Abstract
It is believed that memory reactivation transiently renders consolidated memory labile and that this labile or deconsolidated memory is reconsolidated in a protein synthesis-dependent manner. The synaptic correlate of memory deconsolidation upon reactivation, however, has not been fully characterized. Here, we show that 3,5-dihydroxyphenylglycine (DHPG), an agonist for group I metabotropic glutamate receptors (mGluRI), induces synaptic depotentiation only at thalamic input synapses onto the lateral amygdala (T–LA synapses) where synaptic potentiation is consolidated, but not at synapses where synaptic potentiation is not consolidated. Using this mGluRI-induced synaptic depotentiation (mGluRI-depotentiation) as a marker of consolidated synapses, we found that mGluRI-depotentiation correlated well with the state of memory deconsolidation and reconsolidation in a predictable manner. DHPG failed to induce mGluRI-depotentiation in slices prepared immediately after reactivation when the reactivated memory was deconsolidated. DHPG induced mGluRI-depotentiation 1 h after reactivation when the reactivated memory was reconsolidated, but it failed to do so when reconsolidation was blocked by a protein synthesis inhibitor. To test the memory-specificity of mGluRI-depotentiation, conditioned fear was acquired twice using two discriminative tones (2.8 and 20 kHz). Under this condition, mGluRI-depotentiation was fully impaired in slices prepared immediately after reactivation with both tones, whereas mGluRI-depotentiation was partially impaired immediately after reactivation with the 20 kHz tone. Consistently, microinjection of DHPG into the LA 1 h after reactivation reduced fear memory retention, whereas DHPG injection immediately after reactivation failed to do so. Our findings suggest that, upon memory reactivation, consolidated T–LA synapses enter a temporary labile state, displaying insensitivity to mGluRI-depotentiation.
Introduction
Once acquired, memory is consolidated over time in a protein synthesis-dependent manner (Kandel, 2001). Consolidated memory had been believed to be persistent and static; however, a series of elegant experiments from many laboratories have provided compelling evidence that consolidated memory becomes labile temporarily upon memory reactivation (Nader et al., 2000; Debiec et al., 2002; Myers and Davis, 2002; Eisenberg et al., 2003; Lee et al., 2004; Inda et al., 2005) (but see Milekic and Alberini, 2002). Microinjection of anisomycin, an inhibitor of protein synthesis, immediately after reactivation of a fear memory impairs long-term retention (>24 h) of the reactivated memory, but not short-term retention (<4 h) (Nader et al., 2000), suggesting that reconsolidation of a labile memory requires protein synthesis, as does initial memory consolidation. Importantly, in vivo field potential recordings from the lateral amygdala (LA) have shown that auditory-evoked field potentials respond consistently with the reconsolidation hypothesis in that a reconsolidation block after memory reactivation reduces the field potentials in an input-specific manner (Doyère et al., 2007).
One of the most important premises for the reconsolidation hypothesis is a prediction that there exists a set of memory-storing synapses that are temporarily “deconsolidated” or “labile” upon memory reactivation (the term “deconsolidation” is defined as a process by which consolidated memory becomes labile). To date, it has been difficult to isolate these deconsolidated synapses, and thus, the state of consolidation can only be inferred by post hoc observation of amnesia after pharmacological interference. The lack of a tool providing a direct readout of synaptic state has hampered in-depth analyses of the memory reconsolidation process. Therefore, it is necessary to find an alternative and more direct approach to detect the state of synapses upon reactivation.
In our previous study (Kim et al., 2007), we reported two forms of depotentiation induced either by paired-pulse low-frequency stimulation (pp-LFS) or by application of 3,5-dihydroxyphenylglycine (DHPG), the group I metabotropic glutamate receptor (mGluR) agonist. Both forms of depotentiation apparently reverse fear conditioning-induced potentiation of excitatory synaptic transmission at thalamic-input synapses onto lateral amygdala (T–LA) synapses. Interestingly, this particular study was performed using rats in which fear memory had been consolidated, indicating that both forms of depotentiation may reverse consolidated potentiation. DHPG-induced mGluRI- depotentiation involves internalization of surface AMPA receptors and appears to recruit a more specific mechanism than pp-LFS-induced depotentiation (which involves activation of not only mGluRI but also NMDA receptors). In any case, both forms of depotentiation can be used to distinguish potentiated synapses, although DHPG-induced mGluRI-depotentiation may be more useful to further dissect different states (e.g., consolidation states) of synaptic potentiation.
In the present study, we tested whether DHPG-induced mGluRI-depotentiation could serve as a marker for consolidated synapses. After establishing mGluRI-depotentiation as a useful marker for consolidated synapses, we tested whether reactivation of fear memory rendered consolidated T–LA synapses labile or deconsolidated. Our findings suggest that consolidated T–LA synapses become labile or deconsolidated upon reactivation, as does a consolidated memory.
Materials and Methods
Animals.
Male Sprague Dawley rats (3–5 weeks of age) were obtained from Orient Bio. Rats were housed in plastic cages and maintained with ad libitum access to food and water under an inverted 12 h light/dark cycle (light off at 9:00 A.M.). Behavioral training was done during the dark portion of the light/dark cycle. All behavioral procedures were approved by Institute of Laboratory Animal Resources of Seoul National University.
Animal surgery and histology.
Rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.). When fully anesthetized, rats were mounted on a stereotaxic apparatus (Stoelting) and implanted bilaterally into the LA (LA; anteroposterior, −2.3 mm; mediolateral, ±5.0 mm; dorsoventral, −6.5 mm from bregma) with 26 gauge stainless-steel cannulas (model C315G; Plastics One). A 32 gauge dummy cannula was inserted into each guide cannula to prevent clogging. Two jewelry screws were implanted over the skull serving as anchors, and the whole assembly was affixed on the skull with dental cement. Rats were given at least 5 d to recover before experiments. To verify the intra-LA placement of the injector cannula tips, rats were anesthetized after completion of the experiments with urethane (1 g/kg, i.p.) and transcardially perfused with 0.9% saline solution, followed by 10% buffered formalin. Brains were removed and postfixed overnight. Coronal sections (80 μm thick) were cut using a vibroslicer (NVSL; World Precision Instruments), stained with cresyl violet, and examined under a light microscope.
Intra-amygdala infusion.
Anisomycin (Sigma-Aldrich) was dissolved in equimolar HCl, diluted with artificial CSF (aCSF), and adjusted to pH 7.4 with NaOH. S-DHPG (Tocris) was dissolved in aCSF. Anisomycin (62.5 μg/0.5 μl/side) or S-DHPG (1 μg/0.5 μl/side) was administrated bilaterally into the LA via a 33 gauge injector cannula (C315I; Plastics One) attached to a 10 μl Hamilton syringe at a rate of 0.25 μl/min. After the drug infusion, the injector cannulas were left in place for an additional minute to diffuse the drug away from the cannula tip. Dummy cannulas were then replaced, and rats were returned to their home cage.
Apparatus.
Fear conditioning was performed in a rectangular Plexiglas chamber (context A) with a metal grid floor connected to an electrical current source (San Diego Instruments or Coulbourn Instruments) in a sound attenuating room. The room with white light on had the sound of a fan serving as a background noise. For the second conditioning shown in Figure 5, a cylindrical Plexiglas chamber (context B) with a metal grid floor, which was slightly slanted, was used in a sound attenuating room in which the light was off. Reactivation and tone testing were performed in the cylindrical Plexiglas chamber (context C) in a sound-attenuating cubicle with a flat black Formica floor (San Diego Instruments). The cubicle with the light off was ventilated with a fan serving as a background noise. A camera was mounted on the top of the chamber for recording.
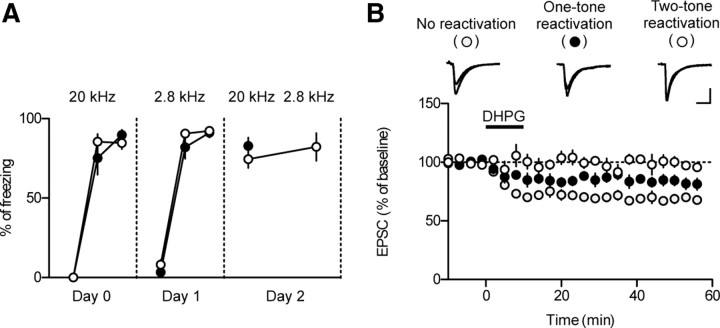
Figure 5.
mGluRI-depotentiation is memory- and synapse-specific. A, Rats were fear conditioned with two distinct tones (20 kHz on day 0 and 2.8 kHz on day 1). Memory reactivation was assessed 24 h after the last conditioning (day 2). Two tones (20 and 2.8 kHz) were sequentially presented in the two-tone group with an interval of 30 s. Slices were prepared immediately after presentation of the last tone on day 2. B, mGluRI-induced depotentiation in each group. Please note that the magnitude of mGluRI-depotentiation in the one-tone group was in between those in the other two groups. To avoid possible bias, the experiments shown in this figure were performed blindly.
General behavior procedures.
For fear conditioning, rats were placed in the conditioning chamber (context A) and left undisturbed for 2 min. A neutral tone [conditioned stimulus (CS); 30 s, 2.8 kHz, 85 dB] coterminating with an electrical footshock [unconditioned stimulus (US); 1.0 mA, 1 s] was then presented three times with an average interval of 100 s. For the experiments shown in Figure 5, rats were additionally conditioned in another conditioning chamber (context B) with a 20 kHz tone. For the experiments shown in Figure 6, a moderate conditioning protocol was used [three pairings of CS (30 s, 2.8 kHz, 85 dB) and US (0.5 mA, 1 s)]. Rats were returned to their home cages 60 s after the last shock was applied. Twenty-four hours after the last conditioning, one tone (30 s, 2.8 kHz, 85 dB) was presented for memory reactivation in context C, except for the Figure 5 experiments in which two tones (20 and 2.8 kHz, respectively) were sequentially presented with an interval of 30 s.
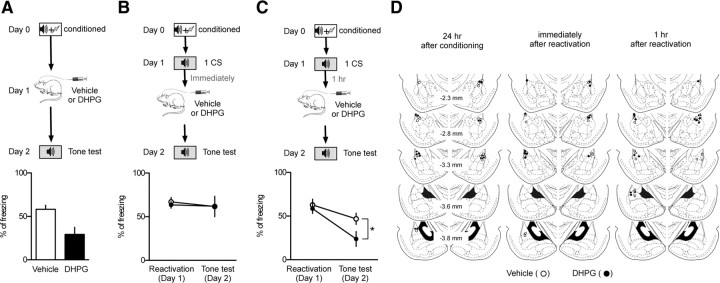
Figure 6.
Microinjection of DHPG into the LA impairs long-term retention of a consolidated memory, but not of a labile memory. A, The behavioral procedure for the experiments (top). Microinjection of DHPG 24 h after fear conditioning impaired fear memory retention compared with vehicle-injected controls (vehicle, 58.3 ± 4.6%, n = 10, open circle; DHPG, 29.3 ± 8.3%, n = 10, filled circle; bottom). B, The behavioral procedure for the experiments (top). Microinjection of DHPG immediately after reactivation had no significant effects on memory retention relative to vehicle-injected controls (vehicle, 61.8 ± 11.7%, n = 7, open circle; DHPG, 62.0 ± 7.6%, n = 9, filled circle; bottom). C, The behavioral procedure for the experiments (top). Microinjection of DHPG 1 h after reactivation impaired fear memory retention compared with vehicle-injected controls (vehicle, 46.9 ± 6.4%, n = 8, open circle; DHPG, 23.8 ± 8.5%, n = 8, filled circle; bottom). *p < 0.05. D, Schematic illustration showing cannula tip placement (DHPG-injected, filled circle; vehicle-injected, open circle). The numbers on the left side indicate the anteroposterior coordinates caudal to bregma. The illustration was adopted from the atlas of Paxinos and Watson (1998).
For the experiments shown in Figure 2, anisomycin (62.5 μg) or vehicle was infused into the LA through injector cannulas at a given time point after reactivation (0, 1, or 6 h after reactivation). Three tones were presented for memory testing in context C 24 h after reactivation, with the first tone presented 4 min after placement of the rats. Freezing level during the first tone presentation was used for both plotting and statistical comparison. For the experiments shown in Figure 3, the same procedure were used, but slices were prepared at a given time point after reactivation (0, 1, or 6 h after reactivation) instead of the drug infusion. For the experiments shown in Figure 4, anisomycin or vehicle was infused into the LA immediately after reactivation, and slices were prepared 1 h after reactivation. For the experiments shown in Figure 6, S-DHPG (1 μg) or vehicle was infused into the LA through injector cannulas at a given time point after reactivation (0 or 1 h after reactivation). Three tones were presented for memory testing in context C 24 h after drug injection, with the first tone presented 4 min after placement of the rats. Freezing level during the first tone presentation was used for both plotting and statistical comparison. Conditioned freezing was defined as immobility except for respiratory movements. Total freezing time during a test period was normalized to the duration of tone presentation.
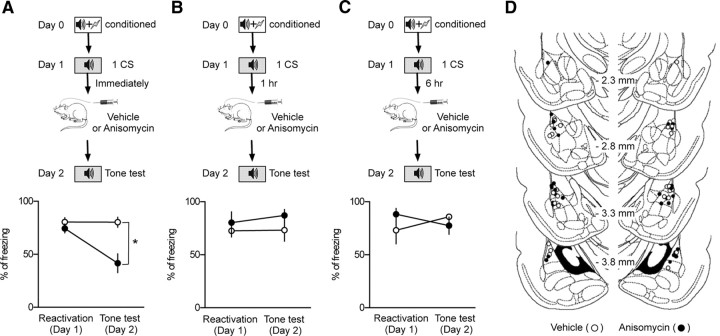
Figure 2.
Reconsolidation after memory reactivation appears to be completed within 1 h after reactivation. A, The behavioral procedure for the experiments (top). Microinjection of anisomycin (41.6 ± 8.9%; n = 7; filled circle) immediately after reactivation induced amnesia compared with vehicle controls (80.3 ± 4.8%; n = 4; open circle; bottom). *p < 0.05. B, The behavioral procedure for the experiments (top). Microinjection of anisomycin (87.1 ± 5.6%; n = 4; filled circle) 1 h after reactivation had no effects on the freezing level measured 24 h after reactivation compared with vehicle controls (73.3 ± 10.4%; n = 5; open circle; bottom). C, The behavioral procedure for the experiments (top). Microinjection of anisomycin (77.4 ± 8.1%; n = 3; filled circle) 6 h after reactivation had no effects on the freezing level measured 24 h after reactivation compared with vehicle controls (85.9 ± 2.5%; n = 5; open circle; bottom). D, Schematic illustration showing cannula tip placement (anisomycin-injected, filled circle; vehicle-injected, open circle). The illustration was adopted from the atlas of Paxinos and Watson (1998).
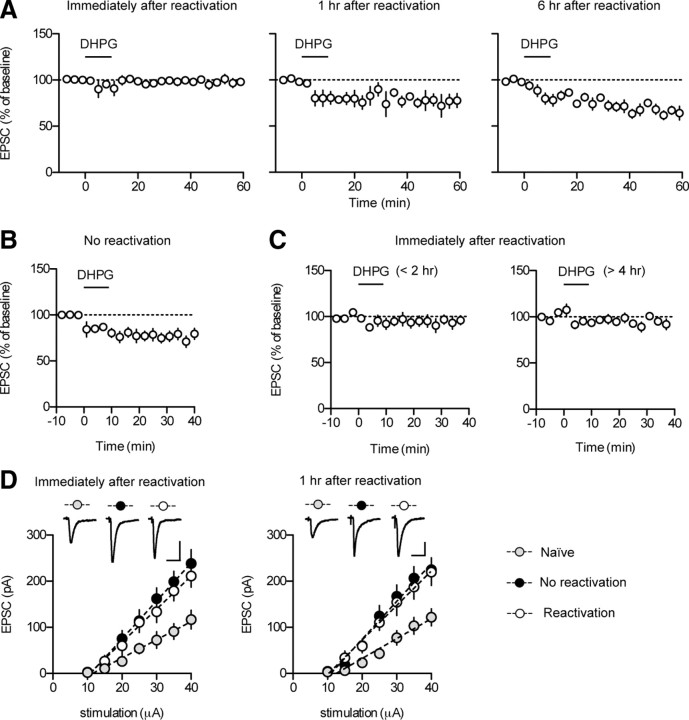
Figure 3.
Application of DHPG induces mGluRI-depotentiation at T–LA synapses in slices prepared after reconsolidation, but not before. A, DHPG application failed to induce the depotentiation in slices prepared immediately after reactivation (left). DHPG application produced a significant reduction in EPSC amplitudes in slices prepared 1 h (middle) or 6 h (right) after reactivation. B, DHPG application produced a significant reduction in EPSC amplitudes in slices prepared after exposure to the reactivation context without tone presentation (no-reactivation group). C, In slices prepared immediately after reactivation, DHPG application at various time points (<2 vs >4 h) after slice preparation does not alter the magnitude of mGluRI-depotentiation. DHPG had no discernible effects on T–LA synaptic transmission when applied either <2 or >4 h after slice preparation (<2 h after slice preparation, n = 5, 96.1 ± 5.7% of baseline, paired t test, p > 0.5; >4 h after slice preparation, n = 6, 95.2 ± 3.8% of baseline, paired t test, p > 0.2). Brain slices were prepared from conditioned animals immediately after reactivation. Whole-cell recordings were initiated at the proper times to apply DHPG at the designated time points (<2 vs >4 h). Different slices were used for each experiment. D, Input–output relationship of EPSCs between the naive, reactivation, and no-reactivation group. Amygdala slices were prepared immediately (left) or 1 h (right) after reactivation. Left, In slices prepared immediately after reactivation, synaptic efficacy was enhanced in both reactivation (open circle; n = 13; slope = 7.180 ± 0.79 pA/μA) and no-reactivation groups (filled circle; n = 14; slope = 8.224 ± 0.97 pA/μA) compared with naive controls (gray circle, n = 9, slope = 3.941 ± 0.70 pA/μA; one-way ANOVA, F(2,33) = 5.623, Newman–Keuls posttest, reactivation vs no-reactivation group, p > 0.05; reactivation vs naive group, p < 0.05; no-reactivation vs naive group, p < 0.01). Representative traces were an average of four traces from each group with an input stimulation of 35 μA. Calibration: 20 ms, 100 pA. Right, In slices prepared 1 h after reactivation, synaptic efficacy was enhanced in both reactivation (open circle; n = 9; slope, 7.484 ± 0.91 pA/μA) and no-reactivation groups (filled circle; n = 9; slope = 8.188 ± 0.97 pA/μA) compared with naive controls (gray circle; n = 7; slope, 4.311 ± 0.73 pA/μA; one-way ANOVA, F(2,22) = 4.764, Newman–Keuls posttest, reactivation vs no-reactivation group, p > 0.05; reactivation vs naive group, p < 0.05; no-reactivation vs naive group, p < 0.05). Representative traces were an average of four traces from each group with an input stimulation of 35 μA. Calibration: 20 ms, 50 pA.
Figure 4.
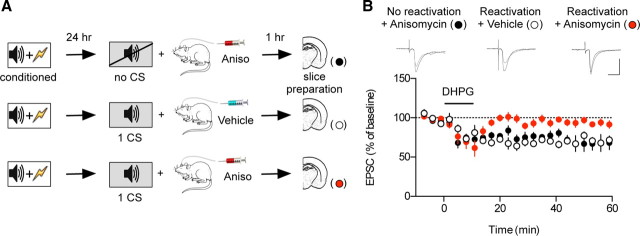
When reconsolidation is blocked, DHPG application induces mGluRI-depotentiation at T–LA synapses in slices prepared 1 h after reactivation. A, The behavioral procedure for the experiments. B, When reconsolidation was blocked by microinjection of anisomycin immediately after reactivation, DHPG application failed to induce mGluRI-depotentiation in slices 1 h after reactivation (red circle). DHPG was able to induce mGluRI-depotentiation in vehicle-injected controls (open circle) and in anisomycin-injected controls without reactivation (filled circle). Anisomycin or vehicle was microinjected immediately after reactivation. To avoid possible bias, the experiments shown in this figure were performed blindly. Representative paired traces were an average of four traces before and after DHPG application. Calibration: 20 ms, 100 pA.
Slice preparation.
Rats were anesthetized with isoflurane and decapitated to remove the brain. The isolated whole brains were placed in a ice-cold modified aCSF solution containing the following (in mm): 175 sucrose, 20 NaCl, 3.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 MgCl2, and 11 d-(+)-glucose. Solutions were then gassed with 95% O2/5% CO2. Coronal slices (300 or 400 μm) including the LA were cut using a vibroslicer (NVSL; World Precision Instruments) and incubated in normal aCSF containing the following (in mm): 120 NaCl, 3.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 MgCl2, 2 CaCl2, and 11 d-(+)-glucose, continuously bubbled at room temperature with 95% O2/5% CO2. Just before transferring a slice to the recording chamber, the cortex overlying the LA was cut away with a scalpel so that cortical epileptic burst discharges would not invade the LA in the presence of picrotoxin (100 μm for whole-cell recordings, 10 μm for field-potential recordings). For field potential recordings, in which afferents were stimulated at a stronger intensity, a lower concentration of picrotoxin was used to preserve inhibitory tone and, thus, to prevent multisynaptic firing.
Afferent stimulation.
We chose brain slices containing a well isolated, sharply defined trunk (containing thalamic afferents) crossing the dorsolateral division of the LA, which is a site of convergence of somatosensory and auditory inputs (Pitkänen et al., 1997). The sizes of the LA and central amygdala were relatively constant in these slices, and the closest trunk to the central nucleus of the amygdala was used when multiple trunks were observed. Thalamic afferents were stimulated using a concentric bipolar electrode (MCE-100; Rhodes Medical Instruments; or CBAEC75; FHC) placed on the midpoint of the trunk between the internal capsule and medial boundary of the LA (Kim et al., 2007). Regions and cells of interest for all recordings were located beneath the midpoint of the trunk spanning the LA horizontally (Kim et al., 2007). We were unable to study cortical input synapses to the LA, another important synapses for fear memory retention (Tsvetkov et al., 2002; Boatman and Kim, 2006), mainly because of the following reasons: (1) Application of DHPG produced a depotentiation only at T–LA synapses but not at cortical input synapses onto the LA (Hong et al., 2009), and (2) pp-LFS produced a depotentiation at cortical input synapses onto the LA both before and after memory consolidation (data not shown) (Hong et al., 2009).
Field potential recordings.
Extracellular field potentials were recorded using a parylene-insulated microelectrode (573210; A-M Systems) in 400-μm-thick slices. The stimulation of thalamic pathways elicited simple negative field potentials that had a constant latency of ∼4 ms and a duration of 5–15 ms. Baseline stimulation (0.017 Hz, 0.2 ms pulse duration) was delivered at an intensity between 100 and 250 μA, which evoked a response that was ∼50% of the maximum evoked response. A submersion-type recording chamber (∼0.5 ml in volume) was continuously superfused with aCSF (34.0 ± 1.0°C) at a flow rate of 1–2 ml/min. Extracellular field potentials were amplified and filtered (low-pass filter, 1 kHz; high-pass filter, 1 Hz; Dam80; World Precision Instruments) and digitized at 1 kHz (ADC-42; Pico Technologies) or at 20 kHz (NAC 2.0 acquisition system; Theta Burst). The digitized signals were stored and analyzed on a personal computer using the LTP program (Anderson and Collingridge, 2001) or NAC Gather software. To obtain stable, long-term recordings, we started a recording >3.5 h after slice preparation. To improve the signal-to-noise ratio, data were averaged using a three-point running average in a subset of the time-lapse experiments.
Whole-cell patch-clamp recordings.
Whole-cell recordings were made using an Axopatch 200A or 700A amplifier (MDS Analytical Technologies). Recordings were obtained using pipettes with a resistance of 2.5–3.5 MΩ and filled with the following solution (in mm): 100 Cs-gluconate, 0.6 EGTA, 10 HEPES, 5 NaCl, 20 TEA (tetraethylammonium), 4 Mg- ATP, 0.3 Na-GTP, and 3 QX-314 [N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide], with the pH adjusted to 7.2 with CsOH and the osmolarity adjusted to ∼297 mmol/kg with sucrose. Recordings were made under infrared–differential interference contrast-enhanced visual guidance from neurons that were three to four cell layers below the surface of 300-μm-thick slices at 32.0 ± 1.0°C. Neurons were voltage-clamped at −70 mV, and solutions were delivered to slices via superfusion driven by gravity at a flow rate of 1.5 ml/min. The pipette series resistance was monitored throughout the experiments. If the series resistance changed by >20%, the data were discarded. Whole-cell currents were filtered at 1 kHz, digitized at up to 20 kHz, and stored on a microcomputer (Clampex 8 software; MDS Analytical Technologies). All recordings were completed within 4.5 h after slice preparation, mainly because of cell viability of the 300-μm-thick slices. The cells were classified as principal neurons based on the pyramidal shape of their somata. A minor portion (<5%) of recorded neurons exhibited spontaneous EPSCs with a faster decay time and larger amplitude (>100 pA), typical characteristics of interneurons in the LA (Mahanty and Sah, 1998), and were excluded from analysis (Kim et al., 2007).
Statistical analyses.
The data from each neuron/slice were treated as independent samples. In all experiments with behaviorally trained rats, the data include samples from three or more animals. Comparisons of data points between behaviorally trained groups were performed using unpaired t test, one-way ANOVA (between-group comparisons by Newman–Keuls posttest) or repeated-measures ANOVA (between-group comparisons by Bonferroni's posttest). In several experiments, a paired t test was used to determine whether synaptic responses after the induction of plasticity differed significantly from baseline responses, and an unpaired t test was used to test the effect of drugs or behavioral manipulation. Values of p < 0.05 were considered significant. All values are expressed as mean ± SEM.
Results
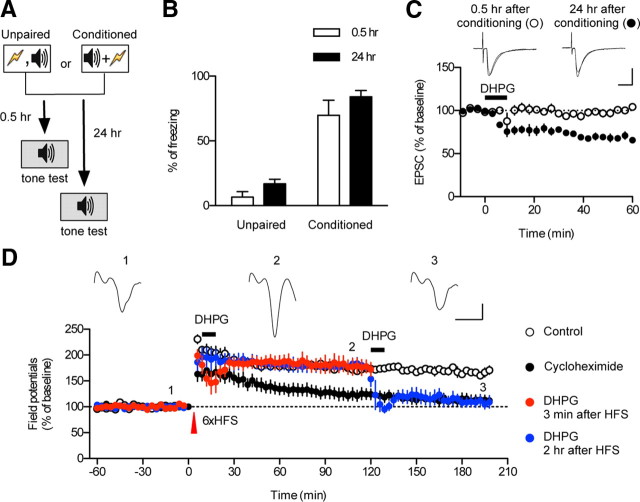
To establish a correlation between synaptic plasticity and reconsolidated memory, it is important to set up a behavioral paradigm and time-matched brain slice preparation to monitor synaptic transmission. We first set up a reproducible and consistent behavioral protocol for ex vivo electrophysiological studies (Fig. 1A,B). Three pairings of tone and shock consistently produced robust freezing monitored either 0.5 h (short-term memory) or 24 h (long-term memory) after conditioning, whereas unpairing of tone and shock failed to do so. We also used the same protocol for experiments using cannulated rats (see Fig. 4). We randomly chose animals for electrophysiological recordings from each group (one set of trained rats was tested for conditioned freezing, whereas another set was killed to prepare brain slices).
Figure 1.
Application of DHPG produces significant depression of excitatory synaptic transmission at T–LA synapses in slices prepared from fear-conditioned rats after fear memory consolidation, but not before. A, The behavioral procedure for the experiments. Freezing was tested at 0.5 or 24 h after conditioning or unpairing. The white and gray colors in the rectangles represent different contexts. B, Freezing responses in each group. Error bars indicate SEM. C, Application of DHPG induced significant depression at T–LA synapses in slices prepared 24 h after conditioning (filled circle), but not in slices prepared 0.5 h after conditioning (open circle). Representative paired traces were an average of four traces before and after DHPG application. Calibration: 10 ms, 50 pA. D, Application of DHPG 2 h after HFS, but not 3 min after HFS, produced significant depression of T–LA synaptic transmission. L-LTP was successfully induced by six trains of HFS (open circle). Application of cycloheximide impaired the persistent maintenance of L-LTP (filled circle). Application of DHPG 2 h after HFS produced an apparent depotentiation of L-LTP (blue circle). Application of DHPG 3 min after HFS, however, had no significant effects on L-LTP (red circle). Representative traces were an average of four traces at each point. Calibration: 5 ms, 0.2 mV.
mGluRI-depotentiation as a marker of consolidated potentiation
Almost all forms of depotentiation have been reported to reverse long-term potentiation (LTP) before consolidation, but not after (Arai et al., 1990; Fujii et al., 1991; O'Dell et al., 1994; Stäubli and Chun, 1996; Xu et al., 1998; Woo and Nguyen, 2003; Zhou et al., 2003; Zhou and Poo, 2004). Based on these findings, it has been proposed that potentiated synapses may have a grace period during which false potentiation can be corrected with a depotentiation mechanism (Zhou et al., 2003). In our previous study, we reported mGluRI-depotentiation in rats in which memory was consolidated (Kim et al., 2007). Therefore, in this study we questioned whether DHPG-induced mGluRI-depotentiation would reverse fear conditioning-induced synaptic potentiation at T–LA synapses in slices prepared before or after consolidation of fear memory. Because consolidation of fear memory or its trace is known to be completed within 24 h (Schafe et al., 2005), we prepared slices 0.5 and 24 h after fear conditioning as preconsolidation and postconsolidation groups, respectively. Application of R,S-DHPG (100 μm; 10 min) or S-DHPG (50 μm; 10 min) induced significant depression of EPSC amplitude at T–LA synapses in slices prepared 24 h after conditioning (filled circle; n = 6; 67.77 ± 2.90% of baseline; paired t test, p < 0.0001), but it had no significant effects on T–LA synaptic transmission in slices prepared 0.5 h after conditioning (open circle; n = 4; 100.5 ± 2.6% of baseline; paired t test, p > 0.8) (Fig. 1C). To test the possibility that different stress levels triggered by the shocks during conditioning may be responsible for the observed effects of DHPG, we examined whether unpaired animals would show a significant depotentiation at the two time points (0.5 and 24 h). We found no significant depotentiation in the two time points tested (0.5 h after unpairing, n = 5, 94.0 ± 3.0% of baseline; paired t test, p > 0.1; 24 h after unpairing, n = 4, 98.6 ± 5.89% of baseline; paired t test, p > 0.8) (data not shown). In addition, we determined whether pp-LFS, which was previously shown to produce a depotentiation of conditioning-induced potentiation in T–LA pathways after consolidation (Kim et al., 2007), would reproduce the observed effect of DHPG. pp-LFS, however, induced a significant depression of field potentials in T–LA pathways in slices prepared 5 min after conditioning (n = 4; 70.1 ± 2.1% of baseline; paired t test, p < 0.001) (data not shown), making the pp-LFS-induced depotentiation not a useful marker of consolidated T–LA synapses. This result is consistent with the idea that pp-LFS may recruit multiple plastic mechanisms compared with DHPG (Cho et al., 2000; Cho and Bashir, 2002). Together, DHPG-induced mGluRI-depotentiation appears to be specific for consolidated potentiation, which is in marked contrast to conventional forms of depotentiation.
To corroborate this unexpected differential effect of DHPG on synaptic strength in fear-conditioned animals, we examined the effects of DHPG on LTP before and after LTP consolidation in naive animals. As described in previous studies (Lee et al., 2002), we could successfully induce late-phase LTP at T–LA synapses with six trains of 100 Hz high-frequency stimulation (HFS) with 1 s duration (open circle; n = 4; 167.6 ± 5.6% of baseline) (Fig. 1D). The same HFS produced a decremental form of long-term potentiation in the presence of cycloheximide, a protein synthesis inhibitor, returning to baseline <2 h after HFS (filled circle; n = 8; 112.9 ± 9.8% of baseline) (Fig. 1D). This is consistent with a previous finding that blockade of protein synthesis impairs LTP consolidation in this pathway (Huang et al., 2000). We chose two time points (3 min or 2 h) after HFS when potentiation persisted or returned to baseline in the presence of cycloheximide, respectively, and these time points were used to examine differential effects of DHPG on potentiated synaptic transmission before and after LTP consolidation. Application of DHPG 3 min after the HFS produced no significant change (red circle; n = 4; 178.97 ± 14.37%; unpaired t test, p > 0.7) (Fig. 1D), whereas DHPG induced a long-lasting depression when applied 2 h after HFS (blue circle; n = 4; 110.55 ± 9.23%; unpaired t test, p < 0.05) (Fig. 1D). This finding indicates that DHPG depresses potentiated responses only after LTP consolidation, consistent with the result shown in Figure 1C. Together, these data show that mGluRI-depotentiation is induced only after consolidation of either fear memory traces or LTP, which led us to use this mGluRI-depotentiation as a marker of consolidated T–LA synapses.
DHPG failed to induce mGluRI-depotentiation in slices prepared immediately after memory reactivation
Having established a new marker of consolidated potentiation, we next examined whether memory reactivation would render consolidated T–LA synapses labile or deconsolidated. Because a labile memory after reactivation is reconsolidated over time, we estimated both lability of a reactivated memory and magnitude of mGluRI-depotentiation as a function of time after reactivation and searched for a relationship between these two parameters. Lability of a reactivated memory was estimated by monitoring the effectiveness of the protein synthesis inhibitor anisomycin, which was microinjected into the LA after memory reactivation (30 s tone presentation). We used anisomycin mainly because it has been a standard drug to block memory reconsolidation in the previous studies, and use of anisomycin gave us an opportunity to compare our data relative to those previous results that had been widely accepted. Anisomycin or vehicle was microinjected into the LA immediately, 1 h, or 6 h after memory reactivation. Freezing was assessed 24 h after memory reactivation. Microinjection of anisomycin immediately after reactivation impaired fear memory compared with vehicle-injected controls (Bonferroni's posttest, p < 0.001) (Fig. 2A), but the same injection 1 or 6 h after reactivation had no significant effects on a long-term fear memory compared with the vehicle-injected groups (p > 0.05 for all pairs, Bonferroni's posttest) (Fig. 2B,C). This indicates that, under our experimental conditions, a labile memory is reconsolidated within 1 h.
Next, we examined the effects of memory reactivation on DHPG-induced mGluRI-depotentiation. Slices were prepared immediately, 1 h, or 6 h after reactivation of consolidated memory (24 h after conditioning). Although application of DHPG produced mGluRI-depotentiation in slices prepared either 1 or 6 h after memory reactivation (1 h post-reactivation group, n = 4, 77.33 ± 6.73% of baseline, paired t test, p < 0.05; 6 h post-reactivation group, n = 5, 64.31 ± 2.29% of baseline, p < 0.0001), it did not produce mGluRI-depotentiation in slices prepared immediately after memory reactivation (n = 6, 98.59 ± 3.91% of baseline, paired t test, p > 0.7) (Fig. 3A). As a control, we included a no-reactivation control in which rats were exposed to the reactivation context for the same duration as in the reactivation group but without tone presentation. Application of DHPG produced mGluRI-depotentiation in no-reactivation controls when slices were prepared immediately after exposure to the reactivation context (n = 6; 75.30 ± 4.28% of baseline; paired t test, p < 0.0001) (Fig. 3B), indicating that a tone presented during reactivation is critical for the observed effect of reactivation on mGluRI-depotentiation. We then determined whether DHPG application at various time points after slice preparation would alter the magnitude of mGluRI-depotentiation. In slices prepared immediately after reactivation, we did not find any significant differences in the magnitude of mGluRI-depotentiation between the recordings in which DHPG was applied either <2 or >4 h after slice preparation (different slices were used for each recording) (Fig. 3C). Thus, mGluRI-depotentiation was not varied with the DHPG treatment time under our experimental conditions. In addition, to rule out the possibility that different stress levels triggered by tone presentation may be responsible for the observed effects of DHPG, we tested whether unpaired animals would show a significant depotentiation immediately or 1 h after tone presentation. We found no significant depotentiation in the two time points tested (immediately after tone presentation, n = 5, 94.7 ± 4.4% of baseline, paired t test, p > 0.2; 1 h after tone presentation, n = 5, 98.6 ± 8.0% of baseline, paired t test, p > 0.8) (data not shown).
The lack of mGluRI-depotentiation might be attributable to the absence of residual synaptic potentiation immediately after reactivation. We therefore determined whether reactivation alters T–LA synaptic strength. Synaptic efficacy (measured as the input–output relationship at T–LA synapses) was compared between the naive, no-reactivation, and reactivation group. We failed to find any significant differences in the input–output relationship of T–LA synaptic transmission between the reactivation group and no-reactivation controls when the input–output relationship was estimated either immediately or 1 h after reactivation (Fig. 3D). The input–output relationship in the reactivation group and no-reactivation controls both differed significantly from that in naive controls (Fig. 3D). In short, T–LA synaptic strength appears not to be altered when measured immediately or 1 h after reactivation. Thus, the insensitivity to mGluRI-depotentiation immediately after reactivation is most likely attributable to temporary deconsolidation or lability of consolidated T–LA synapses.
DHPG failed to induce mGluRI-depotentiation in slices prepared 1 h after memory reactivation when reconsolidation was blocked
If mGluRI-depotentiation marks the reconsolidated memory, it would be expected not to be observed for an extended time window when reconsolidation is blocked by anisomycin. To test this prediction, two controls were included: vehicle-injected controls (vehicle injection after memory reactivation) and anisomycin-injected controls (anisomycin injection alone without memory reactivation). Anisomycin or vehicle was microinjected into the LA bilaterally. Cannulas were carefully removed after decapitation, and brain slices were prepared. We only used animals in which the tip of the injector cannula was placed correctly within the LA of both hemispheres and made whole-cell recordings from the two coronal slices just anterior and posterior to that containing the tip. Slices were prepared 1 h after memory reactivation (Fig. 4A), when memory reactivation alone had no discernible effects on mGluRI-depotentiation, possibly because of reconsolidation (Fig. 3A, middle). Application of DHPG did not induce depotentiation in slices prepared from the group in which anisomycin was injected immediately after reactivation (red circle; n = 7; 92.37 ± 4.12% of baseline; paired t test, p > 0.1) (Fig. 4B). DHPG produced significant depotentiation, however, in slices prepared from the groups either in which a vehicle was injected immediately after reactivation (open circle; n = 7; 70.07 ± 3.72% of baseline; paired t test, p < 0.05) or in which anisomycin was injected without reactivation (filled circle; n = 8; 67.60 ± 8.81%; paired t test, p < 0.05). Compared with vehicle-injected or anisomycin-injected controls, microinjection of a consolidation blocker, anisomycin, immediately after memory reactivation impaired mGluRI-depotentiation in slices prepared 1 h after reactivation (F(2,19) = 4.52, p < 0.05; Newman–Keuls posttest, p < 0.05 for both pairs) (Fig. 4B). These results indicate that lability or deconsolidation of consolidated T–LA synapses upon reactivation persists when memory reconsolidation is blocked by anisomycin.
Memory- and synapse-specificity of mGluRI-depotentiation
Thus far, we have demonstrated that DHPG induces mGluRI-depotentiation in slices prepared only when fear memory is consolidated. We next tested whether the lack of mGluRI-depotentiation immediately after reactivation was specific to a set of synapses that store the reactivated memory. One possibility is that memory reactivation somehow produces a nonspecific block of depotentiation at all synapses in a given neuron. To address this, we developed a behavioral protocol in which two distinct memories were acquired and maintained. We tested different frequencies of tones for discriminative fear conditioning and found the two tones (2.8 and 20 kHz) that were best discriminated. Rats were conditioned with a 20 kHz tone and on a following day, with a 2.8 kHz tone. As shown in Figure 5A, freezing to the first tone used for fear conditioning (2.8 kHz) on the second day was negligible (filled circle, one-tone reactivation group, 0.99 ± 0.58% of freezing; open circle, two-tone reactivation group, 2.43 ± 0.91% of freezing), indicating that a fear memory associated with the 2.8 kHz tone in the first conditioning was not retrieved by presentation of a 20 kHz tone. A tone test was performed 24 h after the second fear conditioning, and freezing was strong in response to either tone (Fig. 5A).
Using this experimental paradigm, three experimental groups were examined: a no-reactivation group, a one-tone (20 kHz) reactivation group, and a two-tone reactivation group (2.8 kHz and 20 kHz). For the two-tone reactivation group, the two tones were presented sequentially with an interval of 30 s. Slices were prepared immediately after reactivation, when memory reactivation has a maximal effect on mGluRI-depotentiation. If presentation of one of the tones differentially deconsolidates a distinct population of consolidated synapses, DHPG would induce partial depotentiation. Indeed, this prediction was in agreement with our findings. The magnitude of mGluRI-depotentiation in the one-tone reactivation group (81.64 ± 1.60% of baseline) was between that in the no-reactivation controls and in the two-tone reactivation group (69.26 ± 0.96 and 97.93 ± 1.15% of baseline, respectively; F(2,12) = 129.1, p < 0.0001; one-tone reactivation group vs the other group, Newman–Keuls posttest, p < 0.05) (Fig. 5B). These results suggest that the reactivation-induced insensitivity to mGluRI-depotentiation is memory- and synapse-specific.
Impairment of fear memory retention by microinjection of DHPG into the LA
We have reasoned that DHPG-induced mGluRI-depotentiation at T–LA synapses would result in weakening of fear memory. If so, can DHPG be used as an in vivo detector for memory deconsolidation and reconsolidation after reactivation? For this, we first determined whether microinjection of DHPG into the LA would impair retention of consolidated fear memory. The effect of DHPG on fear memory retention, however, would be partial at most because DHPG is known to produce a depotentiation only at T–LA synapses, but not at cortical input synapses onto the LA (Hong et al., 2009), other important synapses for fear memory retention (Tsvetkov et al., 2002; Boatman and Kim, 2006). Therefore, to enhance the detection sensitivity of a fear memory test, we established a moderate conditioning protocol in which relationship between synaptic strength and memory output was far from saturation. Microinjection of DHPG impaired fear memory retention assessed 24 h after the injection relative to vehicle controls (unpaired t test, p < 0.05) (Fig. 6A). We then questioned whether DHPG microinjected into the LA after reactivation would have the same pattern of effects on fear memory retention as DHPG exerted on mGluRI-depotentiation ex vivo. Indeed, DHPG microinjected immediately after reactivation failed to impair fear memory retention (Bonferroni's posttest, p > 0.05) (Fig. 6B), but DHPG microinjected 1 h after reactivation impaired fear memory retention (Bonferroni's posttest, p < 0.05) (Fig. 6C). Thus, the effect of DHPG microinjected after reactivation on fear memory retention correlates with the state of memory deconsolidation and reconsolidation estimated either with the anisomycin injection (Fig. 2) or with mGluRI-depotentiation (Fig. 3).
Discussion
In the present study, we have demonstrated that DHPG produces ex vivo depotentiation (mGluRI-depotentiation) at synapses in which synaptic strength is consolidated by fear conditioning or LTP, but not at synapses in which synaptic strength is not consolidated. Thus, the presence of mGluRI-depotentiation appears to be a reliable marker of consolidated synapses, whereas the absence seemingly reflects labile synapses. This is further evidenced by the observation that DHPG fails to produce mGluRI-depotentiation in slices prepared immediately after reactivation when the reactivated memory is labile, whereas it induces mGluRI-depotentiation in slices prepared after reconsolidation (1 or 6 h after reactivation). When reconsolidation is blocked by anisomycin, DHPG fails to produce mGluRI-depotentiation in slices prepared 1 h after reactivation. Furthermore, mGluRI-depotentiation is memory- and synapse-specific, as evidenced by the observation that mGluRI-depotentiation is partially impaired when acquired memories are partially reactivated. In support of the ex vivo results obtained with mGluRI-depotentiation, DHPG impairs fear memory retention when microinjected into the LA 1 h after reactivation, but not immediately after reactivation. Together, our findings are consistent with temporary deconsolidation of the consolidated T–LA synapses upon memory reactivation.
Our experiments provide evidence that the timing of brain slicing, not the DHPG application to the slice, is correlated with our behavioral results as evidenced by the results of DHPG mircroinjection experiment (Figs. 3, 6). These results indicate that synaptic properties that are assessed in brain slices reflect the in vivo synaptic state at the timing of brain slicing. In other words, synaptic changes that have occurred in vivo appear to halt at the time of brain slicing. In support of this idea, the absence of mGluRI-depotentiation in the slices prepared immediately after reactivation persists over 4 h after brain slicing (Fig. 3C). The reconsolidation would have been completed by this period time if it was allowed in vivo (Figs. 2, 6). One possible explanation for this phenomenon is that synaptic deconsolidation and reconsolidation require functional connections from other brain regions that are severed during brain slicing. Support for this idea comes from the previous reports that the consolidation/reconsolidation of amygdala-based memories may require the activity of other brain regions such as the medial prefrontal cortex, locus ceruleus, and nucleus of the solitary tract (Asan, 1998; McGaugh, 2003; Miyashita and Williams, 2003; Debiec and LeDoux, 2004; Hassert et al., 2004; Santini et al., 2004; Rodrigues et al., 2009; Amano et al., 2010).
A battery of previous studies has supported the hypothesis that consolidated memory traces are temporarily labile and reconsolidated over time (Nader et al., 2000; Tronson et al., 2006; Boccia et al., 2007). Importantly, Doyère et al. (2007) have shown that auditory-evoked field potentials in the LA are decreased by injection of another consolidation blocker, U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], immediately after fear memory reactivation in an input-specific manner. In particular, ubiquitin-dependent proteolysis of synaptic proteins upon reactivation has been shown to be critical to induce the lability of the reactivated memory (Lee et al., 2008). This study provides strong evidence for the prevailing hypothesis since the involvement of proteolysis in the lability of a reactivated memory necessitates protein synthesis for the reconsolidation of a reactivated memory. Our findings are consistent with these previous reports and provide additional evidence of synaptic correlates for labile memories after reactivation.
Doyère et al. (2007) have shown that reactivation of a fear memory produces an additional potentiation in auditory-evoked field potentials 3 h after reactivation. We have tested the possibility of reactivation-induced potentiation at T–LA synapses, but at least at T–LA synapses, reactivation-induced potentiation does not seem to play a role. This is evident in our results shown in Figure 3D in which we failed to find any significant differences in input–output relationship of T–LA synaptic transmission between the reactivation group and no-reactivation control when the input–output relationship was estimated either immediately or 1 h after reactivation. The reactivation-induced potentiation of auditory-evoked field potentials, however, could arise from other sources [e.g., plastic changes at cortical input synapses (Tsvetkov et al., 2002; Boatman and Kim, 2006), plastic changes of auditory receptive fields in the MGm/PIN (Weinberger, 1995), etc.].
Although a prevailing hypothesis for memory reactivation and reconsolidation has been generally accepted, there have been several challenges to this hypothesis. First, the widely used protein synthesis inhibitor anisomycin has been shown to exhibit undesirable side effects; anisomycin injection after memory reactivation produces amnesia by enhancing the release of other neurotransmitters such as norepinephrine and dopamine rather than by inhibiting de novo protein synthesis (Canal and Gold, 2007; Canal et al., 2007; Gold, 2008; Qi and Gold, 2009). Second, reconsolidation blockers with memory reactivating stimuli somehow induce synaptic malfunctioning, a reduction in cell excitability, or even cell death (Iordanov et al., 1997, 1998; Curtin and Cotter, 2002; Zhang et al., 2007; Rosenkranz et al., 2009). Finally, memory reactivation may not alter the memory trace itself but may activate inhibitory circuits that quench original memory traces (Alberini, 2008). By directly assaying synaptic status, our study bypasses these side effects of pharmacological interference and provides compelling evidence for the presence of labile and reconsolidated synapses within the excitatory neural circuit supporting fear memory.
In the CA1 region of the hippocampus, it has been reported that application of DHPG induces depotentiation by internalization of surface AMPA receptors (Zho et al., 2002). In marked contrast to our findings, this hippocampal mGluRI-depotentiation appears to reverse HFS-induced LTP only up to 3 min after HFS, but not at later time points after HFS, which is the opposite for our results in amygdala T–LA synapses (compare with Fig. 1D). These opposing results in two different brain regions suggest that the mechanisms underlying mGluRI-depotentiation might be specific to subregions of the brain and that the absence of mGluRI-depotentiation 3 min after HFS in our study may not be attributable to the general mechanisms associated with HFS, including glutamate receptor desensitization or inactivation.
Currently, it is unclear why mGluRI-depotentiation only targets consolidated potentiation. Recent studies have provided evidence that PKMζ is a critical molecule for maintaining a consolidated memory and that the synaptic level of PKMζ proteins determines memory strength (Drier et al., 2002; Pastalkova et al., 2006). Indeed, an inhibitor of PKMζ [ζ inhibitory peptide (ZIP)] has been shown to impair consolidated memories including a fear memory (Shema et al., 2007; Serrano et al., 2008). Furthermore, ZIP impairs conditioned taste aversion associations only when microinjected after memory consolidation, but not before (Shema et al., 2009); this finding is reminiscent of the DHPG effects on fear memory retention in this study. One possible scenario is that activation of mGluRI somehow reduces synaptic expression of PKMζ proteins, which leads to a reduction in both synaptic strength and memory retention.
Alternatively, the composition of postsynaptic AMPA receptors may confer sensitivity to mGluRI-depotentiation. Previous results from the ventral tegmental area have shown that cocaine-induced insertion of GluR1 homomeric AMPA receptors is a prerequisite for mGluR-dependent long-term depression (Bellone and Lüscher, 2006). It may be the case that particular configurations of AMPA receptors required for mGluR-depotentiation are gradually inserted into synapses during memory consolidation. Consolidated synapses may be supported by AMPA receptors that are prone to DHPG- or synaptic activity-induced endocytosis. Intriguingly, memory reactivation has been shown to involve a dynamic exchange of AMPARs at amygdala synapses (Ben Mamou and Nader, 2008), which may explain the temporary insensitivity to mGluRI-depotentiation upon reactivation.
In the present study, we developed a novel marker for consolidated amygdala synapses, namely DHPG-induced mGluRI-depotentiation and impairment of fear memory retention, helping to characterize a change in synaptic properties during the process of memory reactivation. This new assay may be useful to pinpoint molecules that are critical for maintaining consolidated fear memory in the future studies.
Footnotes
This work was supported by Brain Research Center of the 21st Century Frontier Research Program Grant 2009K001261 funded by the Korean Ministry of Science and Technology, and Korea Research Foundation Grant KRF-2007-313-C00679 funded by the Korean Ministry of Education and Human Resource Development (S.C.); by Korea Research Foundation Grant 353-2009-2-C00067 funded by the Korean Government (S.L.); and by Brain Korea 21 Research Fellowships from the Korean Ministry of Education (Jeongyeon Kim, B.S., I.H., Jihye Kim, J.L., S.P.).
References
- Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Arai A, Larson J, Lynch G. Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Res. 1990;511:353–357. doi: 10.1016/0006-8993(90)90184-d. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Nader K. The dynamic nature of memory retrieval: active NMDAR-regulated trafficking of AMPARs in the amygdala, during expression of emotional memory. Soc Neurosci Abstr. 2008;34:295–20. [Google Scholar]
- Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- Boccia M, Freudenthal R, Blake M, de la Fuente V, Acosta G, Baratti C, Romano A. Activation of hippocampal nuclear factor-κB by retrieval is required for memory reconsolidation. J Neurosci. 2007;27:13436–13445. doi: 10.1523/JNEUROSCI.4430-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Gold PE. Different temporal profiles of amnesia after intra-hippocampus and intra-amygdala infusions of anisomycin. Behav Neurosci. 2007;121:732–741. doi: 10.1037/0735-7044.121.4.732. [DOI] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci U S A. 2007;104:12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Bashir ZI. Cooperation between mglu receptors: a depression mechanism? Trends Neurosci. 2002;25:405–411. doi: 10.1016/s0166-2236(02)02228-2. [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Curtin JF, Cotter TG. Anisomycin activates JNK and sensitises DU 145 prostate carcinoma cells to Fas mediated apoptosis. Br J Cancer. 2002;87:1188–1194. doi: 10.1038/sj.bjc.6600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Gold PE. Protein synthesis inhibition and memory: formation vs amnesia. Neurobiol Learn Mem. 2008;89:201–211. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Hong I, Song B, Lee S, Kim J, Kim J, Choi S. Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur J Neurosci. 2009;30:2089–2099. doi: 10.1111/j.1460-9568.2009.07004.x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Delgado-García JM, Carrión AM. Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J Neurosci. 2005;25:2070–2080. doi: 10.1523/JNEUROSCI.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Magun BE. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J Biol Chem. 1998;273:15794–15803. doi: 10.1074/jbc.273.25.15794. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee O, Lee CJ, Choi S. Induction mechanisms for L-LTP at thalamic input synapses to the lateral amygdala: requirement of mGluR5 activation. Neuroreport. 2002;13:685–691. doi: 10.1097/00001756-200204160-00030. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory and emotion. The making of lasting memories. London: Weidenfeld and Nicolson; 2003. [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Enhancement of noradrenergic neurotransmission in the nucleus of the solitary tract modulates memory storage processes. Brain Res. 2003;987:164–175. doi: 10.1016/s0006-8993(03)03323-7. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Systems-level reconsolidation: reengagement of the hippocampus with memory reactivation. Neuron. 2002;36:340–343. doi: 10.1016/s0896-6273(02)01017-6. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: contribution of increased release of norepinephrine, dopamine, and acetylcholine. Learn Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Frick A, Johnston D. Kinase-dependent modification of dendritic excitability after long-term potentiation. J Physiol. 2009;587:115–125. doi: 10.1113/jphysiol.2008.158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Doyère V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25:10010–10014. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci. 2003;23:1125–1132. doi: 10.1523/JNEUROSCI.23-04-01125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cai G, Ni X, Sun J. The role of ERK activation in the neuronal excitability in the chronically compressed dorsal root ganglia. Neurosci Lett. 2007;419:153–157. doi: 10.1016/j.neulet.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Zho WM, You JL, Huang CC, Hsu KS. The group I metabotropic glutamate receptor agonist (S)-3,5-dihydroxyphenylglycine induces a novel form of depotentiation in the CA1 region of the hippocampus. J Neurosci. 2002;22:8838–8849. doi: 10.1523/JNEUROSCI.22-20-08838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]