Abstract
Background
In the United States Hepatitis C virus (HCV) viral clearance is estimated to range between 20 and 30%. The objective of this study was to estimate the frequency of HCV clearance and identify correlates of viral clearance among patients newly identified as HCV antibody positive in a large urban health system in Los Angeles, California.
Methods
We identified patients between November 2015 and September 2017 as part of a newly implemented HCV screening and linkage-to-care program at University of California Los Angeles (UCLA) Health System. All patients were eligible for screening, though there were additional efforts to screen patients born between 1945 and 1965. We reviewed Medical records to categorize anti-HCV antibody positive patients as having spontaneously cleared HCV infection (HCV RNA not detected) or not (HCV RNA detected). We excluded those with a prior history of anti-HCV positivity or history of HCV treatment. We compared differences between those with and without detectable HCV RNA using chi-square test, Fisher’s exact test, and t-test as appropriate. We assessed factors associated with HCV clearance using logistic regression analysis.
Results
Among the 320 patients included in this study, 56% were male. Baby boomers (52–72 years of age) comprised the single largest age group (62%). We found spontaneous HCV clearance in 58% (n = 185). HCV viral clearance was slightly higher among women as compared to men (63% vs. 53%; p value = 0.07) and varied by race/ethnicity: clearance among Blacks/African Americans was 37% vs. 58% among whites (p value = 0.02). After adjusting for age, race/ethnicity, and sex we found that those diagnosed with chronic kidney disease had a tendency of decreased HCV viral clearance (adjusted OR = 0.34; 95% CI 0.14–1.03).
Conclusion
Of those patients newly identified as anti-HCV positive, 58% had cleared HCV virus, while the rest showed evidence of active infection. In addition, we found that clearance varied by race/ethnicity and clinical characteristics.
Keywords: Hepatitis C, Epidemiology, Spontaneous clearance
Background
In the United States the proportion of those with spontaneous hepatitis C virus (HCV) clearance varies between studies. Historically it’s been estimated that clearance occurs in 15–20% of patients infected with HCV [1]. Recent data suggest that spontaneous clearance of HCV infection in the absence of treatment is higher than previously estimated [1–3]. In one of the first studies to estimate spontaneous HCV clearance –defined on the basis of HCV RNA assessments within 24 months of diagnosis – the frequency of clearance was 26% [4].
Why certain groups clear HCV infection without antiviral treatment remains unclear [5–8]. Studies suggest host innate immune system or genetic factors may play a role [9–11]. Factors such as sex, race/ethnicity, young age, HLA type, IL28B genotype, HIV-infection status, and chronic hepatitis B infection are known to affect clearance [2, 4, 10, 12–21]. However, most studies on spontaneous HCV viral clearance are limited by small sample size, heterogeneous definitions of cases, and study inclusion criteria, including study populations that are limited to high-risk groups such as illicit substance users and men who have sex with men [4, 7, 12, 13]. Little is known about screening populations, in particular baby boomers, a birth cohort known to have increased risk of HCV infection [22–25]. Our aim was to identify the frequency of and factors associated with spontaneous HCV clearance among patients participating in a hepatitis C screening program at a large urban health system in Los Angeles, California.
Methods
Study population
Patients seeking primary care services at UCLA Health were screened for HCV infection between November 2015 and September 2017. As per the US preventive task force recommendations for HCV screening, testing was initiated with the HCV antibody test (anti HCV Ab) and was targeted to high risk groups, as well as one-time testing of adults born between 1945 and 1965 regardless (regardless of their HCV risk factors) [26]. The program has been previously described [27]. Briefly, a structural intervention using electronic medical record prompts was implemented to promote HCV screening among patients seeking care at UCLA health. The electronic medical record (EMR) prompts were specifically developed to not only capture high risk groups for HCV infection, who had never tested for HCV, but also to target screening towards “baby boomers” (i.e., those born between 1945 and 1965) without any prior history of HCV testing.
In this study, we reviewed the medical records of patients who were HCV antibody positive during the screening period (“newly identified cases”). We also conducted interviews to confirm history of treatment or testing history for HCV. Patients who reported that they were under treatment or had received treatment for HCV in the past were excluded from this study. Patients who reported positive HCV antibody or RNA test results before the screening period were also excluded.
Measures
HCV antibody testing was conducted by the UCLA microbiology laboratory, using standard protocols for the ADVIA Centaur XP System (Siemens Medical Solutions USA, Inc., Malven, PA). The ADVIA Centaur HCV assay is an in vitro diagnostic immunoassay for the qualitative determination of immunoglobulin G (IgG) antibodies to hepatitis C virus in human serum and plasma using the ADVIA Centaur® System. The assay was used in conjunction with HCV RNA testing to determine HCV infection status. Specifically, HCV antibody reactive tests were tested for HCV RNA viral load using the COBAS® Ampliprep/ COBAS® TaqMan® HCV test (Qualitative and Quantitative, v2.0; Pleasanton, California). We collected patient demographic and clinical characteristics including age, sex, race/ethnicity, and laboratory values including liver function tests (aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, bilirubin levels) and, human immunodeficiency virus (HIV) infection status. In cases where these laboratory tests were not conducted on the same day as the HCV testing, we matched the laboratory testing data to the HCV testing data such that only testing data within 3 months of HCV antibody screening was included. We used ICD-10 codes (ICD-10 code for CKD: ICD-10-CM: N18* and ICD-10 code for DM: E11* and E10*) to determine whether patients were diagnosed with chronic kidney disease (CKD) or diabetes mellitus. These clinical markers were included in the study given the scientific literature indicating the association between CKD, diabetes, and chronic HCV infection [28–31].
Statistical analysis
We defined HCV clearance as evidence of HCV Ab reactivity and HCV RNA non-detectable test results in the absence of treatment. We examined differences between those who cleared their HCV infection and those who remained chronically infected using chi-square test, Fisher’s exact test, and t-tests. We used logistic regression analysis to examine factors associated with HCV clearance including sociodemographic and clinical characteristics. We conducted all analyses using SAS 9.4 (NC, USA).
Human subjects
The UCLA Institutional Review Board (IRB) approved the study activities (IRB# 15–001226).
Results
Study population characteristics
Three hundred and eighty-six patients were considered newly identified HCV Ab Positive. Among this group, 135 patients had detectable HCV RNA indicating current active infection. Of the 251 HCV antibody positive/HCV RNA undetectable patients, four had a false positive anti-HCV antibody test result, ten had a prior history of successful HCV treatment with clearance of HCV infection due to treatment (i.e., not spontaneous clearance), and 52 could not be reached for verification of their prior history of HCV treatment (Fig. 1). These 66 patients were excluded from the study resulting in a total of 320 study patients – 135 HCV antibody positive/HCV RNA positive (i.e., infection not cleared) and 185 HCV antibody positive/ HCV RNA negative (i.e., spontaneous clearance). The majority of the study population was born between 1945 and 1965 (62%) and 56% were male. Whites/Caucasians represented the single largest race/ethnicity group (64%), followed by Black/African American (13%) and Hispanic/Latino (9%) (Table 1). Among our study population of newly identified HCV antibody positive patients participating in a HCV screening program 58% spontaneously cleared HCV.
Fig. 1.
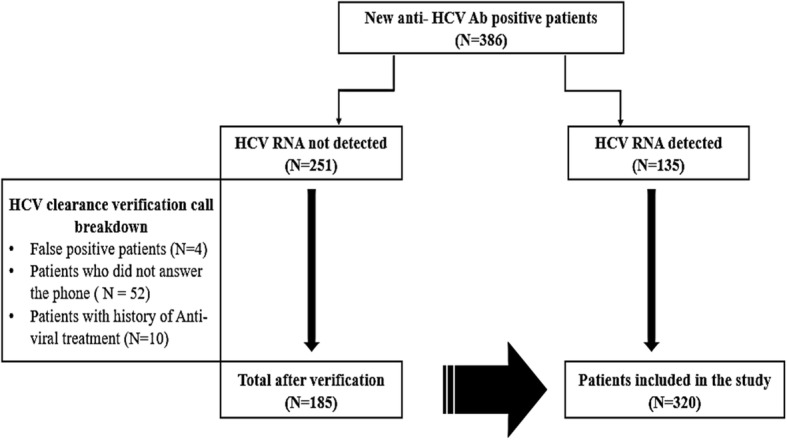
HCV viral clearance population breakdown
Table 1.
Demographic and clinical characteristics of newly identified HCV antibody positive patients, UCLA Health system, November 2015–September 2017
| HCV Antibody Positivea (n = 320) | ||
|---|---|---|
| n | % | |
| Age group (years) | ||
| 18–52 | 99 | 31.4 |
| 52–72 (“Baby Boomers”) | 198 | 61.5 |
| ≥ 73 | 23 | 7.1 |
| Sex | ||
| Male | 179 | 55.9 |
| Female | 140 | 44.1 |
| Race/Ethnicity | ||
| Asian or Pacific Islander | 5 | 1.8 |
| Black or African American | 38 | 13.4 |
| Hispanic or Latino | 25 | 8.8 |
| Otherb | 33 | 11.6 |
| White or Caucasian | 183 | 64.4 |
| Clinical Characteristics | ||
| Hepatitis C RNA detected | 135 | 42 |
| HIV Positive | 10 | 3.1 |
| Chronic Kidney Diseasec | 23 | 7.2 |
| Diabetes Mellitus | 45 | 14.2 |
| Laboratory Results d | ||
| ALT (U/L) | 25 (17–47) | |
| AST(U/L) | 26 (20–39) | |
| Platelet count | 232 (188–271) | |
| Bilirubin (mg/dL) | 0.5 (0.3–0.7) | |
Abbreviations: ALT Alanine Transaminase, AST Aspartate Transaminase
aSum may not equal total because of missing information
bincludes participants self-identifying as multiracial
cCKD based on ICD-10 codes
ddata presented as median and interquartile range
Prevalence of HCV clearance
The prevalence of HCV clearance varied by a number of demographic and clinical characteristics with HCV clearance being somewhat higher among women (63% vs. 53%; p value = 0.07), although not meeting statistical significance, and varying by race/ethnicity (Table 2). Specifically, we note that HCV clearance was lowest among those who identified as Black/African American (37%).
Table 2.
Prevalence and factors associated with absence of HCV RNA among newly identified HCV antibody positive patients, UCLA Health system, November 2015–2017
| HCV RNA negative | P value | Unadjusted OR (95% Cl) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|
| n | % | ||||
| Total | 185 | 58.0 | – | – | – |
| Age group (years) | 0.17 | ||||
| 18–52 | 58 | 58.6 | Ref | Ref | |
| 52–72 (“Baby Boomers”) | 118 | 59.6 | 1.04 (0.64–1.70) | 1.35 (0.75–2.39) | |
| ≥ 73 | 9 | 39.1 | 0.45 (0.18–1.15) | 0.45 (0.16–1.30) | |
| Sex | 0.07 | ||||
| Male | 95 | 53.1 | Ref | Ref | |
| Female | 88 | 63.3 | 1.53 (0.97–2.40) | 1.53 (0.91–2.56) | |
| Race/Ethnicity | 0.02 | ||||
| Asian | 3 | 60.0 | 1.08 (0.18–6.59) | 1.00 (0.14–5.44) | |
| Black or African American | 14 | 36.8 | 0.42 (0.21–0.86) | 0.43 (0.21–0.92) | |
| Hispanic or Latino | 10 | 40.0 | 0.48 (0.20–1.12) | 0.63 (0.26–1.57) | |
| Othera | 27 | 82.0 | 3.87 (1.43–10.51) | 4.38 (1.54–12.44) | |
| White or Caucasian | 106 | 58.2 | Ref | Ref | |
| Clinical Characteristics | |||||
| HIV Positive | 0.33 | ||||
| Yes | 4 | 40.0 | 0.48 (0.13–1.73) | – | |
| No | 180 | 58.3 | Ref | ||
| Chronic Kidney Diseaseb | 0.06 | ||||
| Yes | 9 | 39.1 | 0.45 (0.19–1.06) | 0.37 (0.14–1.03) | |
| No | 173 | 59.0 | Ref | Ref | |
| Diabetes Mellitus | 0.76 | ||||
| Yes | 25 | 55.6 | 0.91 (0.48–1.71) | – | |
| No | 157 | 57.9 | Ref | ||
Abbreviations: OR odds ratio, CI confidence interval
aincludes participants self-identifying as multiracial
bCKD based on ICD-10 codes
Factors associated with spontaneous HCV clearance
After adjusting for age and sex, we found that race/ethnicity was independently associated with spontaneous HCV clearance. Those who identified as African American/Black had a 57% decreased odds of HCV clearance as compared to Whites [adjusted odds ratio (aOR) =0.43; 95% confidence interval (CI) =0.21–0.92) (Table 2). Moreover, those diagnosed with chronic kidney disease (aOR = 0.34; 95% CI 0.14–1.03) had a tendency to decreased likelihood of spontaneous HCV clearance.
Discussion
We investigated the frequency of and factors associated with spontaneous HCV viral clearance among patients participating in a hepatitis C screening program at a large urban health system in Los Angeles. In this study, 58% of patients with newly identified infection had evidence of HCV viral clearance. It should be noted that we only included newly identified HCV Ab positive patients with prior testing and verified treatment history through both chart review and patient interviews, which may explain the difference compared to other published studies [1, 4, 30]. Among our patient population, spontaneous HCV viral clearance varied by race/ethnicity and was somewhat less likely to occur among those with chronic kidney disease (CKD).
Consistent with previous studies, clearance was greater among white patients compared to their black counterparts [1, 4]. Previous studies on genetic markers perhaps could explain the increased proportion of spontaneous viral clearance among non-black patients [10, 32–34]. Key modification with natural killer (NK) cells populations, HLA class II alleles and IL28B polymorphism have been suggested to predict the relationship between ethnic characteristics and HCV clearance [1, 32, 35–37]. A study conducted by Golden-Mason et al. proposed that the proportion of NKp46 expression was lower among African Americans compared to their white counterparts [1, 35]. NK cells are known to be the immune system’s first line of defense against viral pathogens [38]. NK cells do this work by eliminating virus-infected cells directly via cytolytic mechanisms or indirectly by secreting cytokines [38, 39]. Moreover, HLA type II alleles have been suggested to have some conflicting roles however, research suggests their role with HCV clearance includes the fact that African Americans have weaker HCV specific immunity [37]. Furthermore, IL28B polymorphism is known to be the strongest host gene predictor of HCV clearance. Previous studies discuss that IL28B allele rs12979860 were less likely to be observed among persons of African descent compared to European descent. Study by Ge et al., outlines the fact that HCV clearance occurred in 36.4% among non-blacks compared to 9.3% among black persons [10]. The suggested mechanism along with previous studies provides some evidence that NK cells and other genetic markers can play a protective role in patients exposed to hepatitis C. However, despite studies on predictors of HCV clearance, the association between ethnic characteristics and HCV viral clearance is not well understood and should be explored further.
Our study also found that patients with chronic kidney disease were somewhat less likely to clear HCV infection compared to those without chronic kidney disease. Due to several clinical implications, in 2017, the Kidney Disease Improving Global Outcome organization drafted specific guidelines to increase HCV screening among chronic kidney disease patients [40]. Earlier studies propose that HCV infection may influence the development of chronic kidney disease by stimulating a series of immune reactions that targets the kidney, which ultimately leads to glomerulonephritis [29, 41]. According to Azmi et al., the mechanism related to glomerulonephritis and HCV infection is known to be immune-complex mediated. Researchers believe that deposition of the immune complexes can trigger glomerulonephritis [41]. Overall, we also observed that HCV was associated with chronic kidney disease, although from our cross-sectional study we cannot determine causation.
There are a number of limitations in our study including: (1) our modest sample size limits our ability for extensive analyses; (2) given the lack of information on the precise timing of HCV exposure and HCV infection we are unable to determine time to HCV clearance; (3) exclusion of patients whose HCV clearance status could not be verified could have biased our estimates of HCV clearance; (4) lack of information on viral subtype limited our ability to determine whether HCV subtype is associated with clearance; and (5) we included only “newly identified cases” of HCV, excluding patients with previous HCV positive results, thus our results are not generalizable to other populations nor do they represent an unbiased population estimate of HCV clearance. Finally, we were unable to verify a specific Race/Ethnicity for patients included in the “Other” category during data extraction, which could have potentially affected our conclusions on the associations between HCV clearance and Race/ethnicity. Despite those limitations, this is one of a few studies exploring difference in HCV spontaneous clearance among a screening population, with rigorous strategies employed to exclude those with previous treatment history or false positive testing status.
Future studies should explore on the social regulation of gene expression. According to Cole et al., social regulation of gene expression is a conceptual relationship between genes and social world [42]. Recent studies suggest clear associations between social factors and the regulations of the human genome [42–44]. Perhaps the decreased expression of NKp46 or other genetic factors observed among black patients can be attributed to their exposure to poor environmental, social and economic conditions. Asking and attempting to answer those questions might enable a better understanding of what drives genetic differences at the molecular level.
Conclusions
Overall, this study confirms a higher frequency of HCV spontaneous clearance. In addition, this study identified certain subgroups more likely to clear HCV infection. Individuals who are at greater risk for HCV infection such as those who identify as African American and patients with chronic kidney disease should be prioritized for HCV screening and treatment initiatives.
Acknowledgements
The authors wish to thank you Youssef Challita and Dr. El-Kabbany for reviewing draft of the manuscript.
Abbreviations
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- CKD
chronic kidney disease
- HCV
Hepatitis C
- HIV
Human Immunodeficiency Virus
- ICD-10
International classification of diseases
- UCLA
University of California, Los Angeles
Authors’ contributions
All authors have read and approved the manuscript before submission. MK – data acquisition, drafting, writing manuscript and critically thinking about manuscript content. MJ – results interpretation, data/statistical analysis, and critically thinking about manuscript content. KC– Provided valuable insights for revising manuscript. CS – provided valuable insights for revising the manuscript and interpretation. DH – Data acquisition, drafting manuscript. SR – Data acquisition specifically calling and verifying HCV treatment history/ HCV spontaneous clearance. YB– data/statistical analysis. SS – UCLA Pledger Liver institute attending and provided valuable insights for revising manuscript. JDK – Principal investigator and provided valuable insights in obtaining funding, study design, analysis and manuscript preparation.
Funding
This work was supported in part by a grant to Dr. Klausner from Gilead Sciences, Incorporated as Frontlines of communities in the United States (FOCUS) program partners (20173211). Gilead Sciences had no role in the study design, analysis, interpretation of the data or manuscript preparation.
Availability of data and materials
All data generated or analyzed during this study are available from the principal investigator on reasonable request.
Ethics approval and consent to participate
Study activities were reviewed by the UCLA International Review Board (IRB) under IRB# 15–001226.
Consent for publication
Not applicable.
Competing interests
KWC has received a research grant to the institution from Merck Sharpe & Dohme, Corp. The other authors do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mabel Michille Kimble, Email: mskimble0214@gmail.com.
Marjan Javanbakht, Email: javan@g.ucla.edu.
Kara W. Chew, Email: KChew@mednet.ucla.edu
Chrysovalantis Stafylis, Email: cstafylis@mednet.ucla.edu.
Di He, Email: dihe7@ucla.edu.
Samantha Ramirez, Email: SDRamirez@mednet.ucla.edu.
Yeonsoo Baik, Email: ybaik10@ucla.edu.
Sammy Saab, Email: Ssaab@mednet.ucla.edu.
Jeffrey D. Klausner, Email: Jdklausner@mednet.ucla.edu
References
- 1.Aisyah D. N., Shallcross L., Hully A. J., O'Brien A., Hayward A. Assessing hepatitis C spontaneous clearance and understanding associated factors-A systematic review and meta-analysis. Journal of Viral Hepatitis. 2018;25(6):680–698. doi: 10.1111/jvh.12866. [DOI] [PubMed] [Google Scholar]
- 2.Seaberg EC, et al. Spontaneous clearance of the hepatitis C virus among men who have sex with men. Clin Infect Dis. 2015;61(9):1381–1388. doi: 10.1093/cid/civ562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsum AM, et al. Spontaneous clearance of hepatitis C virus infection among human immunodeficiency virus-infected men who have sex with men. Open Forum Infect Dis. 2017;4(2):ofx090. doi: 10.1093/ofid/ofx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdelwahab SF. Cellular immune response to hepatitis-C-virus in subjects without viremia or seroconversion: is it important? Infect Agent Cancer. 2016;11:23. doi: 10.1186/s13027-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285(30):22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grebely J, et al. Genetics of spontaneous clearance of hepatitis C virus infection: a complex topic with much to learn. Hepatology. 2014;60(6):2127–2128. doi: 10.1002/hep.27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Post JJ, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189(10):1846–1855. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- 9.Ge D, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DL, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X, et al. IL28B genetic variation is associated with spontaneous clearance of hepatitis C virus, treatment response, serum IL-28B levels in Chinese population. PLoS One. 2012;7(5):e37054. doi: 10.1371/journal.pone.0037054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebely J, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–120. doi: 10.1002/hep.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grebely J, et al. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol. 2007;21(7):447–451. doi: 10.1155/2007/796325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui JI, et al. The effects of alcohol on spontaneous clearance of acute hepatitis C virus infection in females versus males. Drug Alcohol Depend. 2016;169:156–162. doi: 10.1016/j.drugalcdep.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks-Davis R, et al. Hepatitis C virus reinfection and spontaneous clearance of reinfection--the InC3 study. J Infect Dis. 2015;212(9):1407–1419. doi: 10.1093/infdis/jiv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, et al. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107(3):892–897. doi: 10.1182/blood-2005-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch MP, et al. Correlates of hepatitis C virus (HCV) RNA negativity among HCV-seropositive blood donors. Transfusion. 2006;46(3):469–475. doi: 10.1111/j.1537-2995.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Garten RJ, et al. Factors influencing a low rate of hepatitis C viral RNA clearance in heroin users from southern China. World J Gastroenterol. 2008;14(12):1878–1884. doi: 10.3748/wjg.14.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melendez-Morales L, et al. Chronic hepatitis B and other correlates of spontaneous clearance of hepatitis C virus among HIV-infected people with hemophilia. AIDS. 2007;21(12):1631–1636. doi: 10.1097/QAD.0b013e32826fb6d9. [DOI] [PubMed] [Google Scholar]
- 20.Bulteel N, et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J Hepatol. 2016;65(2):266–272. doi: 10.1016/j.jhep.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Deutsch M, et al. Clinical characteristics, spontaneous clearance and treatment outcome of acute hepatitis C: a single tertiary center experience. Saudi J Gastroenterol. 2013;19(2):81–85. doi: 10.4103/1319-3767.108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konerman Monica A., Thomson Mary, Gray Kristen, Moore Meghan, Choxi Hetal, Seif Elizabeth, Lok Anna S.F. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology. 2017;66(6):1805–1813. doi: 10.1002/hep.29362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geboy AG, et al. High hepatitis C infection rate among baby boomers in an urban primary care clinic: results from the HepTLC initiative. Public Health Rep. 2016;131(Suppl 2):49–56. doi: 10.1177/00333549161310S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younossi ZM, et al. Implementation of baby boomer hepatitis C screening and linking to care in gastroenterology practices: a multi-center pilot study. BMC Gastroenterol. 2016;16:45. doi: 10.1186/s12876-016-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner BJ, et al. Implementing hospital-based baby boomer hepatitis C virus screening and linkage to care: strategies, results, and costs. J Hosp Med. 2015;10(8):510–516. doi: 10.1002/jhm.2376. [DOI] [PubMed] [Google Scholar]
- 26.Chou Roger, Cottrell Erika Barth, Wasson Ngoc, Rahman Basmah, Guise Jeanne-Marie. Screening for Hepatitis C Virus Infection in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2013;158(2):101. doi: 10.7326/0003-4819-158-2-201301150-00574. [DOI] [PubMed] [Google Scholar]
- 27.Castrejon M, et al. Implementation of a large system-wide hepatitis C virus screening and linkage to care program for baby boomers. Open Forum Infect Dis. 2017;4(3):ofx109. doi: 10.1093/ofid/ofx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barsoum RS, William EA, Khalil SS. Hepatitis C and kidney disease: a narrative review. J Adv Res. 2017;8(2):113–130. doi: 10.1016/j.jare.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusnir J, Roth D. Direct-acting antiviral agents for the hepatitis C virus-infected chronic kidney disease population: the Dawn of a new era. Semin Dial. 2016;29(1):5–6. doi: 10.1111/sdi.12456. [DOI] [PubMed] [Google Scholar]
- 30.Dai CY, et al. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol. 2015;30(5):879–884. doi: 10.1111/jgh.12313. [DOI] [PubMed] [Google Scholar]
- 31.Jadoul M, Martin P. Hepatitis C treatment in chronic kidney disease patients: the kidney disease improving global outcomes perspective. Blood Purif. 2017;43(1–3):206–209. doi: 10.1159/000452730. [DOI] [PubMed] [Google Scholar]
- 32.Zheng MH, et al. Interleukin-28B rs12979860C/T and rs8099917T/G contribute to spontaneous clearance of hepatitis C virus in Caucasians. Gene. 2013;518(2):479–482. doi: 10.1016/j.gene.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Sousa MA, et al. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6. doi: 10.1186/1741-7015-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkar M, et al. Racial/ethnic differences in spontaneous HCV clearance in HIV infected and uninfected women. Dig Dis Sci. 2013;58(5):1341–1348. doi: 10.1007/s10620-012-2486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golden-Mason L, et al. Race- and gender-related variation in natural killer p46 expression associated with differential anti-hepatitis C virus immunity. Hepatology. 2012;56(4):1214–1222. doi: 10.1002/hep.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah DP, et al. Demographics, socio-behavioral factors, and drug use patterns: what matters in spontaneous HCV clearance? J Med Virol. 2012;84(2):235–241. doi: 10.1002/jmv.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen HR, et al. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology. 2007;46(2):350–358. doi: 10.1002/hep.21714. [DOI] [PubMed] [Google Scholar]
- 38.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76(12):2421–2438. [PubMed] [Google Scholar]
- 40.(KDIGO), K.D.I.G.O. Clinical Practice guideline on the prevention, diagnosis, evaluation, and treatment of hepatitic C in CKD. 2017 [cited 2018 April 12]; Available from: https://kdigo.org/guidelines/hepatitis-c-in-ckd/.
- 41.Azmi AN, Tan SS, Mohamed R. Hepatitis C and kidney disease: an overview and approach to management. World J Hepatol. 2015;7(1):78–92. doi: 10.4254/wjh.v7.i1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole SW. Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health. 2013;103(Suppl 1):S84–S92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole SW, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18(3):132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available from the principal investigator on reasonable request.


