Abstract
In the past years, claims of cognitive and attentional function of the cerebellum have first been raised but were later refuted. One reason for this controversy might be that attentional deficits only occur when specific cerebellar structures are affected. To further elucidate this matter and to determine which cerebellar regions might be involved in deficits of covert visual attention, we used new brain imaging tools of lesion mapping that allow a direct comparison with control patients. A total of 26 patients with unilateral right-sided cerebellar infarcts were tested on a covert visual attention task. Eight (31%) patients showed markedly slowed responses, especially in trials in which an invalid cue necessitated reorienting of the focus of attention for target detection. Compared with the 18 patients who performed within the range of healthy control subjects, only the impaired patients had lesions of cerebellar vermal structures such as the pyramid. We suggest that these midcerebellar regions are indirectly involved in covert visual attention via oculomotor control mechanisms. Thus, specific cerebellar structures do influence attentional orienting, whereas others do not.
Introduction
The cerebellum has been traditionally viewed as a structure that contributes primarily to the regulation of motor coordination. In the past years, claims for an additional cognitive function of the cerebellum have been raised [for review, see Haarmeier and Thier (2007), Thach (2007), Timmann and Daum (2007), Baillieux et al. (2008), and Glickstein and Doron (2008)], and the traditional belief of the motor dominant role is challenged by studies demonstrating that the cerebellum is involved in a variety of nonmotor functions (Akshoomoff and Courchesne, 1992; Courchesne et al., 1994; Fiez, 1996; Townsend et al., 1999; Cabeza and Nyberg, 2000; Allen and Courchesne, 2003; Schweizer et al., 2007).
With regard to visuospatial attention tasks, functional neuroimaging and lesion studies yielded inconsistent results as to the role of the cerebellum, and the question arises whether the cerebellum is per se or only secondary involved in such tasks. With respect to the latter it was argued that impairment in attentional tasks is rather based on oculomotor or motor deficits induced by lesions of the cerebellum (Ravizza and Ivry, 2001; Glickstein, 2007). Support for a primary role of the cerebellum in spatial attentional processes comes from functional imaging and older lesion studies (Courchesne et al., 1994; Townsend et al., 1999; Nobre et al., 2000; Mao et al., 2007). One study examined patients with autism and focal cerebellar lesions (Townsend et al., 1999) who performed a spatial target detection and target discrimination task (Posner et al., 1980, 1984). An analysis of the patients with focal lesions revealed that those whose posterior vermis was involved had the strongest orienting deficits. The authors assumed that the deficits reflect a disruption of gaze shift programming that delays covert orienting and suggested that the cerebellum has an anticipatory function (Courchesne, 1997; Courchesne and Allen, 1997) by coordinating rapid shifts of attention (Courchesne et al., 1994).
These findings have been challenged by studies that did not show any spatial attention deficit in patients with cerebellar lesions (Yamaguchi et al., 1998; Schoch et al., 2004; Golla et al., 2005; Hokkanen et al., 2006).
To summarize, data regarding the role of the cerebellum in attention and cognition are conflicting, and the role of the cerebellum in cognitive function is still a matter of debate (Glickstein, 2007). One reason for this confusion might be insufficient control of anatomical details as only specific cerebellar structures might sustain attentional processes, whereas cerebellar lesions sparing these structures might not result in impaired attention. To test this hypothesis and to determine which cerebellar structures might be critical for covert visual attention, we used new brain imaging tools of lesion mapping that allow a direct comparison with unaffected control patients (Rorden and Karnath, 2004). The present study thus defines the key area(s) typically associated with deficient covert orientating in humans by performing a lesion-subtraction analysis that compares a group of cerebellar patients with normal to those with deficient performance in a visuospatial attention task. For reasons of homogeneity, only patients with right-sided lesions were included in the study.
Materials and Methods
Patients.
We investigated a series of 26 patients with mainly unilateral right cerebellar damage due to stroke as demonstrated by magnetic resonance imaging (MRI). To achieve group homogeneity, only patients with right-sided lesions participated in the study. Twenty-four patients were right handed and two left handed. Patients with diffuse brain damage as well as patients with tumors or patients with insufficient communication abilities were not included. None of the patients took psychoactive drugs 24 h before the examination. Subjects gave informed consent for their participation in the study, which was performed in accordance with the ethical standards laid down in the 1962 Declaration of Helsinki. Clinical and demographic variables of all patients are shown in Table 1.
Table 1.
Demographic and clinical data of all patients with cerebellar lesions subdivided into patients with normal and abnormal RTs
| Normal RT group | Abnormal RT group | p value | |
|---|---|---|---|
| Number | 18 | 8 | |
| Sex | 5 f; 13 m | 2 f; 6 m | 0.883† |
| Etiology | 17 infarct, 1 hemorrhage | 8 infarct | |
| Age (years) [median (range)] | 68 (37–81) | 68 (40–79) | 0.935* |
| Time interval lesion–testing (d) [median (range)] | 10 (4–186) | 14 (9–274) | 0.125* |
| Lesion volume (% cerebellar volume) [median (range)] | 3.2 (0.3–23.1) | 11.2 (1.9–20.8) | 0.238* |
| Number of characters in the tapping task [median (range)] | 113 (74–163) | 102 (55–135) | 0.129* |
| Body sway (closed eyes) (% present) | 56 | 75 | 0.347† |
| Dysarthria (% present) | 17 | 25 | 0.619† |
| Dysmetria (% present) | 44 | 38 | 0.741† |
| Saccadic smooth pursuit (% present) | 44 | 75 | 0.149† |
| Smooth pursuit gain [median (range)] | 0.93 (0.70–1.0) | 0.80 (0.69–0.95) | 0.128* |
| OKN gain [median (range)] | 0.79 (0.39–0.98) | 0.61 (0.39–0.81) | 0.152* |
| Saccadic mean peak velocity (°/s) [median (range)] | 454 (392–514) | 431 (402–448) | 0.183* |
| Impaired vestibulo-ocular reflex suppression (% present) | 33 | 50 | 0.420† |
| Gaze-evoked nystagmus (% present) | 56 | 75 | 0.347† |
f, Female; m, male;
*statistical comparison using Mann–Whitney U test;
†statistical comparison using χ2 test.
Eight of the 26 patients (31%) showed a mean reaction time in the covert visual attention (CVA) paradigm (see below) that was 2 SDs above the mean of an age-matched control group (mean reaction time 0.434 ± 0.459 ms) and were considered as “abnormal reaction time (RT) group.” In contrast, the other 18 (69%) patients showed mean reaction times within the mean ± 2 × SD of the control group, i.e., did not differ in their performance in the covert visual attention paradigm compared with the age-matched control group and were considered “normal RT group.” To avoid that simple motor deficits would underlie prolonged reaction times during the attention task, a tapping task was conducted to control this possible confound. Every patient was instructed to tap with the index finger of the leading hand on a space key as quickly as possible for 30 s. The dependent variable was the number of characters. This task required the identical motor performance as in the CVA task. Furthermore, clinical symptoms were assessed (Table 1). Patients were classified as having dysarthria if their speech was slurring and words not understandable. The intactness of the vestibulo-ocular reflex suppression (VORS) was tested clinically by an experienced neuro-ophthalmologist who rotated the patient slowly side to side in a rotating chair while the patient clasped his/her hands in front and fixated the thumb. The unambiguous presence of corrective saccades signified impairment. To test smooth pursuit, patients were instructed to follow a slow lateral moving light. The occurrence of saccadic pursuit was considered pathological. Gaze-evoked nystagmus was tested by looking laterally at the examiner's finger. None of the patient had a spontaneous nystagmus. To assess body sways, patients had to stand with their feet together, their eyes alternately open and closed (Romberg's test). A positive sign was noted when a swaying, sometimes irregular swaying, and even toppling over occurs. The finger-to-nose test was applied to test dysmetria. The presence of an oscillating and dysmetric movement was considered abnormal. In addition, DC electrooculography (EOG) with monocular horizontal channels from electrode pairs (silver-chloride electrodes, diameter 4 mm) placed lateral to the outer canthi and with a vertical channel from an electrode pair above and below one eye was performed in the majority of patients. EOG included a smooth pursuit eye movement analysis in 21 out of the 26 patients (81%), a saccadic eye movement recording in 20 out of the 26 patients (76%), and an optokinetic nystagmus (OKN) recording in 19 out of the 26 patients tested (73%). At the time of EOG all patients were alert, cooperative, and attentive. Patients were seated in the center of a darkened room with the head fixed in front of a curved projection screen at a distance of 1.5 m. Before recording, a calibration procedure was performed whereby the patient had to look back and forth between two fixation points (calibration angle 40°). For the sinusoidal smooth pursuit test, subjects were instructed to follow a computer-controlled laser dot (diameter 5 mm) moving sinusoidally with a peak velocity of 20°/s (0.16 Hz) for 60 s. Signals were low-pass filtered (cutoff frequency 100 Hz). All data were controlled and analyzed offline on a computer running interactive eye movement analysis software (Toennies/Erich Jaeger). The calculation of the total smooth pursuit gain (ratio of eye velocity/target velocity) was based on a Fourier transformation of the derivation, i.e., the velocity trace over the entire recording time (Toennies/Erich Jaeger, 2000) (see Table 1). For the OKN, an alternating black and white stripe pattern of equal width of 7° was moved at constant velocity in a horizontal plane across a semispherical screen with a peak stimulus velocity of 60°/s. The subjects were asked to look at the stripes passing by without following them to the periphery. The gain of the subjects' slow-phase velocity of the OKN was evaluated by comparing the velocity of the stimulus and the target trace, i.e., the gain was defined as peak slow-phase eye velocity divided by peak stimulus velocity (60°/s). Due to the gradual build-up in slow-phase velocity, eye velocity was measured 30 s after OKN onset (Baloh and Honrubia, 2001). Horizontal saccades were visually triggered by moving the laser dot in a square-wave pattern 30° to the right and left sides. Subjects were asked to follow the light that stepped between the eccentric horizontal positions.
Both groups were comparable with respect to age, performance in the tapping task, time since lesion and size of lesion. Although the abnormal RT group tended to show more clinical symptoms such as saccadic pursuit eye movements, the respective comparisons did not reveal significant differences. This lack of an effect, however, is limited by group size, and as will be outlined in the discussion, oculomotor and attention deficits may be strongly related.
The control group consisted of 15 healthy subjects without any record of a neurological disorder (8 males, 7 females; mean age 61 years, SD 12.6 years). The control group neither differed with respect to age from the “abnormal RT” nor from the “normal RT” group (one-way ANOVA p = 0.876). To rule out the possibility that mere hospitalization may have affected the patients' performance, an additional control group of seven age-matched (one-way ANOVA p = 0.557) hospitalized patients (4 males, 3 females; mean age 57 years, SD 7.7 years) without any disease affecting the brain (five patients with neuropathy, one with a history of lumbar spinal stenosis and one with Lambert-Eaton syndrome) was tested. No difference was observed between the 15 healthy controls and the seven hospitalized controls with regard to the overall reaction times (one-way ANOVA p = 0.872), so that it seemed safe to use the initial control group for further analyses.
Experimental paradigm.
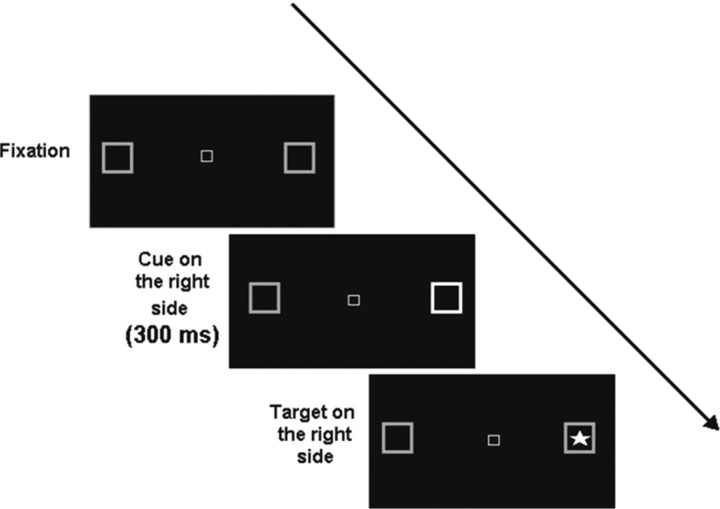
The covert visual attention task was designed after the original paradigm used by Posner et al. (1984) and programmed on Berisoft Cooperation Software, Frankfurt, Germany. The experiments were performed in a darkened and sound-attenuated room. To control for saccades, eye movements were recorded and analyzed offline using an infrared eye tracker and processed with a dedicated software package (Wineye). Trials with saccades were removed from the analysis, thus, in the control group 3% of the trials were removed, whereas in the “normal RT group” 8% and in the “abnormal RT” group 19% of the trials had to be removed. Head movements were minimized by use of a chin rest. Subjects underwent the experiment at a fixed viewing distance of 50 cm from the monitor. They were instructed to keep their gaze fixed on the center of the screen where a white central outline box comprising a visual angle of 0.5° was shown. The central box was flanked on the right and the left by square boxes placed at 8° from the center on a black screen (Fig. 1). Before the target, one of the peripheral boxes brightened for 300 ms. This cue indicated the correct location of the upcoming target on 80% of trials (valid trials), in 20% of trials the target appeared in the opposite box (invalid trials). After varying cue–target latencies of 0, 150, 550, or 1000 ms [interstimulus interval (ISI)], the target, a bright asterisk comprising 3°, was presented in either the left or the right peripheral box until a response was made (Fig. 1) for a maximum of 5 s. In <1% of trials responses were not made within this time window; therefore, these misses were not analyzed further. The task was to press the space bar as quickly as possible with the index finger of the dominant hand. The sequence of the 160 trials (left, right, valid, invalid, different ISIs) was completely randomized. Participants were told to respond as fast as possible, and they were urged to avoid saccades.
Figure 1.
Experimental design: single trials started with the presentation of a central fixation box, followed by the cue, i.e., a brightening of the peripheral box for 300 ms before the target (bright asterisk) appeared.
The RTs to the target were measured as a function of ISI, presentation side, group, and validity. For the data analysis a statistical software package (SPSS, version 15.0) was used.
Lesion analysis.
Lesion analysis was done by established brain imaging lesion techniques (Rorden and Karnath, 2004). The lesions of all patients were documented by MRI. The MRI protocol used diffusion-weighted (DWI) and T2-weighted fluid-attenuated inversion-recovery (FLAIR) imaging. Scans were obtained on a 1.5 T echoplanar imaging (EPI)-capable system (Magnetom Vision, Siemens). The FLAIR sequence was acquired with 19 axial slices (slice thickness 5 mm) with an interslice gap of 1 mm, a field of view (FOV) of 175 × 230 mm2, a repetition time (TR) of 9000 ms, and an echo time (TE) of 108 ms. DWI was performed with a single-shot EPI spin echo sequence (TR 5000 ms, TE 137 ms, FOV 256 × 256 mm2; matrix 64 × 64 pixels; slice thickness 5 mm, gap 1 mm). MRI lesions were defined on FLAIR sequences and verified by DWI sequences. The initial scanning was optionally repeated during the following days until a firm diagnosis could be made, and the infarcted area became clearly demarcated. These late scans were used in the present study to avoid possible artifacts due to edema or intracranial pressure. Furthermore, lesion-mapping plots were carefully examined for possible brain edema, which is also visible in the MRI scan and might distort the lesion mapping.
The median time between lesion and the MRI scans used for the present analysis was 4 d (range 1–10 d). Lesions were mapped with MRIcro software (www.sph.sc.edu/comd/rorden/mricro.html) onto slices of a T1-weighted template MRI scan from the Montreal Neurological Institute (MNI) (www.bic.mni.mcgill.ca/cgi/icbm_view). This template is approximately oriented to match Talairach space (Talairach and Tournoux, 1988). Lesions were mapped onto the slices that correspond to z coordinates −55, −49, −43, −37, −31, −25, −19, and −13 mm in Talairach coordinates. Statistical analysis using χ2 test was conducted to test whether the lesions of the corresponding anatomical structures between the two groups (i.e., the abnormal RT and the normal RT group) differed or not.
Results
Behavioral data
A repeated-measure ANOVA with the within-subjects factors target side (right vs left), validity (valid vs invalid), and ISI (0, 150, 550, 1000 ms) and the between-subjects factor group (abnormal RT group, normal RT group, controls) revealed a significant main effect of group (F(2,38) = 38.28; p < 0.01). This main effect reflects that performers with abnormal RTs displayed an overall worse performance than the controls and the normal RT group. Other main effects were validity (F(1,38) = 8.23; p = 0.007) and ISI (F(3,114) = 9.50; p < 0.01), indicating that RTs were shorter in valid trials and in trials with longer ISIs. Presentation side had no significant effect (F(1,38) = 1.37; p = 0.251). The only significant interaction with group was seen for validity (F(2,38) = 3.48; p = 0.043), indicating that the abnormal RT group was especially impaired in invalid trials (Figs. 2, 3).
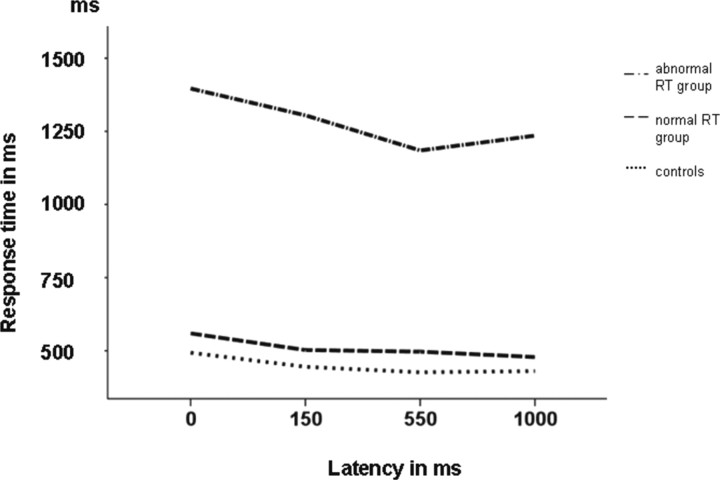
Figure 2.
Performance of the patients and of the age-matched controls in the CVA paradigm plotted against cue to target delay. Normal controls achieved the fastest response, below 500 ms (dotted line). The abnormal RT group showed responses above 1 s (dashed-dotted line), whereas the normal RT group showed nearly normal response times (dashed line).
Figure 3.
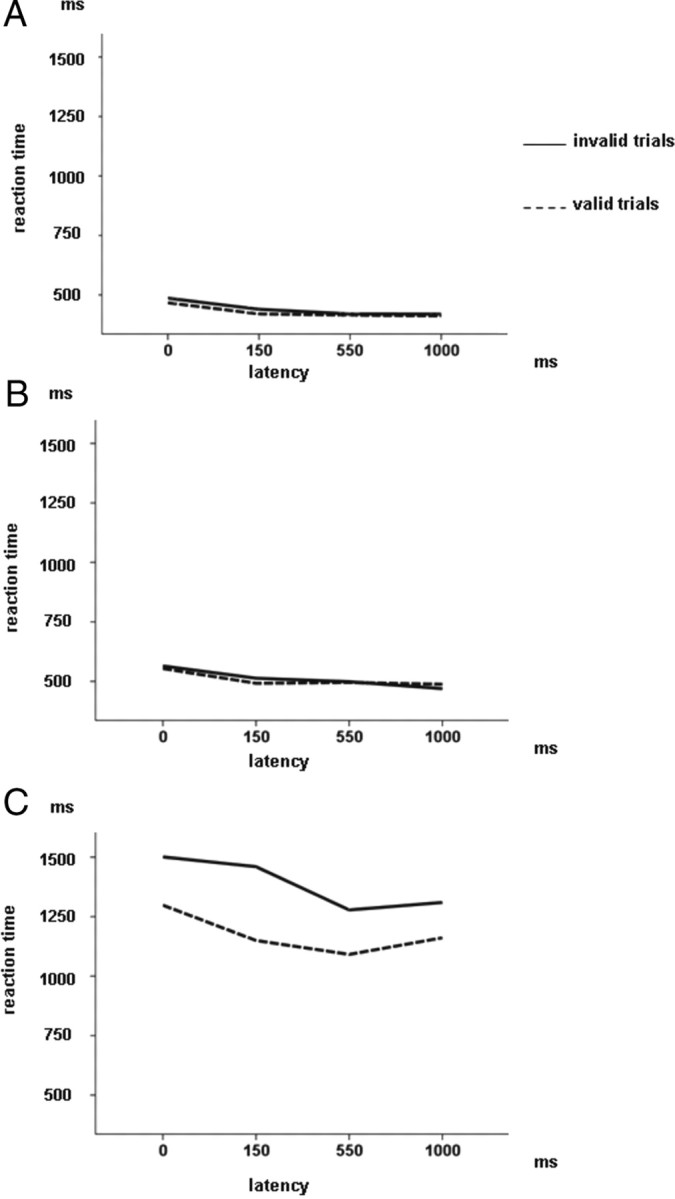
A, Reaction time of the valid and invalid cue–target conditions for the 15 healthy controls plotted against latencies of 0, 150, 550, and 1000 ms. B, Reaction time of the cue–target conditions for the 18 patients with normal RTs plotted against latencies. C, Reaction time of the cue–target conditions for the eight patients with abnormal RTs plotted against latencies.
When the analysis was restricted to the two patient groups, the same effects as above could be observed, i.e., main effects of validity (F(1,24) = 5.86; p < 0.023), latency (F(3,72) = 5.71; p = 0.001), and group (F(1,24) = 42.61; p < 0.01), indicating that assignment to the normal RT group, valid trials, and longer latencies were associated with shorter reaction times. Analogous to the ANOVA that included all three groups, only the interaction between validity and group was significant (F(1,24) = 5.28; p < 0.03), reflecting that the abnormal RT group displayed not only worse overall performance but that they were especially impaired when invalid cues were used so that they had to redirect attention from the cue to the target in the opposite visual hemifield.
Anatomical data
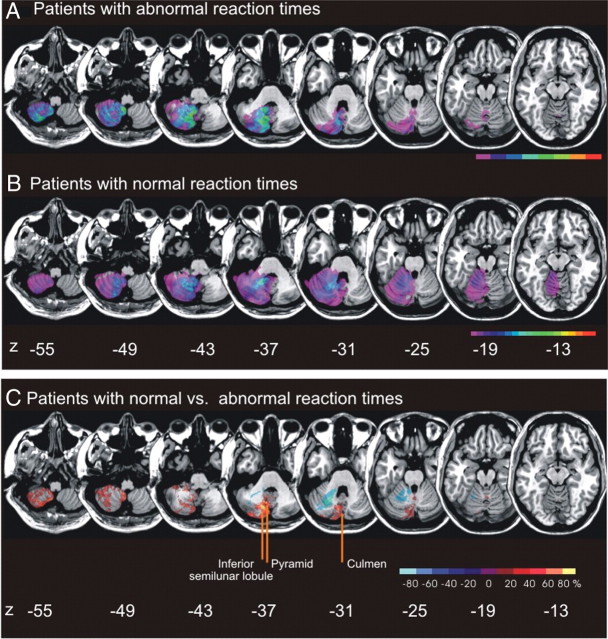
In our sample of 26 admitted stroke patients with right cerebellar brain damage, we contrasted the eight patients with abnormal RTs to the 18 patients with normal RTs, i.e., with reaction times within 2 × SD of the healthy controls. The two groups were comparable with respect to age, motor performance, acuity of lesion, and size of lesion (Table 1). Figure 4, A and B, illustrates lesion density plots for both groups. To identify the structures that were commonly damaged in patients with abnormal RTs but were typically spared in patients with normal RTs, we subtracted the superimposed lesions of the abnormal RT group from the overlap image of the normal RT group and vice versa [for details concerning the lesion subtraction technique, see Rorden and Karnath (2004)]. Figure 4C illustrates that the maximum overlap of areas specifically related to the abnormal RT group in the CVA paradigm were the culmen (x = 4, y = −73, z = −31), the pyramid of the vermis (x = 10, y = −64, and z = −37), and parts of the inferior semilunar lobule. All but one of the performers with abnormal RTs had lesions affecting the inferior semilunar lobule and a lesion of the pyramid (88%). Four patients (50%) had lesions of the culmen. Thus, the pyramid of the vermis was more affected in the abnormal RT group than in patients with normal RTs (χ2 = 7.95; p = 0.005). There was a tendency, indicating that lesions of the culmen and of the inferior semilunar lobule were more frequent in the abnormal RT group (for the inferior semilunar lobule χ2 = 4.21; p = 0.04; for the culmen χ2 = 3.13; p = 0.077). Despite the fact that lesion volume did not differ between the abnormal and normal RT group (see Table 1), we conducted a correlation analysis between lesion volume and performance to reveal a potential association. Neither in the abnormal nor in the normal RT group was a significant correlation obtained (abnormal RT group: r = −0.210; n = 8; p = 0.618; normal RT group: r = −0.004; n = 18; p = 0.988); thus, abnormal RTs seem not to depend on greater lesion volume.
Figure 4.
A, Overlay lesion plots of patients with reaction times above the mean ± 2 × SD of the healthy control group (abnormal RT group). The number of overlapping lesions is illustrated by different colors coding increasing frequencies from violet (n = 1) to red (n = 8). B, Overlay lesion plot of the comparison patients with right cerebellar lesions with reaction times below the mean ± 2 × SD of the healthy control group (n = 18) (normal RT). The number of overlapping lesions is illustrated by different colors coding increasing frequencies from violet (n = 1) to red (n = 18). C, Overlay plot of the subtracted superimposed lesions of the patients with abnormal RTs minus the comparison group, i.e., patients with normal RTs and vice versa. The percentage of overlapping lesions of the group who performed worse after subtraction of the control group is illustrated by five different colors coding increasing frequencies from dark red (difference 1–20%) to white-yellow (difference 81–100%). Each color represents 20% increments. The colors from dark blue (difference −1 to −20%) to light blue (difference −81 to −100%) indicate regions damaged more frequently in the comparison group than in patients who performed worse in the covert visual attention task. Talairach z coordinates of each transverse slice are given (Talairach and Tournoux, 1988). The figure illustrates that the anatomical areas related to longer reaction times, i.e., abnormal RTs, are the pyramid of the vermis, the culmen, and partly the inferior semilunar lobule.
To exclude the possibility that cerebellar output nuclei were differently affected in patients with abnormal and normal RTs, we analyzed whether the dentate nucleus, which presents the major cerebellar efferent node, was more affected in patients with abnormal RTs or not. However, no difference was seen between the two groups (χ2 = 0.348; p = 0.555) at x = 19, y = −55, and z = −36, corresponding to the maximum overlap site of the dentate/interposed nucleus based on a probabilistic three-dimensional MRI atlas (Dimitrova et al., 2006).
Discussion
The present data revealed that 8 (31%) out of the 26 patients with unilateral right-sided cerebellar lesions had reaction times in the covert visual attention paradigm that were outside the range of healthy controls by 2 SDs (abnormal RT group). These patients did not only show prolonged overall responses, moreover, they were specifically impaired in invalid trials, i.e., when the target appeared on the opposite side of the cue so that reorienting of attention was necessary. The damaged areas associated with this behavioral deficit were in the first place the pyramid of the vermis, the culmen, and partly the inferior semilunar lobule.
To our knowledge, this is the first study on covert attention orienting in patients with focal cerebellar lesion that applies a modern state-of-the-art lesion-mapping technique. This mapping technique significantly reduces the anatomical uncertainty brought in by the procedures used in previous studies where only rough anatomical landmarks could be taken into consideration and where no direct visual and/or statistical comparisons between patients with and without deficient covert visual attention could be performed (Rorden and Karnath, 2004). In contrast to previous data published by Townsend et al. (1999), eye movements as well as motor action, i.e., tapping speed of the index finger, were controlled in the present study; thus, it seems unlikely, that the impairment in the covert visual attention task in the abnormal RT group reflected a basal motor deficit.
Thus, two firm conclusions may be drawn from the present data: First, cerebellar lesions can result in deficits in covert visuospatial attention. This notion is based on the observation that the eight patients showed longer mean reaction times in the attention paradigm while no motor deficits were present. It is further supported by the finding that these patients were especially impaired in trials where the cue was invalid. In these trials, attention had to be shifted twice: first, from the fixation square to the cue, then from the cue to the target located in the other visual hemifield. Hence, their deficit in attention orienting came into play twice and, therefore, prolonged responses profoundly in these “invalid” trials. In addition, this observation also argues against a simple motor deficit since such a deficit should not affect invalid trials more than valid trials. The latter observation also speaks against a disconnection hypothesis that would relate the deficits to lesions of the cerebellum's output nuclei. Such lesions should not have differential effects on different tasks. In addition, nearly half of the patients with normal RT data had lesions affecting the cerebellum's most important output nucleus, the dentate nucleus.
The second conclusion is that cerebellar lesions do not need to affect visuospatial attention: 18 (69%) of the 26 patients did not show any behavioral difference compared with the controls. Their lesions were restricted to the cerebellar hemispheres and mostly spared the midcerebellar vermal structures. This finding is in line with the Golla et al. (2005) study that failed to reveal attention deficits in their patients who mostly had hemispheric cerebellar lesions. That is, these patients were comparable to our normal RT group. Likewise, Yamaguchi et al. (1998) claimed that the lateral cerebellum is not involved in visuospatial attention shifts. With the limitations of our study kept in mind (none of our patients had an isolated lesion of the vermis, one patient with impaired performance had no vermal lesion, structural MRI scans might not necessarily show the full functional extent of a lesion), it seems possible to hypothesize that other parts than the cerebellar hemispheres, namely the pyramid of the vermis (vermal lobule VIII), the culmen (vermal lobule VI), and partly the inferior semilunar lobule, are necessary for attentional orienting. This assumption is largely supported by Townsend et al. (1999), who showed that cerebellar patients whose lesions involved the posterior vermis had the strongest orienting deficits. These cerebellar midline structures are also known to be involved in the control of saccadic eye movements (Ron and Robinson, 1973; Leigh and Zee, 1991; Bötzel et al., 1993; Vahedi et al., 1995; Barash et al., 1999; Robinson and Fuchs, 2001). For example, lesions of parts of the vermis such as, e.g., the pyramid have been reported to induce deficient saccadic eye movements (Leigh and Zee, 1991; Bötzel et al., 1993; Vahedi et al., 1995; Barash et al., 1999). Hence, the vermis has been suggested to contribute to the accuracy of saccades (Takagi et al., 1998; Ettinger et al., 2005) and might be responsible for a time-dependent saccadic adaptation (Thier et al., 2002).
Although in this study we used a covert visual attention paradigm in which relevant stimuli are attended in the absence of overt orienting eye movements, which bring targets of interests to the fovea (Moore et al., 2003; Awh et al., 2006), there is indirect evidence suggesting that neural mechanisms of saccadic programming and covert visual attention overlap (Rizzolatti et al., 1987; Corbetta et al., 1998; Nobre et al., 2000). In accordance with this model, our data suggest an anatomical and behavioral link between oculomotor vermal structures and impaired covert visual attention—like that suggested by Townsend et al. (1999) 10 years earlier. Note that our performers with abnormal RTs seem to have had more difficulties controlling their eye movements as they made more illicit saccades. Also, the clinical and EOG examination revealed more oculomotor deficits in this patient group whereby this difference failed to reach significance. Hence, our results support the concept of “premotor theory of attention” (Rizzolatti et al., 1987) that hypothesized that covert attention arises from latent eye movement commands even when eye movements are not made. Therefore, and considering the cerebellum as the most important link between visual and motor areas of the cerebral cortex (Glickstein, 2007) the vermis might also be part of a neural cerebrocerebellar circuitry involved in spatial attention and saccade generation, including the frontal eye field (FEF) (Astafiev et al., 2003; Müller et al., 2003; Müller and Kleinschmidt, 2007), the superior colliculus (Müller et al., 2005), and cortical parietal areas (Posner et al., 1984; Wardak et al., 2004).
Not only on the cortical but also on the cerebellar level, attentional and saccadic control seem to be tightly related. Similar to the FEF (Moore and Armstrong, 2003), vermal structures such as the pyramid might be involved in the process of saccade preparation and thus facilitate visual selection and covert visual attention shifts. Alternatively, lesions of the vermis might indirectly affect cortical areas considered to be involved in attention as well as oculomotor processes via diaschisis effects.
The limitations of the present study should be kept in mind, since structural MRI scans might not necessarily show the full functional extent of a lesion. Despite the fact that lesion-mapping plots were thoroughly examined for possible brain edema, we cannot entirely exclude a later change in lesion volume, although it has been recently shown that lesion volumes calculated from baseline images give a very good estimate of final lesion size in ischemic stroke patients (Rivers et al., 2006). In addition, areas that appear structurally intact in anatomical scans may not necessarily be functioning normally due to an abnormal perfusion. Therefore, perfusion-weighted imaging, which measures the amount and latency of blood flow in certain regions, provides a promising new tool to address these issues in future studies.
Another limitation might be that performance in acute stroke patients seems to be vulnerable to other factors such as, e.g., concentration. However, patients were thoroughly selected to include only vigilant patients in the study. Furthermore, the time between testing and lesion did not differ between the two groups, minimizing the risk of confounding variables. And last but not least, testing patients in the chronic phase of their disease would have introduced another, possibly more severe confound: reorganization of brain function and structure. Nevertheless, to control for possible reorganization processes, we are planning to perform a follow-up examination—provided that the patients agree to participate.
Thus, testing patients in the acute phase of disease may allow more valid conclusions regarding the cerebellum's function in the normal brain.
In our view, the present study underlines the necessity of human lesion studies even in the era of advanced functional neuroimaging. Only lesion studies can differentiate between areas that are involved and those that are necessary for a task (Rorden and Karnath, 2004; Müller and Knight, 2006). For example, a previous fMRI study failed to demonstrate cerebellar involvement in attentional shifts and concluded that cerebellar damage would only affect the motor component during attention shifting tasks (Bischoff-Grethe et al., 2002). Here we show that damage to the vermis of the cerebellum does indeed induce a deficit in shifting of attention while leaving motor function intact.
Footnotes
We are grateful to thank Sonja Wicht for data assessment.
References
- Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Mariën P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Honrubia V. Ed 3. New York: Oxford UP; 2001. Clinical neurophysiology of the vestibular system. [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–10939. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Ivry RB, Grafton ST. Cerebellar involvement in response reassignment rather than attention. J Neurosci. 2002;22:546–553. doi: 10.1523/JNEUROSCI.22-02-00546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bötzel K, Rottach K, Büttner U. Normal and pathologic saccadic dysmetria. Brain. 1993;116:337–353. doi: 10.1093/brain/116.2.337. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Prediction and preparation; anticipatory role of the cerebellum in diverse neurobehavioural functions. Behav Brain Sci. 1997;20:248–249. [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, Beck A, Aurich V, Forsting M, Timmann D. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30:12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Antonova E, Crawford TJ, Mitterschiffthaler MT, Goswani S, Sharma T, Kumari V. Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. Neuroimage. 2005;24:487–494. doi: 10.1016/j.neuroimage.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Glickstein M. What does the cerebellum really do? Curr Biol. 2007;17:R824–R827. doi: 10.1016/j.cub.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Doron K. Cerebellum: connections and functions. Cerebellum. 2008;7:589–594. doi: 10.1007/s12311-008-0074-4. [DOI] [PubMed] [Google Scholar]
- Golla H, Thier P, Haarmeier T. Disturbed overt but normal covert shifts of attention in adult cerebellar patients. Brain. 2005;128:1525–1535. doi: 10.1093/brain/awh523. [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Thier P. The attentive cerebellum—myth or reality? Cerebellum. 2007;6:177–183. doi: 10.1080/14734220701286187. [DOI] [PubMed] [Google Scholar]
- Hokkanen LS, Kauranen V, Roine RO, Salonen O, Kotila M. Subtle cognitive deficits after cerebellar infarcts. Eur J Neurol. 2006;13:161–170. doi: 10.1111/j.1468-1331.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. Philadelphia: F.A. Davis; 1991. The neurology of eye movements. [Google Scholar]
- Mao L, Zhou B, Zhou W, Han S. Neural correlates of covert orienting of visual spatial attention along vertical and horizontal dimensions. Brain Res. 2007;1136:142–153. doi: 10.1016/j.brainres.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG, Kleinschmidt A. Temporal dynamics of the attentional spotlight: neuronal correlates of attentional capture and inhibition of return in early visual cortex. J Cogn Neurosci. 2007;19:587–593. doi: 10.1162/jocn.2007.19.4.587. [DOI] [PubMed] [Google Scholar]
- Müller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Müller NG, Donner TH, Bartelt OA, Brandt SA, Villringer A, Kleinschmidt A. The functional neuroanatomy of visual conjunction search: a parametric fMRI study. Neuroimage. 2003;20:1578–1590. doi: 10.1016/s1053-8119(03)00416-6. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. J Cogn Neurosci. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Ron S, Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973;36:1004–1022. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schoch B, Gorissen B, Richter S, Ozimek A, Kaiser O, Dimitrova A, Regel JP, Wieland R, Hövel M, Gizewski E, Timmann D. Do children with focal cerebellar lesions show deficits in shifting attention? J Neurophysiol. 2004;92:1856–1866. doi: 10.1152/jn.00185.2004. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Alexander MP, Cusimano M, Stuss DT. Fast and efficient visuotemporal attention requires the cerebellum. Neuropsychologia. 2007;45:3068–3074. doi: 10.1016/j.neuropsychologia.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Thieme; 1988. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. [Google Scholar]
- Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6:163–167. doi: 10.1080/14734220701373530. [DOI] [PubMed] [Google Scholar]
- Thier P, Dicke PW, Haas R, Thielert CD, Catz N. The role of the oculomotor vermis in the control of saccadic eye movements. Ann N Y Acad Sci. 2002;978:50–62. doi: 10.1111/j.1749-6632.2002.tb07555.x. [DOI] [PubMed] [Google Scholar]
- Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6:159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Toennies/Erich Jaeger. Operating manual of Nystagliner. Höchberg, Germany: Toennies/Erich Jaeger; 2000. [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci. 1999;19:5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K, Rivaud S, Amarenco P, Pierrot-Deseilligny C. Horizontal eye movement disorders after posterior vermis infarctions. J Neurol Neurosurg Psychiatry. 1995;58:91–94. doi: 10.1136/jnnp.58.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S. Visuospatial attention shift and motor responses in cerebellar disorders. J Cogn Neurosci. 1998;10:95–107. doi: 10.1162/089892998563806. [DOI] [PubMed] [Google Scholar]