | 0.550 | |||
| Home | 134/275 (49) | 47/100 (47) | 87/175 (50) | |
| Workplace | 10/275 (4) | 3/100 (3) | 7/175 (4) | |
| Sport | 6/275 (2) | 5/100 (5) | 1/175 (1) | |
| Street | 65/275 (24) | 19/100 (19) | 46/175 (26) | |
| Public building | 56/275 (20) | 24/100 (24) | 32/175 (18) | |
| Nursing home | 4/275 (1) | 2/100 (2) | 2/175 (1) | |
| Witnessed arrest | 207/275 (75) | 80/100 (80) | 127/175 (73) | 0.170 |
| Bystander CPR | 161/275 (59) | 58/100 (58) | 103/175 (59) | 0.890 |
| CPR time ≥ 20 min | 135/242 (56) | 34/86 (40) | 101/156 (65) | < 0.001 |
| Shockable rhythm | 82/264 (31) | 45/95 (47) | 37/169 (22) | < 0.001 |
| Adrenaline dose ≥ 4 mg | 61/225 (27) | 11/71 (16) | 50/154 (33) | 0.008 |
| Post-resuscitation | ||||
| Shock | 186/275 (68) | 44/100 (44) | 142/175 (81) | < 0.001 |
| Coronary angiography | 64/275 (23) | 34/100 (34) | 30/175 (17) | 0.001 |
| Target temperature = 33 °C | 210/275 (76) | 82/100 (82) | 128/175 (73) | 0.096 |
| TTM duration = 24 h | 224/275 (82) | 84/100 (84) | 140/175 (80) | 0.412 |
| Outcomes | ||||
| Survival discharge | 142/275 (52) | 80/100 (80) | 62/175 (35) | < 0.001 |
| MRS score 0 to 3 at discharge | 72/275 (26) | 47/100 (47) | 25/175 (14) | < 0.001 |
P < 0.05 are presented in italics
AKI acute kidney injury, CPR cardiopulmonary resuscitation, MRS modified Rankin Scale, RRT renal replacement therapy, TTM targeted temperature management
Table 2.
Baseline characteristics of the study population according to the recovery of acute kidney injury
| Variable | All patients with AKI (n = 175) | AKI non-recovery group (n = 106) | AKI recovery group (n = 69) | P value |
|---|---|---|---|---|
| Characteristics of AKI | ||||
| AKI stage (initial) | < 0.001 | |||
| Stage 1 | 120/175 (69) | 57/106 (54) | 63/69 (91) | |
| Stage 2 | 25/175 (14) | 20/106 (19) | 5/69 (7) | |
| Stage 3 | 30/175 (17) | 29/106 (27) | 1/69 (2) | |
| AKI stage (highest) | < 0.001 | |||
| Stage 1 | 68/175 (39) | 16/106 (15) | 52/69 (75) | |
| Stage 2 | 30/175 (17) | 20/106 (19) | 10/69 (15) | |
| Stage 3 | 77/175 (44) | 70/106 (66) | 7/69 (10) | |
| Duration of AKI ≥ 4 days | 88/175 (50) | 66/106 (62) | 22/69 (32) | < 0.001 |
| RRT frequency | 45/175 (26) | 41/106 (39) | 4/69 (6) | < 0.001 |
| RRT duration ≥ 4 days | 23/45 (51) | 22/41 (54) | 1/4 (25) | 0.346 |
| †RRT requirements at discharge | 4/8 (50) | 4/6 (67) | 0/2 (0) | 0.429 |
| Demographics | ||||
| Male sex | 117/175 (67) | 70/106 (66) | 47/69 (68) | 0.775 |
| Age ≥ 60 years | 99/175 (57) | 68/106 (64) | 31/69 (45) | 0.012 |
| Weight ≥ 60 kg | 115/174 (66) | 71/105 (68) | 44/69 (64) | 0.600 |
| Medical history | ||||
| Heart failure | 7/172 (4) | 6/104 (6) | 1/68 (2) | 0.247 |
| Hypertension | 84/173 (49) | 56/105 (53) | 28/68 (41) | 0.118 |
| Diabetes mellitus | 50/173 (29) | 37/105 (35) | 13/68 (19) | 0.022 |
| Chronic kidney disease | 10/172 (6) | 9/104 (9) | 1/68 (2) | 0.091 |
| Resuscitation | ||||
| Arrest cause | 0.520 | |||
| Medical or uncertain | 141/175 (81) | 88/106 (83) | 53/69 (77) | |
| Trauma | 4/175 (2) | 2/106 (2) | 2/69 (3) | |
| Poisoning | 2/175 (1) | 0/106 (0) | 2/69 (3) | |
| Drowning | 0/175 (0) | 0/106 (0) | 0/69 (0) | |
| Asphyxia | 28/175 (16) | 16/106 (15) | 12/69 (17) | |
| Arrest location | 0.680 | |||
| Home | 87/175 (50) | 57/106 (54) | 30/69 (44) | |
| Workplace | 7/175 (4) | 1/106 (1) | 6/69 (9) | |
| Sport | 1/175 (1) | 0/106 (0) | 1/69 (1) | |
| Street | 46/175 (26) | 27/106 (26) | 19/69 (28) | |
| Public building | 32/175 (18) | 19/106 (18) | 13/69 (19) | |
| Nursing home | 2/175 (1) | 2/106 (2) | 0/69 (0) | |
| Witnessed arrest | 127/175 (73) | 76/106 (72) | 51/69 (74) | 0.748 |
| Bystander CPR | 103/175 (59) | 61/106 (58) | 42/69 (61) | 0.662 |
| CPR time ≥ 20 min | 101/156 (65) | 71/96 (74) | 30/60 (50) | 0.002 |
| Shockable rhythm | 37/169 (22) | 19/101 (19) | 18/68 (27) | 0.238 |
| Adrenaline dose ≥ 4 mg | 50/154 (33) | 40/96 (42) | 10/58 (17) | 0.002 |
| Post-resuscitation | ||||
| Shock | 142/175 (81) | 96/106 (91) | 46/69 (67) | < 0.001 |
| Coronary angiography | 30/175 (17) | 13/106 (12) | 17/69 (25) | 0.034 |
| Target temperature = 33 °C | 128/175 (73) | 76/106 (72) | 52/69 (75) | 0.593 |
| TTM duration = 24 h | 140/175 (80) | 83/106 (78) | 57/69 (83) | 0.486 |
| Outcomes | ||||
| Survival discharge | 62/175 (35) | 17/106 (16) | 45/69 (65) | < 0.001 |
| MRS score 0 to 3 at discharge | 25/175 (14) | 3/106 (3) | 22/69 (32) | < 0.001 |
P < 0.05 are presented in italics
†Thirty-seven patients receiving renal replacement therapy (RRT) died before discharge. Therefore, whether or not RRT was required could only be assessed in eight patients
AKI acute kidney injury, RRT renal replacement therapy, CPR cardiopulmonary resuscitation, TTM targeted temperature management, MRS modified Rankin Scale
Descriptive data
The median age of patients was 66 (55–73) years, and weight was 65 ± 12 kg, with most patients being male (187/275, 68%). The median time interval from call to arrival of emergency medical service was 7 (5–11) min. The median duration of CPR was 23 (12–32) min, and median dose of adrenaline (epinephrine) was 2 (1–4) mg.
Clinical course of AKI
AKI developed in 175/275 (64%) patients and 69/175 (39%) patients recovered from AKI. In total, 169/175 (97%) patients were diagnosed with AKI based on serum creatinine level and 6/175 (3%) were diagnosed based on the urine output criteria. In most cases, AKI developed within three days of ROSC [155/175 (89%), median time to AKI development, 1 (1–2) day] and patients recovered within seven days of ROSC [59/69 (86%), median time to AKI recovery, 3 (2–7) days]. The median duration of AKI was 4 (2–7) days. Duration of AKI was significantly longer in the AKI non-recovery group than in the AKI recovery group [5 (2–9) vs. 1 (1–5) days; P < 0.001]. Most patients were diagnosed with AKI stage 1 initially [120/175 (69%)]. However, the number of stage 3 AKI patients increased from 30/175 (17%) to 77/175 (44%) after the initial diagnosis of AKI (Table 2). In addition, the proportions of patients at different AKI stages varied significantly between AKI non-recovery and recovery groups (Table 2 and Fig. 2; P < 0.001). RRT was conducted in 45/175 (26%) patients. The median duration of RRT was 4 (1–8) days. Use of RRT was significantly higher in the AKI non-recovery group than in the AKI recovery group [41/106 (39%) vs. 4/69 (6%); P < 0.001]. RRT requirements at discharge could only be evaluated in eight patients (4/8 [50%]), because 37 patients receiving RRT did not survive till discharge (Table 2). Four of these patients required RRT at discharge, two had fully recovered by discharge, and two were diagnosed with chronic kidney disease.
Fig. 2.
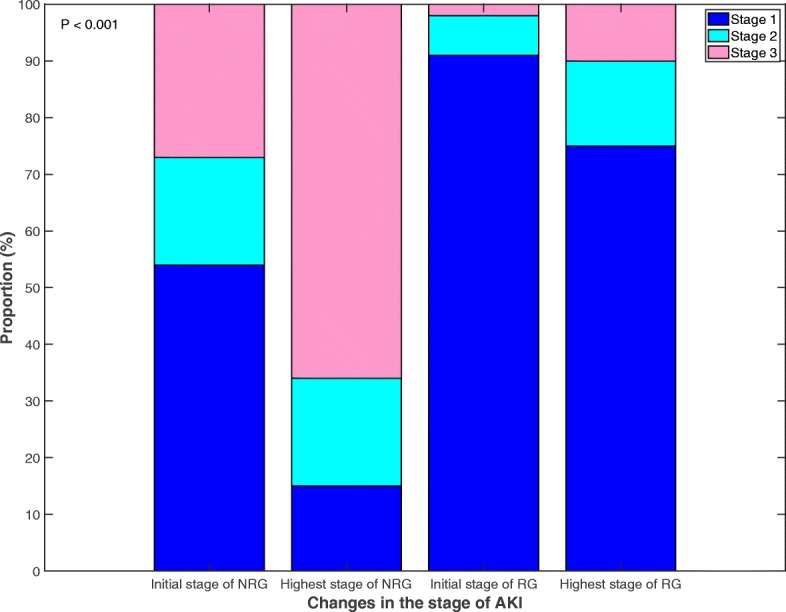
Changes in the stage of acute kidney injury after out-of-hospital cardiac arrest. AKI, acute kidney injury; NRG, non-recovery group; RG, recovery group
Factors associated with AKI development and recovery
In all patients, hypertension and shock were potent predictors for the development of AKI (Table 3). Among patients with AKI, hypertension, CPR time ≥ 20 min, AKI stage 3 (highest), and shock were negatively associated with recovery from AKI (Table 3).
Table 3.
Factors associated with acute kidney injury development and recovery in multivariate analysis
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| AKI development | |||
| Hypertension | 3.011 | 1.434, 6.322 | 0.004 |
| Shock | 5.654 | 2.750, 11.626 | < 0.001 |
| AKI recovery | |||
| Hypertension | 0.357 | 0.137, 0.930 | 0.035 |
| CPR time ≥ 20 min | 0.275 | 0.104, 0.730 | 0.010 |
| AKI stage 3 (highest) | 0.130 | 0.040, 0.420 | 0.001 |
| Shock | 0.173 | 0.049, 0.605 | 0.006 |
P < 0.05 are presented in italics
AKI acute kidney injury, CPR cardiopulmonary resuscitation
AKI development: Hosmer and Lemeshow test: chi-square = 0.537; df = 4; P = 0.970
AKI recovery: Hosmer and Lemeshow test: chi-square = 8.980; df = 7; P = 0.254
Impact of target temperature on the AKI
After adjusting for age, medical history, witnessed arrest, CPR time, shockable rhythm, epinephrine dose, shock, and coronary angiography, the target temperature was not associated with the development of AKI (the target temperature was eliminated in step 5 of a stepwise backward selection of variables; OR, 0.670; 95% CI, 0.280–1.601; P = 0.367). In addition, the target temperature was also not associated with recovery from AKI (OR, 1.207; 95% CI, 0.605–2.411; P = 0.593).
Impact of development of and recovery from AKI on outcomes
Survival discharge and good neurological outcome at discharge (MRS scores, 0–3) were observed in a significantly higher number of non-AKI patients than in AKI patients [survival discharge 80/100 (80%) vs. 62/175 (35%); P < 0.001; good neurological outcome, 47/100 (47%) vs. 25/175 (14%); P < 0.001, Table 1]. In addition, the proportion of patients with survival discharge and good neurological outcome at discharge was significantly higher in the AKI recovery group than in the AKI non-recovery group [survival discharge, 45/69 (65%) vs. 17/106 (16%); P < 0.001; good neurological outcome 22/69 (32%) vs. 3/106 (3%); P < 0.001].
In multivariate analysis, the development of AKI was negatively associated with survival discharge (adjusted OR, 0.238; 95% CI, 0.102–0.555; P = 0.001; Table 4). In patients with AKI, recovery from AKI was a potent predictor of survival discharge (adjusted OR, 8.308; 95% CI, 3.120–22.123; P < 0.001; Table 4). Development of AKI was not associated with good neurological outcome at discharge; however, recovery from AKI was a potent predictor of good neurological outcome at discharge (adjusted OR, 36.822; 95% CI 4.097–330.926; P = 0.001; Table 5).
Table 4.
Factors associated with survival discharge in multivariate analysis
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| All patients | |||
| Adrenaline dose ≥ 4 mg | 0.428 | 0.187, 0.978 | 0.044 |
| Shock | 0.089 | 0.034, 0.232 | < 0.001 |
| Coronary angiography | 10.955 | 3.412, 35.172 | < 0.001 |
| AKI | 0.238 | 0.102, 0.555 | 0.001 |
| AKI patients | |||
| Shock | 0.178 | 0.051, 0.626 | 0.007 |
| Coronary angiography | 12.084 | 3.116, 46.856 | < 0.001 |
| AKI recovery | 8.308 | 3.120, 22.123 | < 0.001 |
P < 0.05 are presented in italics
AKI acute kidney injury
All patients: Hosmer and Lemeshow test: chi-square = 8.954; df = 7; P = 0.256
AKI patients: Hosmer and Lemeshow test: chi-square = 5.315; df = 4; P = 0.256
Table 5.
Factors associated with good neurological outcome in multivariate analysis
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| All patients | |||
| Shockable rhythm | 3.085 | 1.057, 9.006 | 0.039 |
| CPR time ≥ 20 min | 0.235 | 0.081, 0.679 | 0.007 |
| Shock | 0.160 | 0.055, 0.465 | 0.001 |
| Coronary angiography | 14.087 | 4.291, 46.248 | < 0.001 |
| AKI patients | |||
| Coronary angiography | 12.644 | 2.712, 58.952 | 0.001 |
| AKI recovery | 36.822 | 4.097, 330.926 | 0.001 |
P < 0.05 are presented in italics
AKI acute kidney injury, CPR cardiopulmonary resuscitation
All patients: Hosmer and Lemeshow test: chi-square = 5.207; df = 6; P = 0.518
AKI patients: Hosmer and Lemeshow test: chi-square = 0.733; df = 2; P = 0.693
Subgroup analysis in patients with good neurological outcomes
There were no significant differences in the distribution of MRS scores according to AKI development, recovery, and RRT in the cohort with good neurological outcomes at discharge (see Additional file 1, Tables S1 to S3). Only two patients (MRS 2 and 3) were treated with RRT (Additional file 1: Table S3), one of whom (MRS 3) still required RRT at discharge.
Recovery rate after AKI according to the stages of AKI
Recovery rate after AKI was significantly higher in patients with stage 1 AKI than in those with stage 2 or 3 AKI [initial stage: stage 1, 63/120 (53%); stage 2, 5/25 (20%); stage 3, 1/30 (3%); P < 0.001 and highest stage: stage 1, 52/68 (77%); stage 2, 10/30 (33%); stage 3, 7/77 (9%); P < 0.001).
Survival analysis
Cox regression analysis with time-varying covariate of AKI showed that patients with AKI had a higher risk of death than those without AKI (hazard ratio, 2.813; 95% confidence interval, 1.628–4.859; P < 0.001; Table 6).
Table 6.
Cox regression analysis with time-varying covariate of development of acute kidney injury showing the effect of five variables on the risk of death
| Variable | Coefficient | Standard Error | Wald | P value | Hazards ratio | 95% CI |
|---|---|---|---|---|---|---|
| Witnessed arrest | −0.485 | 0.218 | 4.968 | 0.026 | 0.615 | 0.402, 0.943 |
| Adrenaline dose ≥ 4 mg | 0.611 | 0.211 | 8.419 | 0.004 | 1.843 | 1.219, 2.784 |
| Coronary angiography | −1.629 | 0.468 | 12.138 | < 0.001 | 0.196 | 0.078, 0.490 |
| Shock | 1.446 | 0.374 | 14.976 | < 0.001 | 4.244 | 2.041, 8.826 |
| †T_COV_AKI | 1.034 | 0.279 | 13.746 | < 0.001 | 2.813 | 1.628, 4.859 |
P < 0.05 are presented in italics
†T_COV_AKI means time-varying covariate of AKI development
AKI acute kidney injury, CI confidence interval
Discussion
To the best of our knowledge, the present study is the first to investigate the recovery rate after AKI and the association between the recovery from AKI and outcomes after OHCA in patients treated with TTM. Although the natural course of AKI and the relationship between AKI and clinical outcomes in other critical care areas such as sepsis have been previously investigated, the clinical outcomes in post-cardiac arrest care are still uncertain [14].
The overall incidence of AKI in our cohort (64%) was slightly higher than the weighted mean prevalence of AKI (52%; range, 37–81%) in a previous systematic review because the proportion of patients with a non-shockable rhythm in our cohort was high (69%) [3]. In South Korea, non-shockable rhythms occupy a large proportion of initial rhythms [15]. Because shockable rhythms are associated with a significantly lower risk of developing AKI, the incidence of AKI in our cohort might be higher than that in previous reports [4, 6]. The same reason may have affected the low recovery rate after AKI in our cohort (39%); however, the proportions of shockable rhythms were not significantly different between the AKI non-recovery and recovery groups [19/101 (19%) vs. 18/68 (27%); P = 0.238]. A previous study reported that the renal function returned to normal before hospital discharge in 67/83 (81%) survivors [3]. In our results, the recovery rate increased up to 73% when we considered only the patients with survival discharge (45/62 patients). However, we could not determine whether the recovery rate after AKI would be different depending on the proportion of shockable rhythms. Thus, further studies including higher proportion of shockable rhythms are needed.
In contrast to the negative association between the development of AKI and survival discharge, there was a strong positive association between recovery from AKI and outcomes (acceptance of our hypothesis). This association may be caused by changes in the AKI stages after the initial diagnosis of AKI because an increase in the proportion of patients with stage 3 AKI led to an increase in mortality in this study. In the case of the AKI non-recovery group, the proportion of patients with stage 3 AKI increased from 27 to 66% (Fig. 2).
The association between the development of AKI and neurological outcome had been reported several times [1, 4]. Although the development of AKI was significantly associated with survival at discharge, it was not associated with good neurological outcome at discharge. This might be attributed to small number of participants. However, multivariate analysis showed that recovery from AKI was a potent predictor of survival and good neurological outcome at discharge. Naturally, we should interpret these results carefully because the characteristics of the OHCA patients are significantly different from critical care patients, such as those with severe sepsis [14, 16]. Whole body ischaemia and reperfusion leads to post-cardiac arrest syndrome, including brain injury, myocardial dysfunction, systemic ischaemia, and reperfusion response [16]. Various factors, including the duration of ischaemia, cause of cardiac arrest, OHCA interventions, and the patient’s baseline health status, could affect neurological outcomes in OHCA patients. Therefore, to determine whether the development of and recovery from AKI could affect the long-term neurological and functional outcomes of OHCA patients, further studies are warranted. In addition, patients’ death before recovery of AKI could exaggerate the outcome of the AKI recovery group compared to that of the AKI non-recovery group. In addition, patients’ death before the recovery of AKI might be caused by reasons (competing risk problems) other than AKI [17]. To decrease competing risk problems, we conducted subgroup analysis after excluding patients who died within 48 h since ROSC (Additional file 1: Table S4). Development of and recovery from AKI were significant predictors of survival discharge in the multivariate analysis even after excluding patients who died within 48 h (Additional file 1: Table S5). In addition, recovery from AKI was a potent predictor of good neurological outcome at discharge in patients with AKI (Additional file 1: Table S6). Most of the results of the multivariate analysis did not change after excluding patients who died within 48 h since ROSC.
Neither the incidence of AKI nor recovery of AKI was significantly influenced by target temperature (33 °C vs. 36 °C) in our cohort. However, the exact effect of target temperatures on the development of AKI could not be determined in this study because the target temperature was not randomised in our cohort. Recently, the post hoc analysis of TTM trial (randomised clinical trial for comparing the TTM 33 °C versus 36 °C after OHCA) [10] for evaluating the effect of target temperature on the development of AKI has been reported [18, 19]. Although the incidence of AKI was higher in the TTM-33 group compared to that in the TTM-36 group (49% versus 40%), there was no association between the target temperature and development of AKI based on the logistic regression analysis [18]. We could not determine whether there was a difference in the recovery rate of AKI according to the target temperature because the recovery rate according to the target temperature had not been reported in that study.
The appropriate time to initiate RRT in a critical care setting has not been defined [20]. Studies on the issues of early versus delayed initiation of RRT show heterogeneous results in a critical care setting [21–23]. In the case of OHCA treated with TTM, studies on early initiation of RRT have not yet been conducted. In our cohort, RRT was mostly conducted after the patient reached Stage 3 AKI (95%). Therefore, we could not verify the preventive effect of RRT in our study. Another study including a large cohort is warranted to verify the effect of RRT on the outcomes of the OHCA treated with TTM.
Limitations
The present study has several limitations. First, there was a higher risk of selection bias because the data of the present study were collected retrospectively. A prospective study will be needed to confirm our findings. Second, although the present study was conducted using a multicentre cohort, the size of the cohort was relatively small. In addition, all study sites were located in South Korea. Therefore, our results cannot be representatives of all OHCA patients. Third, determining the development of and recovery from AKI based on serum creatinine level could lead to underestimation of the development of AKI or overestimation of recovery from AKI compared to an approach based on the estimated glomerular filtration rate calculated from cystatin C [24]. In addition, decreased muscle metabolism in critically ill patients could also lead to an overestimation of renal function [25]. Therefore, the incidence and recovery rates of AKI in our cohort might be affected by the use of serum creatinine levels to diagnose AKI in most patients. Fourth, we could not determine the effect of RRT in the present study because most of the patients (80%) treated with RRT did not survive to discharge. Further studies are needed to evaluate the efficacy of RRT in OHCA patients treated with TTM. Fifth, although we determined the development of AKI by considering both serum creatinine level and hourly urine output, we did not record the data on hourly urine output. Therefore, serial changes in hourly urine output could not be presented in the results. Sixth, only a small number of patients were included from the screened OHCA patients (275/3,697 [7%]). In South Korea, the emergency medical service providers do not have the legal right to declare death. All patients with OHCA are transported to the emergency to confirm death, with or without CPR, even if they show obvious signs of death (e.g. postmortem lividity and rigour mortis). Therefore, 71% (2619/3697) of the screened patients were initially excluded from our cohort. The important exclusion was the absence of treatment with TTM (443/3697 patients [12%]). There were several reasons for this exclusion. Most of the institutions in South Korea use surface cooling or intravascular cooling devices for strict temperature control, including rewarming speed. However, the disposable components of these devices are not covered by the South Korean national health insurance system. Approximately 1500 to 3500 USD is needed for TTM treatment, in addition to the basic costs of post-cardiac arrest care and intensive care. Therefore, some of the patients’ legal surrogates did not agree to TTM. In addition to the financial issue, TTM itself has several contraindications. For example, refractory shock that does not respond to fluid or vasopressors, severe sepsis, poor neurological condition before cardiac arrest, pregnancy, arrhythmia that does not respond to treatment or coronary vasospasm, active bleeding or thrombolytic use, terminal illness before cardiac arrest, DNAR orders, and comatose patients with other aetiologies. The above reasons might have limited patient inclusion in the present study.
Conclusions
In our cohort of adult OHCA patients treated with TTM (n = 275), the recovery rate from AKI after OHCA was 39%, and recovery from AKI was a potent predictor of survival and good neurological outcome at discharge.
Additional file
Table S1. The distribution of modified Rankin Scale scores according to the development of acute kidney injury in patients with good neurological outcomes at discharge. Table S2. The distribution of modified Rankin Scale scores according to the recovery from acute kidney injury in patients with good neurological outcomes at discharge. Table S3. The distribution of modified Rankin Scale scores according to renal replacement therapy in patients with good neurological outcomes at discharge. Table S4. Baseline characteristics of the study population according to the recovery of acute kidney injury after excluding patients who died within 48 h since return of spontaneous circulation. Table S5. Factors associated with survival discharge in multivariate analysis after excluding patients who died within 48 h since return of spontaneous circulation. Table S6. Factors associated with good neurological outcome in multivariate analysis after excluding patients who died within 48 h since return of spontaneous circulation. (DOCX 22 kb)
Acknowledgements
We specially thank our researcher (Yiseul Jeon, RN) for her help in continuing this project.
Abbreviations
- AKI
Acute kidney injury
- CI
Confidence interval
- CPR
Cardiopulmonary resuscitation
- DNAR
Do-not-attempt resuscitation
- ESRD
End-stage renal disease
- KDIGO
Kidney Disease: Improving Global Outcomes
- MRS
Modified Rankin scale
- OHCA
Out-of-hospital cardiac arrest
- OR
Odds ratio
- ROSC
Return of spontaneous circulation
- RRT
Renal replacement therapy
- TTM
Targeted temperature management
Authors’ contributions
Oh JH contributed to study conception and design. Park YS, Choi YH, Oh JH, Cho IS, Cha KC and You JS contributed to data acquisition. Park YS, Choi YH, Oh JH, Cho IS, Cha KC and You JS contributed to data analysis and interpretation. Choi BS contributed to statistical analysis and revision. Oh JH contributed to acquisition of funding. Park YS, Choi YH, Choi BS and Oh JH contributed to the drafting of the manuscript and its critical revision for important intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant no. NRF-2018R1C1B5082969).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study design and plan were approved by the institutional review boards (IRB) of each hospital (Approval No. Chung-Ang University Hospital IRB: 1810-010-16212, Yonsei University Health System Severance Hospital IRB: 4-2018-0857, Ewha Womans University Mokdong Hospital IRB: EUMC-2018-11-004, Hanil General Hospital IRB: HGH-2018-OTH-017, Yonsei University Wonju Severance Cristian Hospital IRB: CR318103, and Yonsei University Gangnam Severance Hospital IRB: 3-2018-0298). Written informed consent was waived by the IRB.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yoo Seok Park and Yoon Hee Choi contributed equally to the writing of this article as first authors.
Contributor Information
Yoo Seok Park, Email: pys0905@yuhs.ac.
Yoon Hee Choi, Email: unii@ewha.ac.kr.
Je Hyeok Oh, Phone: + 82 2 6299 2550, Email: jehyeokoh@cau.ac.kr.
In Soo Cho, Email: mensa@hanmail.net.
Kyoung-Chul Cha, Email: chaemp@yonsei.ac.kr.
Byung-Sun Choi, Email: bschoi@cau.ac.kr.
Je Sung You, Email: youjsmd@yuhs.ac.
References
- 1.Storm C, Krannich A, Schachtner T, Engels M, Schindler R, Kahl A, Otto NM. Impact of acute kidney injury on neurological outcome and long-term survival after cardiac arrest - a 10year observational follow up. J Crit Care. 2018;47:254–259. doi: 10.1016/j.jcrc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Cornell TT, Selewski DT, Alten JA, Askenazi D, Fitzgerald JC, Topjian A, Holubkov R, Page K, Slomine BS, Christensen JR, Dean JM, Moler FW. Acute kidney injury after out of hospital pediatric cardiac arrest. Resuscitation. 2018;131:63–68. doi: 10.1016/j.resuscitation.2018.07.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandroni C, Dell'anna AM, Tujjar O, Geri G, Cariou A, Taccone FS. Acute kidney injury after cardiac arrest: a systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016;82(9):989–999. [PubMed] [Google Scholar]
- 4.Beitland S, Nakstad ER, Staer-Jensen H, Draegni T, Andersen GO, Jacobsen D, Brunborg C, Waldum-Grevbo B, Sunde K. Impact of acute kidney injury on patient outcome in out-of-hospital cardiac arrest: a prospective observational study. Acta Anaesthesiol Scand. 2016;60(8):1170–1181. doi: 10.1111/aas.12753. [DOI] [PubMed] [Google Scholar]
- 5.Tujjar O, Mineo G, Dell'Anna A, Poyatos-Robles B, Donadello K, Scolletta S, Vincent JL, Taccone FS. Acute kidney injury after cardiac arrest. Crit Care. 2015;19:169. doi: 10.1186/s13054-015-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geri G, Guillemet L, Dumas F, Charpentier J, Antona M, Lemiale V, Bougouin W, Lamhaut L, Mira JP, Vinsonneau C, Cariou A. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med. 2015;41(7):1273–1280. doi: 10.1007/s00134-015-3848-4. [DOI] [PubMed] [Google Scholar]
- 7.Section 2: AKI Definition. Kidney Int Suppl (2011). 2012; 2(1):19–36. [DOI] [PMC free article] [PubMed]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettila V, Prowle JR, Schetz M, Joannidis M. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, TTMT I. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 11.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, Deakin CD, Finn JC, Grasner JT, Hazinski MF, Iwami T, Koster RW, Lim SH, Ma MH, BF MN, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Ong ME, Travers AH, Nolan JP, Utstein C. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2015;96:328–340. doi: 10.1016/j.resuscitation.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Haywood K, Whitehead L, Nadkarni VM, Achana F, Beesems S, Bottiger BW, Brooks A, Castren M, Ong MEH, Hazinski MF, Koster RW, Lilja G, Long J, Monsieurs KG, Morley PT, Morrison L, Nichol G, Oriolo V, Saposnik G, Smyth M, Spearpoint K, Williams B, Perkins GD, Collaborators C COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the international liaison committee on resuscitation. Resuscitation. 2018;127:147–163. doi: 10.1016/j.resuscitation.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Peters E, Antonelli M, Wittebole X, Nanchal R, Francois B, Sakr Y, Vincent JL, Pickkers P. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from The Intensive Care Over Nations audit. Crit Care. 2018;22(1):188. doi: 10.1186/s13054-018-2112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BK, Park KN, Kang GH, Kim KH, Kim G, Kim WY, Min JH, Park Y, Park JB, Suh GJ, Son YD, Shin J, Oh JS, You YH, Lee DH, Lee JS, Lim H, Jang TC, Cho GC, Cho IS, Cha KC, Choi SP, Choi WJ, Han C, Korean Hypothermia Network I Outcome and current status of therapeutic hypothermia after out-of-hospital cardiac arrest in Korea using data from the Korea Hypothermia Network registry. Clin Exp Emerg Med. 2014;1(1):19–27. doi: 10.15441/ceem.14.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassager C, Nagao K, Hildick-Smith D. Out-of-hospital cardiac arrest: in-hospital intervention strategies. Lancet. 2018;391(10124):989–998. doi: 10.1016/S0140-6736(18)30315-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5(3):47. doi: 10.21037/atm.2016.08.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rundgren M, Ullen S, Morgan MPG, Glover G, Cranshaw J, Al-Subaie N, Walden A, Joannidis M, Ostermann M, Dankiewicz J, Nielsen N, Wise MP. Renal function after out-of-hospital cardiac arrest; the influence of temperature management and coronary angiography, a post hoc study of the target temperature management trial. Crit Care. 2019;23(1):163. doi: 10.1186/s13054-019-2390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spoelstra-de Man AME, Oudemans-van Straaten HM. Acute kidney injury after cardiac arrest: the role of coronary angiography and temperature management. Crit Care. 2019;23(1):193. doi: 10.1186/s13054-019-2476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Section 5: Dialysis Interventions for Treatment of AKI. Kidney Int Suppl (2011). 2012; 2(1):89–115. [DOI] [PMC free article] [PubMed]
- 21.Moreira FT, Palomba H, Chaves RCF, Bouman C, Schultz MJ, Serpa NA. Early versus delayed initiation of renal replacement therapy for acute kidney injury: an updated systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Rev Bras Ter Intensiva. 2018;30(3):376–384. doi: 10.5935/0103-507X.20180054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyere R, Lebert C, Bohe J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP, Investigators I-IT, the CTN Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med. 2018;379(15):1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 23.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, Boanta A, Gerss J, Meersch M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 24.Hasslacher J, Barbieri F, Harler U, Ulmer H, Forni LG, Bellmann R, Joannidis M. Acute kidney injury and mild therapeutic hypothermia in patients after cardiopulmonary resuscitation - a post hoc analysis of a prospective observational trial. Crit Care. 2018;22(1):154. doi: 10.1186/s13054-018-2061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schetz M, Gunst J, Van den Berghe G. The impact of using estimated GFR versus creatinine clearance on the evaluation of recovery from acute kidney injury in the ICU. Intensive Care Med. 2014;40(11):1709–1717. doi: 10.1007/s00134-014-3487-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The distribution of modified Rankin Scale scores according to the development of acute kidney injury in patients with good neurological outcomes at discharge. Table S2. The distribution of modified Rankin Scale scores according to the recovery from acute kidney injury in patients with good neurological outcomes at discharge. Table S3. The distribution of modified Rankin Scale scores according to renal replacement therapy in patients with good neurological outcomes at discharge. Table S4. Baseline characteristics of the study population according to the recovery of acute kidney injury after excluding patients who died within 48 h since return of spontaneous circulation. Table S5. Factors associated with survival discharge in multivariate analysis after excluding patients who died within 48 h since return of spontaneous circulation. Table S6. Factors associated with good neurological outcome in multivariate analysis after excluding patients who died within 48 h since return of spontaneous circulation. (DOCX 22 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


