Abstract
Animal models have demonstrated that mild hearing loss caused by acoustic trauma results in spontaneous hyperactivity in the central auditory pathways. This hyperactivity has been hypothesized to be involved in the generation of tinnitus, a phantom auditory sensation. We have recently shown that such hyperactivity, recorded in the inferior colliculus, is still dependent on cochlear neural output for some time after recovery (up to 6 weeks). We have now studied the capacity of an intrinsic efferent system, i.e., the olivocochlear system, to alter hyperactivity. This system is known to modulate cochlear neural output. Anesthetized guinea pigs were exposed to a loud sound and after 2 or 3 weeks of recovery, single-neuron recordings in inferior colliculus were made to confirm hyperactivity. Olivocochlear axons were electrically stimulated and effects on cochlear neural output and on highly spontaneous neurons in inferior colliculus were assessed. Olivocochlear stimulation suppressed spontaneous hyperactivity in the inferior colliculus. This result is in agreement with our earlier finding that hyperactivity can be modulated by altering cochlear neural output. Interestingly, the central suppression was generally much larger and longer lasting than reported previously for primary afferents. Blockade of the intracochlear effects of olivocochlear system activation eliminated some but not all of the effects observed on spontaneous activity, suggesting also a central component to the effects of stimulation. More research is needed to investigate whether these central effects of olivocochlear efferent stimulation are due to central intrinsic circuitry or to coactivation of central efferent collaterals to the cochlear nucleus.
Introduction
It is well established that after lesions of the peripheral auditory receptor, the cochlea, increased spontaneous activity (hyperactivity) develops in central auditory nuclei. This plasticity has been demonstrated in a wide range of animal models, using either mechanically, acoustically, or drug-induced cochlear lesions (Kaltenbach et al., 2000; Seki and Eggermont, 2003; Kaltenbach et al., 2004; Ma et al., 2006; Brozoski et al., 2007; Bauer et al., 2008; Dong et al., 2009, 2010; Mulders and Robertson, 2009). Hyperactivity has been suggested to be involved in the generation of tinnitus, an auditory phantom perception (Brozoski et al., 2002; Kaltenbach et al., 2004; Bauer et al., 2008). This hypothesis is supported by the fact that the hyperactivity seems restricted to tonotopic regions broadly corresponding to the area of hearing loss as shown in cochlear nucleus (CN), the central nucleus of the inferior colliculus (CNIC), and the auditory cortex (Kaltenbach et al., 2000; Komiya and Eggermont, 2000; Seki and Eggermont, 2003; Ma et al., 2006; Dong et al., 2009; Mulders et al., 2009) and the observation in human studies that there is a strong correlation between the tinnitus pitch and the hearing loss frequencies (Mühlnickel et al., 1998; Norena et al., 2002; Eggermont and Roberts, 2004).
Although it is generally believed that central hyperactivity is intrinsic to the nuclei involved and is not dependent on increased activity of the auditory nerve (Eggermont and Roberts, 2004), we have recently shown in a guinea pig model of hyperactivity caused by unilateral acoustic trauma that up to 6 weeks later, hyperactivity can be abolished by silencing cochlear activity (Mulders and Robertson, 2009). If indeed hyperactivity is involved in the generation of tinnitus, this finding may open up possible avenues for therapy. In the paper by Mulders and Robertson (2009), the methods used for silencing cochlear output were either irreversible, causing complete deafness (cochlear ablation), or artificial (cochlear perfusion with kainate), which may provide proof of principle but would be unsuitable for clinical application.
It is of interest to know whether intrinsic mechanisms that have the capacity to modulate cochlear activity, for example, the olivocochlear system, would also cause modulation of hyperactivity. Activation of the olivocochlear system, which originates in the brainstem and terminates in the cochlea (Warr and Guinan, 1979; White and Warr, 1983) and CN (Benson and Brown, 1990; Brown, 1993; Benson et al., 1996), is well known to have a suppressive effect on sound-evoked and spontaneous cochlear neural output (Guinan and Gifford, 1988; Rajan, 1988). Therefore, in the present paper we have investigated the effects of activation of the olivocochlear system on the level of hyperactivity caused by acoustic trauma in CNIC neurons.
Materials and Methods
Animals.
Twenty-three adult pigmented guinea pigs of both sexes were used, weighing between 317 and 420 g at the time of acoustic trauma or sham surgery. The experimental protocols conformed to the Code of Practice of the National Health and Medical Research Council of Australia, and were approved by the Animal Ethics Committee of The University of Western Australia.
Acoustic trauma and sham surgery.
Animals received a subcutaneous injection of 0.1 ml of atropine sulfate (0.6 mg/ml), followed by diazepam (5 mg/kg, i.p.) and Hypnorm (0.315 mg/ml fentanyl citrate and 10 mg/ml fluanisone; 1 ml/kg, i.m.). When deep anesthesia was obtained as assessed by the foot withdrawal reflex, animals were placed on a heating blanket in a soundproof room and mounted in hollow ear bars. A small opening was made in the bulla to place an insulated silver wire on the bony shelf close to the round window. A compound action potential (CAP) audiogram (Johnstone et al., 1979) for the frequency range 4–24 kHz was used to assess the animals' cochlear sensitivity using a closed sound system. All sound stimuli were presented through a ½ inch condenser microphone driven in reverse as a speaker (Bruel and Kjaer, type 4134). Pure tone stimuli were synthesized by a computer equipped with DIGI 96 soundcard connected to an analog/digital interface (ADI-9 DS, RME Intelligent Audio Solution). Sample rate was 96 kHz. The interface was driven by a custom-made computer program (Neurosound, MI Lloyd), which was also used to collect single-neuron data. CAP signals were amplified, filtered (100 Hz-3 kHz bandpass) and recorded with a second data acquisition system (Powerlab 4SP, AD Instruments). When cochlear sensitivity was determined to be within the normal range the contralateral ear was blocked with plasticine and the animal was subjected to a continuous pure tone of 10 kHz at 124 dB SPL for 1 h (acoustic trauma). After the acoustic trauma the CAP audiogram was again measured, the incision was sutured, and buprenorphine (0.05 mg/kg, s.c.) was given postoperatively as analgesic. Four animals were not subjected to acoustic trauma and served as sham controls. Survival times ranged from 13 to 28 d.
Surgery for efferent experiments.
For single-neuron recordings in the inferior colliculus, anesthesia was induced by an injection with 0.1 ml of atropine followed by an intraperitoneal injection of Nembutal (pentobarbitone sodium, 30 mg/kg) and a 0.15 ml intramuscular injection of Hypnorm. The maintenance anesthesia regime consisted of full Hypnorm doses every hour and half doses of Nembutal every 2 h. Animals were placed on a heating blanket in a sound proof room and artificially ventilated on carbogen (95% O2 and 5% CO2). Animals were paralyzed with 0.1 ml of pancuronium bromide (2 mg/ml, i.m.). The electrocardiogram was continuously monitored and heart rate never increased over preparalysis levels at any stage of the experiments. At the end of the experiments, animals were killed with 0.3 ml of Lethabarb (sodium pentobarbitone 325 mg/ml, Virbac Australia). After mounting the animals in hollow ear bars, the left cochlea was exposed and an insulated silver wire was placed on the round window to record another CAP audiogram for the frequency range 4–24 kHz.
After a craniotomy exposing the cerebellum and the caudal part of the visual cortex, the cerebellum overlying the floor of the IVth ventricle was aspirated. This enabled placement under visual control of the stimulating electrodes on the olivocochlear bundle (OCB) (platinum-iridium concentric bipolar electrodes with a tip diameter between 3 and 4 μm, World Precision Instruments, connected to an isolated stimulator output, AM Systems Model 2100). To keep the stimulating current necessary to evoke a large OCB effect as low as possible and to minimize the chance of stimulating the dorsal acoustic stria, the stimulating electrodes need to be in very close proximity to the decussation of the OC fibers. To achieve this the stimulating electrodes were placed on the midline and the point was determined where the threshold current to evoke a facial twitch by single shocks was lowest (Seluakumaran et al., 2008). The animals were then paralyzed and proper placement of the stimulating electrodes on the OCB was confirmed by measuring the suppression of the CAP and increase in cochlear microphonic (CM) receptor potential after electrical stimulation using trains of biphasic current pulses (100 ms duration, 0.1 ms pulses, 300 Hz, repetition interval 1/s). To determine the magnitude of the OC-induced suppression, the CAP amplitude after OCB stimulation was matched to the CAP amplitude in response to a tone of a lower intensity. The difference in sound intensity needed to match the amplitudes was used as a measure of the strength of the OC effect (Desmedt, 1962). OCB effects were measured at 10 kHz, except when the audiogram showed a threshold loss at this frequency. In these instances, the effect of OCB stimulation was determined at 8 kHz. OCB effects measured in this study, were comparable with published data in normal guinea pigs (Mulders et al., 2002) and ranged from 14 to 20 dB equivalent suppression with current strengths ranging from 150 to 400 μA. CM responses were measured in the same manner as the CAP using 1 kHz tones at reasonably high sound levels ranging from 94 to 100 dB SPL to obtain a smooth CM trace. Effects of OC stimulation on cochlear gross potentials were verified regularly throughout the experiment and the experiment was terminated if effects on cochlear gross potentials could not be detected.
To record extracellular single-neuron responses in the CNIC, glass-insulated tungsten microelectrodes (Merrill and Ainsworth, 1972) were advanced vertically through the visual cortex into the contralateral CNIC using a stepping motor microdrive. Electrode placement in the CNIC (∼2.5 mm ventral to the cortical surface) was indicated by the onset of strong sound-driven activity with a short latency and a systematic progression from low to high characteristic frequencies (CFs) with increasing depth. The craniotomy was covered with 5% agar in saline to improve mechanical stability. When a single unit was isolated, its CF and threshold at CF were determined audiovisually, by continuously varying the frequency and intensity of a pure tone stimulus and estimating the lowest intensity that evoked a just-detectable increase in firing rate (Shore et al., 2003; Bleeck et al., 2006; Sayles and Winter, 2008). Audiovisual estimates of neuronal CF using the same software as in the present study have been shown to agree closely with those obtained from response area measurements (Ingham et al., 2006). The spontaneous firing rate was measured for a period of 10 s. During all spontaneous rate measurements the speaker was turned off. This abolished the possibility that the spontaneous activity recorded was driven activity caused by uncontrolled background noise from the sound delivery system.
When baseline spontaneous rate was established the effects of electrical stimulation of the OCB on the neuron's spontaneous rate was measured (OCB parameters as for optimal cochlear effects: 100 ms duration, 0.1 ms pulses, 300 Hz, 50–100 repetitions). Repetition rate of OCB stimulation was 0.2 s−1 for most neurons (n = 63) and 1 s−1 for an additional nine neurons.
Cochlear perfusions.
In 10 animals, the efferent stimulation was combined with cochlear perfusions of strychnine to block the intracochlear effects of the OCB stimulation. In these animals, a hole was made in the cochlear apex using a hooked pick. The tip of a glass perfusion pipette was carefully inserted using a micromanipulator through a small hole in the scala tympani wall of the basal cochlear turn. The perfusion pipette contained artificial perilymph (127 mm NaCl, 5 mm KCl, 1 mm MgCl2 · 6H2O, 1 mm NaH2PO4 · H2O, 12 mm NaHCO3, 11 mm glucose, and 2 mm CaCl2 at pH 7.4) with 20 μm strychnine added. Perfusion rate was 2 μl/min. To monitor cochlear effects, CAP amplitudes were monitored as before with and without efferent stimulation. The effects of efferent stimulation on the neurons were measured before strychnine perfusion, after block of the peripheral effects as assessed by CAP measurements and after recovery of the peripheral effects to before perfusion levels. Recovery of the cochlear efferent effect from strychnine block took ∼30 min.
CNIC field potentials measurements.
To assess the effect of electrical stimulation of the OCB on the CNIC and the potential for current spread to the dorsal acoustic stria, in each animal field potential measurements were performed at different depths in the CNIC and with a range of shock strengths applied to the OCB. These measurements were made using the same microelectrode as used for single-neuron recordings. For each individual neuron it was ascertained that a field potential was not observed at the shock strength used.
Results
Hearing loss
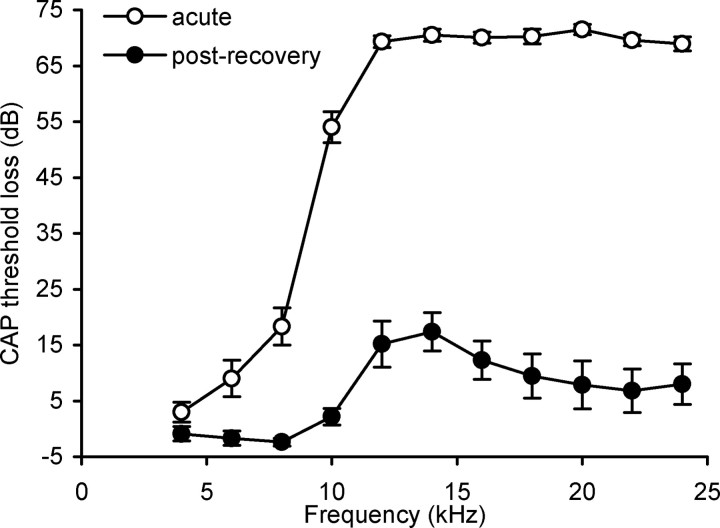
Immediately after acoustic trauma the audiogram revealed a large hearing loss from 6 kHz upward, with the largest loss (∼70 dB) from 12 kHz to 24 kHz (Fig. 1). However, after 13–28 d of recovery the hearing loss recovered substantially, showing a restricted hearing loss (∼16 dB) peaking at 12 and 14 kHz (Fig. 1), in agreement with previous studies (Dong et al., 2009; Mulders and Robertson, 2009).
Figure 1.
Loss of cochlear sensitivity measured as CAP threshold change recorded in the cochlea after acute acoustic trauma (open circles; n = 16) and after recovery from acoustic trauma (black circles; n = 16). Excluded from this figure are three animals in which only the audiogram after recovery was measured. Data are mean ± SEM.
CNIC neurons
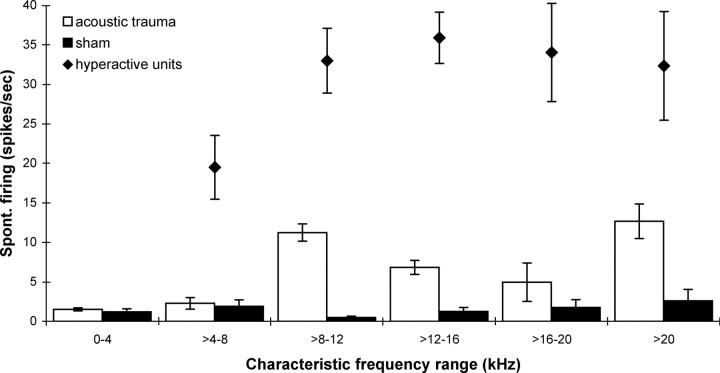
In a previous study (Mulders and Robertson, 2009) it was established that in normal animals 95% of neurons in the CNIC have a spontaneous rate <8 spikes/s. Figure 2 illustrates the low spontaneous firing rates found in all CF regions for the group of sham operated animals in the present study. These data are compared with the average spontaneous rates in the same CF regions seen 2 weeks after acoustic trauma (data from Mulders and Robertson, 2009). The increased incidence of neurons with a high spontaneous firing rate after acoustic trauma encountered in our experiments is in agreement with other studies (Wang et al., 1996, 2002; Imig and Durham, 2005; Ma et al., 2006; Brozoski et al., 2007; Bauer et al., 2008; Dong et al., 2009; Mulders and Robertson, 2009). In the present study, only neurons with a spontaneous firing rate >8 spikes/s were selected for testing the effect of efferent stimulation. The average spontaneous firing rate of the neurons selected is also shown in Figure 2. It is apparent that these firing rates are markedly elevated relative to the sham animals.
Figure 2.
Averaged spontaneous firing rates per CF range in CNIC neurons in sham operated animals (black bars, n = 4) and in animals 2 weeks after acoustic trauma [open bars, n = 5, data taken from Mulders and Robertson (2009)]. Solid diamonds show averaged spontaneous firing rates of neurons selected for investigation of OCB effects. All data are mean ± SEM.
The effects of electrical stimulation of the OCB were recorded in a total of 72 CNIC neurons 13–28 d after acoustic trauma. The CFs of these neurons ranged from 4.6 to 32 kHz with an average of 12.9 ± 0.7 kHz and their spontaneous rate ranged from 9.4 to 92.4 spikes/s with an average of 31.2 ± 2.3 spikes/s. In 44 of these neurons, only effects of efferent stimulation were assessed (CF range between 5.1 and 32 kHz, mean 12.9 ± 0.8 kHz, spontaneous rate range between 9.5 and 84.6, mean 32.9 ± 2.8), but in 28 neurons efferent effects were recorded before and after block of medial olivocochlear peripheral effects by cochlear strychnine perfusions (CF range between 4.6 and 28 kHz, mean 12.8 ± 1 kHz, spontaneous rate range between 9.4 and 92.4, mean 28.6 ± 3.4).
Effects of efferent stimulation without strychnine application
In each animal, the level of efferent (OCB) stimulation was adjusted to result in a near-maximal effect on the CAP amplitude recorded from the cochlea. Stimulation levels ranged from 150 to 400 μA. In all animals, the suppression of CAP amplitude was equivalent to a decrease in intensity of between 14 and 20 dB SPL. OCB activation was further confirmed by observing an increase in CM amplitude.
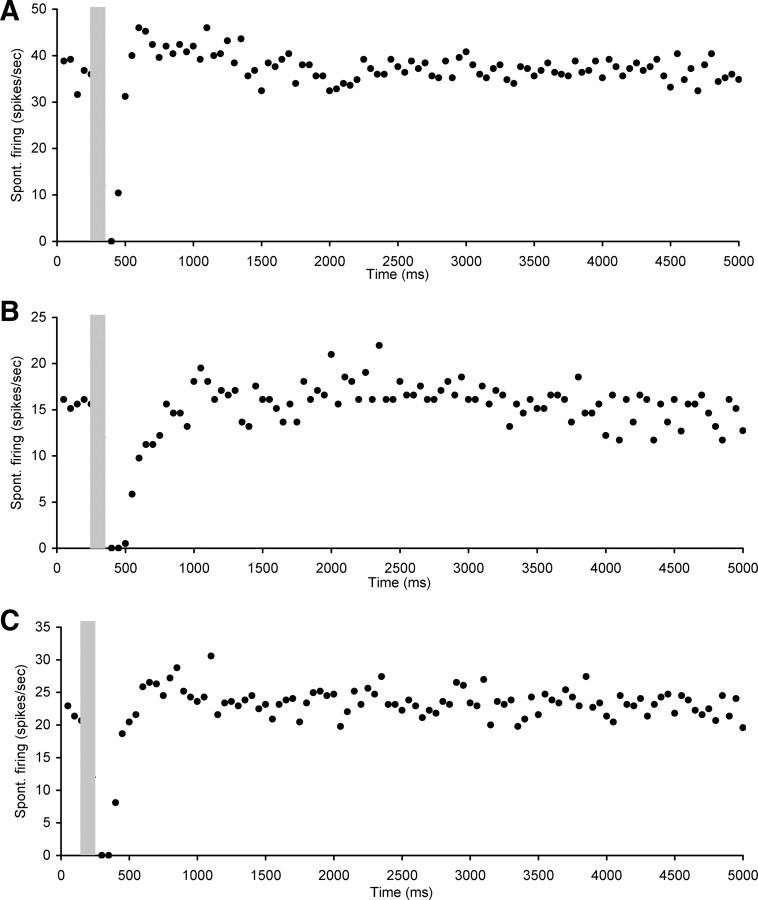
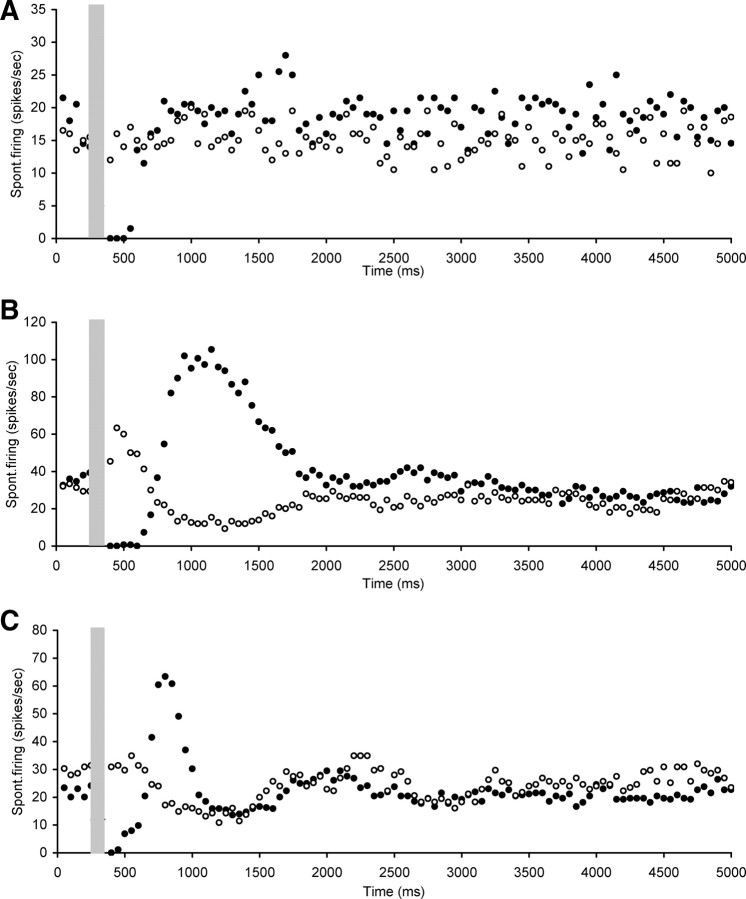
Electrical stimulation of the OCB resulted in marked changes in the level of spontaneous firing rates of hyperactive CNIC neurons. The effects were diverse but could be divided into four main categories. The first category comprised neurons (n = 20) that showed a short-lasting (ranging from 100 to 400 ms) but complete suppression of spontaneous rate followed by a return to baseline spontaneous rate levels (Fig. 3). The mean CF of neurons in this category was 11.2 ± 1.3 kHz (ranging between 6 and 28 kHz) and mean spontaneous firing rate was 22.8 ± 2.7 spikes/s (ranging between 9.4 and 50.7 spikes/s).
Figure 3.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), showing examples of short (ranging from 100 to 400 ms) suppression of spontaneous rate followed by a return to baseline spontaneous rate levels. A, Neuron CF 28 kHz, threshold 60 dB SPL. B, Neuron CF 6 kHz, threshold 22 dB SPL. C, Neuron CF 7.8 kHz, threshold 17 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A and B was 250 μA and in C 400 μA.
The second category comprised neurons (n = 45) that showed a short (50–450 ms duration) suppression of spontaneous rate followed by more complex changes. These changes included sharp overshoots after the initial suppression followed by a return to baseline levels which could be either fast or slow, sharp overshoots followed by another period of suppression before return to baseline or oscillatory patterns (Fig. 4). The mean CF of neurons in this category was 13.6 ± 0.8 kHz (ranging between 4.6 and 32 kHz) and their mean spontaneous firing rate was 34.3 ± 3.2 spikes/s (ranging between 9.5 and 92.4 spikes/s).
Figure 4.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), showing examples of short or nondetectable (ranging from 0 to 450 ms) suppression of spontaneous rate followed by more complex changes. A, Neuron CF 21.2 kHz, threshold 21 dB SPL. B, Neuron CF 10.9 kHz, threshold 29 dB SPL. C, Neuron CF 18.3 kHz, threshold 75 dB SPL. D, Neuron CF 9.7 kHz, threshold 28 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A was 350 μA, in B and C 250 μA, and in D 400 μA.
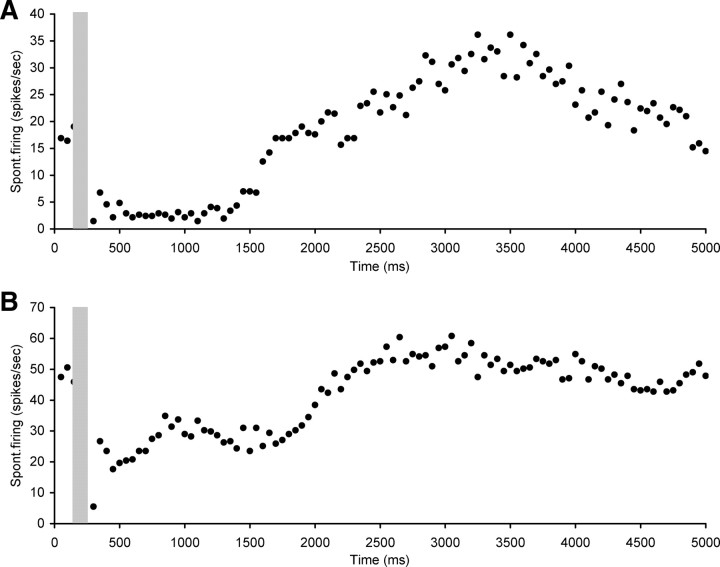
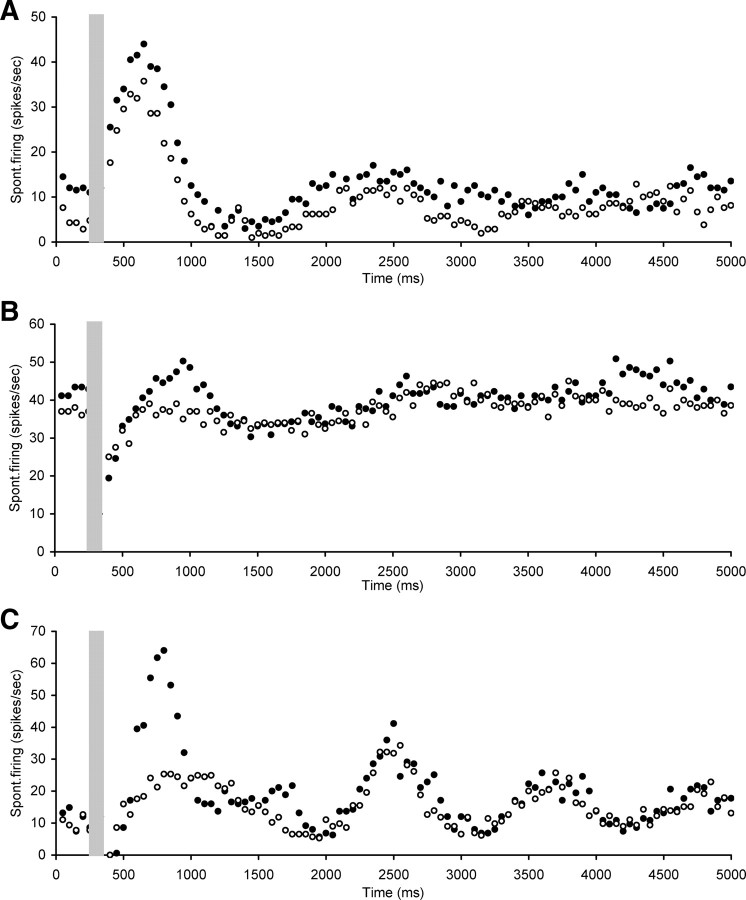
The third category consisted of five neurons that showed a long suppression after stimulation (ranging from 500 to 2550 ms) with either some oscillation visible after the suppression or a slow return to baseline levels (Fig. 5). The mean CF of neurons in this category was 12.3 ± 1.8 kHz (ranging between 9 and 18.6 kHz) with a mean spontaneous firing rate of 35.8 ± 6.0 spikes/s (ranging between 19.6 and 51.3 spikes/s).
Figure 5.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), showing examples of long suppression after stimulation (ranging from 500 to 2550 ms) with either some late oscillation visible or a slow return to baseline levels. A, Neuron CF 18.6 kHz, threshold 82 dB SPL. B, Neuron CF 10.9 kHz, threshold 56 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A and B was 250 μA.
The fourth category (examples not shown) consisted of two neurons in which OCB stimulation had no effect on spontaneous rate. The CF of these neurons was 17.4 and 16.7 kHz with spontaneous rates of 22.5 and 34.7 spikes/s, respectively. Nonetheless, there was a significant effect of OCB activation in the periphery (15 dB equivalent at 10 kHz and ∼6 dB equivalent at 17 kHz).
There were no statistically significant differences regarding CF and spontaneous firing rate between the four categories (Kruskall–Wallis test).
Effects of efferent stimulation with strychnine application
Twenty-eight neurons were held long enough to obtain measurements of spontaneous activity before and after intracochlear strychnine perfusion. Fifteen of these neurons were selected for analysis. Selection criteria were a total loss of peripheral inhibition with electrical stimulation of the OCB as established with CAP measurements and no evidence of a field potential in CNIC with electrical stimulation of the OCB at the current strength used while measuring the effects on spontaneous activity.
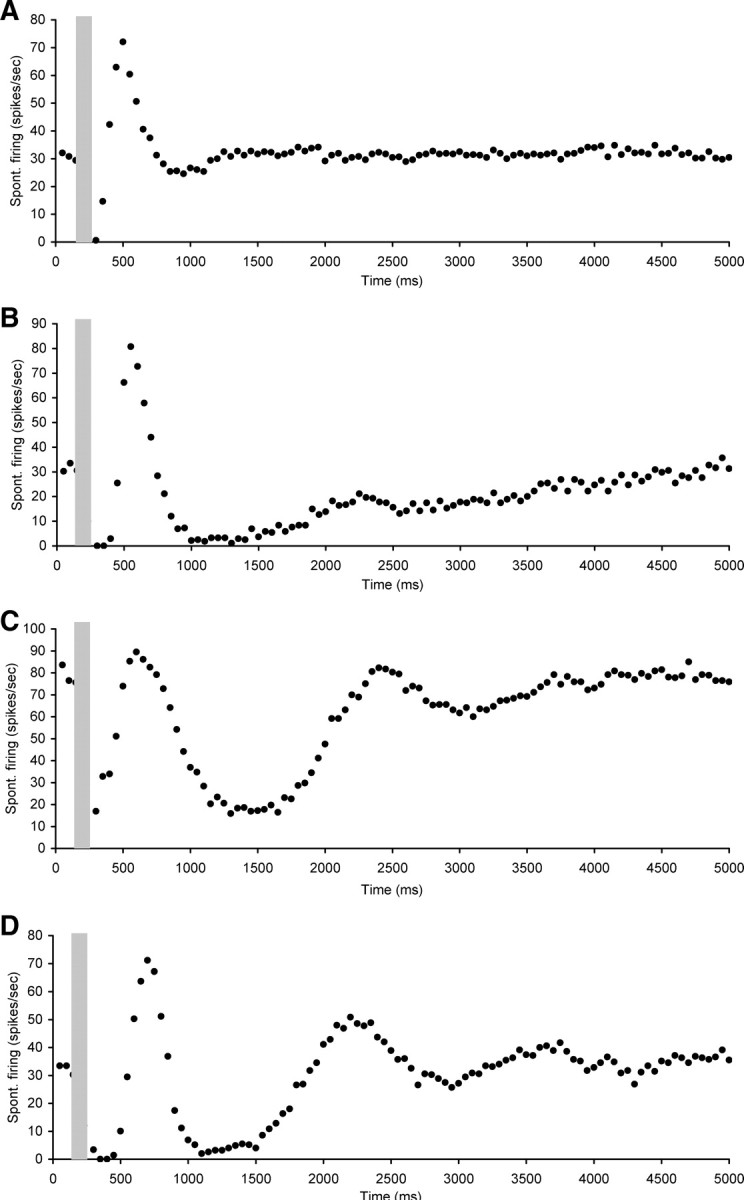
In 8 of these 15 neurons, the initial suppression (before application of strychnine) caused by electrical stimulation of the OCB (varying in these neurons from 150 to 600 ms) was completely abolished by strychnine perfusion. Two of these neurons were of category 1, in which only suppression was observed with OCB activation, and the strychnine perfusion therefore abolished all effects on these neurons (Fig. 6A). The remaining six neurons belonged to category 2 (short suppression followed by complex changes). In all these neurons, the short suppression was abolished but other more complex changes could still be observed after intracochlear strychnine perfusion. Figure 6B illustrates an example of one of the category 2 neurons showing that intracochlear strychnine perfusion resulted in an absence of the immediate suppression following OCB stimulation. However, an immediate overshoot followed by a small suppression was still present. Figure 6C shows another example of a category 2 neuron in which OCB stimulation after intracochlear strychnine perfusion led to late suppression followed by some very small oscillations.
Figure 6.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), recorded both before (dark circles) and after (open circles) strychnine perfusion in the cochlea. Illustrations of neurons where the immediate suppression was abolished (A–C), but sometimes later effects remained (B, C). A, Neuron CF 7.8 kHz, threshold 20 dB SPL. B, Neuron CF 9.2 kHz, threshold 21 dB SPL. C, Neuron CF 14.5 kHz, threshold 22 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A and B was 300 μA and in C 150 μA.
In 4 of the 15 neurons, the immediate suppression observed after OCB stimulation was not completely abolished by cochlear strychnine perfusion, but its duration was reduced from 250, 500, 650, or 1350 ms before strychnine to 200, 300, 150, and 600 ms after strychnine, respectively (Fig. 7A–D). Figure 7C shows a neuron that at first sight (before strychnine perfusion) seems to show some suppression followed by a large long-lasting overshoot before returning to baseline. However, in this figure panel the neuron's spontaneous rate without electrical stimulation or strychnine perfusion is also indicated as a dotted line. This illustrates that in this particular neuron the OCB activation causes both an immediate suppression, but also a late component suppression (starting ∼2.5 s after electrical stimulation) which lasts beyond the 5 s trial, decreasing the “prestimulation” spontaneous rate value. In between the early and late suppression there was also a small excitatory effect. Strychnine perfusion in the cochlea seemed to eliminate or reduce the early suppression, but still shows some small excitatory effect and a reduced late suppression. Figure 7D shows a neuron with long-lasting initial suppression before strychnine perfusion. Cochlear perfusion with strychnine eliminated the immediate suppression but a later inhibitory effect was still apparent, starting ∼300 ms after stimulation.
Figure 7.
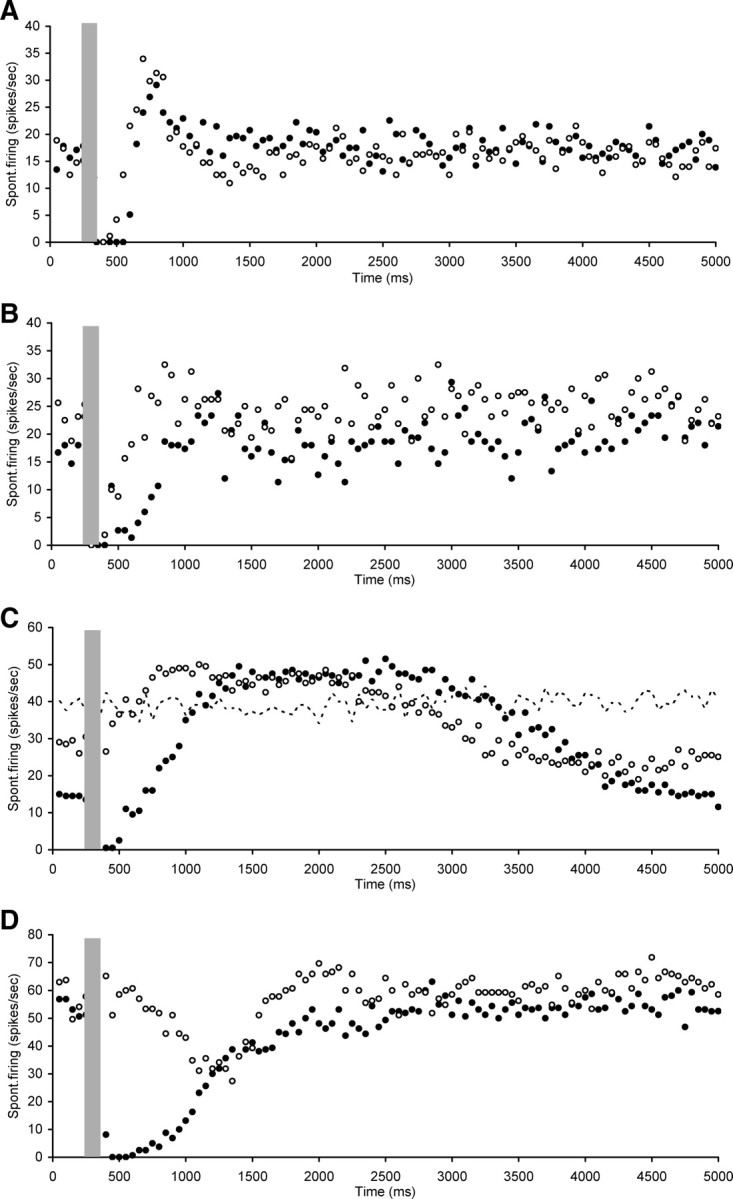
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), recorded both before (dark circles) and after (open circles) strychnine perfusion in the cochlea. Illustrations of neurons in which the immediate suppression observed after OCB stimulation was not completely abolished by cochlear strychnine perfusion but its duration was reduced. In C also indicated is the spontaneous firing rate without OCB stimulation or strychnine perfusion (dotted line). A, Neuron CF 8.5 kHz, threshold 43 dB SPL. B, Neuron CF 9.2 kHz, threshold 67 dB SPL. C, Neuron nonresponsive to sound. D, Neuron CF 9 kHz, threshold 61 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A and D was 250 μA and in B and C 300 μA.
The final three neurons are shown in Figure 8. One of these neurons (Fig. 8A) showed no immediate suppression with electrical activation of the OCB, even though the OCB effect in the cochlea was equivalent to 15 dB at 8 kHz. Instead there was an immediate overshoot followed by suppression and some oscillation. Strychnine perfusion did not change these effects. Finally, there were two neurons in which strychnine perfusion in the cochlea had no effect on the suppression observed (200 and 250 ms), but in both instances decreased the overshoot that followed the suppression while leaving the other later effects unchanged (Fig. 8B,C).
Figure 8.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), recorded both before (dark circles) and after (open circles) strychnine perfusion in the cochlea. A, Illustration of neuron in which strychnine perfusion did not change effects of OCB stimulation. B, C, Two neurons in which strychnine perfusion in the cochlea did not affect the suppression, but did decrease the following overshoot. A, Neuron CF 22.9 kHz, threshold 44 dB SPL. B, Neuron CF 18 kHz, threshold 66 dB SPL. C, Neuron CF 16.8 kHz, threshold 36 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A was 250 μA, in B 350 μA, and in C 150 μA.
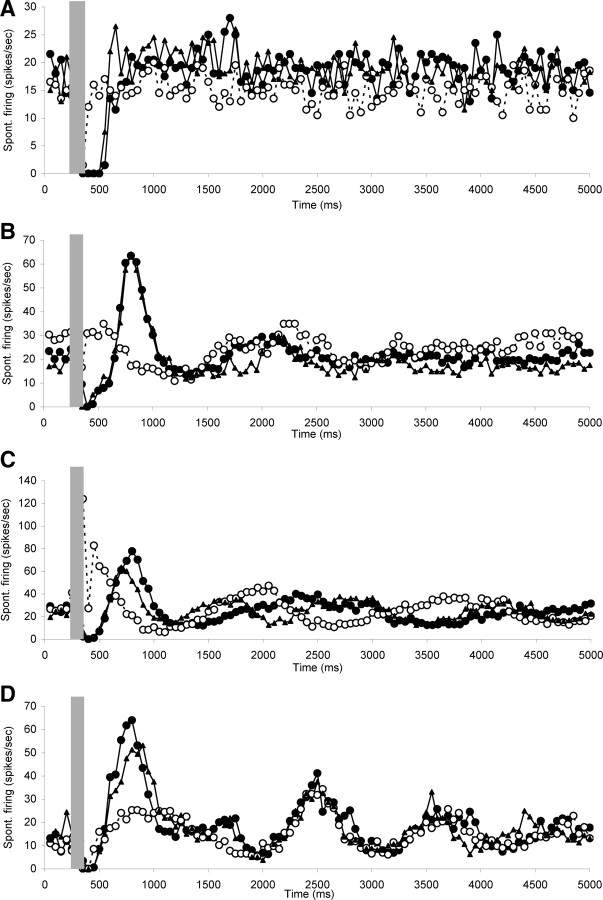
In eight neurons, the effects of OCB stimulation were observed before and immediately after strychnine blockade as well as after recovery. Figure 9 shows four examples, illustrating that the effects were highly reversible and repeatable.
Figure 9.
Scatter plots of spontaneous firing of hyperactive CNIC neurons with electrical stimulation of the OCB (gray bar), recorded before (dark circles) and after (open circles) strychnine perfusion in the cochlea, as well as after recovery from the strychnine effects (dark triangles). Note reversibility of the strychnine effects and the repeatability of all components of the OCB stimulation effects before blockade and after recovery. A, Neuron CF 7.8 kHz, threshold 20 dB SPL. B, Neuron CF 14.5 kHz, threshold 23 dB SPL. C, Neuron CF 13.9 kHz, threshold 39 dB SPL. D, Neuron CF 16.8 kHz, threshold 36 dB SPL. Stimulation parameters: 100 ms duration, 0.1 ms pulses, 300 Hz. Stimulation strength in A was 250 μA and in B–D 150 μA.
Discussion
The present data demonstrate for the first time that activation of the intrinsic efferent system, the OC system, can alter the abnormally high levels of spontaneous activity of CNIC neurons that are present 2–4 weeks after acoustic trauma. Some of the effects of OCB stimulation were caused by intracochlear action, but other effects must be the result of direct effect of the stimulation on central pathways since they remained after intracochlear blockade of the receptors of the medial OC system. The relationship between these OCB effects on spontaneous hyperactivity after acoustic trauma and the effects on spontaneous activity in normal animals is unknown. In normal animals with the anesthetic regime used in the present study spontaneous firing rates of CNIC neurons are so low (Torterolo et al., 2002; Mulders and Robertson, 2009) that any OCB effects are difficult to quantify.
The observation that the increased spontaneous activity of CNIC neurons can be modulated by OCB stimulation is consistent with our recent findings that silencing the auditory nerve suppresses the high spontaneous activity of CNIC neurons up to 6 weeks after acoustic trauma (Mulders and Robertson, 2009), and the well described effects of OCB stimulation on auditory afferent firing. Electrical stimulation of the OCB at the floor of the IVth ventricle is thought to stimulate the medial OC system (Gifford and Guinan, 1987), which terminates onto the cochlear outer hair cells (Liberman and Brown, 1986; Dallos et al., 1997). In addition to modulating the electro-mechanical gain of the cochlea (Dallos et al., 1997), medial system activation also results in a reduction of spontaneous firing of auditory afferents (Wiederhold and Kiang, 1970; Guinan and Gifford, 1988). This is most likely caused by a decrease in the endocochlear potential, arising from a shunt in current through the OHC when the medial system is activated (Housley and Ashmore, 1991; Blanchet et al., 2000). The reduced endocochlear potential causes hyperpolarization of the inner hair cells and a reduction of the spontaneous neurotransmitter release onto the auditory afferent fibers, reducing spontaneous firing of the afferents (Brown and Nuttall, 1984; Sewell, 1984).
It is noteworthy that the suppression of spontaneous firing rate reported in primary afferents after electrical activation of the OCB is qualitatively different from the suppression shown in the present data. The maximum amount of suppression observed in primary afferents was ∼50% (Wiederhold and Kiang, 1970; Guinan and Gifford, 1988), whereas in most instances there was a 100% reduction of spontaneous firing observed in CNIC neurons in the present experiments. Nonetheless, in many neurons a complete elimination of this suppression was produced after blockade of the intracochlear effects of OCB stimulation with strychnine. This indicates that the striking effects of OCB stimulation on central hyperactivity were due to the intracochlear effects of OCB stimulation on the primary afferents. The explanation for the discrepancy in magnitude of suppression is unknown. An earlier study on the effects of OCB stimulation on CNIC neurons in control animals described a similar difference in the magnitude of effect on spontaneous activity (Seluakumaran et al., 2008), whereas in the CN of control animals the effects of OCB stimulation on spontaneous activity are small (Mulders et al., 2008). These observations suggest that local intrinsic circuitry between CN and CNIC is capable of increasing the effect of OCB stimulation on spontaneous activity.
Another notable aspect of the suppression in hyperactive CNIC neurons after OCB stimulation was its duration (up to 2.5 s after stimulation). The strychnine experiments indicate that at least 450 ms of suppression can be attributed to intracochlear effects of stimulation. Whether this is a direct translation of long-lasting effects on primary afferents or again due to further modulation by intrinsic circuitry is unknown. Starr and Wernick (1968) recording in CN of normal cats also showed that electrical stimulation of the OCB could result in suppression of spontaneous rate for up to 500 ms in duration after stimulation ceased. There is no compelling evidence for such long-lasting suppression of primary afferent spontaneous firing by OCB stimulation. Studies on the effects of OCB stimulation on primary afferents in cats describe mainly effects during stimulation (Wiederhold and Kiang, 1970; Guinan and Gifford, 1988). These studies do not describe the precise duration of effects after stimulation ceased, but only describe the effects as disappearing rapidly.
In two neurons, the blockade of intracochlear effects with strychnine perfusion had no effect on the immediate suppression but it did eliminate the overshoot after the suppression. Overshoots have been reported to occur in primary afferent as well after OCB stimulation (Wiederhold and Kiang, 1970; Guinan and Gifford, 1988). Since in the present data the overshoot could be eliminated without an effect on the suppression, this suggests it is not just a rebound of inhibition but rather a late excitatory effect caused by electrical stimulation of the OC system.
When the intracochlear effects of medial OC system were blocked by strychnine, electrical stimulation of the OCB still resulted in a variety of effects on spontaneous rate, including late inhibition and excitation. There are three possible explanations for these persistent effects. The first possibility is that some of the effects are caused by activation of the lateral OC system, whose axons run close to the site of stimulation (Brown, 1993; Warr et al., 1997) and which project to the primary afferent dendrites underneath the inner hair cells in the cochlea (Warr et al., 1997). Since the α9 and α10 subunits of the nicotinic acetylcholine receptor are specific for the medial system (Vetter et al., 1999; Lustig, 2006), the blockade by strychnine perfusion would not alter any lateral effects on primary afferents. However, activation of the lateral system by electrical stimulation at the floor of the IVth ventricle has never been confirmed and is considered unlikely in view of the unmyelinated axons of the lateral system (Brown, 1987; Warr et al., 2002).
A second possibility is that the electrical stimulation not only stimulated the medial OC system but also axons projecting out of the CN. These could include contralaterally projecting neurons (Cant and Gaston, 1982; Schofield and Cant, 1996), whose axons run close to the surface of the floor of the IVth ventricle (Arnott et al., 2004; Smith et al., 2005). Stimulation of these axons could cause either orthodromic or antidromic effects in CN which would flow on to IC (Cant and Benson, 2003). These effects could then be manifested as the excitatory or inhibitory changes observed in the present experiments that remained after strychnine perfusions. It is also possible that the electrical stimulation resulted in unintentional activation of the dorsal acoustic stria, comprised of axons from the dorsal CN projecting to the IC (Smith et al., 2005). However, we placed our stimulating electrodes at the point of the lowest shock threshold to elicit facial twitch (before paralysis), and we have shown previously that this should not result in activation of ascending axons when stimulating current is kept low (Seluakumaran et al., 2008). To minimize the possibility involvement of the dorsal acoustic stria, we excluded neurons from analysis when a field potential could be detected in IC.
A third possibility is that the medial OC system provides direct input to CN neurons. Medial OC neurons are known to have collaterals projecting to the CN (Winter et al., 1989; Benson and Brown, 1990; Benson and Potashner, 1990; Brown, 1993; Benson et al., 1996; Horváth et al., 2000), and we have previously shown direct excitatory effects of these collaterals on CN neurons, in particular onset chopper neurons (Mulders et al., 2002, 2003, 2007, 2009). These neurons are thought to provide wide band inhibition to other CN neurons (Verhey et al., 2003; Arnott et al., 2004; Neuert et al., 2004) and could be responsible for the modulation of spontaneous activity remaining after cochlear strychnine. Further investigations are required to determine the exact cause of the extensive effects on spontaneous rate when the peripheral effects of the OC system are blocked.
Our present results clearly indicate a strong effect of stimulation of the medial OC system on hyperactivity caused by acoustic trauma. This demonstration that an intrinsic control system can modify maladaptive plastic phenomena in the auditory pathway, could have important clinical implications. If spontaneous hyperactivity is indeed involved in the generation of tinnitus (Brozoski et al., 2002; Kaltenbach et al., 2004; Bauer et al., 2008), then our results could indicate a beneficial effect of OC system activation on tinnitus. Our observations that the suppressive effects on spontaneous activity lasted after the stimulation had ceased, is consistent with a role for the OC system in residual inhibition, a temporary reduction of tinnitus experienced in tinnitus patients that persists for a few seconds after masking sounds are turned off (Vernon and Meikle, 2003; Roberts et al., 2008). Furthermore, activation of the OC system could be a contributory mechanism to the often beneficial effects of masking sounds on the perception of tinnitus (Jastreboff, 2007; Lugli et al., 2009), since the OC system itself can be activated by sound (Robertson and Gummer, 1985; Robertson and Winter, 1988; Thompson and Thompson, 1991; Brown et al., 2003; Lugli et al., 2009). Finally, focal and specific pharmacological manipulation of the peripheral action of the OC system might offer therapeutic possibilities for the treatment of tinnitus without the complications of unwanted central side effects.
Footnotes
This work was supported by grants from the Royal National Institute for Deaf People (United Kingdom), the Medical Health and Research Infrastructure Fund (Western Australia), and The University of Western Australia. We thank Mr. Christo Bester for help with acquisition of sham control data.
References
- Arnott RH, Wallace MN, Shackleton TM, Palmer AR. Onset neurones in the anteroventral cochlear nucleus project to the dorsal cochlear nucleus. J Assoc Res Otolaryngol. 2004;5:153–170. doi: 10.1007/s10162-003-4036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CG, Potashner SJ. Retrograde transport of [3H]glycine from the cochlear nucleus to the superior olive in the guinea pig. J Comp Neurol. 1990;296:415–426. doi: 10.1002/cne.902960307. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J Comp Neurol. 1990;295:52–70. doi: 10.1002/cne.902950106. [DOI] [PubMed] [Google Scholar]
- Benson TE, Berglund AM, Brown MC. Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol. 1996;365:27–41. doi: 10.1002/(SICI)1096-9861(19960129)365:1<27::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Gentamicin blocks ACh-evoked K+ current in guinea-pig outer hair cells by impairing Ca2+ entry at the cholinergic receptor. J Physiol. 2000;525:641–654. doi: 10.1111/j.1469-7793.2000.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeck S, Sayles M, Ingham NJ, Winter IM. The time course of recovery from suppression and facilitation from single units in the mammalian cochlear nucleus. Hear Res. 2006;212:176–184. doi: 10.1016/j.heares.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:605–618. doi: 10.1002/cne.902600412. [DOI] [PubMed] [Google Scholar]
- Brown MC. Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brainstem. J Comp Neurol. 1993;337:600–613. doi: 10.1002/cne.903370406. [DOI] [PubMed] [Google Scholar]
- Brown MC, Nuttall AL. Efferent control of cochlear inner hair cell responses in the guinea-pig. J Physiol. 1984;354:625–646. doi: 10.1113/jphysiol.1984.sp015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, de Venecia RK, Guinan JJ., Jr Responses of medial olivocochlear neurons. Specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res. 2003;153:491–498. doi: 10.1007/s00221-003-1679-y. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Gaston KC. Pathways connecting the right and left cochlear nuclei. J Comp Neurol. 1982;212:313–326. doi: 10.1002/cne.902120308. [DOI] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. Auditory-evoked potentials from cochlea to cortex as influenced by activation of the efferent olivocochlear bundle. J Acoust Soc Am. 1962;34:1478–1496. [Google Scholar]
- Dong S, Mulders WH, Rodger J, Robertson D. Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience. 2009;159:1164–1174. doi: 10.1016/j.neuroscience.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea pig auditory brainstem. Eur J Neurosci. 2010;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. II. Spontaneous rate. Hear Res. 1988;33:115–127. doi: 10.1016/0378-5955(88)90024-x. [DOI] [PubMed] [Google Scholar]
- Horváth M, Kraus KS, Illing RB. Olivocochlear neurons sending axon collaterals into the ventral cochlear nucleus of the rat. J Comp Neurol. 2000;422:95–105. [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc Biol Sci. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490:391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Ingham NJ, Bleeck S, Winter IM. Contralateral inhibitory and excitatory frequency response maps in the mammalian cochlear nucleus. Eur J Neurosci. 2006;24:2515–2529. doi: 10.1111/j.1460-9568.2006.05134.x. [DOI] [PubMed] [Google Scholar]
- Jastreboff MM. Sound therapies for tinnitus management. Prog Brain Res. 2007;166:435–440. doi: 10.1016/S0079-6123(07)66042-7. [DOI] [PubMed] [Google Scholar]
- Johnstone JR, Alder VA, Johnstone BM, Robertson D, Yates GK. Cochlear action potential threshold and single unit thresholds. J Acoust Soc Am. 1979;65:254–257. doi: 10.1121/1.382244. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Lugli M, Romani R, Ponzi S, Bacciu S, Parmigiani S. The windowed sound therapy: a new empirical approach for an effectiv personalized treatment of tinnitus. Int Tinnitus J. 2009;15:51–61. [PubMed] [Google Scholar]
- Lustig LR. Nicotinic acetylcholine receptor structure and function in the efferent auditory system. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:424–434. doi: 10.1002/ar.a.20302. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972;10:662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Winter IM, Robertson D. Dual action of olivocochlear collaterals in the guinea pig cochlear nucleus. Hear Res. 2002;174:264–280. doi: 10.1016/s0378-5955(02)00701-3. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Paolini AG, Needham K, Robertson D. Olivocochlear collaterals evoke excitatory effects in onset neurones of the rat cochlear nucleus. Hear Res. 2003;176:113–121. doi: 10.1016/s0378-5955(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Harvey AR, Robertson D. Electrically evoked responses in onset chopper neurons in guinea pig cochlear nucleus. J Neurophysiol. 2007;97:3288–3297. doi: 10.1152/jn.01148.2006. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Seluakumaran K, Robertson D. Effects of centrifugal pathways on responses of cochlear nucleus neurons to signals in noise. Eur J Neurosci. 2008;27:702–714. doi: 10.1111/j.1460-9568.2008.06046.x. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Paolini AG, Needham K, Robertson D. Synaptic responses in cochlear nucleus neurons evoked by activation of the olivocochlear system. Hear Res. 2009;256:85–92. doi: 10.1016/j.heares.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Neuert V, Verhey JL, Winter IM. Responses of dorsal cochlear nucleus neurons to signals in the presence of modulated maskers. J Neurosci. 2004;24:5789–5797. doi: 10.1523/JNEUROSCI.0450-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena A, Micheyl C, Chéry-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7:358–369. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- Rajan R. Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I. Dependence on electrical stimulation parameters. J Neurophysiol. 1988;60:549–568. doi: 10.1152/jn.1988.60.2.549. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9:417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear Res. 1985;20:63–77. doi: 10.1016/0378-5955(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Robertson D, Winter IM. Cochlear nucleus inputs to olivocochlear neurones revealed by combined anterograde and retrograde labelling in the guinea pig. Brain Res. 1988;462:47–55. doi: 10.1016/0006-8993(88)90583-5. [DOI] [PubMed] [Google Scholar]
- Sayles M, Winter IM. Reverberation challenges the temporal representation of the pitch of complex sounds. Neuron. 2008;58:789–801. doi: 10.1016/j.neuron.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Origins and targets of commissural connections between the cochlear nuclei in guinea pigs. J Comp Neurol. 1996;375:128–146. doi: 10.1002/(SICI)1096-9861(19961104)375:1<128::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Seluakumaran K, Mulders WH, Robertson D. Effects of medial olivocochlear efferent stimulation on the activity of neurons in the auditory midbrain. Exp Brain Res. 2008;186:161–174. doi: 10.1007/s00221-007-1219-2. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The relation between the endocochlear potential and spontaneous activity in auditory nerve fibres of the cat. J Physiol. 1984;347:685–696. doi: 10.1113/jphysiol.1984.sp015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Sumner CJ, Bledsoe SC, Lu J. Effects of contralateral sound stimulation on unit activity of ventral cochlear nucleus neurons. Exp Brain Res. 2003;153:427–435. doi: 10.1007/s00221-003-1610-6. [DOI] [PubMed] [Google Scholar]
- Smith PH, Massie A, Joris PX. Acoustic stria: anatomy of physiologically characterized cells and their axonal projection patterns. J Comp Neurol. 2005;482:349–371. doi: 10.1002/cne.20407. [DOI] [PubMed] [Google Scholar]
- Starr A, Wernick JS. Olivocochlear bundle stimulation: effects on spontaneous and tone-evoked activities of single units in cat cochlear nucleus. J Neurophysiol. 1968;31:549–564. doi: 10.1152/jn.1968.31.4.549. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Posteroventral cochlear nucleus projections to olivocochlear neurons. J Comp Neurol. 1991;303:267–285. doi: 10.1002/cne.903030209. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Falconi A, Morales-Cobas G, Velluti RA. Inferior colliculus unitary activity in wakefulness, sleep and under barbiturates. Brain Res. 2002;935:9–15. doi: 10.1016/s0006-8993(02)02235-7. [DOI] [PubMed] [Google Scholar]
- Verhey JL, Pressnitzer D, Winter IM. The psychophysics and physiology of comodulation masking release. Exp Brain Res. 2003;153:405–417. doi: 10.1007/s00221-003-1607-1. [DOI] [PubMed] [Google Scholar]
- Vernon JA, Meikle MB. Tinnitus: clinical measurement. Otolaryngol Clin North Am. 2003;36:293–305. vi. doi: 10.1016/s0030-6665(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Salvi RJ, Powers N. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. J Neurophysiol. 1996;75:171–183. doi: 10.1152/jn.1996.75.1.171. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ., Jr Efferent innervation of the organ of Corti: two separate systems. Brain Res. 1979;173:152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Warr WB, Boche JB, Neely ST. Efferent innervation of the inner hair cell region: origins and terminations of two lateral olivocochlear systems. Hear Res. 1997;108:89–111. doi: 10.1016/s0378-5955(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Warr WB, Beck Boche JE, Ye Y, Kim DO. Organization of olivocochlear neurons in the cat studied with the retrograde tracer cholera toxin-B. J Assoc Res Otolaryngol. 2002;3:457–478. doi: 10.1007/s10162-002-2046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JS, Warr WB. The dual origins of the olivocochlear bundle in the albino rat. J Comp Neurol. 1983;219:203–214. doi: 10.1002/cne.902190206. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Cole KS. Descending projections from auditory brainstem nuclei to the cochlea and cochlear nucleus of the guinea pig. J Comp Neurol. 1989;280:143–157. doi: 10.1002/cne.902800110. [DOI] [PubMed] [Google Scholar]