Abstract
Odor identity is coded in spatiotemporal patterns of neural activity in the olfactory bulb. Here we asked whether meaningful olfactory information could also be read from the global olfactory neural population response. We applied standard statistical methods of dimensionality-reduction to neural activity from 12 previously published studies using seven different species. Four studies reported olfactory receptor activity, seven reported glomerulus activity, and one reported the activity of projection-neurons. We found two linear axes of neural population activity that accounted for more than half of the variance in neural response across species. The first axis was correlated with the total sum of odor-induced neural activity, and reflected the behavior of approach or withdrawal in animals, and odorant pleasantness in humans. The second and orthogonal axis reflected odorant toxicity across species. We conclude that in parallel with spatiotemporal pattern coding, the olfactory system can use simple global computations to read vital olfactory information from the neural population response.
Introduction
In the olfactory system, several odors may be coded by specific olfactory receptors or glomeruli that are alone sufficient to generate an innate response (Suh et al., 2004; Kobayakawa et al., 2007; Kurtovic et al., 2007; Semmelhack and Wang, 2009). Whereas this labeled-line coding scheme is clearly applicable to some odors, it is unlikely to be the general rule, as it does not scale to the millions of discernable odors. These are likely represented by temporally evolving patterns of activity across a large population of olfactory neurons, either receptors or glomeruli (Friedrich and Korsching, 1997; Hildebrand and Shepherd, 1997; Malnic et al., 1999; Rubin and Katz, 1999; Ma and Shepherd, 2000; Uchida et al., 2000; Belluscio and Katz, 2001; Firestein, 2001; Laurent et al., 2001; Meister and Bonhoeffer, 2001; Spors and Grinvald, 2002; Leon and Johnson, 2009). The clear advantage of spatiotemporal pattern coding is that the large number of olfactory receptor types (Buck and Axel, 1991) provides a vast coding space where odorants can be discriminated despite differing by only minute molecular properties. How patterns in this extremely high dimensional space are translated into practical olfactory decisions, however, is not well understood (Mainen, 2006).
Here we hypothesized that in parallel with labeled-line and spatiotemporal combinatorial representations in olfaction, the olfactory system may also rely on simple features of neural population activity to reduce the high dimensionality of olfactory space and extract meaningful information. Several studies have demonstrated that dimensionality-reduction, whether simple (Scott and Mark, 1987; Laubach et al., 1999) or sophisticated (Friston et al., 1993; Aflalo and Graziano, 2006), may provide viable approximations of brain-calculations applied to neural ensembles (McClurkin et al., 1991). Perhaps the simplest approach to dimensionality reduction is Principal Component Analysis, where an n-dimensional space is linearly transformed into a set of ordered orthogonal axes such that the first axis (PC1) captures the maximal variability in the data, and each successive axis captures the maximal remaining variability (Methods and Supporting Section 1). Guided by studies of other sensory modalities (Chapin and Nicolelis, 1999), here we applied PCA to each of 12 previously published datasets reporting the olfactory neural response of seven species to multiple odorants (Table 1), and asked whether the main PCA axes of the neural olfactory space were correlated with olfactory behavior and perception.
Table 1.
The list of datasets used in this analysis and their parameters
| ID | Reference | Species | No. of odors | No. of neurons | Neuron type | Measurement method | Dilution |
|---|---|---|---|---|---|---|---|
| 1 | Hallem and Carlson (2006) | Drosophila | 110 | 24 | OR | Spike recording | 1/100 in mineral oil |
| 2 | Kreher et al. (2008) | Larva | 27 | 21 | OR | Spike recording | 1/100 in mineral oil |
| 3 | Bhandawat et al. (2007) | Drosophila | 18 | 7 | OR | Spike recording | 1/100 in mineral oil |
| 4 | Bhandawat et al. (2007) | Drosophila | 18 | 7 | PN | Spike recording | 1/100 in mineral oil |
| 5 | Manzini et al. (2007) | Tadpole | 13 | 67 | GLO | Imaging | 200 μm |
| 6 | Friedrich and Korsching (1997) | Zebrafish | 17 | 14 | GLO | Imaging | 10 μm |
| 7 | Takahashi et al. (2004a) | Rat | 35 | 53 | GLO | Imaging | 1/50 in mineral oil |
| 8 | Takahashi et al. (2004b) | Rat | 61 | 84 | GLO | Imaging | Acids, diketones, ketones, 1/10; aldehydes 1/50 in mineral oil |
| 9 | Uchida et al. (2000) | Rat | 35 | 30 | GLO | Imaging | As above |
| 10 | Saito et al. (2009) | Human and mouse | 62 | 63 | OR | EC50 | N/A |
| 11 | Soucy et al. (2009) | Mouse | 100 | 137 | GLO | Imaging | 1/100 in mineral oil |
| 12 | Wang et al. (2003) | Drosophila | 16 | 23 | GLO | Imaging | 10% SV |
SV, Saturated vapor concentration; OR, olfactory receptor; PN, projection neuron; GLO, glomeruli; N/A, not applicable.
We found that the first two principal components of the olfactory neural population response accounted for more than half of the variance in neural activity. Furthermore, these two axes predicted behavior and perception across species. The first axis predicted the behavior of approach or withdrawal in animals, as well as the perception of odorant pleasantness in humans. The second axis predicted odorant toxicity across species. These findings suggest that vital olfactory information may be extracted reliably and fast by simple linear computations on the neural population response.
Materials and Methods
General
Neural response datasets (Table 1), vapor pressure values (supplemental Table 5, available at www.jneurosci.org as supplemental material), behavioral odor response data (supplemental Table 1, available at www.jneurosci.org as supplemental material) and LD50 scores (supplemental Table 3, available at www.jneurosci.org as supplemental material) were obtained from published sources or from the original authors. Our inclusion criteria for PCA derivation was datasets with >12 neurons and 12 odorants, to allow correlation analysis. We augmented this with two datasets that were smaller (datasets 3 and 4), but the authors provided us the raw data, allowing in depth analysis. Similarly, our inclusion criteria for correlation with behavior was at least 12 odors with behavioral data. One dataset was excluded (Sachse et al., 1999) because its PC1 alone accounted for 68% of the variance, suggesting that from the point of view of our analysis the information in this dataset was limited (note that including this dataset would have strengthened our later results). This manuscript revolves around the use of PCA analysis, here conducted using Matlab. For a detailed example of applying PCA to this type of data, see supplemental materials section 1. Unless stated otherwise, we used the Spearman correlation formula to calculate correlations.
Neural responses
All neural responses were taken from the published papers. In cases where the data were not fully detailed, we contacted the authors to obtain the data directly. In dataset 10, the total neural response was estimated by the number of activated receptors. This was done following the author's advice (J. Mainland, personal communication). If using the published EC50 values instead, the later result was in fact slightly stronger in the same direction.
Normalization
To normalize the total neural response we counted the number of olfactory neurons that were excited or inhibited. For datasets 1 and 2 we used the threshold defined by the authors (50 spikes/s for excitation). We did not normalize the total response of datasets that reported the neural response as a color-coded table (datasets 7–10).
Simulation
To simulate the response of a population of olfactory neurons to different levels of odor concentration (see Fig. 8) we generated 30 olfactory neurons with different tuning curves as in Figure 8 B. We assumed two very responsive neurons (225 ± 10 spike/s), three high response neuron (175 ± 10 spike/s), five with moderate response (125 ± 10 spike/s) and the rest with weak response (i.e., 75 + 10) that are recruited gradually as the concentration increases.
Figure 8.
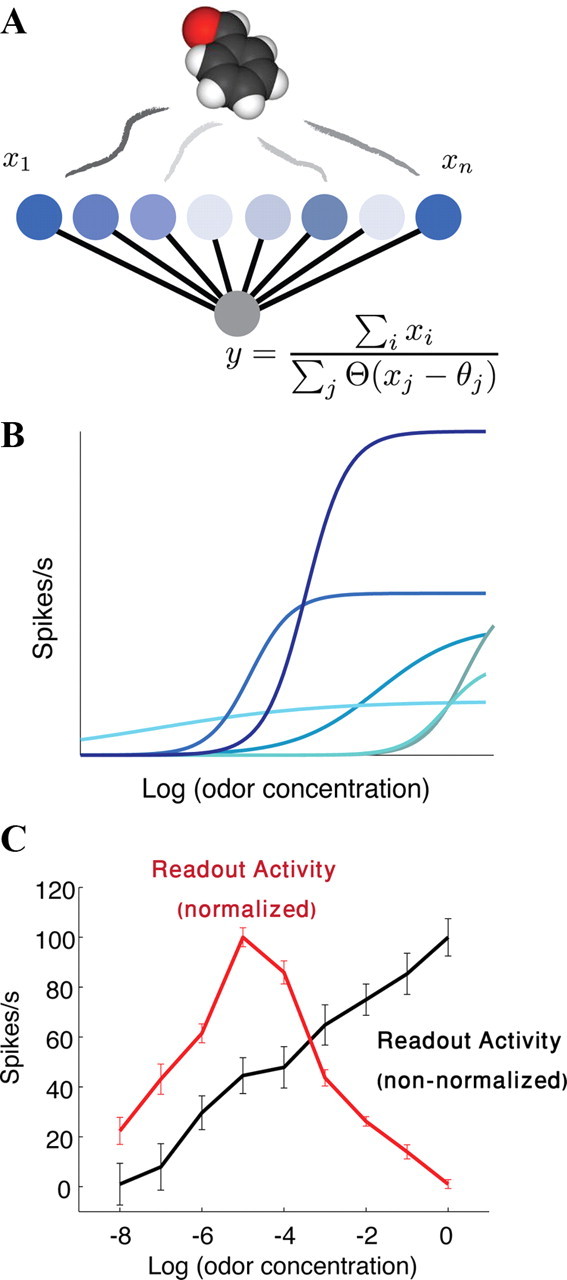
A normalized readout model for a population of olfactory neurons. A, Olfactory neurons (x_1,x_2,...,x_n), responding to a presented odor, according to their individual receptive field properties (in different shades of blue), send their projections to a readout neuron, y (in gray). Neuron y calculates the weighted sum of all neurons converging to it, which is then normalized by the number of neurons exceeding a threshold. θ is the Heaviside function, and θi is the individual threshold for each neuron. B, A schematic of the neural response curves of the x i neurons, as a function of the odorant concentration. C, We simulated a population of 30 receptor neurons (Fishilevich et al., 2005) with different noisy tuning curves (see Materials and Methods). The activity of the normalized response sum readout model (red) increases with concentration, and then decreases above a certain concentration value—where sensitive receptors saturate, and receptors that are weakly tuned for this odor are now recruited (and contribute to the normalization term). For comparison, we show the non-normalized response sum (black), whose response is monotonic in the odor concentration. Error bars are SE values of 30 repeated simulations of the neural response.
Human estimates
Subjects.
Eighteen healthy normosmic subjects (12 females) ranging in age from 23 to 40 years participated in the study after providing informed consent to procedure approved by the Helsinki committee.
Odorant ratings.
All experiments were conducted in a room specially designed for olfaction experiments. The walls of this room are coated in stainless steel to prevent odor adhesion, and the room is supplied by dedicated Carbon and HEPA filters. All interaction with subjects was by computer-generated digital voice. Each subject ranked the pleasantness and intensity of each odorant on a visual analog scale (VAS). Each odorant was presented twice to each subject. In total, we had an average of 25 ratings per odorant, as a few subjects did not want to rate for the second time. Odorants were diluted 1/100 in mineral oil. The pleasantness of an odorant was calculated by taking the median of all subjects' average ratings.
Deriving PC2 of human perception
We applied PCA to the data from Dravnieks' (1985) Atlas of Odor Character Profiles, wherein ∼150 experts (perfumers and olfactory scientists) ranked (from 0 to 5, reflecting “absent” to “extremely” representative—supplemental Fig. 2, available at www.jneurosci.org as supplemental material) 160 odorants (144 monomolecular species, and 16 mixtures) against each of the 146 verbal descriptors. The methods we used here to calculate PC2 of perception have been used by us before to calculate PC1 of perception (Khan et al., 2007), and a perceptual space derived of these PCs can be navigated at the odor space link at http://www.weizmann.ac.il/neurobiology/worg/.
Results
To identify simple informative features of the olfactory neural code, we applied PCA to the neural response data from 12 experiments that studied seven species (Table 1). Four of these studies contained data from olfactory receptor neurons, and seven studies contained data from glomeruli. Notably, olfactory system architecture implies that the receptive range of a glomerulus typically reflects the receptive range of a single receptor type (Mombaerts et al., 1996). Dataset 4 contained data from projection neurons.
The principal axis of olfactory neural responses was strongly related to the total neural response
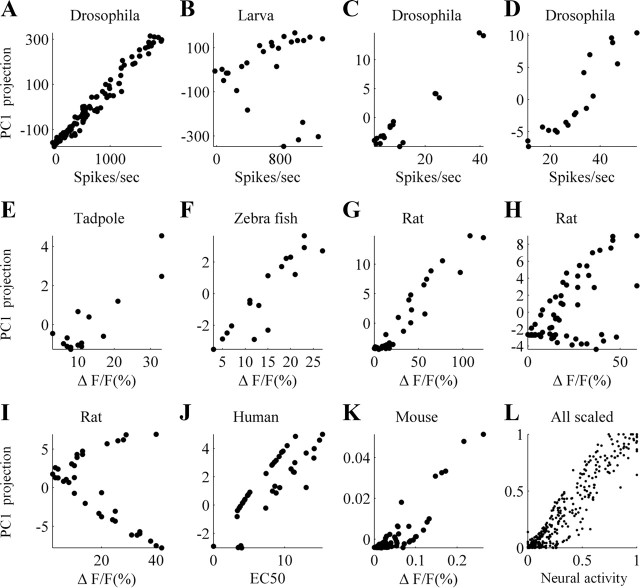
In nine of the 12 datasets we analyzed, we found a strong correlation between PC1 of neural response space and the summed activity of the sampled population, whether spike rates or optical signal (ΔF/F), with r values ranging between 0.73 and 0.98 (all p < 0.001; Fig. 1 A,C–G,J,K). In the other three datasets (Fig. 1 B,H,I), the relation between PC1 and total response bifurcated into two branches, where each branch reflected the response to odors from a restricted chemical group (aromatics in dataset 2; alkyl aldehyde, acids and aromatic in dataset 8; aldehydes, acids and two esters in dataset 9). Considering each branch separately, the generally observed high correlation between the first PC and the total neural response was retained (r > 0.7, p < 0.01 in all three datasets; see supplemental Fig. 1, available at www.jneurosci.org as supplemental material). We note that these strong correlations were evident despite the differences across species and the variety of recording methods used (Table 1, last three columns).
Figure 1.
The primary axis of neural population activity was highly correlated with the total neural response. A–K, Correlation between the projection on PC1 and the sum of total neural activity. Every dot represents one odor. L, Overlay of all data from A–K. Both total activity and projection values were normalized to the range [0,1]. (For clarity, we show in L only the upper branch of the data from B, H, and I.)
The strong relation between PC1 and total neural sum was not a byproduct of PCA
The relation between PC1 and total neural sum was not imposed by PCA: Randomly shuffling each odorant's response pattern did not influence the relation between PC1 and total neural response sum. Conversely, randomly shuffling each neuron's response value did negate the relation between PC1 and total neural response (average r = 0, average p = 0.5; Fig. 2 A).
Figure 2.
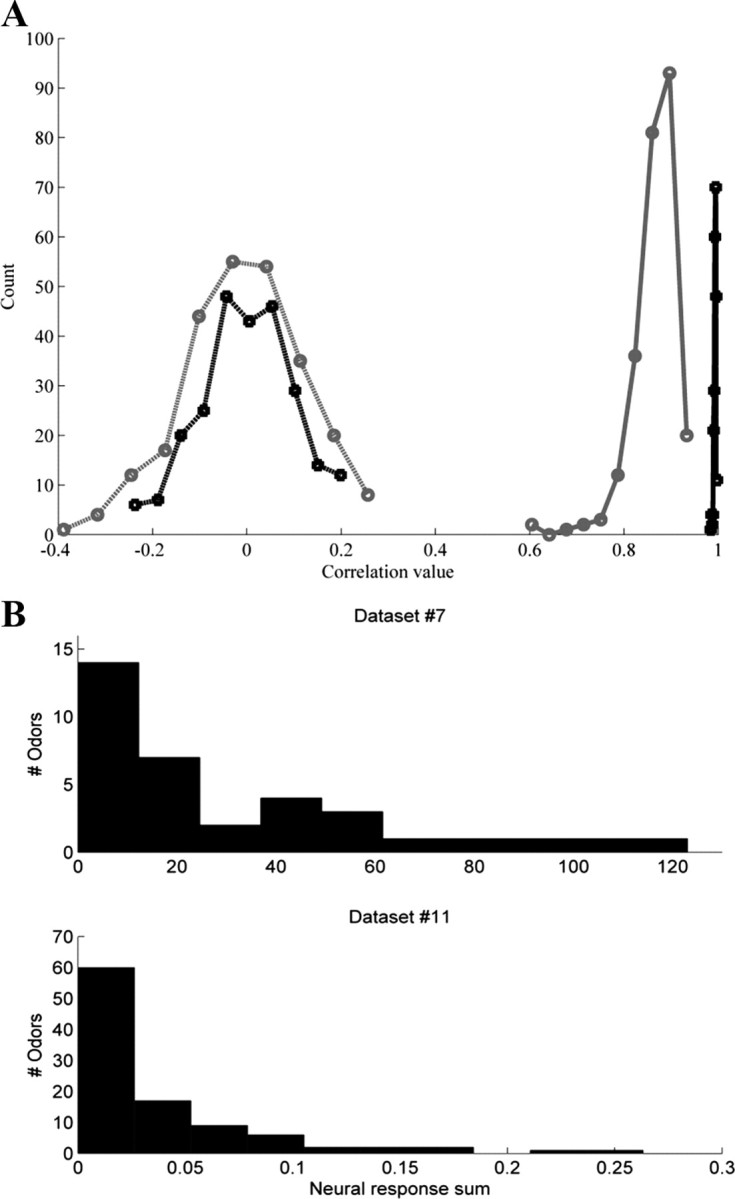
The strong relation between PC1 and total neural sum was not a byproduct of PCA. A, Two examples of correlation distributions between PC1 and total neural sum when we randomly shuffled the dataset's values. Black, Dataset 1; gray: datasets 10. Solid line, Correlation distribution when we permuted the neural response of each odor (i.e., the total neural response elicited by each odor did not change). Dashed line, Correlation distribution when we permuted each neuron's response (i.e., the total neural response that each odor elicited did change). The average correlation in this shuffling analysis was centered on zero. Correlations were calculated using Pearson correlation. B, Total neural response reflected an exponential distribution.
To better understand what type of response distribution may have generated the observed relation between PC1 and the total neural response, we further explored individual properties of the different datasets. Because in several datasets the distribution of the total neural response was exponential (Fig. 2 B), we simulated an odor response matrix such that each odor activated a different number of receptors according to an exponential distribution, and found that PC1 was highly correlated to the total neural response (average r > 0.98, p < 0.001 in 100 simulations). In contrast, random matrices generated according to several other distributions (Poisson, Normal, Beta, Rayleigh, Weibull, and Uniform distributions with several different parameters and different matrix sizes) yielded correlation values that were distributed normally around zero (with SD ranging from 0.26 to 0.37 in 200 simulations). Together, these results suggested that the relation between PC1 and the total neural response may reflect an exponential neural population activity distribution in olfaction. Notably, this exponential distribution of the total neural response dovetails with the previously identified exponential distribution of specific olfactory receptor responses (Hallem and Carlson, 2006; Carey et al., 2010).
PC1 was unlikely to be a reflection of odor concentration alone
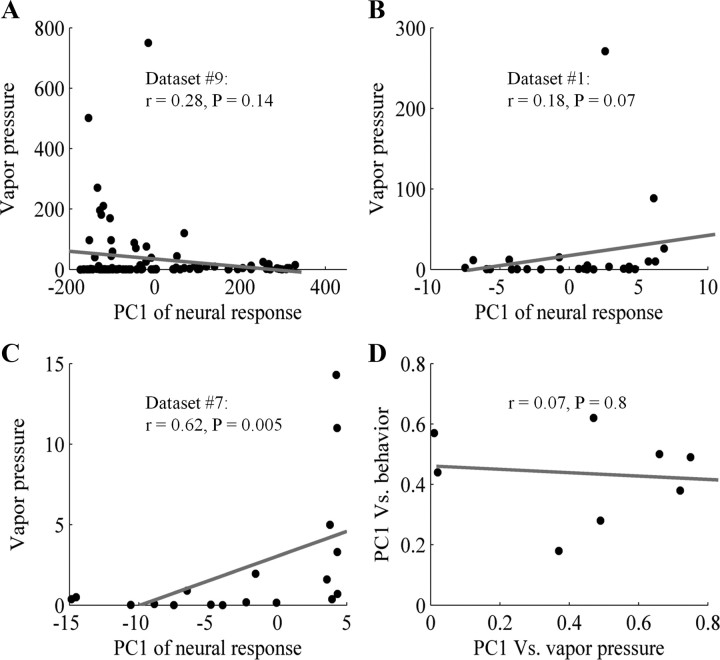
The high correlation between PC1 and the total neural response may suggest that PC1 reflected odorant concentration. We suggest, however, that PC1 captured more than odorant concentration alone. We note in this respect that most of the experiments analyzed here were conducted at equal concentrations, and this should guard against a primary influence of odorant concentration. Still, despite equal concentrations in the liquid phase, different odorant vapor pressures may have resulted in different vapor concentrations at the receptor (Cometto-Muñiz et al., 2003). We therefore tested the relation between odorant vapor pressure (a good indicator of vapor concentration) and PC1 values in nine datasets (datasets 5, 6, and 10 used odors in aqueous phase, and were therefore not used for this analysis). Whereas in four datasets there was a significant correlation between odorant vapor pressure and PC1 (datasets 2, 4, 7 and 11 p < 0.05), in the remaining five datasets we found no correlations (|r| < 0.38, p > 0.07, Fig. 3 A–C). The latter was in agreement with previous results where the vapor concentration of 20 odorants diluted by 1/100 in mineral oil was not correlated with the receptor response (Pelz et al., 2006). Finally, the results obtained later in this manuscript further suggested that PC1 indeed captured more than odorant concentration alone, and this is addressed in the discussion.
Figure 3.
PC1 was unlikely to be a reflection of odor intensity alone. A, B, The correlation between PC1 of neural response and vapor pressure in two different datasets. Each dot shows the odor PC1 value and its vapor pressure in mmHg (supplemental Table 1, available at www.jneurosci.org as supplemental material). C, A third dataset is a case where there was a correlation. D, Correlation between the correlation of PC1 with behavior and PC1 with vapor pressure across datasets.
Redundancy in the olfactory neural response
Our analysis was based on the sampled subset of the animal's total receptor repertoire. While there was no obvious or reported bias in these samples, one may suggest that it may be too small of a sample to reflect the true structure of the olfactory code. We find, however, that the covariance matrices of the neural activity (Fig. 4 A) show high interneuron correlations reflecting the common overlap in neural receptive fields in the olfactory code. To quantify this redundancy, or how well the PC1 of a subset of receptor neurons reflects the PC1 of the total receptor population, we compared PC1 derived for subsets of dataset 1, to PC1 of the entire dataset (Dataset 1 contains 24 neurons which is more than half of the total Drosophila receptor repertoire). As can be seen in Figure 4 B, even when using <5% of the receptors, the projections on PC1 of the subset were still highly correlated with the projections values on PC1 of the complete set. Thus, we suggest that PC1 in the datasets we analyzed is likely to be a good reflection of the PC1 in the complete repertoire. We note that this redundancy is consistent with the notion of olfactory evolution through gene duplications (Lancet and Ben-Arie, 1993; Glusman et al., 1996; Trask et al., 1998; Gilad et al., 2003, 2005; Niimura and Nei, 2003).
Figure 4.
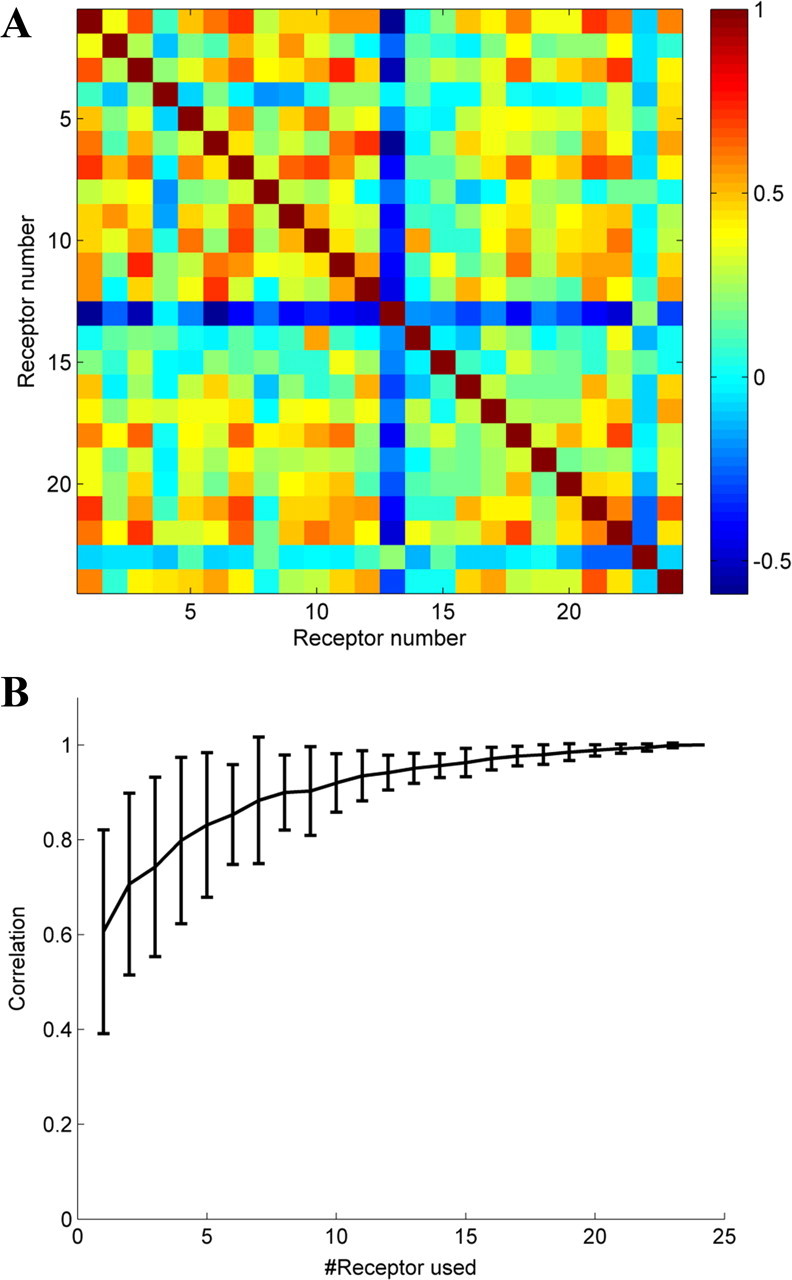
Olfactory coding redundancy. A, The covariance matrix of dataset 1. As can be seen, a substantial number of receptors are cross-correlated. B, Correlation analysis of PC1 to total neural response of dataset 1. The abscissa shows the number of receptors used of the 24 available to calculate the PC1.
Because PC1 alone accounted on average for 38% of the variance in neural activity, its relation to total neural response was not an artifact (Fig. 2), it likely represented more than odor concentration alone (Fig. 3), and was likely a good reflection of PC1 from the entire receptor repertoire (Fig. 4), we next asked whether this principle axis of olfactory neural activity carried behavioral information.
The first principal axis of neural activity predicted odorant attraction
A primary olfactory-driven behavior is approach or withdrawal. Given that total neural response predicted approach/withdrawal from odors in Drosophila larvae (Kreher et al., 2008), and was here correlated to PC1 (Fig. 1), we set out to test whether PC1 predicted this behavior, and whether such predictive value extended to other species.
Whereas the correlation between total neural response and approach/withdrawal in the study by Kreher et al. (2008) was r = 0.54 (Spearman correlation), the correlation between PC1 and approach/withdrawal in the same dataset was r = 0.76 (p < 0.001; Spearman correlation, Fig. 5 A). Furthermore, after augmenting the behavioral data of the larvae with separately reported Drosophila adult odor preferences for seven additional odors (Stensmyr et al., 2003) (supplemental Table 1, available at www.jneurosci.org as supplemental material), we tested whether PC1 of Drosophila adult's neural response space was similarly related to odor preferences. Despite obtaining behavioral data and neural activity from independent studies, and different labs, we found significant correlation in two cases, and nearly so in the third (dataset 1, r = 0.36, p = 0.07; dataset 3, r = 0.72, p = 0.005; dataset 4, r = 0.66, p = 0.01). Together, the results show that PC1 was significantly correlated with behavior in three of four datasets from larvae and adult Drosophila, and nearly so in the fourth (p = 0.07). The probability of obtaining three significant correlations (p < 0.01) when testing four datasets is less than one in 10−5. To conclude, PC1 of neural activity predicted olfactory behavior in Drosophila larvae and adults, and was a better predictor of behavior than was total neural response alone.
Figure 5.
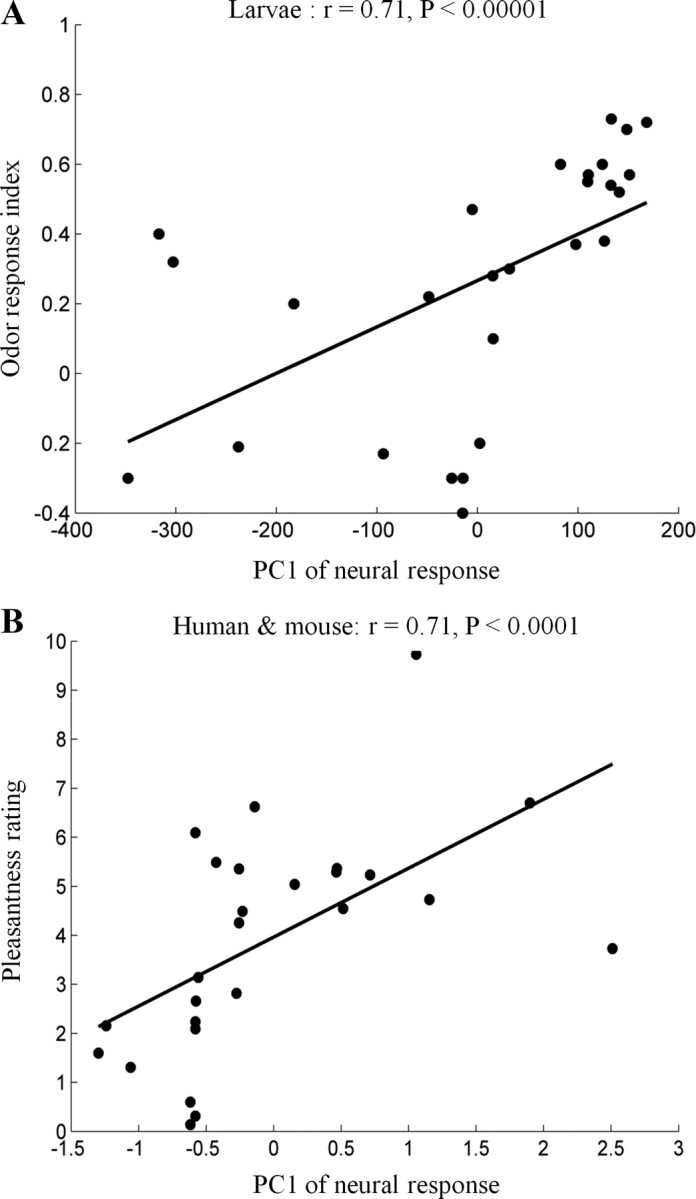
The principal axes of neural space reflected olfactory behavior and perception. A, Correlation between PC1 of neural population activity and the odor preferences of Drosophila larvae (dataset 2). Every dot represents a single odor. B, Correlation between PC1 of neural space in humans and mice with human odor pleasantness (dataset 10). Every dot represents a single odor.
Whereas olfactory behavioral data were available for Drosophila, we could not find relevant (i.e., same odorants) behavioral data for the other species for which we had neural data, namely tadpole, zebrafish, human, mouse and rat. We therefore conducted experiments to study the perceptual estimates of humans. Dataset 10 reported the neural response of 10 human neurons and 53 mouse neurons to a set of 62 odorants. We asked 18 human subjects to rate the odorant pleasantness of 26 odorants randomly selected from those tested by Saito et al. (2009) (see Materials and Methods). The correlation between human receptor PC1 and odorant pleasantness was 0.49 (p = 0.009, supplemental Table 2, available at www.jneurosci.org as supplemental material). To conclude, we found that PC1 of the neural response of larvae, Drosophila and humans reflected odor preferences. We next tested our model in rodents (datasets 7–11).
Several lines of evidence suggest similarity in rodent and human odor preferences. Rodents, like humans, avoid smells associated with spoilage such as aliphatic acids (Hebb et al., 2002, 2004), aliphatic aldehydes (Wood and Coleman, 1995) and alkyl amines (Dielenberg and McGregor, 2001). Correspondingly, mouse investigation time and the time mice choose to spend near an odor was longer for food odors such as peanut butter and shorter for odors considered repellent by humans such as Hexanal (Kobayakawa et al., 2007). Furthermore, directly measuring mouse odor preferences by odor investigation time, revealed a moderate but significant correlation between human and mouse odor preferences that extended beyond food odors alone (Mandairon et al., 2009). We therefore compared the human pleasantness estimates to PC1 of mouse neuronal response (dataset 10, 52 neurons, 63 odorants), and obtained a correlation of r = 0.7 (p < 5 × 10−4). Using both human and mouse neurons together, gave r = 0.75, p < 1.7 × 10−5 (Fig. 5 B). To reiterate, independent measurements of neural activity from receptors in a dish predicted odorant pleasantness for human subjects tested here (Fig. 5 B).
We also tested for a relationship between human odor preferences and rat PC1 of neural activity (datasets 7–9). We found a correlation that was significant in two cases, and nearly so in the third (r = 0.44 to 0.49, average r = 0.47 and p = 0.06, 0.03, and 0.01 respectively). The probability of obtaining significant correlations in three datasets of the five we tested was less than one in 10−3. Thus, we conclude that PC1 predicted odor preferences not only in Drosophila but also in humans. Furthermore, assuming correlation between rodent and human odor preferences (Mandairon et al., 2009), PC1 predicted odor preferences in mice and rats as well.
A significant difference between PC1 and total neural response
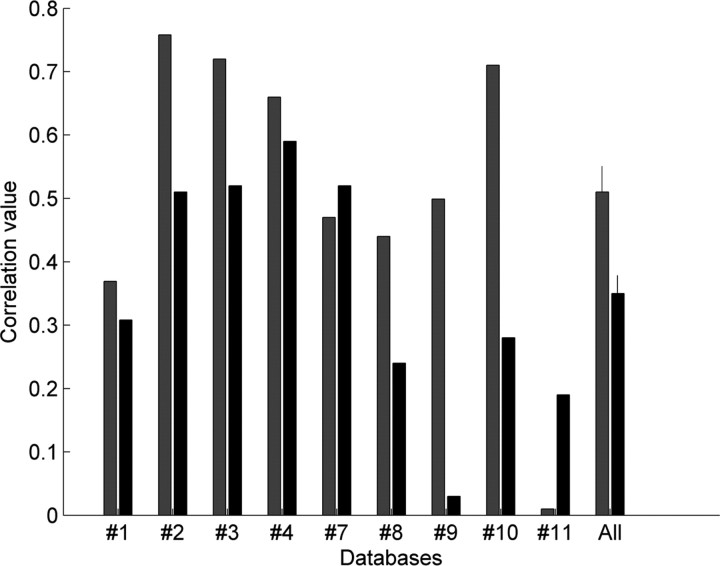
Because Kreher, Carlson, and colleagues had clearly demonstrated that total neural response predicted odor attraction in Drosophila larvae (Kreher et al., 2008), one may raise the possibility that PC1 predicted odor preferences merely because it was a good representation of total neural response. However, we found that PC1 was in fact a significantly better predictor of odor preference than was total neural response sum, generating a stronger correlation in seven of the nine datasets we used for behavioral testing (t(8) = 2.34, p < 0.05) (Fig. 6). Furthermore, as noted earlier, for the dataset of Kreher et al. (2008), PC1 was far more correlated with the behavior than was total activity, and the total neural response and PC1 in that case were poorly correlated (a bifurcated dataset, Fig. 1 B). Together, these results suggest, instead, that the relation between total neural response and approach/withdrawal results from total neural response being a good representation of PC1 and not the other way around.
Figure 6.
A comparison between PC1 and total neural response as predictors of behavior. PC1 (gray) and total neural response sum (black) correlations to odor preference in the seven datasets with behavioral data (Table 1). In most cases, PC1 was a better predictor of behavior.
The second principal axis of neural activity predicted an olfactory signature of toxicity
Having characterized PC1 of neural activity and linking it to behavior in different species, we set out to investigate what behavioral information may be conveyed in the second principal component of olfactory neural space (PC2). PC2 is by definition orthogonal to PC1, and can be approximated as the difference in the activity of two subpopulations of neurons (see supplemental Section 2 for investigation of neural correlates of PC2). The primary neuronal dimension (PC1) reflected the behavior of approach or withdrawal. Once approached, another important olfactory-based decision an animal may take is whether the odor-source is edible or poisonous. We therefore tested the relation between PC2 of the neural response space and all available oral toxicity values (LD50 in mg/kg) for the odors reported in the rat and mouse datasets (supplemental Table 3, available at www.jneurosci.org as supplemental material). Strikingly, we found a significant correlation between neural response PC2 and rat oral toxicity (supplemental Table 3, available at www.jneurosci.org as supplemental material column 3) in 2 of 3 rat datasets (datasets 7–8, r = 0.47 and 0.58; p = 0.01 and 0.0009 respectively, Fig. 7 A), and between neural response PC2 and mouse oral toxicity (supplemental Table 3, available at www.jneurosci.org as supplemental material column 2) in one of two mouse datasets (r = 0.56, p = 0.001, dataset 10; r = 0.4, p = 0.03 when restricting to only the mouse receptors). Again, the probability of obtaining all these correlations combined by chance was less than one in 10−3. We conclude that PC2 of neural activity was a good predictor of toxicity in two species across the majority of the datasets we tested. Finally, to ask whether the correlations we identified were unique, we recomputed the correlations after switching contingencies. We found that the average correlation between PC2 and odor preferences was r = 0.22, p = 0.26 (all r < 0.36 and p > 0.14), and the average correlation between PC1 and odor toxicity was r = 0.15, p = 0.43 (all r < 0.3 and p > 0.09). In other words, PC1 was correlated to odor preferences but not to toxicity, and PC2 was correlated to odor toxicity but not to odor preferences.
Figure 7.
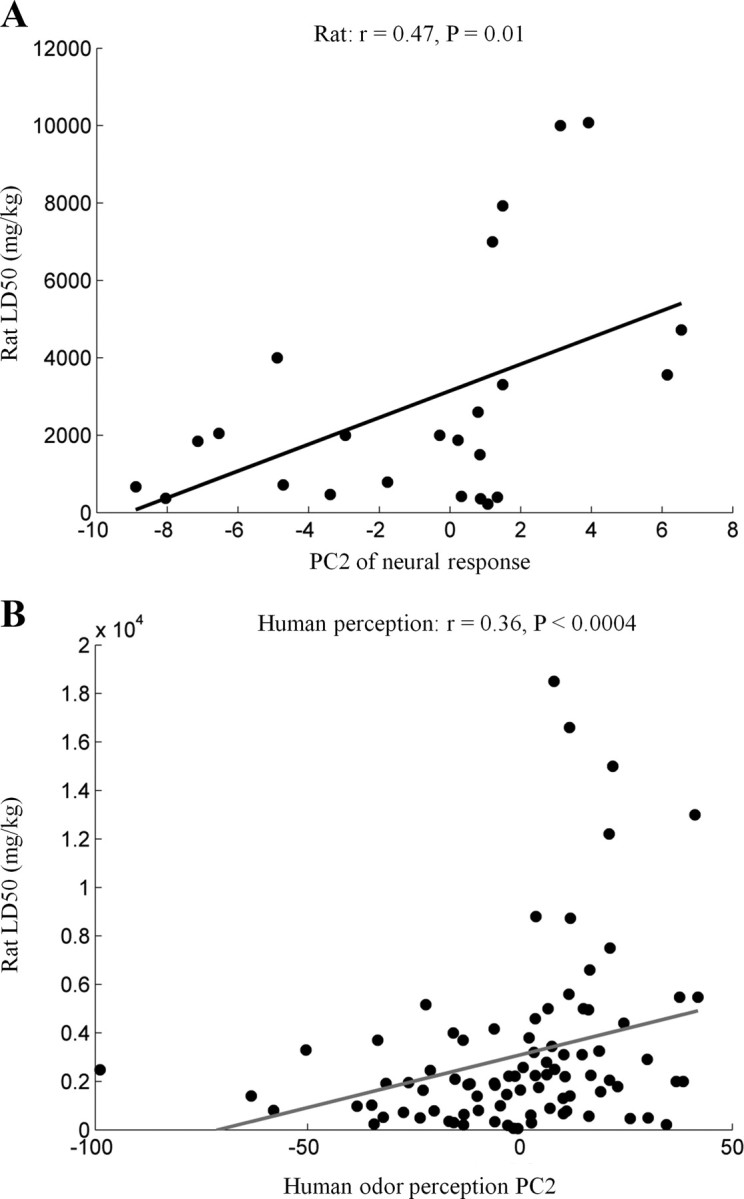
PC2 of neural activity predicted an olfactory signature of toxicity. A, Correlation between PC2 of neural population activity and oral toxicity for rats (LD50 values in mg/kg; dataset 7). Every dot represents an odor. B, Correlation between PC2 of human perceptual space and LD50 values of rats. The dots represent odors that were both used in the Dravnieks experiment (Dravnieks, 1982, 1985) and have available LD50 values (supplemental Table 3, available at www.jneurosci.org as supplemental material).
The first two PCs of olfactory neural activity reflected the first two PCs of human olfactory perception
To complement our analysis of the main axes of olfactory neural space, we conducted a similar analysis of human olfactory perceptual space. Human odor perception space can be described by representing each odor with the average ranking assigned to it using a large set of verbal odor descriptors (see supplemental Fig. 2, available at www.jneurosci.org as supplemental material for an example). Several groups have performed similar analyses in the past, and using this and other methods have universally found that the principal axis of human olfactory perception is odorant pleasantness (Berglund et al., 1973; Schiffman, 1974; Schiffman et al., 1977; Steiner, 1979; Engen, 1982; Richardson and Zucco, 1989; Khan et al., 2007; Zarzo, 2008; Yeshurun and Sobel, 2010). Together, these results imply that PC1 of olfactory neural space is strongly related to PC1 of olfactory perceptual space.
Here we also found that PC2 of rodents' neural space reflected toxicity (Fig. 7 A). This raises the possibility that similar to the relation between the first principal components of neural response and perceptual space, PC2 of the neural response is related to PC2 of perceptual space. To test this, we used the Dravnieks atlas of human odor character profiles (Dravnieks, 1982, 1985) to calculate PC2 of human odor perception space (see Materials and Methods).
Consistent with our prediction, we found a significant correlation between PC2 of human olfactory perceptual space and rat LD50 (r = 0.36, p < 0.0008, n = 90; corrected for two comparisons) (Fig. 7 B, supplemental Table 4, available at www.jneurosci.org as supplemental material) (there are obviously no human LD50 values, and rat LD50 is typically used to infer toxicity to humans). Notably, an independent analysis of human verbal olfactory descriptors suggested that PC2 of human olfactory perceptual space was edibility (Zarzo, 2008). Edibility can be considered the flip-side of toxicity, i.e., an axis ranging from toxic and nonedible at one end to edible and nontoxic at the other. Notably, the second axis of smell identified here corresponds with what may be the primary axis of taste, as identified using multidimensional scaling (Scott and Mark, 1987). To conclude, the first two PCs of olfactory neural space were related to odor attraction/repulsion and toxicity across species. Moreover, in humans, the two first PCs of olfactory perceptual space (found by two different groups using two different verbal descriptor databases (Khan et al., 2007; Zarzo, 2008) were similarly related to odor pleasantness and toxicity/edibility. This suggests that these two axes are conserved across both neural and perceptual olfactory spaces.
Discussion
We found that the two major axes of the neural response in different species and across several datasets predicted olfactory behavior and perception. The first axis, which was highly correlated with the total sum of odor-induced neural activity, reflected the animal behavior of approach or withdrawal, as well as the human perception of odorant pleasantness. The second axis, which is orthogonal to the first, reflected odorant toxicity (thus overlapping with the primary axis of taste (Scott and Mark, 1987)). In humans, these two major axes of neural activity reflected the two major axes of odor perception. These findings suggest a parallel odor coding strategy that is based on linear decomposition of the high dimensional olfactory coding space.
We think that the power of our findings lies more in their cross-species and cross-study repeatability, than in the extent of the correlations observed. Indeed, we note that the predictive relation between the Principal Components of neural activity and behavior was found albeit inherent limitations that may have obscured such correlations. For example, most of the raw data consisted of average spike rates or fluorescent change, thus masking temporal information (Laurent et al., 2001; Bathellier et al., 2008). Moreover, in some cases the data were collected from only a small portion of the olfactory system, and under high odor concentration that might distort the neural response. Finally, PCA performs a linear decomposition and will obscure more complicated nonlinear relations between neural response and behavior (Durbin and Mitchison, 1990; Roweis and Saul, 2000; Tenenbaum et al., 2000). The significant correlations we observed repeatedly, despite all these potential limitations, suggest that PCA uncovered a fundamental link between population neural activity and olfactory behavior and perception.
We emphasize, however, that our finding of simple and behaviorally meaningful components of the global olfactory code does not preclude pattern-based coding. In fact, the combination of the PC1, and PC2 itself, can both be thought of as a form of pattern, albeit not in the manner that the term pattern coding is typically applied in olfaction. Together these two principal components explained on average ∼58% of the overall neural variance (ranging between 34% and 76% across datasets), and the correlations we uncovered with behavior and perception were in the range of 0.36–0.76. This leaves a large proportion of olfactory information that is not coded in the global population response alone.
PC1 and odor concentration
Intuitively, one expects PC1 of neural activity in a sensory system to capture the amplitude of sensory stimulation. This is often the case in vision and audition, but may not be so simple in olfaction, where magnitude plays a unique role in perception. Whereas in other sensory systems magnitude conveys primarily quantity, in olfaction magnitude significantly impacts quality. In many cases, increasing odorant concentration does not just smell like “more of the same,” but instead smells like something entirely different. For example, molecules such as indole and skatole may smell flowery and pleasant at low concentrations yet fecal and decidedly unpleasant at high concentrations (Gross-Isseroff and Lancet, 1988). This unique interplay between quantity and quality, especially the quality of pleasantness, had led to the suggestion that the combination of intensity and pleasantness are a single dimension in olfaction (Henion, 1971). It was since demonstrated that olfactory intensity and pleasantness are dissociable in both perception (Doty, 1975; Moskowitz et al., 1976) and neural activity (Anderson et al., 2003), yet they nevertheless remain intertwined.
The tight link between olfactory intensity and pleasantness implies that it is nearly impossible that PC1 of the neural activity will reflect odor pleasantness yet remain unrelated to odor concentration. However, if concentration was the key source of the relation between PC1 and behavior, one would expect that in those cases where PC1 was highly correlated with behavior, PC1 would also be highly correlated with vapor pressure. This was not the case (Fig. 3 D). Furthermore, in the experiments that we conducted on human perception, we used odorants that were differentially diluted to be of equal perceived intensity. Despite this manipulation of the odorants, PC1 of neural activity still predicted odorant pleasantness.
The link between PC1 of neural activity and pleasantness may provide a framework for explaining the relationship between odor intensity and pleasantness. As noted, when the concentration of an attractive odor is increased, it often becomes less attractive (Doty, 1975; Moskowitz et al., 1976; Stensmyr et al., 2003; Kreher et al., 2008). We propose a model where the first principal component of the neural response is normalized by the number of olfactory neurons that were activated (or inhibited). This way the normalized PC1 value will increase with concentration up to a certain value, beyond which it will start to decrease. This model is consistent with behavior and perception (Fig. 8). Notably, retesting the relation between the normalized PC1 of neural space and odor preferences did not significantly influence the average correlation between PC1 and odor preferences.
Olfactory robustness and speed
Our results help explain the puzzling olfactory behavior whereby animals remain able to discriminate between odors following ablation of the substrate for combinatorial coding (Slotnick et al., 1997; Fishilevich et al., 2005; DasGupta and Waddell, 2008). This retained functionality is somewhat inconsistent with a spatial combinatorial code alone (the exact code is broken when part of the bulb is lesioned), but remains possible when discriminating using global population activity, as the principal component is likely to remain robust to lesion (supplemental Section 3).
An additional phenomenon consistent with our model is the apparent speed of olfactory discrimination: whereas combinatorial codes can develop over hundreds of milliseconds (Friedrich and Laurent, 2001; Spors and Grinvald, 2002), olfactory discriminations can be made within <200 milliseconds (Johnson et al., 2003; Uchida and Mainen, 2003; Abraham et al., 2004; Rinberg et al., 2006; Wesson et al., 2008). Such time frames are consistent with the coding scheme described here (Bathellier et al., 2008). Although the phenomena of robustness and speed can be explained under the spatiotemporal coding scheme alone, we submit that the coding scheme we described provides a solution that is both feasible and simple.
Global coding versus local coding
Recent studies have identified odorants such as CO2 and a male-specific pheromone, that activate a single glomerulus to trigger innate avoidance (Suh et al., 2004), or female courtship (Kurtovic et al., 2007), respectively. By manipulating activity in the cognate receptor neurons, the activation of these single ORN channels was shown to be necessary and sufficient to produce the behavior, suggesting that these receptors are hardwired to specific behavioral outputs. A more recent study in Drosophila investigated the neural code for the odor of vinegar and found that of the six glomeruli activated under low odor concentration only two were important for attraction. Under higher odor concentration, a seventh, newly recruited, glomerulus was responsible for generating an aversion response (Semmelhack and Wang, 2009). Together, these studies suggest a labeled-line like coding of specific odors, or more local neural computations involved in olfactory decisions. In turn, the PC analysis, by construction, relies on a linear combination of all the olfactory neural responses, and is thus “global” rather than local. We note that Kreher et al. (2008), who identified the previously noted relation between total neural response and approach/withdrawal (Kreher et al., 2008), also found that a linear combination of the activity of only five receptors (of 21 measured) accounted for 55% of the test set variance. Again, we suggest that these different coding schemes can work together, and may even work synergistically.
Summary
We found that two important behavioral decisions regarding an odor can be answered in part using simple and robust features of neural activity in the olfactory system. Similar to the population vector coding of directional information in motor cortex (Georgopoulos et al., 1986), our results can be viewed as reflecting preferred “olfactory directions” that can be read as major axes in the population code. Like the rest of the brain, however, the olfactory system also solves even simpler problems through labeled-line triggering of innate responses (Suh et al., 2004; Kurtovic et al., 2007; Semmelhack and Wang, 2009), and far more complicated problems through spatiotemporal pattern coding (Friedrich and Korsching, 1997; Hildebrand and Shepherd, 1997; Malnic et al., 1999; Rubin and Katz, 1999; Ma and Shepherd, 2000; Uchida et al., 2000; Belluscio and Katz, 2001; Firestein, 2001; Laurent et al., 2001; Meister and Bonhoeffer, 2001; Spors and Grinvald, 2002; Leon and Johnson, 2009). We therefore suggest that the neural code of large populations in the olfactory system carries information on different levels of complexity: Whereas detailed information may be carried by the fine spatiotemporal patterns of activity, vital behavioral information can be read out in parallel using simple fast and robust features of the population response.
Footnotes
This work was funded by European Research Council Grant FP7 #200850. N.S. was also supported by the James S. McDonnell Foundation. R.K. is a Visiting Scholar at the Department of Neurobiology, The Weizmann Institute of Science. We thank Arak Elite.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Aflalo TN, Graziano MS. Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array. J Neurosci. 2006;26:6288–6297. doi: 10.1523/JNEUROSCI.0768-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund B, Berglund U, Engen T, Ekman G. Multidimensional analysis of 21 odors. Scand J Psychol. 1973;14:131–137. doi: 10.1111/j.1467-9450.1973.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae . Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MA. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–140. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003;28:467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- DasGupta S, Waddell S. Learned odor discrimination in Drosophila without combinatorial odor maps in the antennal lobe. Curr Biol. 2008;18:1668–1674. doi: 10.1016/j.cub.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Doty RL. An examination of relationships between the pleasantness, intensity and concentration of 10 odorous stimuli. Percept Psychophysics. 1975;17:492–496. [Google Scholar]
- Dravnieks A. Odor quality: semantically generated multi-dimensional profiles are stable. Science. 1982;218:799–801. doi: 10.1126/science.7134974. [DOI] [PubMed] [Google Scholar]
- Dravnieks A. Atlas of odor character profiles. Philadelphia: ASTM; 1985. [Google Scholar]
- Durbin R, Mitchison G. A dimension reduction framework for understanding cortical maps. Nature. 1990;343:644–647. doi: 10.1038/343644a0. [DOI] [PubMed] [Google Scholar]
- Engen T. The perception of odors. New York: Academic; 1982. [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila . Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS. Principal component analysis learning algorithms: a neurobiological analysis. Proc Biol Sci. 1993;254:47–54. doi: 10.1098/rspb.1993.0125. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Gilad Y, Man O, Pääbo S, Lancet D. Human specific loss of olfactory receptor genes. Proc Natl Acad Sci U S A. 2003;100:3324–3327. doi: 10.1073/pnas.0535697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Man O, Glusman G. A comparison of the human and chimpanzee olfactory receptor gene repertoires. Genome Res. 2005;15:224–230. doi: 10.1101/gr.2846405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusman G, Clifton S, Roe B, Lancet D. Sequence analysis in the olfactory receptor gene cluster on human chromosome 17: recombinatorial events affecting receptor diversity. Genomics. 1996;37:147–160. doi: 10.1006/geno.1996.0536. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Lancet D. Concentration-dependent changes of perceived odor quality. Chem Sens. 1988;13:191–204. [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Dominguez H, Trudel F, Laforest S, Drolet G. Odor-induced variation in anxiety-like behavior in mice is associated with discrete and differential effects on mesocorticolimbic cholecystokinin mRNA expression. Neuropsychopharmacology. 2002;27:744–755. doi: 10.1016/S0893-133X(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur J Neurosci. 2004;20:2415–2429. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- Henion KE. Odor pleasantness and intensity: a single dimension? J Exp Psychol. 1971;90:275–279. doi: 10.1037/h0031549. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, Sobel N. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Lancet D, Ben-Arie N. Olfactory receptors. Curr Biol. 1993;3:668–674. doi: 10.1016/0960-9822(93)90064-u. [DOI] [PubMed] [Google Scholar]
- Laubach M, Shuler M, Nicolelis MA. Independent component analyses for quantifying neuronal ensemble interactions. J Neurosci Methods. 1999;94:141–154. doi: 10.1016/s0165-0270(99)00131-4. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Is there a space-time continuum in olfaction? Cell Mol Life Sci. 2009;66:2135–2150. doi: 10.1007/s00018-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Shepherd GM. Functional mosaic organization of mouse olfactory receptor neurons. Proc Natl Acad Sci U S A. 2000;97:12869–12874. doi: 10.1073/pnas.220301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF. Behavioral analysis of olfactory coding and computation in rodents. Curr Opin Neurobiol. 2006;16:429–434. doi: 10.1016/j.conb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Poncelet J, Bensafi M, Didier A. Humans and mice express similar olfactory preferences. PLoS One. 2009;4:e4209. doi: 10.1371/journal.pone.0004209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini I, Brase C, Chen TW, Schild D. Response profiles to amino acid odorants of olfactory glomeruli in larval Xenopus laevis . J Physiol. 2007;581:567–579. doi: 10.1113/jphysiol.2007.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin JW, Optican LM, Richmond BJ, Gawne TJ. Concurrent processing and complexity of temporally encoded neuronal messages in visual perception. Science. 1991;253:675–677. doi: 10.1126/science.1908118. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Moskowitz HR, Dravnieks A, Klarman LA. Odor intensity and pleasantness for a diverse set of odorants. Percept Psychophysics. 1976;19:122–128. [Google Scholar]
- Niimura Y, Nei M. Evolution of olfactory receptor genes in the human genome. Proc Natl Acad Sci U S A. 2003;100:12235–12240. doi: 10.1073/pnas.1635157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz D, Roeske T, Syed Z, de Bruyne M, Galizia CG. The molecular receptive range of an olfactory receptor in vivo (Drosophila melanogaster Or22a) J Neurobiol. 2006;66:1544–1563. doi: 10.1002/neu.20333. [DOI] [PubMed] [Google Scholar]
- Richardson JT, Zucco GM. Cognition and olfaction: a review. Psychol Bull. 1989;105:352–360. doi: 10.1037/0033-2909.105.3.352. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;3:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Roweis ST, Saul LK. Nonlinear dimensionality reduction by locally linear embedding. Science. 2000;290:2323–2326. doi: 10.1126/science.290.5500.2323. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci. 1999;11:3970–3982. doi: 10.1046/j.1460-9568.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S, Robinson DE, Erickson RP. Multidimensional-scaling of odorants-examination of psychological and physiochemical dimensions. Chem Sens Flavour. 1977;2:375–390. [Google Scholar]
- Schiffman SS. Physicochemical correlates of olfactory quality. Science. 1974;185:112–117. doi: 10.1126/science.185.4146.112. [DOI] [PubMed] [Google Scholar]
- Scott TR, Mark GP. The taste system encodes stimulus toxicity. Brain Res. 1987;414:197–203. doi: 10.1016/0006-8993(87)91347-3. [DOI] [PubMed] [Google Scholar]
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Bell GA, Panhuber H, Laing DG. Detection and discrimination of propionic acid after removal of its 2-DG identified major focus in the olfactory bulb: a psychophysical analysis. Brain Res. 1997;762:89–96. doi: 10.1016/s0006-8993(97)00357-0. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Steiner JE. Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav. 1979;13:257–295. doi: 10.1016/s0065-2407(08)60349-3. [DOI] [PubMed] [Google Scholar]
- Stensmyr MC, Giordano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–724. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila . Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Nagayama S, Mori K. Detection and masking of spoiled food smells by odor maps in the olfactory bulb. J Neurosci. 2004a;24:8690–8694. doi: 10.1523/JNEUROSCI.2510-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004b;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Tenenbaum JB, de Silva V, Langford JC. A global geometric framework for nonlinear dimensionality reduction. Science. 2000;290:2319–2323. doi: 10.1126/science.290.5500.2319. [DOI] [PubMed] [Google Scholar]
- Trask BJ, Massa H, Brand-Arpon V, Chan K, Friedman C, Nguyen OT, Eichler E, van den Engh G, Rouquier S, Shizuya H, Giorgi D. Large multi-chromosomal duplications encompass many members of the olfactory receptor gene family in the human genome. Hum Mol Genet. 1998;7:2007–2020. doi: 10.1093/hmg/7.13.2007. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RW, Coleman JB. Behavioral evaluation of the irritant properties of formaldehyde. Toxicol Appl Pharmacol. 1995;130:67–72. doi: 10.1006/taap.1995.1009. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 2010;61:219–241. C1–C5. doi: 10.1146/annurev.psych.60.110707.163639. [DOI] [PubMed] [Google Scholar]
- Zarzo M. Psychologic dimensions in the perception of everyday odors: pleasantness and edibility. J Sens Stud. 2008;23:354–376. [Google Scholar]