Abstract
Hemopressin is a short, nine amino acid peptide (H-Pro-Val-Asn-Phe-Lys-Leu-Leu-Ser-His-OH) isolated from rat brain that behaves as an inverse agonist at the cannabinoid receptor CB1, and is shown here to inhibit agonist-induced receptor internalization in a heterologous cell model. Since this peptide occurs naturally in the rodent brain, we determined its effect on appetite, an established central target of cannabinoid signaling. Hemopressin dose-dependently decreases night-time food intake in normal male rats and mice, as well as in obese ob/ob male mice, when administered centrally or systemically, without causing any obvious adverse side effects. The normal, behavioral satiety sequence is maintained in male mice fasted overnight, though refeeding is attenuated. The anorectic effect is absent in CB1 receptor null mutant male mice, and hemopressin can block CB1 agonist-induced hyperphagia in male rats, providing strong evidence for antagonism of the CB1 receptor in vivo. We speculate that hemopressin may act as an endogenous functional antagonist at CB1 receptors and modulate the activity of appetite pathways in the brain.
Introduction
Hemopressin is a product of the hemoglobin α chain, discovered in rat brain using an enzyme-substrate capture technique and so named as it can cause small decreases in blood pressure (Rioli et al., 2003; Lippton et al., 2006). Subsequently, hemopressin was found also to have nonopioid antinociceptive effects (Dale et al., 2005). In vitro studies show that the peptide acts as a CB1 receptor inverse agonist, and can interact with both peripheral and central pain pathways in vivo (Heimann et al., 2007). To date, all known endogenous cannabinoids, such as 2-arachidonoylglycerol and anandamide, are fatty acid derivatives (Bisogno, 2008; Petrosino et al., 2009). These endocannabinoids are released by postsynaptic neurons “on demand,” following the Ca2+ influx produced in response to postsynaptic depolarization or activation of metabotropic receptors (Kano et al., 2009). When released into the synaptic cleft, endocannabinoids activate presynaptic CB1 receptors, and impart an inhibitory action on further presynaptic transmission. The administration of exogenous CB1 agonists, such as Δ9-tetrahydrocannabinol (THC), the active ingredient of Cannabis sativa, or the synthetic compounds CP55940 [(−)-cis-3-[2-hydroxy-4-(1-1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol] and WIN 55212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone mesylate], increase food intake by increasing motivational reward (Cota et al., 2003a; Pagotto et al., 2006). By comparison, the synthetic compound, rimonabant (SR141716A), is an inverse agonist at the CB1 receptor and is capable of producing weight-reducing effects over extended periods in rodents and humans (Van Gaal et al., 2005; Di Marzo, 2008). The action of rimonabant to reduce specifically motivational appetite is relatively short lived, and any continued weight loss is thought to be mediated mainly via peripheral CB1 interaction with lipid mobilization pathways in adipose tissue and liver, energy expenditure and cellular glucose uptake (Di Marzo, 2008; Nogueiras et al., 2008; Kunos et al., 2009).
We hypothesize that hemopressin may be a naturally occurring inverse agonist of brain CB1 receptors, capable of antagonizing central orexigenic pathways.
Materials and Methods
Cell culture and transfection.
COS-7 Monkey Kidney Fibroblasts cells (Invitrogen) were grown on coverslips in a 24-well plate, in a medium of DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin. Throughout the experiment cells were kept under 5% CO2 in air at 37°C and passage numbers P1–P20 of undifferentiated cells were used for experiments. At ∼90% confluence, cells were transfected with pEGFP-N1-CB1 plasmid (mouse CB1 cDNA was cloned into a pEGFP-N1 vector which encodes the GFPmut1 variant (Clontech Labs), leading to an eGFP fusion at the C terminus of CB1) using Lipofectamine according to the manufacturer's protocol (Invitrogen). Following an overnight transfection, the growth medium was changed, and cells were treated with vehicle (0.25% DMSO), 100 nm AM251, 100 nm, 10 μm, and 100 μm hemopressin, in the absence, or presence of 100 nm WIN 55212-2 (all Tocris Bioscience). Cells were stimulated with drugs for 2 h, and then fixed in an ice-cold solution of 4% paraformaldehyde, 4% sucrose in 0.1 m phosphate buffer for 45 min. Slides were coverslipped using VectorShield hard set (Vector Labs) containing 4′,6-diamidino-2-phenylindole (DAPI) to stain cell nuclei. Images of transfected cells were viewed by an experimenter blinded to treatment group using an Olympus BX51 upright microscope with a 60×/1.4 UPlanApo objective. Images were captured at random using a Coolsnap ES camera (Photometrics) through MetaVue Software (Molecular Devices). Specific bandpass filter sets for DAPI (excitation λ, 360–370 nm, emission λ, 420–460), and eGFP (excitation λ, 480/40 nm, emission λ, 535/50) were used to prevent bleed through from one channel to the next. Fifty cells per treatment group were analyzed to quantify the number of internalized endosomes per cell. To determine an IC50 value for hemopressin, we set up a similar experiment, but cells were treated with hemopressin over a nine point log dilution series (100 μm, 10 μm, 1 μm, 100 nm, 10 nm, 1 nm, 100 pm, 10 pm, 1 pm) in the presence of 100 nm WIN 55212-2. Forty cells per treatment group were analyzed to quantify the number of internalized endosomes per cell. The percentage inhibition of internalization was calculated relative to the control situation of 100 nm WIN 55212-2 alone.
Animals.
All experiments (except those using ob/ob or CB1 receptor knock-outs) were performed on adult, male outbred CD1 mice and male, outbred Sprague Dawley rats (Charles River Laboratories Inc). The male ob/ob mice, homozygous for the obese spontaneous mutation, Lepob, are backcrossed with a C57BL/6N background (B6.V-Lepob/J, Jackson Laboratories). CB1+/+ and CB1−/− littermate mice were obtained by breeding of heterozygotes that had been backcrosssed six times to a C57BL/6N background, as described previously (Marsicano et al., 2002). All animals were housed under a 12:12 h light/dark cycle (lights on 8:00 A.M. to 8:00 P.M.), at 22°C ± 1°C and 45 ± 10% humidity. Pelleted food (Beekay International) and water were available ad libitum unless stated otherwise. Experimental procedures were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and local ethical review. The experiment involving the CB1 knock-out mice was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the local Government of Rheinland-Pfalz, Germany.
Intracerebroventricular surgery.
Under 2% isoflurane (Concord Pharmaceuticals Ltd) in 1 l/min oxygen, mice and rats were implanted stereotaxically with guide cannulae into the right lateral ventricle (0.2 mm posterior, 1 mm lateral from bregma for mice, and 0.8 mm posterior, 1.5 mm lateral from bregma for rats) according to the atlas of Paxinos and Watson (1998). The tip of the guide cannula was positioned 1 mm above the injection site [1 mm (mice), and 3 mm (rats) ventral to the surface of the skull]. All animals were allowed to recover from surgery for 5–7 d before the start of experiments.
Hemopressin effects on nocturnal feeding behavior in mice and rats.
All mice and rats were housed singly at least 3 d before the experiment and food was restricted 3 h before the experiment was due to start. At lights out (8:00 P.M.), animals were fed preweighed chow ad libitum.
In one experiment, 18 mice (31 ± 1.8 g, n = 6) were assigned randomly to receive intraperitoneal injection of vehicle (0.9% w/v NaCl, 10% DMSO, 20% 2-hydroxypropyl-β-cyclodextrin), 500 nmol/kg hemopressin, or 5.4 μmol/kg AM251 (3 mg/kg, based on dose described by Tallett et al., 2007b). Injections were made in a volume of 2 ml/kg body weight. Food intake was determined 1, 2, 4, and 24 h after injection. Results are presented as mean ± SEM for food intake at each time point. Treatments were compared using a one-way ANOVA followed by Dunnett's multiple-comparison post hoc test using the GraphPad Prism statistical package (GraphPad Software). In a second experiment, 24 intracerebroventricularly cannulated CD1 mice (30 ± 1.4 g, n = 6) were assigned randomly to receive intracerebroventricular injection of vehicle (0.9% w/v NaCl) or 1, 5, or 10 nmol hemopressin. Injections were made in a volume of 2 μl per animal. Treatments were compared using a one-way ANOVA followed by Dunnett's multiple-comparison post hoc test. In a third experiment, 12 intracerebroventricularly cannulated rats (320 ± 12 g, n = 6) were assigned randomly to receive vehicle (0.9% w/v NaCl) or 10 nmol hemopressin intracerebroventricularly. Injections were made in a volume of 2 μl per animal. Treatments were compared using a two-tailed t test.
Effects of hemopressin on feeding behavior in CB1 knock-out mice.
Twelve male CB1−/− mice and 12 wild-type littermates (26 ± 2.1 g) were housed singly 1 week before the experiment. Since the two genotypes normally display significantly different body weights (Cota et al., 2003b) and, therefore, food intake, all the mice were fasted overnight before the start of the experiment. One hour after lights on (8:00 A.M.) CB1−/− and CB1+/+ animals were assigned randomly to receive intraperitoneal injection of either vehicle or 500 nmol/kg hemopressin in a volume of 2 ml/kg (n = 5/6). Food intake was determined 1, 2, 4, and 12 h after injection. Treatments were compared using a two-way ANOVA followed by Bonferroni's multiple-comparison post hoc test.
Effects of hemopressin on feeding behavior in leptin-deficient (ob/ob) mice.
Fourteen obese ob/ob mice (38 ± 3.5 g, n = 7) were assigned randomly to receive intraperitoneally vehicle (0.9% w/v NaCl) or 500 nmol/kg hemopressin. Injections were made in a volume of 2 ml/kg body weight. Food intake was determined 1, 2, 4, and 24 h after injection. Treatments were compared using a two-tailed t test.
Hemopressin effects CB1 agonist (CP55940)-induced hyperphagia in rats.
Twenty-four Sprague Dawley rats (320 ± 18 g, n = 5/6) were cannulated into the lateral ventricle under recovery anesthesia 1 week before experimentation. Rats were housed singly at least 3 d before the experiment and food was restricted 3 h before the experiment was due to start. At lights off (8:00 P.M.) animals were assigned randomly to receive vehicle (0.9% w/v NaCl) or 10 nmol hemopressin intracerebroventricularly. Injections were made in a volume of 2 μl per animal. Twenty minutes later, rats received, intraperitoneally, vehicle (0.9% w/v NaCl, 2.5% ethanol) or 0.06 mg/kg CP55940 (Tocris Bioscience Ltd., Brighton, UK) in a volume of 1 ml/kg. The dose of CP55940 was determined in previous published experiments (Dodd et al., 2009). Upon second injection, animals were fed preweighed chow ad libitum. Treatments were compared using a one-way ANOVA followed by Bonferroni's multiple-comparison post hoc test.
Behavioral satiety sequence.
CD1 mice were transferred to transparent cages 3 d prior and fasted overnight before the start of the experiment. In one experiment, 16 mice (32 ± 1 g, n = 8) were assigned randomly to receive intraperitoneally vehicle or 500 nmol/kg hemopressin in a volume of 2 ml/kg. In a second experiment, 14 mice (30 ± 1.4 g, n = 7) were assigned randomly to receive intracerebroventricular injection of either vehicle or 10 nmol hemopressin in a volume of 2 μl per animal. In a third experiment, 18 mice (32 ± 1.6 g, n = 6) were assigned randomly to receive intraperitoneal injection of vehicle (0.9% w/v NaCl, 10% DMSO, 20% 2-hydroxypropyl-β-cyclodextrin), 500 nmol/kg hemopressin, or 5.4 μmol/kg AM251. Following injections, preweighed food was presented and the animals were left undisturbed for 90 min. Behavior was scored using momentary time sampling, every 30 s for the 90 min period, after which point food intake was measured (Lawrence et al., 2002; Scott et al., 2005). The behaviors were scored, 0 or 1, according to the following classifications: feeding (animal at hopper trying to obtain food, chewing, or gnawing), drinking (animal licking at the water spout), grooming (animal scratching, biting or licking any part of its anatomy), resting (animal curled up, resting head with eyes closed), active (animal showing activity, including locomotion, sniffing, rearing), or inactive (animal immobile when aware, or signs of sickness behavior). Data were collected into 5 min period bins for display of the group behavior. Several variables were analyzed: food intake, latency to rest (i.e., the time at which animals first displayed resting), the transition from eating to resting (the time bin when the frequency of eating within the group matches the frequency of resting) and the average percentage of time the animals spent in each of the recorded behaviors.
Results
Hemopressin blocks agonist (WIN 55212-2)-induced eGFP-CB1 receptor internalization
Previous receptor internalization studies on cultured cells have demonstrated that tagged CB1 receptors, the vast majority of which are expressed on the plasma membrane under unstimulated conditions, show rapid and persistent endocytosis in response to stimulation with a CB1 receptor agonist (Hsieh et al., 1999; Coutts et al., 2001; Daigle et al., 2008; Blair et al., 2009). This receptor internalization can be blocked by cotreatment with CB1 receptor inverse agonists (Hsieh et al., 1999; Coutts et al., 2001). In the present study, we confirmed a direct action of hemopressin on CB1 receptors by in vitro eGFP-CB1 internalization assay, in which we compared the action of hemopressin with the well characterized CB1 inverse agonist, AM251, in antagonizing the actions of the agonist WIN 55212-2 (Hsieh et al., 1999) (Fig. 1). Two hours' treatment of transfected cells with WIN 55212-2 caused a significant internalization of eGFP-CB1 receptor into endosomes (p < 0.01; Fig. 1b,e). This effect was blocked by coadministration of either AM251 or increasing doses of hemopressin (IC50 = 1.55 μm, Fig. 1d,e; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). This result supplements other in vitro models demonstrating the action of hemopressin on CB1 receptors (Heimann et al., 2007). Treatment of transfected cells with either AM251 or hemopressin alone did not cause any internalization of eGFP-CB1 receptor into endosomes (Fig. 1b,e).
Figure 1.
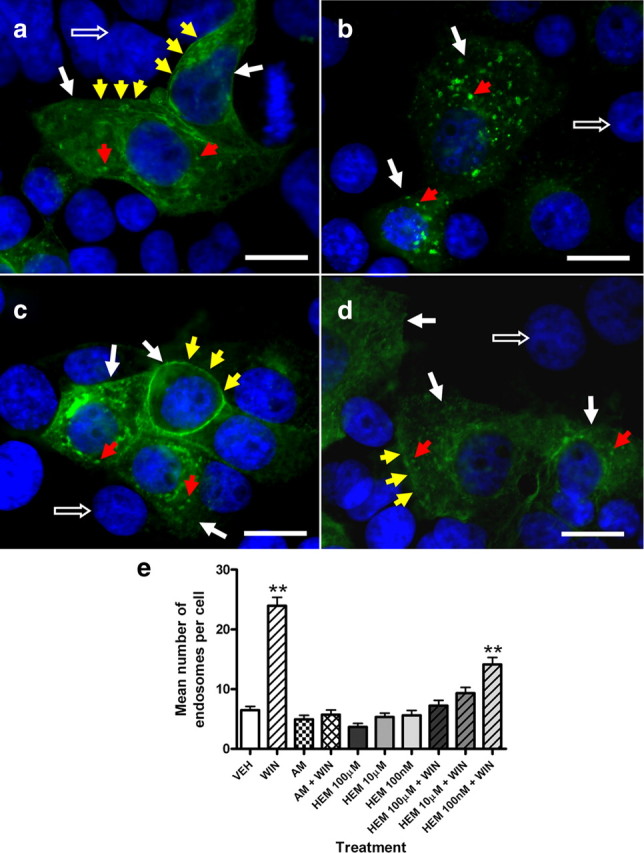
Effect of hemopressin on CB1 agonist (WIN 55212-2)-induced eGFP-CB1 receptor internalization. a, Vehicle (0.25% DMSO); b, 100 nm WIN 55212-2 alone; c, 10 μm hemopressin alone; d, 10 μm hemopressin plus 100 nm WIN 55212-2. Scale bar, 20 μm. White arrows, Transfected cells; open arrows, nontransfected cells (nuclei stained with DAPI); red arrows, internalized endosomes containing eGFP-CB1 receptor; yellow arrows, plasma membrane expressing eGFP-CB1 receptor. e, Histogram showing the mean number of endosomes per cell following stimulation with corresponding treatment. Bars represent mean and SEM; n = 50 cells per treatment. **p < 0.01, one-way ANOVA/Dunnett's post hoc test. AM, AM251; HEM, hemopressin; VEH, vehicle; WIN, WIN 55212-2.
Centrally administered hemopressin results in marked hypophagia in rats and mice
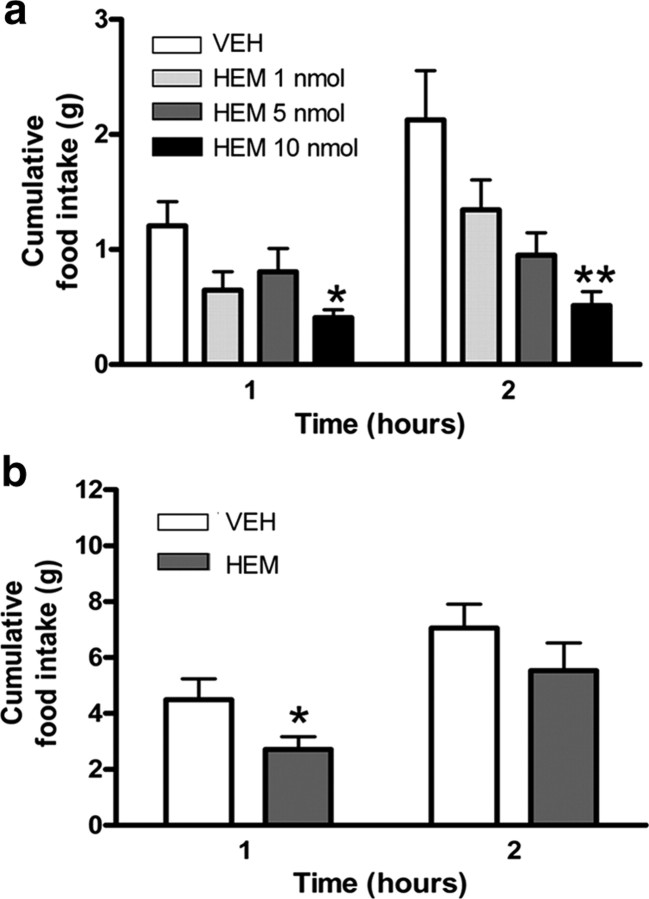
Rimonabant is a well characterized inverse agonist at the CB1 receptor and can act in the brain to reduce appetite (Colombo et al., 1998; Di Marzo et al., 2001; Pagotto et al., 2006; Nogueiras et al., 2008). Thus, we proposed that hemopressin might have the same effect. We found that intracerebroventricular administration of hemopressin caused a dose-dependent decrease of night-time food intake in freely behaving, outbred mice and rats. For mice, a dose of 10 nmol per animal, significantly decreased food intake 1 (p < 0.05), 2 (p < 0.01), and 4 h after injection (p < 0.05, Fig, 2a; supplemental Fig. 2, available at www.jneurosci.org as supplemental material), whereas for rats, the same dose significantly decreased food intake 1 h after injection (p < 0.05; Fig. 2b). For both species, these doses of hemopressin caused no medium-term adverse effects on feeding behavior, as cumulative food intake normalized over the following 12 h period (for mice and rats, respectively, see supplemental Figs. 2, 3, available at www.jneurosci.org as supplemental material).
Figure 2.
a, Hemopressin caused a dose-dependent decrease in normal, night-time feeding when administered intracerebroventricularly to outbred mice (n = 6). The response to 10 nmol/animal was significant within 1 h (*p < 0.05, **p < 0.01; ANOVA/Dunnett's post hoc test). b, A similar, rapid decrease in food intake was measured in outbred rats when the peptide was injected intracerebroventricularly (n = 6, *p < 0.05; t test). Data from additional time points are available in supplemental Figures 1 and 2, available at www.jneurosci.org as supplemental material. HEM, Hemopressin; VEH, vehicle.
Hypophagia produced by systemic administration of hemopressin is absent in CB1−/− mice
Since hemopressin is a relatively small peptide and appears to be able to cross the blood–brain barrier (Heimann et al., 2007), we next tried systemic (intraperitoneal) administration in outbred mice. Again, hemopressin caused a decrease in normal, nocturnal feeding with a significant effect at 2 h postinjection, comparable to that of the synthetic CB1 inverse agonist AM251 (hemopressin p < 0.05, AM251 p < 0.01; Fig. 3a). This slight delay in action of hemopressin was observed in repeated experiments and might reflect the peptide accessing sites of action within the brain. Cumulative food intake normalized over the following 12 h period (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). The dose of AM251 was based on behavioral effects (Tallett et al., 2007b), rather than on comparative CB1 efficacy.
Figure 3.
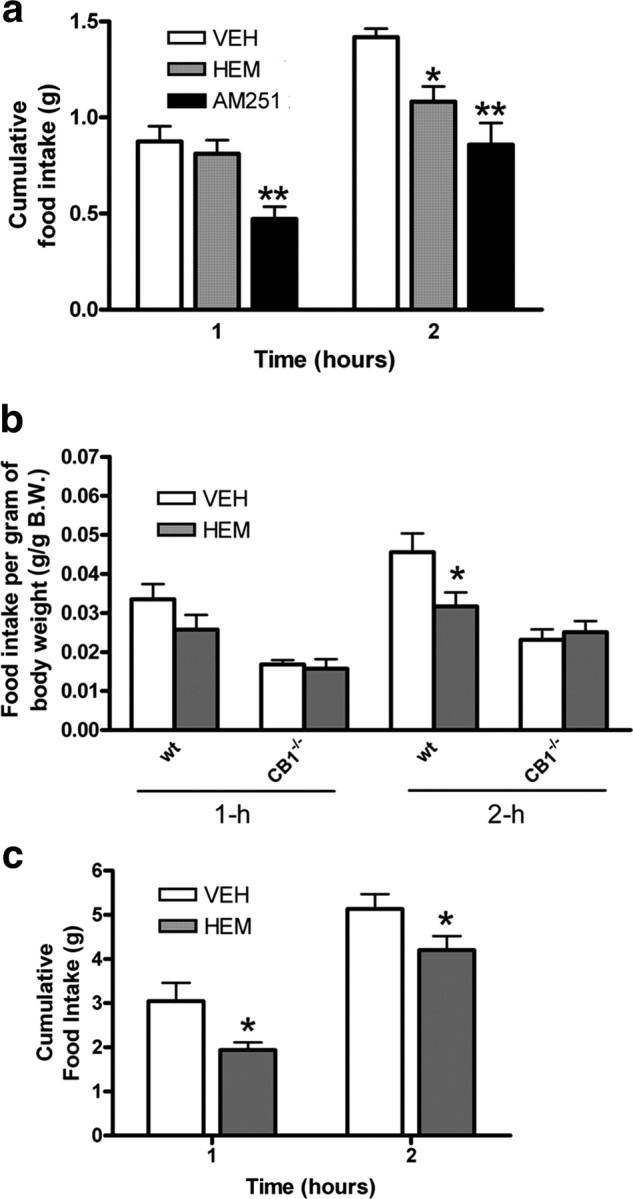
a, When mice were injected with hemopressin (500 nmol/kg, i.p.) there was a slight delay to full effect, which was not significant until 2 h (n = 6, *p < 0.05; one-way ANOVA/Dunnett's post hoc test). Hemopressin hypophagic effects are comparable to those of the synthetic CB1 inverse agonist, AM251 (5.4 μmol/kg, i.p.) at 2 h postinjection (**p < 0.01; one-way ANOVA/Bonferroni's post hoc test). b, To demonstrate that the effect of hemopressin is mediated by cannabinoid receptors, 500 nmol/kg was injected intraperitoneally into wild-type (wt, CB1+/+) and null mutant (CB1−/−) littermates (n = 6). As the two mouse genotypes have significantly different body weights, the data are expressed as grams of food eaten per gram body weight. Hemopressin reduced food intake in the wild-type, but not CB1−/− knock-out mice (*p < 0.05; two-way ANOVA/Bonferroni). c, The hypophagic effects of hemopressin (500 nmol/kg, i.p.) are also present when administered systemically to leptin-deficient, obese ob/ob mice at both 1 and 2 h postinjection (n = 7, *p < 0.05; two-tailed t test). AM, AM251; HEM, hemopressin; VEH, vehicle.
This systemic dosing was repeated in over-night fasted, wild-type (CB1+/+) and CB1 receptor knock-out (CB1−/−) mouse littermates (Marsicano et al., 2002). Since the two genotypes have significantly different average body weights, results are expressed as food intake per gram body weight. The fact that intraperitoneal hemopressin decreased food intake in fasted wild-type mice 2 h after injection (p < 0.05; Fig. 3b), shows that it is capable of overcoming a powerful, natural orexigenic drive. This response is lost in the CB1−/− mice (Fig. 3b), demonstrating that the effect is mediated in vivo by CB1 cannabinoid receptors. Cumulative food intake normalized over the following 12 h period (supplemental Fig. 5, available at www.jneurosci.org as supplemental material).
Systemic administration of hemopressin causes hypophagia in ob/ob mice
Homozygous ob/ob mice are deficient in leptin and express an obese, hyperglycemic and hypophagic phenotype, with elevated endocannabinoid tone in the hypothalamus (Di Marzo et al., 2001). Like Rimonabant in previous studies (Di Marzo et al., 2001), systemic administration of hemopressin causes marked hypophagia at both 1 and 2 h postinjection (p < 0.05; Fig. 3c). Cumulative food intake normalized over the following 12 h period (supplemental Fig. 6, available at www.jneurosci.org as supplemental material).
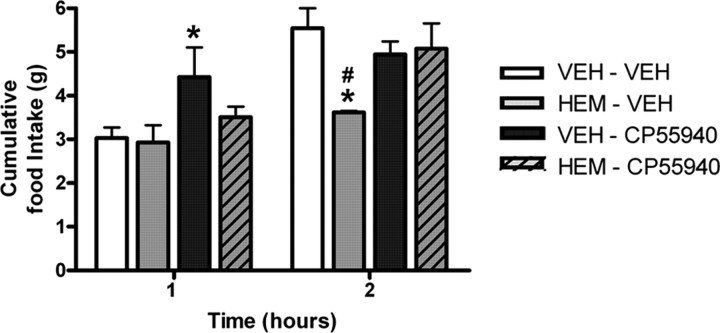
Hemopressin can functionally antagonize CB1 agonist (CP55940)-induced hyperphagia
We and others have shown previously that the CB1 receptor inverse agonist, rimonabant, can functionally antagonize the orexigenic effect of CB1 receptor agonists, such as CP55940 (Dodd et al., 2009). To avoid complications with repeated injections in mice, this experiment was performed in rats. A significant increase in food intake was seen 1 h following CP55940 administered alone (p < 0.05; Fig. 4) and a marked attenuation of this orexigenic drive was observed in the presence of hemopressin. At 2 h postinjection, a significant decrease in food intake was noted following hemopressin administration when compared with controls at the same time point (p < 0.05; Fig. 4), and this was significantly attenuated in the presence of CP55940. Cumulative food intake normalized over the following 12 h period (supplemental Fig. 7, available at www.jneurosci.org as supplemental material).
Figure 4.
To demonstrate that the feeding effect of a CB1 receptor agonist can be blocked pharmacologically, 10 nmol hemopressin (i.c.v.) was coadministered with 0.06 mg/kg CP55940 (i.p.). (n = 5/6, *p < 0.05 compared with vehicle/vehicle group, #p < 0.05 compared with hemopressin/CP55940 group; one-way ANOVA/Bonferroni test). HEM, Hemopressin; VEH, vehicle.
Hemopressin does not disrupt the behavioral satiety sequence
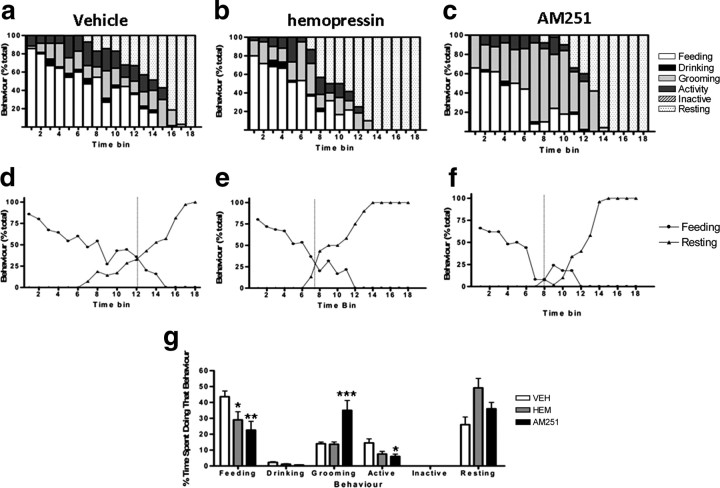
To demonstrate that hemopressin is reducing food intake without causing any adverse effects, such as nausea, aversion or sedation, we demonstrated that treated mice display a normal behavioral satiety sequence (BSS). Singly housed mice, which have their food temporarily removed, display a stereotypic sequence of behaviors when food is returned: eating and drinking, through exploration and grooming, before curling up to sleep (Halford et al., 1998). Any factor reducing appetite because of an abnormal, adverse effect will disrupt this sequence, whereas a natural satiety factor will maintain the sequence but shift it “leftwards.” Indeed, there is evidence that rimonabant and its derivative, AM251, reduce food intake, but also increase scratching in rodent models, probably by an off-target action on opioid receptors (Tallett et al., 2007a,b, 2008) (also see Fig. 6).
Figure 6.
Effects of intraperitoneal hemopressin and AM251 on the BSS. a–c, Overnight fasted mice were presented with food following systemic administration of either vehicle (a), hemopressin (500 nmol/kg) (b), or AM251 (5.4 μmol/kg) (c). Behavior was then monitored every 30 s for 90 min and grouped into feeding, drinking, active, grooming, inactive and resting. Data were collated into 5 min time bins and are presented as percentage of total behavior. d–f, Crossover graphs indicating the point of transition from eating to resting for mice treated with vehicle (d), hemopressin (e), and AM251 (f). The dashed line represents the time bin in which groups spent an equivalent amount of time eating and resting. g, Histogram showing the percentage time spent undertaking a particular behavior over the 90 min period. Hemopressin and AM251 cause a significant decrease in feeding behavior, while AM251 causes a significant increase in grooming (scratching) and decrease in other activities. Bars represent mean and SEM; n = 6. *p < 0.05, **p < 0.01, ***p < 0.001, ANOVA, Dunnett's post hoc test. HEM, Hemopressin; VEH, vehicle.
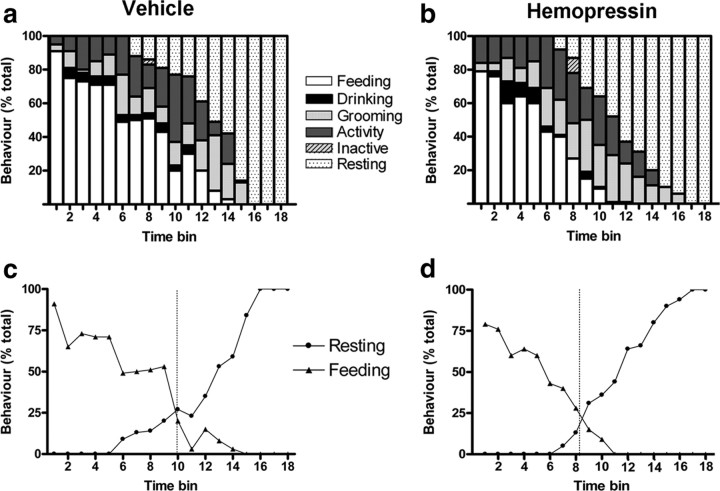
During the 90 min test period, mice treated intraperitoneally with hemopressin spent significantly less time feeding and ate significantly less food than controls (both p < 0.05; supplemental Table 1, available at www.jneurosci.org as supplemental material). No differences were seen between the groups for the average percentage of time spent in the other recorded behaviors. Furthermore, no other unusual behaviors (e.g., excessive scratching, immobility or sickness behavior) were noted. Similar results were recorded for intracerebroventricular injection of hemopressin, though here the reduction in time spent feeding did not reach statistical significance (supplemental Table 1, available at www.jneurosci.org as supplemental material). When plotted against time, the group receiving vehicle intraperitoneally displayed a normal BSS (Fig. 5a). Importantly, hemopressin did not disrupt the sequence, suggesting that it is not reducing feeding by causing any adverse reactions. However, as previously noted for natural satiety factors (Lawrence et al., 2002; Scott et al., 2005), there was an apparent shift of the sequence to the left following hemopressin (Fig. 5c,d). The point of transition from eating to resting took place in time bin 8 for mice given hemopressin compared with time bin 10 for controls. The average latency to rest for mice given hemopressin intraperitoneally was found to be significantly shorter than controls (vehicle, 73 ± 2 min vs hemopressin, 53 ± 5 min; p < 0.01; supplemental Table 1, available at www.jneurosci.org as supplemental material). The maintenance of the BSS and its shift leftwards are important, therefore we wished to compare this result to that of AM251 which, as with rimonanbant, is reported to have an off-target adverse effect in rodents (Tallett et al., 2007a,b, 2008). Both hemopressin and AM251 caused a decrease in feeding (Fig. 6). However, as reported previously, AM251 caused a significant increase in scratching. No such unusual behaviors were recorded following hemopressin administration.
Figure 5.
Effects of intraperitoneal hemopressin on the BSS. a, b, Overnight fasted mice were presented with food following systemic administration of either vehicle (a) or hemopressin (b) (500 nmol/kg, n = 8). Behavior was then monitored every 30 s for 90 min and registered as feeding, drinking, active, grooming, inactive and resting. Data were collected into 5 min time bins and are presented as percentage of total behavior. c, d, Crossover graphs indicating the point of transition from eating to resting for mice treated with vehicle (c) and hemopressin (d). The dashed line represents the time bin in which groups spent an equivalent amount of time eating and resting.
Discussion
Our results demonstrate that hemopressin, a peptide which acts selectively as an inverse agonist at the CB1 receptor (Heimann et al., 2007) can: (1) antagonize CB1 agonist-induced internalization of the CB1 receptor in vitro; (2) induce hypophagia in vivo when administered centrally; (3) induce hypophagia in vivo when administered systemically, but only in mice with functional CB1 receptors; (4) overcome powerful orexigenic drives in fasted or obese mice; and (5) reduce feeding in a behaviorally specific manner.
The endocannabinoid system has diverse roles in cognition, memory, anxiety, motor behavior, nociception and appetite (Svízenská et al., 2008). Numerous studies have described the orexigenic action of the lipid-based endogenous CB1 agonists, such as anandamide and 2-arachidonoylglycerol, on feeding behavior and appetite regulation (Williams and Kirkham, 1999; Hao et al., 2000; Jamshidi and Taylor, 2001; Kirkham et al., 2002). An abundance of synthetic compounds also have been synthesized to interfere with cannabinoid CB1 transmission in attempts to exploit the therapeutic potential offered by targeting this diverse neurotransmitter system. For example, rimonabant has acute central effects on appetite and continuing actions on body weight probably via peripheral interaction with lipid mobilization pathways in white adipose tissue and with cellular glucose uptake systems (Colombo et al., 1998; Di Marzo et al., 2001; Nogueiras et al., 2008). However, the US Food and Drug Administration rejected rimonabant because clinical trials suggested a higher incidence of depression, anxiety and suicidality following prolonged administration (Christensen et al., 2007; Nissen et al., 2008). Furthermore, in this and in previous studies assessing the behavioral satiety sequence after either rimonabant or its derivative, AM251, reductions in feeding have been associated with off-target actions (probably opioid mediated) leading to excessive scratching (Tallett et al., 2007a,b, 2008). By comparison, our behavioral studies have not found any similar adverse reactions in response to hemopressin, either in the short or medium term. Further studies will need to be performed to determine whether hemopressin has any long-term deleterious effects on motivation, or advantageous effects on peripheral metabolism.
Our findings are consistent with other reports showing that synthetic receptor inverse agonists can exhibit hypophagic effects mediated via CB1 receptors, when administered either centrally or systemically (Arnone et al., 1997; Simiand et al., 1998; Di Marzo et al., 2001; Rowland et al., 2001; Verty et al., 2004a; Ward and Dykstra, 2005). Hemopressin, like rimonabant, can functionally antagonize CB1 agonist-induced hyperphagia (Williams and Kirkham, 2002; Dodd et al., 2009) and it is capable of overcoming powerful orexigenic drives in fasted animals. Like rimonabant, hemopressin can also overcome the orexigenic drive produced in leptin-deficient, ob/ob mice (Di Marzo et al., 2001). As either fasted mice or leptin-deficient mice are known to have elevated hypothalamic endocannabinoid levels (Di Marzo et al., 2001; Kirkham et al., 2002), the possibility remains that hemopressin may be acting as an neutral antagonist against heightened endocannabinoid tone rather than as an inverse agonist.
The central mechanisms underlying CB1-mediated effects on appetite are unclear. However, a large body of evidence suggests that CB1 receptors may interact not only directly with the known feeding-related circuitry of the hypothalamus but, also, may impinge on dopaminergic and opioid signaling in the striatum which are known to mediate the motivational and rewarding aspects of feeding behavior (Cota et al., 2003a, 2006; Kirkham, 2009). This is further suggested by the ability of CB1 ligands and fatty acid amide hydrolase inhibitors, to elicit robust feeding responses when administered directly into the nucleus accumbens or into nuclei of the hypothalamus (Williams and Kirkham, 1999; Kirkham et al., 2002; Verty et al., 2005; Soria-Gómez et al., 2007). Interestingly, a number of these studies found no effects of intra-accumbens injection of rimonabant or AM251 on food intake, suggesting that that feeding-related effect of CB1 inverse agonism may depend substantially on an integrated response throughout the forebrain (Werner and Koch, 2003; Verty et al., 2004a,b). A recent functional magnetic resonance imaging study in rats showed that regions of the orbitofrontal cortex, striatum (particularly the nucleus accumbens) and the hypothalamus, are functionally responsive to orexigenic or anorectic doses of opposing CB1 ligands (Dodd et al., 2009). An interesting result from the current study is that hemopressin may also act on satiety pathways, perhaps in the brainstem, or via peripheral CB1 receptors in the gut, since it caused a slight advance (leftwards shift) of the behavioral satiety sequence (Gómez et al., 2002).
The expression and functional profile of hemopressin in the brain is yet to be fully elucidated. Recent studies have described the location of hemoglobin α chain mRNA and protein in rat and human neurons, including those in the dopaminergic system (Richter et al., 2009; Schelshorn et al., 2009). Therefore, it is possible that hemopressin, which is derived from the hemoglobin α chain gene (Rioli et al., 2003; Lippton et al., 2006; Heimann et al., 2007), may be produced within pathways involved in motivated behavior. Furthermore, a very recent paper has described N-terminally extended hemopressin sequences which can act as CB1 agonists in vitro (Gomes et al., 2009), while a precedent has already been set for functional opioidergic peptides derived from the hemoglobin β chain (Nyberg et al., 1997). Such biologically active peptides are not processed by the vesicular secretory pathway, so it is yet to be determined whether their release can be regulated. As the known, lipid-based endocannabinoids are produced “on demand,” similar processes may regulate the production of small, bioactive peptides, as has been seen with some interleukins (Simi et al., 2007).
Hemopressin is a novel bioactive peptide found in the brain that is capable of functionally antagonizing the actions of endogenous cannabinoid receptor agonists and may be placed to act as a natural suppressant of hedonically motivated eating. Indeed, the precedent for mutually antagonistic pathways containing receptor agonists and inverse agonists that can subtly modulate food intake (viz α-MSH and agouti-related peptide which antagonize each other at melanocortin receptors) already exists (Lu et al., 1994; Ollmann et al., 1997; Pritchard et al., 2004), and may indicate the existence of such mutual antagonism as a common feature in central appetite regulatory systems.
Footnotes
G.T.D. holds a Biotechnology and Biological Sciences Research Council priority postgraduate studentship in the area of Integrative Biology. We thank the British Society for Neuroendocrinology for funding G.T.D.'s research visits between the United Kingdom and Germany, Dr. Nadia Luheshi for her invaluable help with the cell culture, and Dr. Tina Ivanov for help with plasmid preparation. A grant from the European Foundation for the Study of Diabetes to G.M. is also acknowledged.
References
- Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Elphick MR, Martin BR, DeLorenzo RJ. Prolonged exposure to WIN55,212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology. 2009;57:208–218. doi: 10.1016/j.neuropharm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003a;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003b;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CS, Pagano Rde L, Rioli V, Hyslop S, Giorgi R, Ferro ES. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides. 2005;26:431–436. doi: 10.1016/j.peptides.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Stark JA, McKie S, Williams SR, Luckman SM. Central cannabinoid signaling mediating food intake: a pharmacological-challenge magnetic resonance imaging and functional histology study in rat. Neuroscience. 2009;163:1192–1200. doi: 10.1016/j.neuroscience.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodríguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61:159–168. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Hao S, Avraham Y, Mechoulam R, Berry EM. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol. 2000;392:147–156. doi: 10.1016/s0014-2999(00)00059-5. [DOI] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21:163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Bátkai S, Sharkey KA, Makriyannis A. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KL, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002;143:360–367. doi: 10.1210/endo.143.2.8609. [DOI] [PubMed] [Google Scholar]
- Lippton H, Lin B, Gumusel B, Witriol N, Wasserman A, Knight M. Hemopressin, a hemoglobin fragment, dilates the rat systemic vascular bed through release of nitric oxide. Peptides. 2006;27:2284–2288. doi: 10.1016/j.peptides.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodés-Cabau J, Cannon CP, Deanfield JE, Despres JP, Kastelein JJ, Steinhubl SR, Kapadia S, Yasin M, Ruzyllo W, Gaudin C, Job B, Hu B, Bhatt DL, Lincoff AM, Tuzcu EM. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F, Sanderson K, Glämsta EL. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 1997;43:147–156. doi: 10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Ligresti A, Di Marzo V. Endocannabinoid chemical biology: a tool for the development of novel therapies. Curr Opin Chem Biol. 2009;13:309–320. doi: 10.1016/j.cbpa.2009.04.616. [DOI] [PubMed] [Google Scholar]
- Pritchard LE, Armstrong D, Davies N, Oliver RL, Schmitz CA, Brennand JC, Wilkinson GF, White A. Agouti-related protein (83-132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J Endocrinol. 2004;180:183–191. doi: 10.1677/joe.0.1800183. [DOI] [PubMed] [Google Scholar]
- Richter F, Meurers BH, Zhu C, Medvedeva VP, Chesselet MF. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J Comp Neurol. 2009;515:538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioli V, Gozzo FC, Heimann AS, Linardi A, Krieger JE, Shida CS, Almeida PC, Hyslop S, Eberlin MN, Ferro ES. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. J Biol Chem. 2003;278:8547–8555. doi: 10.1074/jbc.M212030200. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Schelshorn DW, Schneider A, Kuschinsky W, Weber D, Krüger C, Dittgen T, Bürgers HF, Sabouri F, Gassler N, Bach A, Maurer MH. Expression of hemoglobin in rodent neurons. J Cereb Blood Flow Metab. 2009;29:585–595. doi: 10.1038/jcbfm.2008.152. [DOI] [PubMed] [Google Scholar]
- Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol. 2005;17:452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Simi A, Lerouet D, Pinteaux E, Brough D. Mechanisms of regulation for interleukin-1beta in neurodegenerative disease. Neuropharmacology. 2007;52:1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Soria-Gómez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospéro-García O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology (Berl) 2007a;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers JR. Acute anorectic response to cannabinoid CB1 receptor antagonist/inverse agonist AM 251 in rats: indirect behavioural mediation. Behav Pharmacol. 2007b;18:591–600. doi: 10.1097/FBP.0b013e3282eff0a9. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Endogenous opioids and cannabinoids: system interactions in the regulation of appetite, grooming and scratching. Physiol Behav. 2008;94:422–431. doi: 10.1016/j.physbeh.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology. 2004a;47:593–603. doi: 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology. 2004b;145:3224–3231. doi: 10.1210/en.2004-0059. [DOI] [PubMed] [Google Scholar]
- Verty AN, McGregor IS, Mallet PE. Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in Lewis rats. Brain Res. 2003;967:290–292. doi: 10.1016/s0006-8993(02)04274-9. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of delta 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002;71:333–340. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]