Abstract
Introduction:
Cavity disinfectants help to remove the microbial remnants; hence, its use prior to any restoration is valuable, and a search for alternative to chlorhexidine (CHX) is required which may be more efficacious and can overcome the drawbacks of CHX.
Objective:
The aim of this study was to evaluate the effect of application of three different cavity disinfectants in a clinically relevant time period on the immediate and delayed shear bond strengths (SBSs) of an etch-and-rinse adhesive system to dentin.
Materials and Methods:
For SBS testing, flat coronal dentin surfaces were prepared in two hundred extracted human molars. Specimens were randomly assigned to four groups according to the disinfectant used: Group I: Control (no disinfectant), Group II: 2% CHX solution, Group III: Aloe barbadensis miller (Aloe vera) solution, and Group IV: Azadirachta indica (neem) solution. Specimens were bonded using Prime and Bond NT adhesive which was employed according to the manufacturer's instructions. Resin composite cylinder buildups were done in all the samples. The modes of failure were noted after visual examination using a binocular stereomicroscope. Samples were also analyzed under scanning electron microscope for observation of resin-dentin interface. SBS results were analyzed using one-way ANOVA followed by Tukey's post hoc test.
Results:
The results showed that CHX, Aloe vera, and neem had improved bond strengths as compared to the control group for both immediate and delayed SBSs.
Conclusion:
From the results of the study, the authors concluded that Aloe vera and neem can be used as alternative cavity disinfectants to CHX.
Keywords: Antimicrobial efficacy, Azadirachta indica, chlorhexidine, matrix metalloproteinases, microleakage
INTRODUCTION
During cavity preparation, the success of restorative treatment can be affected by microbial remnants in the cavity walls. The degree of success in the elimination of microorganisms during cavity preparation and prior to the insertion of a restoration may increase the longevity of the restoration and therefore the success of the restorative procedure. Attempts at the complete removal of deep carious dentin, by solely mechanical means, may result in pulpal violation and/or gross destruction of the tooth structure and has failed to generate a completely caries-free cavity.[1,2] Therefore, disinfection of the cavity preparation after caries excavation can aid in the elimination of microbial remnants which may survive and multiply, especially in the presence of microleakage that can be responsible for pulpal irritation, risk of recurrent caries, or postoperative sensitivity and therefore failure of the dental restoration.[3]
The fundamental goal of adhesive dentistry is to create an effective, durable union between tooth structure and the restorative material.[4] The resin-to-dentin adhesion occurs through the infiltration into and polymerization of hydrophilic resins within the collagen matrix exposed through acid decalcification of dentin, thus forming a hybrid layer. In case of etch-and-rinse adhesives, it is pertinent that the adhesive resin monomer penetrates the acid-etched exposed dentin collagen fibrils. However, studies have revealed that this goal is rarely accomplished.[5]
Certain mechanisms have been proposed to improve dentin adhesion, i.e., adjunctive collagen pretreatment, matrix metalloproteinase (MMP) inhibitors, and so forth. MMPs and cysteine cathepsins which are found in human dentin contribute to the degradation of denuded collagen within the hybrid layer.
Chlorhexidine digluconate (CHX) is a biguanide biocide that hinders the formation and progression of dental plaque and has been utilized as an oral antimicrobial agent and is considered the “gold standard” of oral antiseptics. It also has an inhibitory effect on the MMPs (against MMPs 2, 8, and 9) and cathepsins in the dentin. These effects can be useful in preventing collagen degradation and disintegration of the bonding interface over time.[6]
To overcome these limitations, alternate cavity disinfectants, which are safe, effective, and economic, are need of the hour. Aloe barbadensis Miller (Aloe vera) is a perennial succulent plant resembling a cactus, with green dagger-shaped fleshy, spiny, and marginated leaves, filled with a clear viscous gel belonging to the Aloeacease family (subfamily of the Asphoelaceae). Aloe vera gel and aloins also were effective inhibitors of stimulated granulocyte MMPs 2 and 9.[7] Furthermore, antimicrobial adequacy of Aloe vera has been demonstrated by many researchers.[8]
Azadirachta indica (neem) is an evergreen tree belonging to the Meliaceae (mahogany) family with potential medicinal value and is still regarded as “village dispensary” in India. Besides the antimicrobial action, this group of compounds also demonstrates anti-inflammatory efficacy. Aqueous extract of neem reduces the ability of streptococci to colonize tooth surfaces.[9] Furthermore, MMPs 2 and 9 were found to be inhibited by neem,[7] so it can be a good herbal alternative to be used as a cavity disinfectant. This is the first study of its kind to test A. indica (neem) for resin-dentin bond stabilization.
The aim and objective of the present study was to comparatively evaluate CHX, Aloe barbadensis Miller (Aloe vera), and A. indica (neem) for resin-dentin bond stabilization when used as cavity disinfectants and to examine the null hypothesis that pretreatment with herbal disinfectants will not produce any significant differences in the immediate and delayed dentin bond strengths.
MATERIALS AND METHODS
Two hundred freshly extracted noncarious, human mandibular molars were thoroughly cleaned and stored in distilled water until use. Teeth were randomly divided into four experimental groups of 50 teeth each according to four different surface pretreatments (n = 50). Tooth crowns were flattened occlusally using a low-speed diamond disc and mandrel (Markus Ink., Michigan, USA) under water irrigation to expose superficial dentin. The samples were embedded in an autopolymerizing resin (DPI-RR, India) at the level of cementoenamel junction with long axes perpendicular to the acrylic resin surface to form cylinders 2.5 cm in diameter and 5 cm high. A standardized smear layer was created with 1200-grit silicon carbide paper (Oakey's Abrasives, John Oakey and Mohan Ltd., India). An adhesive tape with a 3 mm central hole was positioned on the flat dentin surface to demarcate the pretreatment and bonding area. Application of the cavity disinfectants for 30 s, i.e., 2% CHX (Basic Pharma, Gujarat, India), Aloe vera solution (prepared using Aloe vera powder [Bioprex Labs, Pune, Maharashtra, India] of 99% purity and dissolving 20 mg of Aloe vera powder in 10 ml of distilled water),[7] and neem solution (prepared by mixing 15 g of dry powder of neem leaves [The Indian Neem Tree Company, Mumbai] with 100 mL of sterile distilled water in round bottom flask with occasional shaking and were then filtered through muslin cloth for coarse residue and then through a Whatman no. 1 filter paper and kept in an airtight amber-colored container)[9] in Groups 2, 3, and 4, respectively. All the samples were subjected to acid-etching procedure with Scotchbond Universal Etchant (3M ESPE Dental Products, St. Paul, MN, USA) for 15 s, followed by rinsing with water for 10 s and blot dried. Prime & Bond NT (Dentsply, Caulk, Germany) was used as bonding agent and was applied to enamel/dentin surface with applicator tip for 20 seconds, using light brushing motion. It was air dried for 5 seconds and then light cured for 10 seconds. Nanohybrid resin composite was applied in 5–6 increments (Filtek Z350, 3M ESPE, St. Paul, MN, USA) with the aid of polyethylene tubes (TYGON laboratory tubing, Saint Gobain, Akron, OH, USA; 3 mm diameter, 2 mm height, and 0.5 mm thickness)[10] and individually light cured for 40 s using light curing unit Spectrum 800 (Dentsply, Caulk, Milford, USA) with an output of 600 mW/cm2. The tubes were then removed. Half of the specimens (immediate testing group) were then stored in distilled water at 37°C for 24 h for completion of polymerization before immediate testing and scanning electron microscopic (SEM) analysis. The remaining half samples from each group (delayed testing group) were stored in distilled water at 37°C in an incubator for 6 months. Storage solution was changed every 2 weeks.
For shear bond strength (SBS) testing, each tooth was secured in a specially designed attachment jig to hold the specimens to the universal testing machine (Instron, ADMET, Enkay Enterprises, New Delhi, India). Load was applied by the testing machine through a wire loop adjusted to the bonded interface at a crosshead speed of 0.5 mm/min. SBS in MPa was calculated from the peak load at failure divided by the specimen surface area.
After testing, the fracture modes were evaluated under a stereomicroscope (Olympus, Zoom type) and classified according to the predominant mode of fracture as an adhesive fracture at the resin-cement dentin interface, cohesive fracture in the resin cement, cohesive fracture in dentin, or mixed adhesive and cohesive fracture in the resin cement.
RESULTS
Data analysis was performed using the parametric test “one-way ANOVA test” (for comparing more than two groups). Paired t-test was used for comparing two paired readings. Level of statistical significance was set at P < 0.05. Post hoc Tukey's test was used for pairwise comparison of subgroups (ANOVA test is positive, i.e., P is less than the selected significance level) [Table 1]. Fractographical analysis showed that the control group showed more adhesive and CHX, Aloe vera, and neem showed mixed and cohesive failures both [Table 2].
Table 1.
Intragroup comparison: Comparison of immediate and delayed shear bond strengths among four groups
| Group | Cavity disinfectants used | Mean (SD) |
P | |
|---|---|---|---|---|
| Immediate | Delayed | |||
| Group I | Control | 16.04 (1.39) | 8.02 (0.70) | 0.001* |
| Group II | CHX | 20.01 (1.06) | 15.94 (1.38) | 0.001* |
| Group III | Aloe vera | 20.06 (1.01) | 15.40 (1.17) | 0.001* |
| Group IV | Neem | 16.53 (1.00) | 8.27 (0.50) | 0.001* |
By paired t-test, *Significance of relationship at P<0.05. CHX: Chlorhexidine, SD: Standard deviation
Table 2.
Percentage failures of samples treated with cavity disinfectants
| Cohesive failures (%) | Adhesive failures (%) | Mixed failures (%) | |
|---|---|---|---|
| Immediate failures | |||
| Control | 30 | 40 | 30 |
| CHX | 60 | 10 | 30 |
| Aloe vera | 80 | 10 | 10 |
| Neem | 60 | 20 | 20 |
| Delayed failures | |||
| Control | 30 | 40 | 30 |
| CHX | 50 | 20 | 30 |
| Aloe vera | 40 | 10 | 50 |
| Neem | 60 | 20 | 20 |
CHX: Chlorhexidine
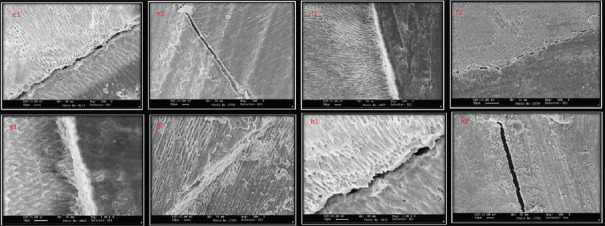
SEM showed that there were more interfacial gaps seen with control group when treated with Prime and Bond NT at both the time intervals. Neem showed weaker interfaces in delayed samples whereas the strongest interfaces were seen with CHX and Aloe vera groups at both time intervals [Figures 1 and 2].
Figure 1.
(a1 and a2) A representative scanning electron microscopic image of bonded interface in control group. Notice the gap between dentin and restoration (×500). (b1 and b2) A representative scanning electron microscopic image of bonded interface in chlorhexidine group where gap-free interface can be observed (×500). (c1 and c2) A representative scanning electron microscopic image of bonded interface inAloe veragroup where gap-free interface can be observed (×500). (d1 and d2) A representative scanning electron microscopic image of bonded interface in neem group where gap-free interface can be observed (×500)
Figure 2.
(e1 and e2) A representative scanning electron microscopic image of bonded interface in control group. Notice the larger gap between dentin and restoration (×500). (f1 and f2) A representative scanning electron microscopic image of bonded interface in chlorhexidine group where gap-free interface can be observed (×500). (g1 and g2) A representative scanning electron microscopic image of bonded interface inAloe veragroup where gap-free interface can be observed (×500). (h1 and h2) A representative scanning electron microscopic image of neem group where interfacial gap can be observed (×1000)
DISCUSSION
MMPs are secreted as proenzymes (zymogens) and are activated by proteinases or some chemical agents, including reactive oxygen species. MMPs can also be activated by low pH,[11] probably through the disruption of cysteine–zinc binding. New strategies to prevent the degradation of dentin bonds may be essential to increase the longevity of bonded restorations. Therefore, the use of exogenous MMP inhibitors, such as CHX, epigallocatechin-3-gallate, gallardin (MMP inhibitor (GM6001)), tetracyclines and analogs, quaternary ammonium salts, and flavonols, may be an effective strategy to enhance the longevity of adhesive restorations.[12,13]
The present in vitro study showed that CHX improved strength with Prime Bond NT. These results are in accordance with studies conducted by Dalli et al.,[14] Sinha et al.,[10] Ahmed et al., and Boiter et al.[15] Using a CHX before etching was shown not to affect bonding to dentin, however, reduced dentin bond strengths usually when a CHX was used after etching because of material interactions. These findings are in agreement with those obtained by Perdigao et al.[16] These results are in contrast with a study conducted by Vieira Rde and da Silva[17] who showed that a cavity disinfectant containing CHX significantly lowered the bond strength. This might be attributed to its inhibitory action on MMPs, thus stabilizing and preserving the integrity of the hybrid layer, thereby inducing a positive effect on bond strength. However, CHX has some inherent side effects that limit its widespread acceptance, for example, contact dermatitis, brown staining of the teeth, and taste alterations which gave emergence to the use of certain herbal cavity disinfectants such as Aloe barbadensis miller (Aloe vera) and A. indica (neem).
The antibacterial effects of Aloe vera have been attributed to various pharmacologically active constituents. The antibacterial action of Aloe barbadensis miller had been proved in a study by Pandey R and Mishra A in 2010. Its bactericidal activity is because of anthraquinone.[18] The presence of coumaric acid was also reported, in the studies of Baranowski et al., to increase the lag phase of the microorganisms, and it was also able to inhibit the enzymatic activity of the microorganisms.[19] Cinnamic acid in Aloe vera gel can inhibit glucose uptake and adenosine triphosphate production in the resting cells of bacteria. Aloe vera also possesses anti-MMP potential, especially against MMP-2 and MMP-9 proven by Barrantes and Guinea. A study conducted by Kudalkar et al.[7] also showed that Aloe vera has an inhibitory effect on MMPs due to the presence of the anthraquinones. In this study, Aloe vera showed a good long-term stabilizing effect on the bond strength. Aloe vera showed good bond strength as it increases the collagen content of the granulation tissue as well as its degree of crosslinking.[20]
The other herbal alternative used in the present study was A. indica (neem). The presence of bioactive constituents such as nimbidin, nimbin, nimbidic acid, nimbolide, gedunin, azadirachtin, mahmoodin, margolone, cyclic trisulfide, alkaloids, glycosides, terpenoids, steroids, tannins, alkaloid margosine, resins, gum, chloride, flouride, silica, sulfur, oils, saponins, flavanoids, calcium, quercetin, and ß-sitosterol which contribute to antimicrobial property.[21,22] Prashant et al. demonstrated that neem stick extract produced a maximum zone of inhibition against Streptococcus mutans at 50% concentration.[23] From the previous studies, MMPs 2 and 9 were also found to be inhibited by neem. The results of the study conducted by Kudalkar et al. showed that mean inhibition of MMPs 2 and 9 by neem was 53.5% and 52.5%, respectively.[7] As pretreatment with the herbal agents used was able to maintain immediate and delayed dentin bond strengths, there is not enough evidence to reject the null hypothesis.
Despite significant improvements of adhesive systems, bonded interface remains the weakest area of tooth-colored restorations. If dentin/adhesive interface is exposed to oral cavity-marginal discoloration, poor marginal adaptation and subsequent loss of retention and restoration are frequent clinical findings. The ideal mode of failure is a 100% cohesive failure in the adhesive layer. In the present study, all the experimental groups exhibited more number of cohesive and mixed failures as compared to adhesive failures.
CONCLUSION
Under the limitations of this in vitro study, it can be concluded that all the cavity disinfectants appear to show promising approach to improve resin-dentin bond durability and thus to enhance the clinical longevity of adhesive composite restorations. Aloe barbadensis miller (Aloe vera) can be used as an alternative to CHX, although long-term in vivo studies are further warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HH. Effects of antibacterial primers with quaternary ammonium and Nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater. 2013;29:462–72. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singla M, Aggarwal V, Kumar N. Effect of chlorhexidine cavity disinfection on microleakage in cavities restored with composite using a self-etching single bottle adhesive. J Conserv Dent. 2011;14:374–7. doi: 10.4103/0972-0707.87201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brännström M. The cause of postrestorative sensitivity and its prevention. J Endod. 1986;12:475–81. doi: 10.1016/S0099-2399(86)80202-3. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca BM, Pleffken PR, Balducci I, Pucci CR, Tay FR, Araujo MA. New trends in dentin bonding: Treatment with chlorhexidine, hyaluronic acid, Vitamin C and green tea. Braz Dent Sci. 2013;16:56–62. [Google Scholar]
- 5.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 6.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 7.Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: An in vitro study. Ayu. 2014;35:85–9. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein RA, Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46:274–81. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 9.Nayak A, Ranganathan N, Sowmya GB, Kishore B, Kudalkar MD. Evaluation of antibacterial and anticandidial efficacy of aqueous and alcoholic effect of neem (Azadirachta indica): An in vitro study. Int J Res Ayurveda Pharm. 2011;2:2305. [Google Scholar]
- 10.Sinha DJ, Jaiswal N, Vasudeva A, Garg P, Tyagi SP, Chandra P. Comparative evaluation of the effect of chlorhexidine and Aloe barbadensis miller (Aloe vera) on dentin stabilization using shear bond testing. J Conserv Dent. 2016;19:406–9. doi: 10.4103/0972-0707.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 12.Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 13.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–32. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Dalli M, Ercan E, Zorba YO, İnce B, Şahbaz C, Bahşi E. Effect of 1% chlorhexidine gel on the bonding strength to dentin. J Dent Sci. 2010;5:8–13. [Google Scholar]
- 15.Boiter CG, Stoica F, Mitariu MC, Burlibasa M, Stef L. In vitro influence of 2% chlorhexidine on links established at the hybrid layer between collagen fibres and Nano adhesives used in adhesive system. Afr J Biotechnol. 2013;12:1438–42. [Google Scholar]
- 16.Perdigao J, Denehy GE, Swift EJ., Jr Effects of chlorhexidine on dentin surfaces and shear bond strengths. Am J Dent. 1994;7:81–4. [PubMed] [Google Scholar]
- 17.Vieira Rde S, da Silva IA., Jr Bond strength to primary tooth dentin following disinfection with a chlorhexidine solution: An in vitro study. Pediatr Dent. 2003;25:49–52. [PubMed] [Google Scholar]
- 18.Pandey R, Mishra A. Antibacterial activities of crude extracts of Aloe barbadensis to clinically isolated bacterial pathogens. Appl Biochem Biotechnol. 2010;160:1356–61. doi: 10.1007/s12010-009-8577-0. [DOI] [PubMed] [Google Scholar]
- 19.Weir TL, Park SW, Vivanco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–9. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol Cell Biochem. 1998;181:71–6. doi: 10.1023/a:1006813510959. [DOI] [PubMed] [Google Scholar]
- 21.Govindachari TR, Suresh G, Gopalakrishnan G, Banumathy B, Masilamani S. Identification of antifungal compounds from the seed oil of Azadirachta indica. Phytoparasitica. 1998;26:109–16. [Google Scholar]
- 22.Ghosh A, Chakrabarti RP, Bhadury S, Nag T, Sarkar S. Bioremidation of heavy metals from neem leaf extract by chelation with dithizone. Asian Pharm Clin Res. 2009;2:87–92. [Google Scholar]
- 23.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]