Abstract
Although fleshy fruit species are economically important worldwide and crucial for human nutrition, the regulation of their fruit metabolism remains to be described finely. Fruit species differ in the origin of the tissue constituting the flesh, duration of fruit development, coordination of ripening changes (climacteric vs. non-climacteric type) and biochemical composition at ripeness is linked to sweetness and acidity. The main constituents of mature fruit result from different strategies of carbon transport and metabolism. Thus, the timing and nature of phloem loading and unloading can largely differ from one species to another. Furthermore, accumulations and transformations of major soluble sugars, organic acids, amino acids, starch and cell walls are very variable among fruit species. Comparing fruit species therefore appears as a valuable way to get a better understanding of metabolism. On the one hand, the comparison of results of studies about species of different botanical families allows pointing the drivers of sugar or organic acid accumulation but this kind of comparison is often hampered by heterogeneous analysis approaches applied in each study and incomplete dataset. On the other hand, cross-species studies remain rare but have brought new insights into key aspects of primary metabolism regulation. In addition, new tools for multi-species comparisons are currently emerging, including meta-analyses or re-use of shared metabolic or genomic data, and comparative metabolic flux or process-based modeling. All these approaches contribute to the identification of the metabolic factors that influence fruit growth and quality, in order to adjust their levels with breeding or cultural practices, with respect to improving fruit traits.
Keywords: amino acids, cross-species, fleshy fruit, inter-species, metabolism regulation, organic acids, primary metabolism, sugars
Introduction
Fresh fruits (866 Mt worldwide in 20161) and their derived products are economically important. Besides their energetic role in human diet linked notably with their carbohydrate contents, they are also crucial for human nutrition and health, especially in relation with their contents in vitamins, anti-oxidants and fibers (Baldet et al., 2014; Rodriguez-Casado, 2016; Wang et al., 2016; Aune et al., 2017; Padayachee et al., 2017). In fruit tissues, primary metabolism can be defined as the biochemical processes that are necessary for growth and development and shared by a large number of taxonomic groups (Verpoorte, 2000), and produces metabolites that are generally essential for organism survival (Aharoni and Galili, 2011). It contributes to flesh growth and ripening, and final fruit quality, particularly sweetness and acidity. Its operation varies according to botanical species and developmental stages. Different botanical species may differ in the composition of the phloem sap originating from leaves and unloaded into the fruit, the occurrence of transient starch accumulation during development, the hormonal orchestration of ripening changes, and the major metabolites accumulated at ripening (Figure 1). All these aspects are linked with primary metabolic pathways involving carbohydrates, organic acids, and amino acids. These pathways are regulated along fruit development that can last from a few dozens to more than 200 days-post-anthesis (DPA), from fruit set to ripe fruit, depending on the species (Table 1). Early development stages after fruit set are usually characterized by a high concentration of organic acids whereas ripening is associated with soluble sugar accumulation (Famiani et al., 2015; Beauvoit et al., 2018). However, the regulation of metabolic pathways is not that simple.
FIGURE 1.
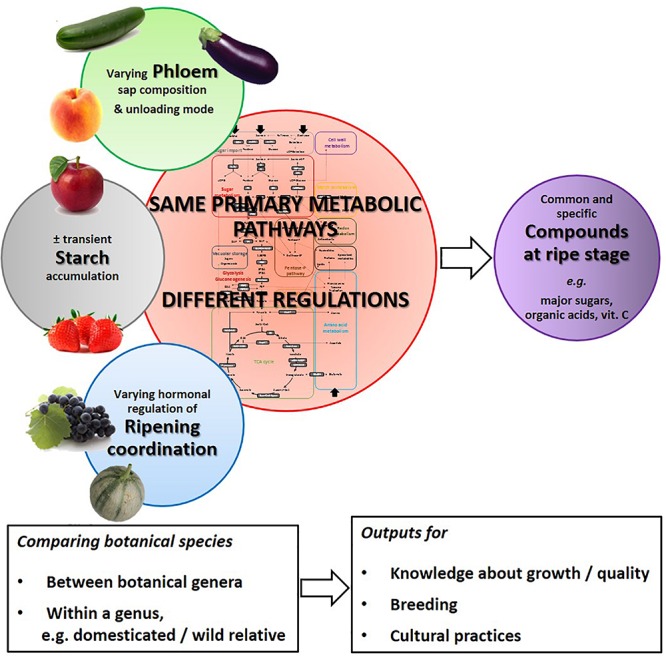
Schema of the major physiological and metabolic differences and similarities between fleshy fruit species leading to varying fruit compositions at ripe stage.
TABLE 1.
Major characters linked with primary metabolism and differing between temperate fruit species.
| Botanical family | Species | Fruit development duration (DPA) | Climacteric ripening a | Major phloem transported sugara | Transient starch storage | Major soluble sugars in ripe fruit | Major organic acids in ripe fruit | Major amino acids in ripe fruit | References |
| Actinidiaceae | Kiwifruit Actinidia deliciosa Actinidia chinensis | 237 | Yes2 | Sucrose | Yes | Glucose/fructose | Quinate/citrate | Aspartate | Chen et al., 2017; Klages et al., 1998; Nardozza et al., 2013; Richardson et al.,2011 |
| Hardy kiwifruit (kiwai) Actinidia arguta | 140 | Yes | Yes | Sucrose | Quinate/citrate | – | Kim et al., 2012; Klages et al., 1998; Mikulic-Petkovsek et al.,2012 | ||
| Cucurbitaceae | Cucumber Cucumis sativus | 20 | No2 | Raffinose/stachyose/sucrose4 | Fructose/glucose | Malate (commercial)/citrate (physiological) | – | Hu et al., 2009; McFeeters et al.,1982 | |
| Melon Cucumis melo | 48 | Yes for cantaloupe2 No for honeydew1 | Raffinose/stachyose/sucrose | Sucrose | Citrate | Glutamate/glutamine/GABA | Mitchell et al., 1992; Wang et al.,1996 | ||
| Watermelon Citrullus lanatus | 55 | No2 | Raffinose/stachyose/sucrose3 | Sucrose | Malate | Gao et al., 2018; Zhang and Ge,2016 | |||
| Ericaceae | Blueberry Vaccinium corymbosum | Yes2 | Glucose/fructose | Citrate | Mikulic-Petkovsek et al.,2012 | ||||
| Grossulariaceae | Blackcurrant Ribes nigrum | Glucose/fructose | Citrate | Glutamine/α-alanine | Burroughs, 1960; Mikulic-Petkovsek et al.,2012 | ||||
| Redcurrant Ribes rubrum | Glucose/fructose | Citrate | Glutamine/α-alanine | Burroughs, 1960; Mikulic-Petkovsek et al.,2012 | |||||
| Rosaceae | Apple Malus domestica | 160 | Yes2 | Sucrose/sorbitol4 | Yes | Fructose | Malate | Asparagine/aspartate/glutamate | Brookfield et al., 1997; Drake and Eisele, 1999; Zhang et al.,2010 |
| Apricot Prunus armeniaca | 65 | Yes2 | Sucrose/sorbitol4 | Glucose/fructose (cv. Harcot) sucrose (cvs Bavinity and Trevatt) | Citrate/malate | Bae et al., 2014; Wills et al.,1983 | |||
| Blackberry Rubus fruticosus | Yes/no | Glucose/fructose | Isocitrate | Asparagine/glutamate | Burdon and Sexton, 1993; Burroughs, 1960; Mikulic-Petkovsek et al., 2012; Whiting,1958 | ||||
| Sweet cherry Prunus avium | No2 | Sucrose/sorbitol4 | Glucose/fructose | Malate | Usenik et al., 2008; Wills et al.,1983 | ||||
| Chokeberry Aronia melanocarpa | Sorbitol/glucose | Malate | Mikulic-Petkovsek et al.,2012 | ||||||
| Eastern shadbush Amelanchier canadensis | Glucose/fructose/sorbitol | Malate | Mikulic-Petkovsek et al.,2012 | ||||||
| Peach Prunus persica | 125 | Yes2 | Sucrose/sorbitol4 | Yes | Sucrose | Malate/citrate | Asparagine/glutamate/proline | Moing et al., 1998; Wills et al.,1983 | |
| Pear Pyrus communis Pyrus pyrifolia | Yes2 | Sucrose/sorbitol4 | Yes | Fructose | Malate/citrate | Asparagine/serine | Chen et al., 2007; Drake and Eisele, 1999; Mesa et al.,2016 | ||
| Prune Prunus domestica | Sucrose/sorbitol4 | Glucose/fructose/sucrose (cultivar dependent) | Malate | Wills et al.,1983 | |||||
| Plum Prunus salicina | 91 | Yes/no | Glucose/fructose | Quinate | Bae et al.,2014 | ||||
| Raspberry Rubus idaeus | No2 | Glucose/fructose | Citrate | Serine/α-alanine | Burroughs, 1960; Mikulic-Petkovsek et al.,2012 | ||||
| Rowanberry Sorbus aucuparia | Sorbitol | Malate | Mikulic-Petkovsek et al.,2012 | ||||||
| Strawberry Fragaria x ananassa | 39 | No2 | Sucrose3 | Yes | Glucose/fructose or glucose/fructose/sucrose cultivar dependent | Citrate | Asparagine/glutamine | Burroughs, 1960; Mikulic-Petkovsek et al., 2012; Moing et al., 2001; Souleyre et al.,2004 | |
| Wild strawberry Fragaria vesca | Glucose/fructose/sucrose | Citrate | Mikulic-Petkovsek et al.,2012 | ||||||
| Rutaceae | Clementine/mandarine Citrus clementina Citrus unshiu | No2 | No | Sucrose | Citrate | Legua et al., 2014; Mehouachi et al.,1995 | |||
| Acidic lemon Citrus limon | 150 | No2 | Glucose/sucrose | Citrate | Albertini et al.,2006 | ||||
| Acidless lemon Citrus limon | 150 | No2 | Fructose | Citrate/malate/quinate | Glutamate/alanine | Albertini et al., 2006; Brückner and Westhauser,1994 | |||
| Acidic lime Citrus latifolia | 150 | No2 | Glucose/sucrose | Citrate | Albertini et al.,2006 | ||||
| Acidless lime Citrus limettioides | 150 | No2 | Fructose | Citrate/malate/quinate | Albertini et al.,2006 | ||||
| Acidic orange Citrus sinensis | 150 | No2 | Glucose/fructose | Citrate | Albertini et al.,2006 | ||||
| Acidless orange Citrus sinensis | 150 | No2 | Sucrose | Fructose | Quinate | Glutamate/alanine | Albertini et al., 2006; Brückner and Westhauser, 1994; Hijaz and Killiny,2014 | ||
| Solanaceae | Eggplant Solanum melongena | Yes/No2 | Glucose/fructose | Malate | Kozukue et al., 1978; Makrogianni et al.,2017 | ||||
| Pepper Capsicum chilense | 70 | Yes/no1 | Glucose/fructose | Citrate (red) Malate (green) | GABA/proline/asparagine | Flores et al., 2012; Osorio et al.,2012 | |||
| Goji berry Lycium barbarum | Glucose/fructose | Citrate/malate | Mikulic-Petkovsek et al.,2012 | ||||||
| cultivated tomato, Solanum lycopersicum | 40–60 | Yes1,2 | Sucrose4 | Yes | Glucose/fructose | Citrate/malate | Glutamate/glutamine | Schaffer and Petreikov, 1997; Schauer et al.,2004 | |
| Wild tomato, S. neorickii, S. chmielewskii, S. habrochaites S. pennellii | 40–60 | Sucrose | Citrate/malate | Tryptophan (S. habrochaites) Aspartate (S. chmielewskii) Pyoglutamate/aspartate (S. neorickii) GABA (S. pennellii) | Schauer et al.,2004 | ||||
| Vitaceae | Grape berry Vitis vinifera | 100–110 | No2 | Sucrose | Little or no | Glucose/fructose | Malate/tartrate | Proline/alanine/GABA | Dai et al., 2013; Guan et al., 2017; Ollat et al., 2002; Swanson and El-Shishiny,1958 |
Fruit development duration in days post-anthesis (DPA), ripening type (climacteric or non-climacteric) and compositional characteristics of phloem sap and ripe fruit. aDefined according to Barry and Giovannoni (2007)1, Paul et al. (2012)2, Rennie and Turgeon (2009)3, Fu et al. (2011)4.
Studies dealing with fruit metabolism include biochemical analyses of metabolites from targeted analyses to metabolomics in tissues or sap, measurement of enzyme activity and regulation, transcriptomics, map-based cloning or genome (re)sequencing. Several of these approaches can be combined for one cultivar across fruit development or use a large collection of genetic resources. This review focuses on comparing species for the programming and integration of primary metabolic pathways with growth and fruit quality, mostly for temperate fruits. Such comparisons should help identifying key regulation points, for instance regarding the trade-offs between fruit yield and quality, and possibly propose hypotheses for breeding or agricultural practices.
Similarities and Dissimilarities Are Noticed in the Composition of Primary Metabolites in Fruits
Fruit taste is strongly influenced by sweetness and acidity, which are associated to sugars and organic acids, respectively. Sugars are abundant in most fleshy fruits; so much that fructose has been named after them (i.e., after the Latin fructus). Besides, several amino acids influence the so-called umami taste. The composition and concentrations of these major constituents of the ripe fruit vary according to species (Table 1).
Soluble Sugars and Organic Acids
Concerning soluble sugars, hexoses are usually more abundant than sucrose. This is the case for most berries, e.g., raspberries, blackberry (Mikulic-Petkovsek et al., 2012) and grape berry (Dai et al., 2013), as well as kiwifruit (Richardson et al., 2011), pepper (Osorio et al., 2012), eggplant (Makrogianni et al., 2017), and cherry (Wills et al., 1983; Usenik et al., 2008). In some species, fructose is more abundant than glucose and sucrose, such as in apple and pear (Drake and Eisele, 1999) or blackcurrant (Mikulic-Petkovsek et al., 2012). However, there are also species in which sucrose is the most abundant sugar, such as mandarin (Legua et al., 2014), peach (Wills et al., 1983), watermelon (Gao et al., 2018), melon (Wang et al., 1996), and hardy kiwi (Klages et al., 1998; Mikulic-Petkovsek et al., 2012). Finally, some species contain sucrose in almost the same proportion as hexoses, for instance several cultivars of litchi (Litchi chinensis) (Wang et al., 2006) and cultivated as well as wild strawberry (Moing et al., 2001; Mikulic-Petkovsek et al., 2012).
Sugar alcohols are a major component for some fruit species. Sorbitol, which is common in Rosaceae trees, is present in notable quantities in the developing peach and apricot fruit (Bae et al., 2014). It is also one of the main sugars in ripe chokeberry, rowanberry and eastern shadbush (Mikulic-Petkovsek et al., 2012). Another sugar-alcohol, myo-inositol, is present in the early stages of kiwifruit and hardy kiwi development (Klages et al., 1998).
Concerning organic acids, a recent review (Famiani et al., 2015) and research study (Mikulic-Petkovsek et al., 2012) listed the main organic acids found in fruits of more than 50 species. Citrate and malate are the major organic acids in many fruit species. Typically, young fruits are likely to accumulate malate, which will tend to be replaced by citrate at ripening (McFeeters et al., 1982; Flores et al., 2012). Thus, species such as lime, orange, raspberry, strawberry, blueberry, and melon (Wang et al., 1996) accumulate high levels of citrate, while other species such as apple, cherry, chokeberry, rowanberry, eastern shadbush, watermelon (Gao et al., 2018), and eggplant (Kozukue et al., 1978) build up in malate. Other species, e.g., pear, apricot, goji berry, and blackcurrant accumulate both organic acids. In some cases, other organic acids are also overrepresented, as for example isocitrate in blackberry, tartrate in grape berry and lychee, or quinate in kiwifruit and hardy kiwi (Kim et al., 2012).
Large compositional differences for major compounds have been reported between domesticated species and wild relatives. For instance, the tomato domesticated species (Solanum lycopersicum L.) accumulates hexoses whereas several wild species (Solanum neorickii, Solanum chmielewskii, Solanum habrochaites) accumulate sucrose as the major sugar (Yelle et al., 1988; Schauer et al., 2004). Furthermore, wild tomato species (Solanum pennellii, S. neorickii, S. chmielewskii, S. habrochaites) were found to accumulate higher levels of malate and citrate (Schauer et al., 2004; Steinhauser et al., 2010) than cultivated tomatoes. Similarly, the domestication of mandarin led to a strong decrease in citrate (Wang et al., 2018). Large compositional differences may even be found between cultivars of a given species. An example is given for acidic lemon and lime, where glucose and sucrose are the major sugars, and citrate the main organic acid, whilst in acidless lemon and lime, fructose is the major sugar and citrate, malate and quinate are equally present (Albertini et al., 2006).
Amino Acids
Regarding amino acids, there are also differences according to botanical species. For berries such as strawberry, glutamine, and asparagine are the most abundant ones whereas blackberry accumulate asparagine and glutamate. For blackcurrant, orange, and lemon glutamine is the major amino acid besides alanine (Burroughs, 1960; Brückner and Westhauser, 1994). The latter one is also abundant in raspberry with serine (Burroughs, 1960). In grape berry, proline, arginine, glutamine and alanine dominate in berry skin, while proline, alanine and γ-aminobutyric acid (GABA) dominate in berry pulp (Guan et al., 2017). For several Rosaceae trees, asparagine dominates with glutamate and aspartate in apple (Zhang et al., 2010), with serine in pear (Chen et al., 2007), and with glutamate and proline in peach (Moing et al., 1998). Concerning Solanaceae, asparagine, GABA, and proline seem to be the predominant amino acids in pepper (Osorio et al., 2012). Glutamine and glutamate are the major ones in domesticated tomatoes (S. lycopersicum) together with GABA which is also present in high quantities (Schauer et al., 2004). However, two wild tomato species largely differ according to the latter authors: S. habrochaites harbors a high tryptophan content, and S. pennellii a high GABA pool, five times higher than in the domesticated species. In kiwifruit, aspartate is the main amino acid (Nardozza et al., 2013).
Phloem Loading and Unloading Strategies Differ Among Fruits
Fruits are strong sinks attracting plenty of photoassimilates transported from leaves via phloem. From photosynthetic site to sink, photoassimilates need at least three transporting steps, including phloem loading, phloem long-distance transport, and phloem unloading. The strategies of phloem loading, unloading, and the transported forms of carbon are diverse (Rennie and Turgeon, 2009; Braun et al., 2013). Sucrose is the main photoassimilate transported in phloem in most fruit species such as cultivated tomato, grape, sweet orange and cultivated strawberry (Swanson and El-Shishiny, 1958; Rennie and Turgeon, 2009; Fu et al., 2011; Hijaz and Killiny, 2014). However, several fruit species of the Cucurbitaceae family, such as cucumber, watermelon, and melon also transport oligosaccharides including raffinose and stachyose, in higher or equal concentration than sucrose (Mitchell et al., 1992; Rennie and Turgeon, 2009; Fu et al., 2011). Tree species from the Rosaceae family, such as apple, peach, plum or prune, apricot and sweet cherry also transport sugar alcohols (e.g., sorbitol) (Rennie and Turgeon, 2009; Fu et al., 2011). For example, sorbitol can account for 60–90% of the carbon transported in phloem in peach tree (Moing et al., 1997).
Phloem Loading
Sucrose can be loaded into phloem by three loading strategies, including active apoplasmic, active symplasmic (also called polymer trapping), and passive symplasmic routes (Rennie and Turgeon, 2009; Fu et al., 2011). Active apoplasmic loaders normally have low-abundant plasmodesmata in leaf and require the presence of sucrose transporters (SUTs) and hexose and sucrose transporters (SWEETs), such as in tomato leaf (Fu et al., 2011; Jensen et al., 2016; Liesche and Patrick, 2017). In active symplasmic loaders, sucrose diffuses into the companion cells through abundant plasmodesmata, and is enzymatically converted into sugar oligomers (e.g., raffinose and stachyose), which are molecularly larger and cannot diffuse back to phloem parenchyma, forming a polymer trapping mechanism. Fruit species of Cucurbitaceae family, such as cucumber, watermelon and melon, are active symplasmic loaders (Rennie and Turgeon, 2009; Fu et al., 2011). Passive symplasmic loading requires abundant plasmodesmata to allow sucrose diffusion or convection from mesophyll cells to sieve elements following the sugar concentration gradient. Strawberry is a passive loader, and grape is a candidate passive loader (Rennie and Turgeon, 2009). The phloem loading strategies for sugar-alcohols (e.g., sorbitol, mannitol) can be active apoplasmic or passive symplasmic (Reidel et al., 2009). Most fruit trees of Rosaceae family, including apple, apricot, sweet cherry, peach, and pear are passive symplasmic loaders (Reidel et al., 2009; Fu et al., 2011). Multi-species comparison analysis showed that active loading is associated with efficient water conduction and maximized carbon efficiency and growth, while the reverse is true for passive loading (Fu et al., 2011). A meta-analysis of 41 species with a modeling approach further showed that phloem sugar concentrations are in average at 21.1% for active loaders and 15.4% for passive loaders (Jensen et al., 2013). The theoretical optimum sugar concentration in phloem sap proposed was 23.5%. Organic acids are also transported in phloem (Fiehn, 2003), although references are rare. Amino acids, for instance arginine and glycine in grapevine (Gourieroux et al., 2016), also use phloem as the main transport route from source to sink, and are also transported in xylem sap (Tegeder and Hammes, 2018).
Phloem Unloading
In fruit sinks, photoassimilates (sucrose, sugar-alcohols, or oligosaccharides) need to be unloaded following symplasmic or apoplasmic pathways (Braun et al., 2013). In apple and cucumber fruits, phloem unloading is apoplasmic throughout fruit development (Zhang et al., 2004; Hu et al., 2011). In several fruits, shifts between the two phloem unloading strategies can occur during development. Tomato and grape fruits operate symplasmic unloading during early development stage when soluble sugar is low, and switches to apoplasmic unloading during fruit ripening when soluble sugars accumulate (Ruan and Patrick, 1995; Patrick, 1997; Zhang et al., 2006). For kiwifruit, Chen et al. (2017) showed that sucrose phloem unloading occurs mainly through the apoplasmic route along fruit development (44–135 days after blooming). However, Gould et al. (2013), working from 22 to 200 days after anthesis, found that phloem unloading dominantly appeared via symplasmic route in early fruit development, while an apoplasmic route becomes important during the later developmental stages. The dominant symplastic import of sugar at the initial stages of fruit development allows a high inflow of carbon input via mass flow. Shifting from symplasmic to apoplasmic unloading during fruit ripening limits back-flow of assimilates from fruit sink to sieve elements and likely facilitates sugar accumulation to high concentrations in fruit tissues (Ruan and Patrick, 1995; Patrick, 1997; Zhang et al., 2006). For amino acid unloading, most plant species follow a symplasmic process driven by a downhill concentration gradient (Tegeder and Hammes, 2018). The question whether and how loading strategies, carbon loading forms, and unloading strategies influence fruit growth and quality are still under debate.
Primary Metabolism Pathways Are Differentially Regulated
Sugar and Sugar-Alcohol Metabolism
In most fruits, the main source of carbon is imported via phloem in form of sucrose, which can be degraded via the reactions catalyzed by cell wall invertase in the apoplast, neutral invertase or sucrose synthase following symplastic import into the cytosol, or acid invertase following subsequent import into the vacuole. Carbon import patterns are highly variable from one species to another. For example, in sweet pepper both vacuolar and neutral invertases have been proposed as determining carbon import at young stages (Nielsen et al., 1991). This contrasts with kiwifruit, in which sucrose synthase has been proposed as controlling most of the carbon import in growing fruits (Chen et al., 2017). In the same species, the previous measurement of neutral invertase and sucrose synthase cytosolic enzymes seemed in agreement with symplastic phloem unloading throughout fruit development before ripening (Nardozza et al., 2013). Parietal invertase has been found to impact significantly tomato sugar content at maturity (Fridman et al., 2004). A low level of acid invertase activity and the absence of sucrose synthase activity in S. chmielewskii, a wild tomato species, were associated with the high content of sucrose (Yelle et al., 1988). In contrast to S. lycopersicum, the capacity of most enzymes of glycolysis and the tricarboxylic acid (TCA) cycle of S. pennellii, which also accumulates hexoses, is maintained and increases even during the ripening of the fruit, probably reflecting the fact that the fruit continues to grow until maturity (Steinhauser et al., 2010). For sorbitol-transporting fruit species, imported sorbitol is converted into fructose by sorbitol dehydrogenases (Park et al., 2002). In apple, for instance, fructose is stored in the vacuole or metabolized (Berüter et al., 1997). Sorbitol oxidase (Lo Bianco and Rieger, 2002) and sorbitol-6-phosphate dehydrogenase (S6PDH) (Ohkawa et al., 2008) may also play a role in sorbitol metabolism in Rosaceae fruit trees. For Cucurbitaceae, imported raffinose and stachyose are rapidly metabolized via a pathway that includes enzymes of sugar hydrolysis, phosphorylation, transglycosylation, nucleotide sugar metabolism, sucrose cleavage and synthesis, with an initial implication of α-galactosidases (Dai et al., 2011).
In the cytosol, hexoses resulting from import, degradation or export from the vacuole are phosphorylated via the reactions catalyzed by hexokinases (both hexoses) or fructokinases (fructose only). It has been proposed that the high capacities found for these enzymes in young growing fruits promote high fluxes through glycolysis (Biais et al., 2014). Hexoses phosphates are partitioned between cytosol and plastids, although, unlike leaf plastids, fruit plastids are capable of importing hexoses phosphates (Batz et al., 1995; Butowt et al., 2003). Fructose-6-phosphate is phosphorylated via the reaction catalyzed by ATP- or pyrophosphate-dependent phosphofructokinases (only the former is found in both cytosol and plastid), which enables its breakdown via glycolysis in both compartments. Results obtained in banana suggest that these enzymes are inhibited by phosphoenolpyruvate (PEP) via allosteric feedback, indicating that there is a crossed glycolytic flux control between PEP and fructose-6-phosphate, which activates PEP carboxylase (Turner and Plaxton, 2003). In both the cytosol and plastid, glucose-6-phosphate tends to equilibrate with fructose-6-phosphate and glucose-1-phosphate via the reaction catalyzed by phosphoglucose isomerase and phosphoglucomutase, respectively, which are present in both compartments. In the cytosol, glucose-1-phosphate is the precursor of uridine diphosphate glucose (UDP-glucose, via the reaction catalyzed by UDP-glucose pyrophosphorylase), precursor of cell wall (Reiter, 2002, 2008; Mohnen, 2008), ascorbate and sucrose (Reiter and Vanzin, 2001). In the chloroplast, glucose-1-phosphate is converted into adenosine diphosphate glucose (ADP-glucose), the precursor of starch. In most fruits, the acquisition of sweetness at maturation is the result of important metabolic changes leading to sugar accumulation (Bonghi and Manganaris, 2012). Of these, starch degradation is often a major source of sugars and energy as detailed below. Finally, sugar vacuolar storage is probably one of the most important, although overlooked, features regarding fruit sweetness. In particular, modeling sugar metabolism in tomato fruit suggested that tonoplastic sucrose and hexose transporters are major control points that condition fruit sugar content (Beauvoit et al., 2014), in line with dramatic alterations in fruit sugar accumulation provoked by the overexpression of a tonoplast transporters in melon (Cheng et al., 2018).
Organic Acid Metabolism
Malate, citrate, quinate, and tartrate constitute the four main organic acids accumulated to high levels in the vacuoles of fleshy fruits, during their development (DeBolt et al., 2006; Richardson et al., 2011; Tril et al., 2014; Hussain et al., 2017). In fruit, malate is mostly synthetized by the pyruvate kinase bypass, which involves the irreversible carboxylation of phosphenolpyruvate into oxaloacetate (OAA) by phosphenolpyruvate carboxylase, and OAA is subsequently reduced to malate by cytosolic NAD-dependent malate dehydrogenase (Sweetman et al., 2009; Yao et al., 2011). Citrate is produced from OAA by the TCA pathway, operating in a non-cyclic mode, which is known to take place in plants (Sweetlove et al., 2010) and evidenced in citrus fruits (Katz et al., 2011) with the involvement of mitochondrial citrate synthase (Sadka et al., 2001). Quinate is produced at a branch point of the shikimate biosynthesis pathway by the enzyme quinate dehydrogenase (Marsh et al., 2009; Gritsunov et al., 2018). It is a precursor of chlorogenic acids that are major specialized metabolites in a range of fruit species. Tartrate synthesis results from L-ascorbic acid catabolism through the Smirnoff-Weelher pathway (Melino et al., 2009). L-idonate dehydrogenase, which catalyzes a step in this pathway, is present in grape during the green stage of berry development, concomitantly with the tartrate synthesis peak (DeBolt et al., 2006). Once produced, organic acids are stored into flesh cell vacuoles thanks to an acid trap mechanism, which relies on (i) the existence of a strong pH difference between the cytosol (neutral or slightly alkaline) and the vacuole (highly acidic, pH down to 2.5 in citrus) and (ii) the existence of passive di- and tri-anions transporters on the tonoplast (De Angeli et al., 2013; Etienne et al., 2013). For citrate, the existence of a proton coupled active symporter, CsCit1, has also been reported (Shimada et al., 2006). The regulation of vacuolar malate storage has recently begun to be deciphered (Jia et al., 2018). Once the ripening phase starts, organic acids exit the vacuole and are metabolized to (i) fuel the respiration increase linked to climacteric crisis in climacteric fruits (Colombié et al., 2015) or to meet higher energy demand in non-climacteric fruits such as grapes (Sweetman et al., 2009), or (ii) produce hexoses by neoglucogenesis (Walker et al., 2015; Famiani et al., 2016).
Amino Acid Metabolism
Amino acid accumulation in developing fruits is the result of both import from phloem and xylem translocation, and in situ synthesis (Beshir and Mbong, 2017; Wang L. et al., 2017; Mechthild and Hammes, 2018). Several enzymes of amino acid biosynthesis, including, among others, glutamine synthetase, asparagine synthetase, alanine aminotransferase, and methionine synthase have been detected in global proteomic studies in developing grape berries (Wang G. et al., 2017) or by 13C-based flux variance analysis in apple (Beshir and Mbong, 2017). Beside the classical 20 amino acids, fruits can also produce other, non-proteogenic amino acids, such as GABA, which is synthesized through the GABA shunt (Bouché and Fromm, 2004), and possibly β-aminobutyric acid (Thevenet et al., 2017), or citrulline for instance in cucurbits (Fish and Bruton, 2010) including melon (Bernillon et al., 2013) that is produced from arginine (Joshi and Fernie, 2017). Amino acids are not just bricks to build protein in fruits, but also contribute to the global organoleptic qualities of fruits. For example, levels of glutamate contribute to the so-called “umami” taste of tomato (Kurihara, 2015). Amino acid catabolism has been particularly studied in fruits, as it produces numerous quality-related compounds. Phenylalanine leads to the production of polyphenols through the phenylpropanoid pathway, which have antioxidant properties and are health-promoting compounds (Butelli et al., 2008; Cirillo et al., 2014). It is also the starting point of volatile aromas (3-phenylpropanol, 2-phenethylacetate) in melon fruit (Gonda et al., 2018). Isoleucine was shown to be the precursor for 2-methylbutyl ester aromas in strawberry (Pérez et al., 2002) and methoxypyrazines in grape berries (Guillaumie et al., 2013). Thus, amino acid metabolism is a key determinant of fruit quality and palatability.
Cell Walls and Specialized Metabolites
Fruit primary metabolism also provides building blocks for the synthesis of cell-walls, and non-volatile specialized metabolites (Verpoorte, 2000) besides those mentioned above (e.g., flavonoids, alkaloids, anthocyanins, isoprenoids). Primary cell-wall precursors are mainly supplied as nucleoside diphosphate (NDP) derivatives to produce cellulose, hemicelluloses and pectins (Reiter, 2002, 2008; Mohnen, 2008). Secondary cell-wall lignin precursors, monolignols, are produced by the phenylpropanoid pathway (Zhong and Ye, 2015). Flavonoid and anthocyanin precursors are 4-coumaroyl-coenzyme A (4-coumaroyl-CoA) and malonyl-CoA molecules condensed by chalcone synthase (Jaakola, 2013). Alkaloids are a diverse family of specialized metabolites and are synthesized from various precursors. For instance, steroidal alkaloids of tomato fruit derive from cholesterol (Itkin et al., 2013), whereas tropane alkaloids of deadly nightshade come from arginine and ornithine (Sato et al., 2001). Carotenoids come from both the mevalonic (MVA) and the MVA-independent pathway. Their precursor isopentenyl-diphosphate is either produced from acetyl-CoA or pyruvate and glyceraldehyde-3-phosphate (Fraser and Bramley, 2004). Furthermore, most of these specialized metabolites are decorated with sugars and organic acids. Specialized metabolites have a role in plant defense, but their biosynthesis has a metabolic cost. Thus, allocation theory has been developed to explain resource-based trade-off between plant physiological functions (Bazzaz et al., 1987) and was confirmed experimentally at the plant level (Caretto et al., 2015).
Starch Does Not Always Accumulate Transiently During Fruit Development
Starch transient accumulation occurs during fruit development in several fleshy fruits such as strawberry, tomato, banana, kiwifruit, apple, and pear. In strawberry, starch accumulates extremely early in the fruit formation process to 3–5% dry weight, and starch degradation predominates thereafter (Moing et al., 2001; Souleyre et al., 2004). In tomato fruit, starch amount peaks at immature green stage, contributing around 20% dry weight (Schaffer and Petreikov, 1997). In apple, starch accumulation occurs continuously from 4 weeks after anthesis until maximal concentration at about 15–17 weeks, then follows a continuous net degradation (Brookfield et al., 1997). In pear, starch degradation starts several weeks before fruit harvest (Mesa et al., 2016). Though kiwifruit and bananas can accumulate more starch than the abovementioned fruit species during fruit growth, nearly 40 and 70% dry weight, respectively, a similar temporal accumulation/degradation pattern is observed (Zhang et al., 2005; Hall et al., 2013; Li and Zhu, 2017). Because of their conserved temporal profiles, starch levels are used to define a ripening index for fruit harvest in several species including apple (Doerflinger et al., 2015). In all these fruits, in addition to a temporal accumulation, starch also shows spatial distribution patterns. In tomato fruit, starch accumulates more in parenchyma (inner pericarp) than in columella (Schaffer and Petreikov, 1997). For different apple cultivars, along with fruit ripening, different spatial starch accumulation/degradation patterns, such as ring or star-shaped pattern, were observed (Szalay et al., 2013). In bananas, starch is lost from the fruit center to the banana outward (Blankenship et al., 1993). Both the temporal and spatial variations of starch in fruits are linked with sucrose-to-starch metabolic enzyme activities (Schaffer and Petreikov, 1997). For example, Shinozaki et al. (2018) showed that the genes encoding enzymes involved in starch biosynthesis, including ADP-glucose pyrophosphorylase (AGPase) and starch-branching enzyme, showed higher expression in parenchyma, which is coherent with the AGPase enzyme activity and starch amount abundance observed in tomato pericarp. Moreover, the AGPase large subunit allele from S. habrochaites is characterized by increased AGPase activity in line with higher immature fruit starch content, compared to S. lycopersicum. Near-isogenic lines resulting from the interspecific cross of S. habrochaites and S. lycopersicum allowed showing that the high-starch phenotype was related to a temporal extension of transcription of an AGPase large subunit gene that also conferred higher AGPase activity to the high-starch tomato line (Petreikov et al., 2006, 2009).
Starch plays multiple roles during fruit development. At early fruit set, it is suggested to be a carbon reserve, particularly under mild stress conditions (Ruan et al., 2012). A study on kiwifruit suggested that starch turnover occurs at early developmental stage during cell division (Nardozza et al., 2013). When tomato plants were grown under control, shading or water shortage conditions, fruit hexose and sucrose amounts were similar, but fruit starch contents showed large fluctuations during fruit growth, which suggested that starch may play a buffering role for carbon supply under different abiotic stresses (Biais et al., 2014). Fruit species that do not store carbohydrate reserves such as starch, for instance muskmelon, must remain attached to the plant for the accumulation of soluble sugars to occur during ripening (Hubbard et al., 1990). In fruit species that store starch as a reserve of carbohydrates when fruit is ripening, net starch degradation, attributed to the complex actions of a range of enzymes related to starch breakdown at transcriptional and translational levels in banana (Xiao et al., 2018), also contributes to sugar content in banana (Prabha and Bhagyalakshmi, 1998) or kiwifruit (Nardozza et al., 2013). Petreikov et al. (2009) proposed an increase in transient starch accumulation in tomatoes as a valuable strategy for increasing the sink strength of the developing fruit and its final size and sugar levels. However, starch is not always degraded at fruit maturity. A striking example is the Musa genus, where we find dessert bananas characterized by a record degradation of starch (sometimes more than 10% of the dry matter) but also the cooking banana that remains rich in starch at maturity (Hill and Ap Rees, 1994; Jourda et al., 2016).
Several Cross-Species Studies Highlight Domestication Effects as Well as Mechanisms Shared Across Plant Families
Studies comparing two or more fruit species are usually conducted with species belonging to the same genus or family. They rely on approaches ranging from simple biochemical analysis of metabolites to a combination of omics approaches. The use of introgression lines between a cultivated and a wild fruit species will not be considered in this paragraph, although a range of interesting works contributed to decipher the complexity of sugar or carboxylic acid metabolism, especially in tomato (see Ofner et al., 2016 for a summary of S. pennellii introgression lines for instance).
For comparisons within a genus, the parallel study of a cultivated species and one of its close wild relatives may provide insights into the effect of domestication on a primary metabolism pathway and its regulation. For instance, large-scale resequencing of 10 wild and 74 cultivated peach cultivars allowed comparative population genomics that showed an enrichment of gene families related to the carbohydrate metabolic process and TCA cycle within the edible group of peach genotypes (Cao et al., 2014). This work also identified a set of domestication genes, including one encoding a sorbitol-6-phosphate dehydrogenase. The draft genome of peach and whole-genome resequencing of 14 Prunus accessions paved the way to comparative and phylogenetic analyses on manually annotated gene families among peach and other sequenced species, and enabled the identification of members with specific roles in peach metabolic processes for instance for sorbitol metabolism, and stressed common features with other Rosaceae species (The International Peach Genome Initiative, Verde et al., 2013).
Regarding another Rosaceae species, apple, a large-scale biochemical study on several hundreds of accessions, revealed that fruits of wild species showed significantly higher level of ascorbic acid than fruits of cultivated species (Fang et al., 2017). Ascorbic acid content was highly positively correlated with malic acid content, but negatively correlated with fruit weight and soluble solid content. As the expression levels of three genes involved in ascorbic acid accumulation were significantly negatively correlated with ascorbic acid contents in fruits, the latter authors suggested a feedback regulation mechanism in ascorbic acid related gene expression. They attributed the differences observed for fruit ascorbic acid content between the wild and cultivated species to an indirect consequence of human selection for increased fruit size and sweetness and decreased acidity.
For tomato, a combination of genome, transcriptome, and metabolome data from several hundreds of genotypes (wild tomato, S. pimpinellifolium, S. lycopersicum var cerasiforme, and S. lycopersicum accessions) showed how breeding altered fruit metabolite contents (Zhu et al., 2018). During fruit-size targeted selection, the contents of hundreds of metabolites, including primary metabolites, were changed. The authors propose that the increased primary metabolite content between their big-fruit and their small-fruit accession-pools might be the consequence of a larger metabolic sink in domesticated fruits, and that a range of the related metabolic changes may not be caused by the fruit weight genes themselves but rather be the consequence of linked genes. A study involving S. pimpinellifolium, S. lycopersicum var cerasiforme, and S. lycopersicum (Ye et al., 2017) that used a metabolite-based genome-wide association study with linkage mapping and gene functional studies identified a malate transporter (Sl-ALMT9) as being required for malate accumulation during ripening. It also showed that tomato domestication was associated with fixation and extension of favored alleles or mutations that increased malate accumulation.
A comparison of two citrus species, mandarin and orange with a difference in ascorbate content in the pulp (Yang et al., 2011) showed that higher expression of four genes along with lower activity of oxidation enzymes contributes to higher ascorbate in orange. A comparative study of two species of two different genera (Osorio et al., 2012), tomato (climacteric) and pepper (nonclimacteric), based on transcript and metabolite data, unraveled the similarities and differences of the regulatory processes underlying ethylene-mediated signaling in these two fruit types: differences in signaling sensitivity or regulators and activation of a common set of ripening genes influencing metabolic traits.
Finally, an elegant study combining species of three different genera concerns flesh acidity (Cohen et al., 2014). After map-based cloning of Cucumis melo PH gene (encoding a membrane protein) from melon, metabolites that changed in a common and consistent manner between high- and low-acid fruits of three species from three different genera, melon, tomato and cucumber, were searched using metabolic profiling. Functional silencing of orthologous PH genes in the latter two distantly related botanical families led to fruits with low acidity, revealing that the function of PH genes is conserved across plant families.
New Tools Are Emerging for Multi-Species Comparisons
Inter-species comparisons should not be comparing apples and oranges. In this perspective, an early study highlighted the challenge of aligning the different developmental stages (Klie et al., 2014). It could be partially solved by a more systematic use of development ontologies (Jaiswal et al., 2005) for omics approaches, or by the use of metabolic modeling along development and the cross-species comparison of model topologies and model parameters.
Metabolomics profiling has been used to study fruit metabolism within and between species. Thus, comparison by metabolic profiling of 15 peach cultivars pointed to cultivar-dependent and -independent metabolic changes associated with ripening and to the identification of ripening markers (Monti et al., 2016). The latter authors propose that metabolomics, revealing compositional diversity, will help improve fruit quality. Similarly, the profiling of volatile compounds in nine fruit species revealed that differences were mostly qualitative, with only seven common compounds (Porto-Figueira et al., 2015). Classical multivariate analyses such as principal component analysis (PCA), or more elaborated ones such as STATIS, which handles multiple data tables, are being used to mine metabolite data for comparisons between species. This latter statistical analysis was used at the fruit level to compare five species based on the pattern of 16 primary metabolites, and showed that climacteric species most significantly differed from non-climacteric ones with respect to the metabolism of some sugars and amino acids (Klie et al., 2014). However, tools are still required to take full advantage of the metabolomics datasets describing fruit composition that have been or will be, collected in repositories such as MetaboLights (Haug et al., 20132) or the Metabolomics Workbench.3 Although absolute quantitative data are easily reusable and comparable, this is not the gold standard for metabolomics data collected in these repositories, which are generally relative quantification datasets. Normalization methods for appropriate comparison of those data still need to be developed.
The comparative analysis of transcriptomic profiles in varieties of climacteric and non-climacteric melon has highlighted differences, in particular for genes related to ethylene biosynthesis and signaling, but also in gene expression related to sugar metabolism. Indeed, the upward regulation of a soluble (vacuolar) acid invertase could influence the sucrose content of ripe fruit and post-harvest sucrose losses in climacteric fruit, while the upward regulation of invertase inhibitors would explain the high and stable sucrose levels in the non-climacteric variety and could be an important factor in their prolonged shelf-life (Saladié et al., 2015). A comparative study about tomato (climacteric) and pepper (non-climacteric) fruit combined analyses of transcriptomic and metabolic profiles (Osorio et al., 2012). As expected, it showed that genes involved in ethylene biosynthesis were not induced in pepper. However, genes downstream of ethylene perception, such as those implicated in fruit cell wall metabolism or carotenoid biosynthesis, were clearly induced in both Solanaceae species.
For genomics, a computational pipeline has been proposed to identify metabolic enzymes, pathways and gene clusters for about 20 plant species from their sequenced genome including tomato, grapevine and papaya fruit species (Schläpfer et al., 2017). Metabolic pathway databases were generated for 22 species and metabolic gene clusters were identified from 18 species. These vast resources can be used to conduct comparative studies of metabolism regulation between species, with the challenge to decipher organ specificities. Recently, an ambitious study about the evolution of fruit ripening involving transcriptomics, accessible chromatin study and histone and DNA methylation profiling of 11 fruit species revealed three types of transcriptional feedback circuits controlling ethylene-dependent fruit ripening (Lü et al., 2018). Similar approaches could highlight the circuits controlling primary metabolism during fruit growth.
While data on fruit metabolism of different species have been accumulated through years, their use to produce knowledge is now ranging from established statistical approaches to emerging modeling ones (Beauvoit et al., 2018). Modeling approaches involve several tools such as kinetic, stoichiometric or process-based modeling. For tomato, a kinetic metabolic model pointed to the importance of vacuolar storage for sugars (Beauvoit et al., 2014). A stoichiometric model highlighted a climacteric behavior as an emergent property of the metabolic system (Colombié et al., 2015). However, these properties are to be confirmed or infirmed for other fruit species. Recently, process-based models allowed the comparison of sugar concentration in fruits of four species or varieties and showed three species-related modes of sugar concentration control (Dai et al., 2016).
Conclusion
Although different botanical species share the same primary metabolism pathways, the regulation of these pathways is finely tuned along fruit development in particular ways in different species and results in compositional differences of the ripe fruit (Figure 1 and Table 1). These differences result from genetic and epigenetic modifications linked with evolution, adaptation of species to their environment, domestication or breeding. It seems interesting although challenging, to search whether differences between fruit species for fruit development duration are directly or indirectly related to fruit metabolic characteristics as shown for metabolic profiles and lifespan of yeast mutants (Yoshida et al., 2010), or if differences in maturation duration may be related to mitochondrial metabolism as shown for yeast mitochondrial respiration and redox state and lifespan (Barros et al., 2010). Fruit quality improvement remains one of the major objectives of recent years for breeding. Many tools have been developed to achieve this objective, for instance the use of wild genetic material, omics technology, high-throughput phenotyping or biotechnology (Gascuel et al., 2017). Possible targets to improve sugar levels for instance include adjusting the time of shifting from symplasmic to apoplasmic phloem unloading, modifying sugar vacuolar storage, increasing transient starch storage, or increasing early organic acid accumulation and late neoglucogenesis. Most of the latter targets are linked directly with primary metabolism, but fine regulation networks need further attention. In a comparative study of orange varieties (Citrus sinensis), a gene coexpression analysis showed that the sugar/acid ratio-related genes not only encoded enzymes involved in metabolism and transport but also were predicted to be involved in regulatory functions like signaling and transcription (Qiao et al., 2017).
Comparing species helps to identify metabolic factors that influence fruit growth and quality, with a view to manipulating these levels to improve fruit traits. New strategies in species comparison, for instance omics, statistics and modeling, are promising and should continue to be developed in response to the large amount of metabolic data generated by increasingly efficient quantification and identification technologies.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Pierre Pétriacq for critical reading of the manuscript.
Funding. LR was funded by the French “Agence Nationale de la Recherche” (ANR) through the FRIMOUSS project (ANR-15-CE20-0009-01). JW was funded by China Scholarship Council (CSC). We acknowledge the FRIMOUSS, MetaboHUB (ANR-11-INBS-0010) and PHENOME (ANR-11-INBS-0012) projects for further funding.
References
- Aharoni A., Galili G. (2011). Metabolic engineering of the plant primary–secondary metabolism interface. Curr. Opin. Biotechnol. 22 239–244. 10.1016/j.copbio.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Albertini M.-V., Carcouet E., Pailly O., Gambotti C., Luro F., Berti L. (2006). Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 54 8335–8339. 10.1021/jf061648j [DOI] [PubMed] [Google Scholar]
- Aune D., Giovannucci E., Boffetta P., Fadnes L. T., Keum N., Norat T., et al. (2017). Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 46 1029–1056. 10.1093/ije/dyw319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H., Yun S. K., Jun J. H., Yoon I. K., Nam E. Y., Kwon J. H. (2014). Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 87 24–29. 10.5073/jabfq.2014.087.004 [DOI] [Google Scholar]
- Baldet P., Ferrand C., Rothan C. (2014). “Vitamins in Fleshy Fruit,” in Fruit Ripening: Physiology, Signalling and Genomics, eds Nath P., Bouzayen M., Mattoo A. K., Pech J. C. (Wallingford: CABI; ), 127–150. 10.1079/9781845939625.0127 [DOI] [Google Scholar]
- Barros M. H., da Cunha F. M., Oliveira G. A., Tahara E. B., Kowaltowski A. J. (2010). Yeast as a model to study mitochondrial mechanisms in ageing. Mech. Ageing Dev. 131 494–502. 10.1016/j.mad.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Barry C. S., Giovannoni J. J. (2007). Ethylene and fruit ripening. J. Plant Growth Regul. 26 143–159. 10.1007/s00344-007-9002-y [DOI] [Google Scholar]
- Batz O., Scheibe R., Neuhaus H. E. (1995). Purification of chloroplasts from fruits of green pepper (Capsicum anuum L.) and characterization of starch synthesis. Planta 196 50–57. 10.1007/BF00193216 [DOI] [Google Scholar]
- Bazzaz F. A., Chiariello N. R., Coley P. D., Pitelka L. F. (1987). Allocating resources to reproduction and defense. BioScience 37 58–67. 10.2307/1310178 [DOI] [Google Scholar]
- Beauvoit B., Belouah I., Bertin N., Cakpo C. B., Colombié S., Dai Z., et al. (2018). Putting primary metabolism into perspective to obtain better fruits. Ann. Bot. 122 1–21. 10.1093/aob/mcy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvoit B. P., Colombie S., Monier A., Andrieu M.-H., Biais B., Benard C., et al. (2014). Model-assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion. Plant Cell 26 3224–3242. 10.1105/tpc.114.127761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernillon S., Biais B., Deborde C., Maucourt M., Cabasson C., Gibon Y., et al. (2013). Metabolomic and elemental profiling of melon fruit quality as affected by genotype and environment. Metabolomics 9 57–77. 10.1007/s11306-012-0429-1 [DOI] [Google Scholar]
- Berüter J., Feusi M. E. S., Rüedi P. (1997). Sorbitol and sucrose partitioning in the growing apple fruit. J. Plant Physiol. 151 269–276. 10.1016/S0176-1617(97)80252-0 [DOI] [Google Scholar]
- Beshir W. F., Mbong V. B. M. (2017). Dynamic labeling reveals temporal changes in carbon re-allocation within the central metabolism of developing apple fruit. Front. Plant Sci. 8:1785. 10.3389/fpls.2017.01785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biais B., Benard C., Beauvoit B., Colombie S., Prodhomme D., Menard G., et al. (2014). Remarkable reproducibility of enzyme activity profiles in tomato fruits grown under contrasting environments provides a roadmap for studies of fruit metabolism. Plant Physiol. 164 1204–1221. 10.1104/pp.113.231241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship S. M., Ellsworth D. D., Powell R. (1993). A ripening index for banana fruit based on starch content. HortTechnology 3 338–339. 10.21273/horttech.3.3.338 [DOI] [Google Scholar]
- Bonghi C., Manganaris G. A. (2012). “Systems Biology Approaches Reveal New Insights into Mechanisms Regulating Fresh Fruit Quality,” in OMICs Technologies - Tools for Food Science, ed. Benkeblia N. (Boca Raton, FL: CRC Press; ), 201–226. 10.1201/b11534-10 [DOI] [Google Scholar]
- Bouché N., Fromm H. (2004). GABA in plants: just a metabolite? Trends Plant Sci. 9 110–115. 10.1016/j.tplants.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Braun D. M., Wang L., Ruan Y.-L. (2013). Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 65 1713–1735. 10.1093/jxb/ert416 [DOI] [PubMed] [Google Scholar]
- Brookfield P., Murphy P., Harker R., MacRae E. (1997). Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest. Biol. Technol. 11 23–30. 10.1016/S0925-5214(97)01416-6 [DOI] [Google Scholar]
- Brückner H., Westhauser T. (1994). Chromatographic determination of D-amino acids as native constituents of vegetables and fruits. Chromatographia 39 419–426. 10.1007/BF02278756 [DOI] [Google Scholar]
- Burdon J. N., Sexton R. (1993). Fruit abscission and ethylene production of four blackberry cultivars (Rubus spp.). Ann. Appl. Biol. 123 121–132. 10.1111/j.1744-7348.1993.tb04079.x [DOI] [Google Scholar]
- Burroughs L. F. (1960). The free amino-acids of certain British fruits. J. Sci. Food Agric. 11 14–18. 10.1002/jsfa.2740110103 [DOI] [Google Scholar]
- Butelli E., Titta L., Giorgio M., Mock H., Matros A., Peterek S., et al. (2008). Enrichement of tomato fruit with healt-promoting anthocyanins by expression of selected transcription factors. Nat. Biotechnol. 26 1301–1308. 10.1038/nbt.1506 [DOI] [PubMed] [Google Scholar]
- Butowt R., Granot D., Rodríguez-García M. I. (2003). A putative plastidic glucose translocator is expressed in heterotrophic tissues that do not contain starch, during olive (Olea europea L.) fruit ripening. Plant Cell Physiol. 44 1152–1161. 10.1093/pcp/pcg149 [DOI] [PubMed] [Google Scholar]
- Cao K., Zheng Z., Wang L., Liu X., Zhu G., Fang W., et al. (2014). Comparative population genomics reveals the domestication history of the peach, Prunus persica, and human influences on perennial fruit crops. Genome Biol. 15 1–15. 10.1186/s13059-014-0415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretto S., Linsalata V., Colella G., Mita G., Lattanzio V. (2015). Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 16 26378–26394. 10.3390/ijms161125967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Yuan Y., Zhang C., Li H., Ma F., Li M. (2017). Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 255 40–50. 10.1016/j.plantsci.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Chen J., Wang Z., Wu J., Wang Q., Hu X. (2007). Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 104 268–275. 10.1016/j.foodchem.2006.11.038 [DOI] [Google Scholar]
- Cheng J., Wen S., Xiao S., Lu B., Ma M., Bie Z. (2018). Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation. J. Exp. Bot. 69 511–523. 10.1093/jxb/erx440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo G., Curcio M., Vittorio O., Lemma F., Restuccia D., Spirizzi U. G., et al. (2014). Polyphenol conjugates and human health: a perspective review. Crit. Rev. Food Sci. Nutr. 56 326–337. 10.1080/10408398.2012.752342 [DOI] [PubMed] [Google Scholar]
- Cohen S., Itkin M., Yeselson Y., Tzuri G., Portnoy V., Harel-Baja R., et al. (2014). The PH gene determines fruit acidity and contributes to the evolution of sweet melons. Nat. Commun. 5 1–9. 10.1038/ncomms5026 [DOI] [PubMed] [Google Scholar]
- Colombié S., Nazaret C., Bénard C., Biais B., Mengin V., Solé M., et al. (2015). Modelling central metabolic fluxes by constraint-based optimization reveals metabolic reprogramming of developing Solanum lycopersicon (tomato) fruit. Plant J. 81 24–39. 10.1111/tpj.12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N., Cohen S., Portnoy V., Tzuri G., Harel-Beja R., Pompan-Lotan M., et al. (2011). Metabolism of soluble sugars in developing melon fruit: a global transcriptional view of the metabolic transition to sucrose accumulation. Plant Mol. Biol. 76 1–18. 10.1007/s11103-011-9757-1 [DOI] [PubMed] [Google Scholar]
- Dai Z., Wu H., Baldazzi V., van Leeuwen C., Bertin N., Gautier H., et al. (2016). Inter-species comparative analysis of components of soluble sugar concentration in fleshy fruits. Front. Plant Sci. 7:649. 10.3389/fpls.2016.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z. W., Léon C., Feil R., Lunn J. E., Delrot S., Gomès E. (2013). Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 64 1345–1355. 10.1093/jxb/ers396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A., Baetz U., Francisco R., Zhang J., Chaves M. M., Regalado A. (2013). The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 238 283–291. 10.1007/s00425-013-1888-y [DOI] [PubMed] [Google Scholar]
- DeBolt S., Cook D. R., Ford C. M. (2006). L-tartaric acid synthesis from vitamin C in higher plants. Proc. Natl. Acad. Sci. U.S.A. 103 5608–5613. 10.1073/pnas.0510864103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger F. C., Miller W. B., Nock J. F., Watkins C. B. (2015). Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 110 86–95. 10.1016/j.postharvbio.2015.07.012 [DOI] [Google Scholar]
- Drake S. R., Eisele T. A. (1999). Carbohydrate and acid contents of Gala apples and Bartlett pears from regular and controlled atmosphere storage. J. Agric. Food Chem. 47 3181–3184. 10.1021/jf981228x [DOI] [PubMed] [Google Scholar]
- Etienne A., Génard M., Lobit P., Mbéguié-A-Mbéguié D., Bugaud C. (2013). What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 64 1451–1469. 10.1093/jxb/ert035 [DOI] [PubMed] [Google Scholar]
- Famiani F., Battistelli A., Moscatello S., Cruz-Castillo J. G., Walker R. P. (2015). The organic acids that are accumulated in the flesh of fruits: occurrence, metabolism and factors affecting their contents – a review. Rev. Chapingo Ser. Hortic. 21 97–128. 10.5154/r.rchsh.2015.01.004 [DOI] [Google Scholar]
- Famiani F., Farinelli D., Frioni T., Palliotti A., Batistelli A., Moscatello S., et al. (2016). Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol. Plant. 60 155–162. 10.1007/s10535-015-0574-2 [DOI] [Google Scholar]
- Fang T., Zhen Q., Liao L., Owiti A., Zhao L., Korban S. S., et al. (2017). Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 225 132–137. 10.1016/j.foodchem.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Fiehn O. (2003). Metabolic networks of Cucurbita maxima phloem. Phytochemistry 62 875–886. 10.1016/S0031-9422(02)00715-X [DOI] [PubMed] [Google Scholar]
- Fish W. W., Bruton B. D. (2010). Quantification of L-citrulline and other physiologic amino acids in watermelon and various cucurbits. Proc. Am. Soc. Hort. Sci. 10 152–154. [Google Scholar]
- Flores P., Hellín P., Fenoll J. (2012). Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 132 1049–1054. 10.1016/j.foodchem.2011.10.064 [DOI] [Google Scholar]
- Fraser P., Bramley P. M. (2004). The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 43 228–265. 10.1016/j.plipres.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Fridman E., Carrari F., Liu Y., Fernie A. R., Zamir D. (2004). Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305 1786–1789. 10.1126/science.1101666 [DOI] [PubMed] [Google Scholar]
- Fu Q., Cheng L., Guo Y., Turgeon R. (2011). Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 157 1518–1527. 10.1104/pp.111.184820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Zhao S., Lu X., He N., Liu W. (2018). ‘SW’, a new watermelon cultivar with a sweet and sour flavor. HortScience 53 895–896. 10.21273/HORTSCI12857-18 [DOI] [Google Scholar]
- Gascuel Q., Diretto G., Monforte A. J., Fortes A. M., Granell A. (2017). Use of natural diversity and biotechnology to increase the quality and nutritional content of tomato and grape. Front. Plant Sci. 8:652. 10.3389/fpls.2017.00652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda I., Davidovich-Rikanati R., Bar E., Lev S., Jhirad P., Meshulam Y., et al. (2018). Differential metabolism of L-phenylalaninein the formation of aromatic volatiles in melon (Cucumis melo) fruit. Phytochemistry 148 122–131. 10.1016/j.phytochem.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Gould N., Morrison D. R., Clearwater M. J., Ong S., Boldingh H. L., Minchin P. E. (2013). Elucidating the sugar import pathway into developing kiwifruit berries (Actinidia deliciosa). N. Z. J. Crop Hortic. Sci. 41 189–206. 10.1080/01140671.2013.801356 [DOI] [Google Scholar]
- Gourieroux A. M., Holzapfel B. P., Scollary G. R., McCully M. E., Canny M. J., Rogiers S. Y. (2016). The amino acid distribution in rachis xylem sap and phloem exudate of Vitis vinifera ‘Cabernet Sauvignon’ bunches. Plant Physiol. Biochem. 105 45–54. 10.1016/j.plaphy.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Gritsunov A., Peek J., Diaz Caballero J., Guttman D., Christendat D. (2018). Structural and biochemical approaches uncover multiple evolutionary trajectories of plant quinate dehydrogenases. Plant J. 10.1111/tpj.13989 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Guan L., Wu B., Hilbert G., Li S., Gomès E., Delrot S., et al. (2017). Cluster shading modifies amino acids in grape (Vitis vinifera L.) berries in a genotype- and tissue-dependent manner. Food Res. Int. 98 2–9. 10.1016/j.foodres.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Guillaumie S., Ilg A., Réty S., Brette M., Trossat-Magnin C., Decroocq S., et al. (2013). Genetic analysis of the biosynthesis of 2-methox-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant Physiol. 162 604–615. 10.1104/pp.113.218313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. J., Minchin P. E. H., Clearwater M. J., Génard M. (2013). A biophysical model of kiwifruit (Actinidia deliciosa) berry development. J. Exp. Bot. 64 5473–5483. 10.1093/jxb/ert317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K., Salek R. M., Conesa P., Hastings J., de Matos P., Rijnbeek M., et al. (2013). MetaboLights—an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 41 D781–D786. 10.1093/nar/gks1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F., Killiny N. (2014). Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS One 9:1830. 10.1371/journal.pone.0101830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. A., Ap Rees T. (1994). Fluxes of carbohydrate-metabolism in ripening bananas. Planta 192 52–60. 10.1007/BF00198692 [DOI] [Google Scholar]
- Hu L., Sun H., Li R., Zhang L., Wang S., Sui X., et al. (2011). Phloem unloading follows an extensive apoplasmic pathway in cucumber (Cucumis sativus L.) fruit from anthesis to marketable maturing stage. Plant Cell Environ. 34 1835–1848. 10.1111/j.1365-3040.2011.02380.x [DOI] [PubMed] [Google Scholar]
- Hu L.-P., Meng F.-Z., Wang S.-H., Sui X.-L., Li W., Wei Y.-X., et al. (2009). Changes in carbohydrate levels and their metabolic enzymes in leaves, phloem sap and mesocarp during cucumber (Cucumis sativus L.) fruit development. Sci. Hortic. 121 131–137. 10.1016/j.scienta.2009.01.023 [DOI] [Google Scholar]
- Hubbard N. L., Pharr D. M., Huber S. C. (1990). Sucrose metabolism in ripening muskmelon fruit as affected by leaf area. J. Am. Soc. Hortic. Sci. 115 798–802. 10.21273/jashs.115.5.798 [DOI] [Google Scholar]
- Hussain S. B., Shi C.-Y., Guo L.-X., Kamran H. M., Sadka A., Liu Y.-Z. (2017). Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 36 241–256. 10.1080/07352689.2017.1402850 [DOI] [Google Scholar]
- Itkin M., Heinig U., Tzfadia O., Bhide A. J., Shinde B., Cardenas P., et al. (2013). Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341 175–179. 10.1126/science.1240230 [DOI] [PubMed] [Google Scholar]
- Jaakola L. (2013). New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 18 477–483. 10.1016/j.tplants.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Jaiswal P., Avraham S., Ilic K., Kellogg E. A., McCouch S., Pujar A., et al. (2005). Plant ontology (PO): a controlled vocabulary of plant structures and growth stages. Comp. Funct. Genomics 6 388–397. 10.1002/cfg.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. H., Berg-Sørensen K., Bruus H., Holbrook N. M., Liesche J., Schulz A., et al. (2016). Sap flow and sugar transport in plants. Rev. Mod. Phys. 88:035007 10.1103/RevModPhys.88.035007 [DOI] [Google Scholar]
- Jensen K. H., Savage J. A., Holbrook N. M. (2013). Optimal concentration for sugar transport in plants. J. R. Soc. Interface 10:20130055. 10.1098/rsif.2013.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Shen F., Wang Y., Wu T., Xu X., Zhang X., et al. (2018). Apple fruit acidity is genetically diversified by natural variations in three hierarchical epistatic genes: MdSAUR37, MdPP2CH and MdALMTII. Plant J. 95 427–443. 10.1111/tpj.13957 [DOI] [PubMed] [Google Scholar]
- Joshi V., Fernie A. R. (2017). Citrulline metabolism in plants. Amino Acids 49 1543–1549. 10.1007/s00726-017-2468-4 [DOI] [PubMed] [Google Scholar]
- Jourda C., Cardi C., Gibert O., Giraldo Toro A., Ricci J., Mbéguié-A-Mbéguié D., et al. (2016). Lineage-specific evolutionary histories and regulation of major starch metabolism genes during banana ripening. Front. Plant Sci. 7:1778. 10.3389/fpls.2016.01778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Boo K. H., Eigenheer R. A., Phinney B. S., Shulaev V., Negre-Zakharov F., et al. (2011). Label-free shotgun proteomics and metabolite analysis reveal a significant metabolic shift during citrus fruit development. J. Exp. Bot. 62 5367–5384. 10.1093/jxb/err197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. G., Beppu K., Kataoka I. (2012). Physical and compositional characteristics of ‘mitsuko’ and local hardy kiwifruits in Japan. Hortic. Environ. Biotechnol. 53 1–8. 10.1007/s13580-012-0066-7 [DOI] [Google Scholar]
- Klages K., Donnison H., Boldingh H., MacRae E. (1998). myo-Inositol is the major sugar in Actinidia arguta during early fruit development. Aust. J. Plant Physiol. 25 61–67. 10.1071/PP97052 [DOI] [Google Scholar]
- Klie S., Osorio S., Tohge T., Drincovich M. F., Fait A., Giovannoni J. J., et al. (2014). Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species. Plant Physiol. 164 55–68. 10.1104/pp.113.226142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozukue N., Kozukue E., Kishiguchi M., Lee S.-W. (1978). Studies on keeping-quality of vegetables and fruits. III. Changes in sugar and organic acid contents accompanying the chilling-injury of eggplant fruits. Sci. Hortic. 8 19–26. 10.1016/0304-4238(78)90065-1 [DOI] [Google Scholar]
- Kurihara K. (2015). Umami the fith basic taste: history of studies on receptor and role as a food flavor. BioMed. Res. Int. 2015:189402. 10.1155/2015/189402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legua P., Forner J. B., Hernández F., Forner-Giner M. A. (2014). Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 174 60–64. 10.1016/j.scienta.2014.05.004 [DOI] [Google Scholar]
- Li D., Zhu F. (2017). Physicochemical properties of kiwifruit starch. Food Chem. 220 129–136. 10.1016/j.foodchem.2016.09.192 [DOI] [PubMed] [Google Scholar]
- Liesche J., Patrick J. (2017). An update on phloem transport: a simple bulk flow under complex regulation. F1000Res. 6 1–12. 10.12688/f1000research.12577.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco R., Rieger M. (2002). Partitioning of sorbitol and sucrose catabolism within peach fruit. J. Am. Soc. Hortic. Sci. 127 115–121. 10.21273/jashs.127.1.115 12642239 [DOI] [Google Scholar]
- Lü P., Yu S., Zhu N., Chen Y.-R., Zhou B., Pan Y., et al. (2018). Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants 4 784–791. 10.1038/s41477-018-0249-z [DOI] [PubMed] [Google Scholar]
- Makrogianni D. I., Tsistraki A., Karapanos I. C., Passam H. C. (2017). Nutritional value and antioxidant content of seed-containing and seedless eggplant fruits of two cultivars grown under protected cultivation during autumn-winter and spring-summer: properties of seed-containing and seedless eggplants. J. Sci. Food Agric. 97 3752–3760. 10.1002/jsfa.8238 [DOI] [PubMed] [Google Scholar]
- Marsh K. B., Boldingh H. L., Shilton R. S., Laing W. A. (2009). Changes in quinic acid metabolism during fruit development in three kiwifruit species. Funct. Plant Biol. 36 463–470. 10.1071/FP08240 [DOI] [PubMed] [Google Scholar]
- McFeeters R. F., Fleming H. P., Thompson R. L. (1982). Malic and citric acids in pickling cucumbers. J. Food Sci. 47 1859–1861. 10.1111/j.1365-2621.1982.tb12899.x [DOI] [Google Scholar]
- Mechthild T., Hammes U. (2018). The way out and in: phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 43 16–21. 10.1016/j.pbi.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Mehouachi J., Serna D., Zaragoza S., Agusti M., Talon M., Primo-Millo E. (1995). Defoliation increases fruit abscission and reduces carbohydrate levels in developing fruits and woody tissues of Citrus unshiu. Plant Sci. 107 189–197. 10.1016/0168-9452(95)04111-7 [DOI] [Google Scholar]
- Melino V. J., Soole K. L., Ford C. M. (2009). Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol. 9:145. 10.1186/1471-2229-9-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa K., Serra S., Masia A., Gagliardi F., Bucci D., Musacchi S. (2016). Seasonal trends of starch and soluble carbohydrates in fruits and leaves of ‘Abbé Fétel’ pear trees and their relationship to fruit quality parameters. Sci. Hortic. 211 60–69. 10.1016/j.scienta.2016.08.008 [DOI] [Google Scholar]
- Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. (2012). Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 77 1064–1070. 10.1111/j.1750-3841.2012.02896.x [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Gadus M. V., Madore M. A. (1992). Patterns of assimilate production and translocation in muskmelon (Cucumis melo L.). Plant Physiol. 99 959–965. 10.1104/pp.99.3.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11 266–277. 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Moing A., Carbonne F., Zipperlin B., Svanella L., Gaudillère J.-P. (1997). Phloem loading in peach: symplastic or apoplastic? Physiol. Plant. 101 489–496. 10.1111/j.1399-3054.1997.tb01028.x [DOI] [Google Scholar]
- Moing A., Renaud C., Gaudillère M., Raymond P., Roudeillac P., Denoyes-Rothan B. (2001). Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 126 394–403. 10.21273/jashs.126.4.394 [DOI] [Google Scholar]
- Moing A., Svanella L., Rolin D., Gaudillère J.-P., Monet R. (1998). Compositional changes during the fruit development of two peach cultivars differing in juice acidity. J. Am. Soc. Hortic. Sci. 123 770–775. 10.21273/jashs.123.5.770 [DOI] [Google Scholar]
- Monti L. L., Bustamante C. A., Osorio S., Gabilondo J., Borsani J., Lauxmann M. A., et al. (2016). Metabolic profiling of a range of peach fruit varieties reveals high metabolic diversity and commonalities and differences during ripening. Food Chem. 190 879–888. 10.1016/j.foodchem.2015.06.043 [DOI] [PubMed] [Google Scholar]
- Nardozza S., Boldingh H. L., Osorio S., Höhne M., Wohlers M., Gleave A. P., et al. (2013). Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J. Exp. Bot. 64 5049–5063. 10.1093/jxb/ert293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T. H., Skjaerbae H. C., Karlsen P. (1991). Carbohydrate metabolism during fruit development in sweet pepper (Capsicum annuum) plants. Physiol. Plant. 82 311–319. 10.1111/j.1399-3054.1991.tb00099.x [DOI] [Google Scholar]
- Ofner I., Lashbrooke J., Pleban T., Aharoni A., Zamir D. (2016). Solanum pennellii backcross inbred lines (BILs) link small genomic bins with tomato traits. Plant J. 87 151–160. 10.1111/tpj.13194 [DOI] [PubMed] [Google Scholar]
- Ohkawa W., Moriya S., Kanahama K., Kanayama Y. (2008). Re-evaluation of sorbitol metabolism in fruit from rosaceae trees. Acta Hortic. 772 159–166. 10.17660/ActaHortic.2008.772.19 [DOI] [Google Scholar]
- Ollat N., Diakou-Verdin P., Carde J.-P., Barrieu F., Gaudillère J.-P., Moing A. (2002). Grape berry development: a review. J. Int. Sci. Vigne Vin. 36 109–131. 10.20870/oeno-one.2002.36.3.970 [DOI] [Google Scholar]
- Osorio S., Alba R., Nikoloski Z., Kochevenko A., Fernie A. R., Giovannoni J. J. (2012). Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 159 1713–1729. 10.1104/pp.112.199711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayachee A., Day L., Howell K., Gidley M. J. (2017). Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 57 59–81. 10.1080/10408398.2013.850652 [DOI] [PubMed] [Google Scholar]
- Park S. W., Song K. J., Kim M. Y., Hwang J.-H., Shin Y. U., Kim W.-C., et al. (2002). Molecular cloning and characterization of four cDNAs encoding the isoforms of NAD-dependent sorbitol dehydrogenase from the Fuji apple. Plant Sci. 162 513–519. 10.1016/S0168-9452(01)00599-4 [DOI] [Google Scholar]
- Patrick J. W. (1997). Phloem unloading: sieve element unloading and post-sieve element transport. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 191–222. 10.1146/annurev.arplant.48.1.191 [DOI] [PubMed] [Google Scholar]
- Paul V., Pandey R., Srivastava G. C. (2012). The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene: an overview. J. Food Sci. Technol. 49 1–21. 10.1007/s13197-011-0293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez A. G., Olias R., Luaces P., Sanz C. (2002). Biosynthesis of strawberry compounds through amino acid metabolism. J. Agric. Food Chem. 50 4037–4042. 10.1021/jf011465r [DOI] [PubMed] [Google Scholar]
- Petreikov M., Shen S., Yeselson Y., Levin I., Bar M., Schaffer A. A. (2006). Temporally extended gene expression of the ADP-Glc pyrophosphorylase large subunit (AgpL1) leads to increased enzyme activity in developing tomato fruit. Planta 224 1465–1479. 10.1007/s00425-006-0316-y [DOI] [PubMed] [Google Scholar]
- Petreikov M., Yeselson L., Shen S., Levin I., Schaffer A. A., Efrati A., et al. (2009). Carbohydrate balance and accumulation during development of near-isogenic tomato lines differing in the AGPase-L1 allele. J. Am. Soc. Hortic. Sci. 134 134–140. 10.21273/jashs.134.1.134 [DOI] [Google Scholar]
- Porto-Figueira P., Freitas A., Cruz C. J., Figueira J., Câmara J. S. (2015). Profiling of passion fruit volatiles: an effective tool to discriminate between species and varieties. Food Res. Int. 77 408–418. 10.1016/j.foodres.2015.09.007 [DOI] [Google Scholar]
- Prabha T. N., Bhagyalakshmi N. (1998). Carbohydrate metabolism in ripening banana fruit. Phytochemistry 48 915–919. 10.1016/S0031-9422(97)00931-X [DOI] [Google Scholar]
- Qiao L., Cao M., Zheng J., Zhao Y., Zheng Z.-L. (2017). Gene coexpression network analysis of fruit transcriptomes uncovers a possible mechanistically distinct class of sugar/acid ratio-associated genes in sweet orange. BMC Plant Biol. 17:186. 10.1186/s12870-017-1138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel E. J., Rennie E. A., Amiard V., Cheng L., Turgeon R. (2009). Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 149 1601–1608. 10.1104/pp.108.134791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. (2008). Biochemical genetics of nucleotide sugar interconversion reactions. Curr. Opin. Plant Biol. 11 236–243. 10.1016/j.pbi.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Reiter W.-D. (2002). Biosynthesis and properties of the plant cell wall. Curr. Opin. Plant Biol. 5 536–542. 10.1016/S1369-5266(02)00306-0 [DOI] [PubMed] [Google Scholar]
- Reiter W.-D., Vanzin G. F. (2001). “Molecular genetics of nucleotide sugar interconversion pathways in plants,” in Plant Cell Walls, eds Carpita N. C., Campbell M., Tierney M. (Dordrecht: Springer; ), 95–113. 10.1007/978-94-010-0668-2_6 [DOI] [PubMed] [Google Scholar]
- Rennie E. A., Turgeon R. (2009). A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. U.S.A. 106 14162–14167. 10.1073/pnas.0902279106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A. C., Boldingh H. L., McAtee P. A., Gunaseelan K., Luo Z., Atkinson R. G., et al. (2011). Fruit development of the diploid kiwifruit, Actinidia chinensis “Hort16A.”. BMC Plant Biol. 11:182. 10.1186/1471-2229-11-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Casado A. (2016). The health potential of fruits and vegetables phytochemicals: notable examples. Crit. Rev. Food Sci. Nutr. 56 1097–1107. 10.1080/10408398.2012.755149 [DOI] [PubMed] [Google Scholar]
- Ruan Y.-L., Patrick J. W. (1995). The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 196 434–444. 10.1007/BF00203641 [DOI] [Google Scholar]
- Ruan Y.-L., Patrick J. W., Bouzayen M., Osorio S., Fernie A. R. (2012). Molecular regulation of seed and fruit set. Trends Plant Sci. 17 656–665. 10.1016/j.tplants.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Sadka A., Dahan E., Or E., Roose M. L., Marsh K. B., Cohen L. (2001). Comparative analysis of mitochondrial citrate synthase gene structure, transcript level and enzymatic activity in acidless and acid-containing Citrus varieties. Funct. Plant Biol. 28 383–390. 10.1071/PP00136 [DOI] [Google Scholar]
- Saladié M., Cañizares J., Phillips M. A., Rodriguez-Concepcion M., Larrigaudière C., Gibon Y., et al. (2015). Comparative transcriptional profiling analysis of developing melon (Cucumis melo L.) fruit from climacteric and non-climacteric varieties. BMC Genomics 16:440. 10.1186/s12864-015-1649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F., Hashimoto T., Hachiya A., Tamura K.-I., Choi K.-B., Morishige T., et al. (2001). Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 98 367–372. 10.1073/pnas.98.1.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer A. A., Petreikov M. (1997). Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 113 739–746. 10.1104/pp.113.3.739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N., Zamir D., Fernie A. R. (2004). Metabolic profiling of leaves and fruit of wild species tomato: a survey of the Solanum lycopersicum complex. J. Exp. Bot. 56 297–307. 10.1093/jxb/eri057 [DOI] [PubMed] [Google Scholar]
- Schläpfer P., Zhang P., Wang C., Kim T., Banf M., Chae L., et al. (2017). Genome-wide prediction of metabolic enzymes, pathways, and gene clusters in plants. Plant Physiol. 173 2041–2059. 10.1104/pp.16.01942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Nakano R., Shulaev V., Sadka A., Blumwald E. (2006). Vacuolar citrate/H+ symporter of citrus juice cells. Planta 224 472–480. 10.1007/s00425-006-0223-2 [DOI] [PubMed] [Google Scholar]
- Shinozaki Y., Nicolas P., Fernandez-Pozo N., Ma Q., Evanich D. J., Shi Y., et al. (2018). High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 9 1–13. 10.1038/s41467-017-02782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souleyre E. J. F., Iannetta P. P. M., Ross H. A., Hancock R. D., Shepherd L. V. T., Viola R., et al. (2004). Starch metabolism in developing strawberry (Fragaria x ananassa) fruits. Physiol. Plant. 121 369–376. 10.1111/j.0031-9317.2004.0338.x [DOI] [PubMed] [Google Scholar]
- Steinhauser M. C., Steinhauser D., Koehl K., Carrari F., Gibon Y., Fernie A. R., et al. (2010). Enzyme activity profiles during fruit development in tomato cultivars and Solanum pennellii. Plant Physiol. 153 80–98. 10.1104/pp.110.154336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. A., El-Shishiny E. D. H. (1958). Translocation of sugars in the concord grape. Plant Physiol. 33:33. 10.1104/pp.33.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove L. J., Beard K. F. M., Nunes-Nesi A., Fernie A. R., Ratcliffe R. G. (2010). Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci. 15 462–470. 10.1016/j.tplants.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Sweetman C., Deluc L. G., Cramer G. R., Ford C. M., Soole K. L. (2009). Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 70 1329–1344. 10.1016/j.phytochem.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Szalay L., Ordidge M., Ficzek G., Hadley P., Tóth M., Battey N. H. (2013). Grouping of 24 apple cultivars on the basis of starch degradation rate and their fruit pattern. Hortic. Sci. 40 93–101. 10.17221/143/2012-HORTSCI [DOI] [Google Scholar]
- Tegeder M., Hammes U. Z. (2018). The way out and in: phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 43 16–21. 10.1016/j.pbi.2017.12.002 [DOI] [PubMed] [Google Scholar]
- The International Peach Genome Initiative. Verde I., Abbott A. G., Scalabrin S., Jung S., Shu S., et al. (2013). The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 45 487–494. 10.1038/ng.2586 [DOI] [PubMed] [Google Scholar]
- Thevenet D., Pastor V., Baccelli I., Balmer A., Vallat A., Neier R., et al. (2017). The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 213 552–559. 10.1111/nph.14298 [DOI] [PubMed] [Google Scholar]
- Tril U., Fernández-López J., Pérez Alvarez J. A., Vuida-MArtos M. (2014). Chemical, physiochemical, technological, antibacterialand antioxydant properties of rich-fibre powder extract obtained from tamarind (Tamarindus indica L.). Ind. Crops Prod. 55 155–162. 10.1016/j.indcrop.2014.01.047 [DOI] [Google Scholar]
- Turner W. L., Plaxton W. C. (2003). Purification and characterization of pyrophosphate- and ATP-dependent phosphofructokinases from banana fruit. Planta 217 113–121. [DOI] [PubMed] [Google Scholar]
- Usenik V., Fabčič J., Štampar F. (2008). Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 107 185–192. 10.1016/j.foodchem.2007.08.004 [DOI] [Google Scholar]
- Verpoorte R. (2000). “Secondary Metabolism,” in Metabolic Engineering of Plant Secondary Metabolism, eds Verpoorte R., Alfermann A. W. (Dordrecht: Springer; ), 1–29. 10.1007/978-94-015-9423-3_1 [DOI] [Google Scholar]
- Walker R. P., Batistelli A., Moscatello S., Técsi L., Leegood R. C., Famiani F. (2015). Phosphoenolpyruvate carboxykinase and gluconeogenesis in grape pericarp. Plant Physiol. Biochem. 97 62–69. 10.1016/j.plaphy.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Wang G., Xu M., Wang W., Gad G. (2017). Fortifying horticutural crops with essential amino acids: a review. Int. J. Mol. Sci. 18:1306. 10.3390/ijms18061306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Sun X., Weiszmann J., Wolfram W. (2017). System-level and Granger network analysis of integrated proteomic and metabolomic dynamics identitfies key points of grape berry develoment at the interface of primary and secondary metabolism. Front. Plant Sci. 8:1066. 10.3389/fpls.2017.01066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. C., Huang H. B., Huang X. M., Hu Z. Q. (2006). Sugar and acid compositions in the arils of Litchi chinensis Sonn.: cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 81 57–62. 10.1080/14620316.2006.11512029 [DOI] [Google Scholar]
- Wang L., He F., Huang Y., He J., Yang S., Zeng J., et al. (2018). Genome of wild mandarin and domestication history of mandarin. Mol. Plant 11 1024–1037. 10.1016/j.molp.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Wang P.-Y., Fang J.-C., Gao Z.-H., Zhang C., Xie S.-Y. (2016). Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J. Diabetes Investig. 7 56–69. 10.1111/jdi.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wyllie S. G., Leach D. N. (1996). Chemical changes during the development and ripening of the fruit of Cucumis melo (Cv. Makdimon). J. Agric. Food Chem. 44 210–216. 10.1021/jf9503568 [DOI] [Google Scholar]
- Whiting G. C. (1958). The non-volatile organic acids of some berry fruits. J. Sci. Food Agric. 9 244–248. 10.1002/jsfa.2740090411 [DOI] [Google Scholar]
- Wills R. B. H., Scriven F. M., Greenfield H. (1983). Nutrient composition of stone fruit (Prunus spp.) cultivars: Apricot, cherry, nectarine, peach and plum. J. Sci. Food Agric. 34 1383–1389. 10.1002/jsfa.2740341211 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Kuang J., Qi X., Ye Y., Wu Z.-X., Chen J., et al. (2018). A comprehensive investigation of starch degradation process and identification of a transcriptional activator MabHLH6 during banana fruit ripening. Plant Biotechnol. J. 16 151–164. 10.1111/pbi.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-Y., Xie J.-X., Wang F.-F., Zhong J., Liu Y.-Z., Li G.-H., et al. (2011). Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J. Plant Physiol. 168 2196–2205. 10.1016/j.jplph.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Yao Y.-X., Li M., Zhei H., You C.-X., Hao Y.-J. (2011). Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J. Plant Physiol. 168 474–480. 10.1016/j.jplph.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Ye J., Wang X., Hu T., Zhang F., Wang B., Li C., et al. (2017). An InDel in the promoter of Al-ACTIVATED MALATE TRANSPORTER9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 29 2249–2268. 10.1105/tpc.17.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S., Hewitt J. D., Robinson N. L., Damon S., Bennett A. B. (1988). Sink metabolism in tomato fruit?: III. Analysis of carbohydrate assimilation in a wild species. Plant Physiol. 87 737–740. 10.1104/pp.87.3.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Tamura T., Takaoka C., Harada K., Kobayashi A., Mukai Y., et al. (2010). Metabolomics-based systematic prediction of yeast lifespan and its application for semi-rational screening of ageing-related mutants: semi-rational screening of ageing-related mutants. Aging Cell 9 616–625. 10.1111/j.1474-9726.2010.00590.x [DOI] [PubMed] [Google Scholar]
- Zhang H., Ge Y. (2016). Dynamics of sugar-metabolic enzymes and sugars accumulation during watermelon (Citrullus lanatus) fruit developement. Pak. J. Bot. 48 2535–2538. [Google Scholar]
- Zhang L.-Y., Peng Y.-B., Pelleschi-Travier S., Fan Y., Lu Y.-F., Lu Y.-M., et al. (2004). Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 135 574–586. 10.1104/pp.103.036632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Whistler R. L., BeMiller J. N., Hamaker B. R. (2005). Banana starch: production, physicochemical properties, and digestibility—a review. Carbohydr. Polym. 59 443–458. 10.1016/j.carbpol.2004.10.014 [DOI] [Google Scholar]
- Zhang X.-Y., Wang X.-L., Wang X.-F., Xia G.-H., Pan Q.-H., Fan R.-C., et al. (2006). A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 142 220–232. 10.1104/pp.106.081430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li P., Cheng L. (2010). Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 123 1013–1018. 10.1016/j.foodchem.2010.05.053 [DOI] [Google Scholar]
- Zhong R., Ye Z.-H. (2015). Secondary cell walls: biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 56 195–214. 10.1093/pcp/pcu140 [DOI] [PubMed] [Google Scholar]
- Zhu G., Wang S., Huang Z., Zhang S., Liao Q., Zhang C., et al. (2018). Rewiring of the fruit metabolome in tomato breeding. Cell 172 249.e12–261.e12. 10.1016/j.cell.2017.12.019 [DOI] [PubMed] [Google Scholar]


