Abstract
Objectives
This study was aimed to assess the prevalence and risk factors of gastrointestinal parasites on schoolchildren at Bochesa Elementary School around Lake Zwai, Ethiopia. Cross-sectional study was conducted on 384 schoolchildren in May 2016. The gastrointestinal parasites were examined with wet mount and formol-ether concentration techniques. Chi-square (χ2) test was used to evaluate the association between categorical variables and infection prevalence. Binary logistic regression on SPSS version 21 was used, values were considered significant when the p-value was less than 0.05.
Results
The overall prevalence of gastrointestinal parasites was 22.6%. Males, 54 (14.1%) were more infected than females, 32 (8.3%), and 1–4 grade category, 64 (16.7%) were more infected than 5–8 grade category, 22 (5.7%). Age groups of 7–14, 78 (20.3%) were also more infected than > 15, 8 (2.1%); however, the variation was not significant (p > 0.05). In this study, parasitic coinfection was common; however, single gastrointestinal parasites were more dominant. The overall rate of gastrointestinal parasites shows that the environmental conditions where students pass their times are conducive to water-related diseases. Health education on personal and environmental hygiene keeping should be given to schoolchildren and safe wetland playing grounds should be prepared.
Keywords: Children, Lake Zwai, Gastrointestinal, Parasites, Wetlands
Introduction
Many civilizations have been built on the basis of wetland resources [1]; this indicates that wetlands and people are ultimately interdependent with each other [2, 3]. In developing countries, the livelihoods of people are intimately linked with wetlands and these interactions lead to the emergence of gastrointestinal parasitic infections [4].
Faecal-oral contact of infective parasitic stages is important route to human infection by water-related gastrointestinal parasites [5, 6]. Factors like eating raw vegetables, lack of hygiene, unsafe drinking water, lack of toilet facilities, walking barefoot, fishing, irrigation, swimming, playing with moist soil and open defecation may increase the prevalence of gastrointestinal parasites [7] of wetland areas.
The prevalence of gastrointestinal parasites varies from region to region [7]. Prevalence of gastrointestinal parasites has been studied in different countries including Ethiopia [7, 8]. It was found out that these diseases are more prevalent on school age children [9–11]. Hence, the present study was aimed at assessing the prevalence and risk factors of gastrointestinal parasitic infections on schoolchildren at a school located in close proximity to the wetlands of Lake Zwai.
Main text
Methods
Study setting and period
Cross-sectional study was conducted in May 2016 on Bochesa Elementary schoolchildren around Lake Zwai, which is located in the Southwestern direction of lake Zwai, about 167 km South of Addis Ababa, which is the capital of Ethiopia. The school is situated at an altitude of 1636 m above sea level, which is similar to the lake. Students of the school are living under poor socio-economic status and with no adequate safe water supply in their village; and their parents lead their life by cultivation of maize and vegetables using lake water for irrigation and rearing of livestock.
Sample size and sampling techniques
The desired sample size (n) required for the assessment of gastrointestinal parasites and associated risk factors was estimated using standard formula [12].
where n is sample size, z is 1.96, which is z statistics level of confidence, d = 0.05 (absolute precision), and p = expected prevalence of the area.
Since the prevalence of gastrointestinal parasites is not known for this study area on schoolchildren, p-value was taken to be 50%. A 95% confidence interval (z) and a 5% margin of error (d) was used. Therefore, 384 (192 males and 192 were females) students from 7 to 25 years were involved.
Laboratory investigations
Stool samples were collected using applicator sticks in a labeled plastic container.
About 5 g of fresh fecal samples were collected from each students and placed in separate labeled clean plastic stool containers. A small portion of about 2 g sample from each student was examined using wet mount and the remaining about 3 g sample was examined using formol-ether concentration technique [13]. The overall presence of gastrointestinal parasites was confirmed when observed by any of the methods used.
Questionnaire administration
A questionnaire was developed in English and translated into Afan Oromo (the local language) to collect socio demographic data, environmental factors and behavioral habits.
Statistical analysis
The prevalence of infections was reported in proportions. Chi-square (χ2) test was used to evaluate the association between categorical variables and prevalence. For identification of determinant factors, Binary logistic regression was used, and finally the association between independent variables and dependent variables were described on the basis of odd ratio (OR) with 95% confidence interval (CI). Crude OR was estimated by univariate regression analysis and adjusted OR was then estimated by multivariate logistic regression analysis. Values were considered statistically significant when the p-value was less than 0.05.
Results
The overall prevalence of gastrointestinal parasites infection was 22.4% (95% CI 18–26.3%). Different types of parasites like cestodes, nematodes and protozoans with a value of 37 (9.6%), 40 (10.4%) and 29 (7.6%) were identified, respectively from Bochesa Elementary School students. Of these eight species identified, Hymenolepis nana was the predominant parasites detected on 34 (8.9%), and least prevalence was recorded on Taenia spp. with a value of 3 (0.8%) (Table 1).
Table 1.
Different species of gastrointestinal parasites and their prevalence on Bochesa Elementary School
| Parasites | Species obtained | Frequency | Prevalence (%) |
|---|---|---|---|
| Nematodes | A. lumbricoides | 17 | 4.43 |
| T. trichuria | 8 | 2.08 | |
| S. stercoralis | 8 | 2.08 | |
| Hookworm spp. | 14 | 3.65 | |
| Cestodes | H. nana | 34 | 8.85 |
| Taenia spp. | 3 | 0.78 | |
| Protozoa | E. histolytica/dispar | 20 | 5.21 |
| G. lamblia | 18 | 4.69 |
The prevalence of gastrointestinal parasites was statistically significant (χ2 = 7.252; p = 0.005 and χ2 = 6.177; p = 0.008) between sex and grade categories, respectively; whereas insignificant (p > 0.05) variations was observed on the prevalence of gastrointestinal parasites between age groups.
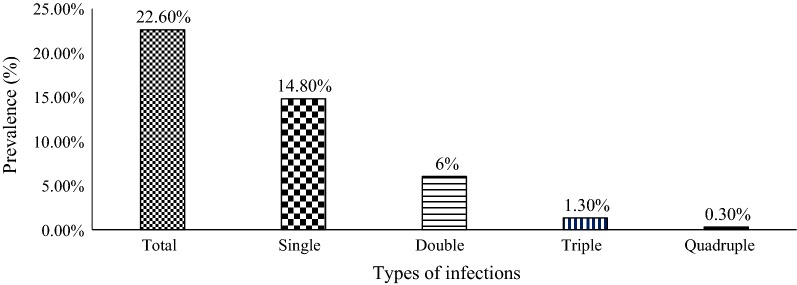
Of these 86 (22.4%) positive cases, infection with single parasite was more dominant (14.8%) than mixed infections, in which double, triple and quadruple parasitic infections with a value of 6.0%, 1.3% and 0.3%, respectively (Fig. 1). The prevalence of single parasitic infection was statistically significant (χ2 = 4.635; p = 0.022) between se; whereas insignificant (p > 0.05) variations were observed with age and grade category.
Fig. 1.
Prevalence of single and mixed parasitic infections among Bochesa Elementary School students
Potential risk factors associated with gastrointestinal parasites
Being male increases the risk of gastrointestinal parasite infection 7.79-folds than being female (AOR: 7.799: 95% CI 1.948–31.222). Students who were from 5 to 8 grade category decreases the risk of gastrointestinal parasite infection by 69.3% than students from 1 to 4 grade category (AOR: 0.307: CI 95% 0.110–0.855). Students who had fishing habits were 3.027 times more likely to be infected by gastrointestinal parasites than their counter parts (AOR: 3.027: CI 95% 1.117–8.206). Students who had raw meat-eating habits were 4.710 times more likely to be infected by gastrointestinal parasites than students who didn’t eat raw meat (AOR: 4.710: CI 95% 1.626–13.643). Similarly, students who had raw vegetable eating habit were 2.8 times more likely to be infected by gastrointestinal parasites than their counter parts (AOR: 2.800: CI 95% 1.126–6.961). Students who had habits of playing with moist soil were 8.571-folds increases the risk of gastrointestinal parasites than students who didn’t play (AOR: 8.571: CI 95% 3.884–18.913). Similarly, students who had sometimes hand washing habit before meals were 4.961-folds increased the risk of gastrointestinal parasites than students who washed their hands before meals (AOR: 4.961: CI 95% 1.773–13.879). Students who practiced open defecation showed increased the risk of gastrointestinal parasite infections with a rate of 6.12 times more than students who used latrines (AOR: 6.118: CI 95% 2.414–15.510) (Table 2).
Table 2.
Binary logistic regression analysis for factors potentially associated with gastro intestinal parasite infection among Bochesa Elementary Schoolchildren
| Risk factors | Category | Positive (%) | Negative (%) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Sex | Male | 51 | 141 | 7.779* (1.948–31.222) |
| Female | 32 | 160 | ||
| Grade category | 1–4 | 61 | 181 | 0.307* (0.110–0.855) |
| 5–8 | 22 | 120 | ||
| Fishing habit | Yes | 53 | 117 | 3.027* (1.117–8.206) |
| No | 30 | 184 | ||
| Raw meat-eating | Yes | 63 | 165 | 4.710* (1.626–13.643) |
| No | 20 | 136 | ||
| Playing with moist soil | Yes | 36 | 34 | 8.571* (3.884–18.913) |
| No | 47 | 267 | ||
| Water for bathing | Lake water | 79 | 255 | 2.125 (0.720–6.274) |
| Ground water | 4 | 46 | ||
| Raw vegetable eating | Yes | 47 | 88 | 2.800* (1.126–6.961) |
| No | 36 | 213 | ||
| Hand washing | Sometimes | 27 | 13 | 4.961* (1.773–13.879) |
| Yes | 56 | 288 | ||
| Finger nail trimming | Yes | 32 | 200 | |
| No | 51 | 101 | 1.869 (0.801–4.361) | |
| Area of defecation | Latrine | 9 | 151 | |
| Open defecation | 74 | 150 | 6.118* (2.414–15.510) | |
| Shoe wearing habit | Yes | 27 | 190 | 2.109 (0.852–5.220) |
| No | 56 | 111 |
*Significant association
Discussion
The present study revealed the occurrence of eight species of gastrointestinal parasite on schoolchildren. The overall prevalence (22.4%) of gastrointestinal parasites observed in this study is comparable with reported cases of Ethiopia [14] and Angola [15].
The present result is also lower when compared with other earlier reports from different parts of the country [7, 9, 16, 17], and outside (Turkey [18] and Egypt [19]). The probable reason for this variation might be attributed to the variation in environmental and living conditions of the study participants.
The occurrence of gastrointestinal helminth parasitic infections among students of Bochesa Elementary School found in Zwai wetlands is an indication of faecal contamination of soil, improper utilization of latrines or absence of them and poor personal hygiene in the area. Similar finding was reported in Zwai Town on pregnant woman [20].
In this study, H. nana (8.9%) was the dominant parasite. Similar findings were reported inside the country [21, 22]. The observed prevalence of 8.6% for H. nana in this study was in agreement with different researchers inside the country [8, 23, 24]; and in Burkina Faso [25]. This result was also relatively higher compared to other studies in different areas of the country [26–31]. This difference might be due to the variation in environmental and living conditions of the study participants.
Higher prevalence of gastrointestinal parasites on males in this study was supported by different researchers inside the country [8, 12, 22, 23] and elsewhere [32, 33]. The probable reason for this variation might be due to more involvement of males in outdoor activities. These activities sometimes carried out bare-footed and this situation predisposing males to infections.
Similarly, higher prevalence of gastrointestinal parasites on lower age groups was in agreement with other reports inside the country [29, 34–36], and Bangalore [37]. The probable reason for this difference might be due to frequent contact and the habit of inserting soil contaminated fingers to their mouth and less awareness of hand washing practices may increase the chance of acquiring gastrointestinal parasites in lower age groups.
Higher prevalence of gastrointestinal parasites on 1–4 grade category was also inline with other studies in Ethiopia [21, 29, 34]. The probable reason for this variation might be due to awareness created to improve personal hygiene in 5–8 grades. The present study revealed that the existence of parasitic co-infection in the wetland of Lake Zwai in Bochesa Elementary schoolchildren. In line with this findings, parasitic co-infections were reported inside the country [7, 9, 16, 29, 38].
The possible reason for the existence of parasitic co-infections in the study area might be associated with Zwai wetland, which is found in front of their residences to make frequent contact for swimming, bathing, fishing and irrigation purpose in the lake and the wetlands around it. In this study, single parasitic infections were more common than parasitic co-infections. These results were comparable with studies conducted elsewhere in Ethiopia [9, 16, 17, 21, 30].
The present study shows that the likelihood of acquiring gastrointestinal parasites in males was 7.79 times higher than in females. In accordance with these findings, agricultural and fishing areas at the lake borders and irrigation canals are common defecation places by males during working times, facilitating the transmission of gastrointestinal parasites would be facilitated. Similar findings were reported in studies conducted elsewhere in Ethiopia [14, 22].
Students having raw-meat eating habits were 4.71 times more likely to be infected by gastrointestinal parasites such as Taenia spp. than students who had no raw-meat eating habits. Recently, the custom of eating raw-meat has grown worldwide in ethnic groups where eating raw-meat was not previously common [5].
Students who had raw vegetable-eating habit were 2.80 times more likely to acquire gastrointestinal parasites than students who had no raw vegetable-eating habits. This finding was supported by studies in Ethiopia [39–42].
Students who played with moist soil were 8.57 times more likely to be infected by gastrointestinal parasites than students who had no such habits. This might be due to the fact that moist soil creates an environment conducive for a high prevalence of intestinal parasites [43, 44].
Students who have practiced open defecation were 6.12 times more likely to acquire gastrointestinal parasites than those who used latrines. Similar findings were reported [8, 27, 41].
Conclusion
The study revealed that the wetland of Lake Zwai are conducive for the survival of water-related gastrointestinal parasites as observed in the schoolchildren of the area. As the weather condition of Zwai wetlands is hot and humid coupled with wet soil is a suitable environment for the occurrence of infective stages of gastrointestinal parasites. These disposing factors to the disease is further increased due to the poor economic status of the community of the area and the nature of students who do not see the risks of infection by gastrointestinal parasites that are out there.
Limitations
We used only wet mount and formol-ether concentration technique to assess the prevalence of gastrointestinal parasites in the collected samples.
Acknowledgements
The authors would like to thank to the Water Thematic Research Project of Addis Ababa University for financially supporting this project, and Adami Tulu Agricultural and Zwai Fisheries Research Centers for allow us access to their laboratory facilities.
Authors’ contributions
AS and BL have designed the proposal, data collection, data analysis, interpretation and later developed this manuscript. Both authors read and approved the final manuscript.
Funding
No funding organization was involved as a grant for this study.
Availability of data and materials
The data used and analyzed in this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Prior to stool collection, a formal letter request of cooperation was submitted to the Adami Tulu Jido Kombolcha Woreda Health Office at Zwai town and the school principal of Bochesa Elementary School. The ethical approval committee of Adami Tulu Jido Kombolcha Woreda Health Office has approved the request. Accordingly, the necessary permissions were obtained from each student, their families and finally, appropriate treatment was given to students who were found positive for gastrointestinal parasites by local nurses. For students who are under the age of 18, parents signature was prepared to be signed through the questionnaire.
Consent for publication
This is not applicable, since any photographs or videos were not taken.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayalew Sisay, Email: ayalewsisay@gmail.com.
Brook Lemma, Email: brklmm2008@gmail.com.
References
- 1.Shine C, De Klemm C. Wetlands, water, and the law: using law to advance wetland conservation and wise use. London: IUCN; 1999. [Google Scholar]
- 2.Brouwer J, Abdoul Kader HA, Sommerhalter T. Wetlands help maintain wetland and dryland biodiversity in the Sahel, but that role is under threat: an example from 80 years of changes at Lake Tabalak in Niger. Biodiversity. 2014;15(2–3):203–219. doi: 10.1080/14888386.2014.934714. [DOI] [Google Scholar]
- 3.Kansiime F, Saunders MJ, Loiselle SA. Functioning and dynamics of wetland vegetation of Lake Victoria: an overview. Wetl Ecol Manag. 2007;15(6):443–451. doi: 10.1007/s11273-007-9043-9. [DOI] [Google Scholar]
- 4.Hailu T. Current prevalence of intestinal parasites emphasis on hookworm and Schistosoma mansoni infections among patients at Workemeda Health Center. Northwest Ethiopia. Clin Microbiol Open Access. 2014;3(4):1–4. [Google Scholar]
- 5.Macpherson CN. Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol. 2005;35(11–12):1319–1331. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Ekong PS, Juryit R, Dika NM, Nguku P, Musenero M. Prevalence and risk factors for zoonotic helminth infection among humans and animals-Jos, Nigeria, 2005–2009. Pan Afr Med J. 2012;12(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Legesse M, Erko B. Prevalence of intestinal parasites among schoolchildren in a rural area close to the southeast of Lake Langano, Ethiopia. Ethiop J Health Dev. 2004;18(116):120. [Google Scholar]
- 8.Haile A, Abera T, Dana D. The prevalence of intestinal parasitic infection and associated factors among primary school children in Gurage zone, South. J Pharm Altern Med. 2016;15:8–15. [Google Scholar]
- 9.Hailegebriel T. Undernutrition, intestinal parasitic infection and associated risk factors among selected primary school children in Bahir Dar, Ethiopia. BMC Infect Dis. 2018;18(1):394. doi: 10.1186/s12879-018-3306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook DM, Swanson RC, Eggett DL, Booth GM. A retrospective analysis of prevalence of gastrointestinal parasites among school children in the Palajunoj Valley of Guatemala. J Health Popul Nutr. 2009;27(1):31. doi: 10.3329/jhpn.v27i1.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omalu IC, Agu N, Ejima IA, Pam DD, Adeniyi KA, Eke SS, Otuu CA, Nnaji CI, Garba Y, Zhiri VG, Makinde HA. Epidemiology of gastrointestinal parasites among school children in Minna, Niger State, Nigeria. Ann Biomed Sci. 2017;16(1):225–235. [Google Scholar]
- 12.Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 6. New York: Wiley; 1995. [Google Scholar]
- 13.Cheesbrough M. District laboratory practice in tropical countries. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 14.Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014;7(1):848. doi: 10.1186/1756-0500-7-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Alegría ML, Colmenares K, Espasa M, Amor A, Lopez I, Nindia A, Kanjala J, Guilherme D, Sulleiro E, Barriga B, Gil E. Prevalence of Strongyloides stercoralis and other intestinal parasite infections in school children in a rural area of Angola: a cross-sectional study. Am J Trop Med Hyg. 2017;97(4):1226–1231. doi: 10.4269/ajtmh.17-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014;14(1):166. doi: 10.1186/1471-2458-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workneh T, Esmael A, Ayichiluhm M. Prevalence of intestinal parasitic infections and associated factors among Debre Elias primary school’s children, East Gojjam Zone, Amhara Region, North West Ethiopia. J Bacteriol Parasitol. 2014;5(1):1. doi: 10.4172/2155-9597.1000181. [DOI] [Google Scholar]
- 18.Doni NY, Gurses G, Simsek Z, Zeyrek FY. Prevalence and associated risk factors of intestial parasites among children of farm workers in the southeastern Anatolian region of Turkey. Ann Agric Environ Med. 2015;20(1):438–442. doi: 10.5604/12321966.1167709. [DOI] [PubMed] [Google Scholar]
- 19.El-sehry NM, Fouda LM, Hassan LWA. Prevalence of parasitic infections and its effect on the health status of primary school children. IOSR J Nurs Health Sci. 2017;6(4):41–52. doi: 10.9790/1959-0604024152. [DOI] [Google Scholar]
- 20.Tefera G. Determinants of anemia in pregnant women with emphasis on intestinal helminthic infection at Sher-Ethiopia Hospital, Ziway, Southern Ethiopia. Immunol Infect Dis. 2014;2(4):33–39. [Google Scholar]
- 21.Gelaw A, Anagaw B, Nigussie B, Silesh B, Yirga A, Alem M, Endris M, Gelaw B. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13(1):304. doi: 10.1186/1471-2458-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tefera E, Mohammed J, Mitiku H. Intestinal helminthic infections among elementary students of Babile town, eastern Ethiopia. Pan Afr Med J. 2015;20(1):1–10. doi: 10.11604/pamj.2015.20.50.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endris M, Lemma W, Belyhun Y, Moges B, Gelaw A, Angaw B. Prevalence of intestinal parasites and associated risk factors among students of Atse Fasil general elementary school Azezo, Northwestern Ethiopia. Ethiop J Health Biomed Sci. 2010;3(1):25–33. [Google Scholar]
- 24.Teklemariam A, Dejenie T, Tomass Z. Infection prevalence of intestinal helminths and associated risk factors among schoolchildren in selected kebeles of Enderta district, Tigray, Northern Ethiopia. J Parasitol Vector Biol. 2014;6(11):166–173. doi: 10.5897/JPVB2014.0173. [DOI] [Google Scholar]
- 25.Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, Shrestha A, Grissoum T, Kaboré A, Schindler C, Utzinger J, Cissé G. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasites Vectors. 2016;9(1):554. doi: 10.1186/s13071-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haftu D, Deyessa N, Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, Southern Ethiopia. Am J Health Res. 2014;2(5):247-4. doi: 10.11648/j.ajhr.20140205.15. [DOI] [Google Scholar]
- 27.Kidane E, Menkir S, Kebede A, Desta M. Prevalence of intestinal parasitic infections and their associations with anthropometric measurements of school children in selected primary schools, Wukro Town, Eastern Tigray, Ethiopia. Sci J Zool. 2013;229(1319):1–6. [Google Scholar]
- 28.Wale M, Wale M, Fekensa T. The prevalence of intestinal helminthic infections and associated risk factors among school children in Lumame town, Northwest, Ethiopia. J Parasitol Vector Biol. 2014;6(10):156–165. doi: 10.5897/JPVB2014.0159. [DOI] [Google Scholar]
- 29.Gebretsadik G. Prevalence of intestinal parasites and associated risk factors among schoolchildren of Homesha district (Woreda) in Benishangul-Gumuz regional state, western Ethiopia. J Fam Med Health Care. 2016;2(4):57–64. doi: 10.11648/j.jfmhc.20160204.16. [DOI] [Google Scholar]
- 30.Abdi M, Nibret E, Munshea A. Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. J Infect Public Health. 2017;10(1):84–92. doi: 10.1016/j.jiph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Lone R, Syed K, Lone A. Recent patterns and risk factors of intestinal helminthes infection among school children in Kashmir. India. Arch Clin Microbiol. 2011;2(3):1–4. [Google Scholar]
- 32.Albonico M, Chwaya HM, Montresor A, Stolfzfus RJ, Tielsch JM, Alawi KS, Savioli L. Parasitic infections in Pemba Island school children. East Afr Med J. 1997;74(5):294–298. [PMC free article] [PubMed] [Google Scholar]
- 33.Baral R, Jha P, Amatya R, Khanal B. Prevalence of intestinal parasitic infections among patients attending in a tertiary care hospital of eastern region of Nepal. A retrospective, laboratory based study. Asian J Med Sci. 2017;8(3):55–59. doi: 10.3126/ajms.v8i3.16909. [DOI] [Google Scholar]
- 34.Meles W, Merid I, Asfaw M. A study of the incidence of intestinal helminthic diseases and their risk factors among school Children in Lumame town, Northwest, Ethiopia. Afr J Parasitol Res. 2015;2(10):152–160. [Google Scholar]
- 35.Norris J, Adelman C, Spantchak Y, Marano K. Social and economic impact review on neglected tropical diseases. Economic Policy/Briefing Paper. Washington DC: Hudson Institute; 2012. [Google Scholar]
- 36.Nyantekyi LA, Legesse M, Belay M, Tadesse K, Manaye K, Macias C, Erko B. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet. Southern Ethiopia. Ethiop J Health Dev. 2010;4(3):185–190. [Google Scholar]
- 37.Golia S, Sangeetha KT, Vasudha CL. Prevalence of parasitic infections among primary school children in Bangalore. Int J Basic Appl Med Sci. 2014;4(1):356–361. [Google Scholar]
- 38.Ayalew A, Debebe T, Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. J Parasitol Vector Biol. 2011;3(5):75–81. [Google Scholar]
- 39.Alamir M, Awoke W, Feleke A. Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Health. 2013;5(10):1697. doi: 10.4236/health.2013.510228. [DOI] [Google Scholar]
- 40.Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, Mathewos B, Birhan W, Gebretsadik S, Gelaw B. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11(1):189. doi: 10.1186/1471-2334-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abera A, Nibret E. Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pac J Trop Med. 2014;7(7):525–530. doi: 10.1016/S1995-7645(14)60088-2. [DOI] [PubMed] [Google Scholar]
- 42.Alelign T, Degarege A, Erko B. Soil-transmitted helminth infections and associated risk factors among schoolchildren in Durbete Town. Northwestern Ethiopia. J Parasitol Res. 2015;2015:641602. doi: 10.1155/2015/641602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abera B, Alem G, Yimer M, Herrador Z. Epidemiology of soil-transmitted helminths, Schistosoma mansoni, and haematocrit values among schoolchildren in Ethiopia. J Infect Dev Ctries. 2013;7(03):253–260. doi: 10.3855/jidc.2539. [DOI] [PubMed] [Google Scholar]
- 44.Feleke BE. Nutritional status and intestinal parasite in school age children: a comparative cross-sectional study. Int J Pediatr. 2016;2016:1962128. doi: 10.1155/2016/1962128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analyzed in this study are available from the corresponding author on reasonable request.