Abstract
The neural substrates of memory for when events occurred are not well described. One reason for this is that the paradigms used to date have permitted isolation of only some of the relevant memory processes. In this experiment, functional magnetic resonance imaging was used to identify for the first time brain regions that support two distinct bases upon which “when” judgments can be made. Seventeen human participants (6 male) completed a continuous recognition memory task where the interval between presentation and re-presentation of words varied between 5 and 25 intervening words (the lag). The task on each trial was to distinguish repeated words from those presented for the first time, and to indicate the lag for repeated words. The inferior parietal lobe showed greater activation for shorter lag judgments, regardless of judgment accuracy. The lingual gyrus, by contrast, was more active for correct than for incorrect lag judgments, regardless of the interval between first and second item presentations. Both of these regions have been linked in previous work to the process of recollection, and the findings described here represent a novel neural dissociation across regions that deploy mnemonic information in fundamentally different ways to support judgments about when events occurred.
Introduction
Episodic memory is considered to support memories for events and the spatiotemporal contexts in which they occurred (Tulving, 1983). Episodic memory thus supports judgments about what happened, as well as where and when events happened (Clayton and Dickinson, 1998; Tulving, 2002). The focus here is on the brain regions that underpin “when” judgments. The work is motivated by an influential cognitive account in which placing an event accurately in time can be done by relying on distance-based as well as location-based processes (Friedman, 1993, 1996, 2001).
Judgments associated with distance-based processes involve an assessment of memory “strength” or “volume.” The key assumption is that memory strength, and/or the number of details that are recovered can be used heuristically to make “when” judgments: strong or detailed memories signal events that are likely to have occurred relatively recently (Hinrichs and Buschke, 1968; Hinrichs, 1970). Location-based processes, by contrast, rely on recovery of relevant contextual details that permit an event to be located accurately in time: I know the event occurred on Friday because I remember that Jim was there and he only works on Fridays.
The definitions of these two classes of process generate predictions for how neural activity will differ if it underpins either location- or distance-based means of making “when” judgments. For distance-based processes, activity should increase as judgments become more recent. For location-based processes, activity should be greater for correct than for incorrect judgments, regardless of how long ago an event actually occurred.
These predictions have not, to our knowledge, been tested using functional magnetic resonance imaging (fMRI) before. Published fMRI studies of memory for when events occurred have, for the most part, required forced-choice judgments between pairs of stimuli that were presented at different time points in prior study phases (Zorrilla et al., 1996; Cabeza et al., 2000; Konishi et al., 2002, 2006; Dobbins et al., 2003; Rajah and McIntosh, 2006; Dudukovic and Wagner, 2007; Kimura et al., 2010; Rajah et al., 2010). These studies contain no reports of analyses that permit a separation between neural activities that might underlie distance- and location-based processes.
Isolating these two classes of process was possible in the experiment described here, where participants completed a continuous verbal recognition memory task in which critical words were repeated after either 10 or 20 intervening words (hereafter referred to as the lag). Participants made old/new judgments, and then a lag judgment for words designated as old. There were five lag judgment options: 5, 10, 15, 20, and 25. This design enables the identification of brain regions supporting distance-based processes, for which greater activity should accompany shorter lag judgments, regardless of the accuracy of the judgment. For regions supporting location-based processes, greater activity should accompany correct lag judgments, regardless of the actual lag of a test word.
Materials and Methods
Participants.
Twenty healthy, right-handed, native English speakers took part in the experiment, receiving payment of £15/h. Data from 17 participants (6 males, mean age = 23, range = 19–35) are presented below. One participant was excluded due to a technical error, and two due to excessive movement artifacts. Informed consent was obtained in a manner approved by Cardiff University.
Materials.
Stimuli were 237 low-frequency nouns obtained from the MRC psycholinguistic database (4–9 letters; Kucera-Francis written frequency, 1–7 per million; imageability range, 500–600; www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm).
Words were presented in two blocks, each containing 109 words. Of these, 102 were repeated within the block. Forty-five were repeated after ten intervening words and 45 after 20 intervening words. A further four words were repeated after 5, 15, or 25 intervening words. An additional seven words were shown only once toward the end of each block. A further 19 words, 14 of which were repeated, were shown in an initial practice session. In total, participants saw 455 stimulus presentations (211 words per block, 33 in the practice session). Two different test lists were constructed to counterbalance the lag (10 vs 20) at which the critical test words were encountered. Word order in each list was determined pseudo-randomly.
Procedure.
Participants completed an initial practice session outside the scanner. In the scanner, stimuli were displayed foveally in uppercase white letters against a black background, subtending maximum visual angles of 6.5° horizontally and 3.2° vertically. Stimuli were back-projected onto a screen viewed by participants through a mirror incorporated into the head coil. E-Prime software (Psychological Software Tool) was used for stimulus presentation and response logging. Responses were made on a magnetic resonance-compatible, five-button response box placed under the right hand.
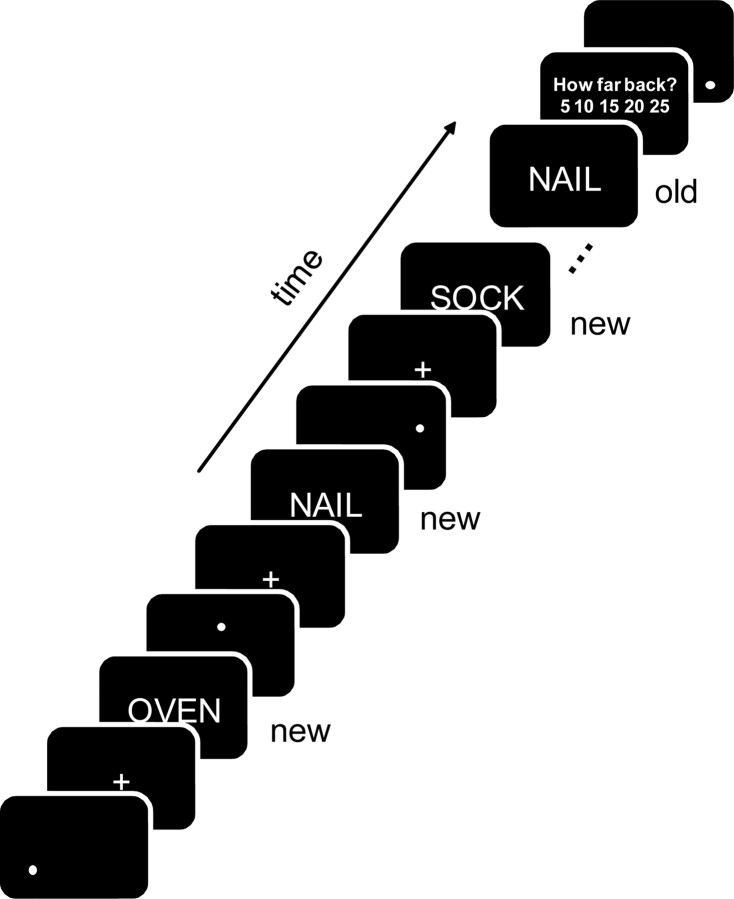
The scanning session was divided into two runs, each lasting ∼20 min. Individual trials were separated by a variable length interval during which a moving dot appeared on the screen (2000 ms average duration, range = 0–4000 ms). Participants were asked to fixate on the moving dot. This task was included to minimize neural activity during rest (Stark and Squire, 2001). Each trial (Fig. 1) began with a fixation cross (+) that was shown for 750 ms and followed by a word (2000 ms) to which participants had to indicate the old/new status via key press. Following “new” responses, a moving dot reappeared on the screen. For “old” responses, a second screen with the prompt “How far back?” appeared for 2500 ms, during which participants indicated whether the word had been represented after 5, 10, 15, 20, or 25 intervening words. A moving dot then reappeared on the screen. Participants were asked to respond as quickly and accurately as possible. Old/new responses were made with the right index and middle fingers (balanced across participants). Lag judgments were made with the same hand using all five keys on the response box, which mapped recency judgments in either ascending or descending order from the leftmost to the rightmost button.
Figure 1.
During scanning, words were presented and then re-presented after different numbers of intervening words (the lag). Participants indicated the old/new status of each word and, following an “old” response, made a lag judgment, estimating the number of words that had intervened between presentation and re-presentation (5, 10, 15, 20, or 25 words).
Data acquisition.
Data were collected on a 3T HDx GE scanner using an eight-channel receiver-only head coil and a gradient-echo echoplanar imaging (EPI) sequence [repetition time (TR)/echo time (TE) = 3000/35 ms, field of view (FOV) = 220 mm, 64 × 64 data matrix, parallel acceleration factor = 2, 90° flip angle]. Forty-six oblique-axial slices were acquired in an interleaved fashion, each 2.6 mm thick with zero slice gap (3.4 × 3.4 × 2.6 mm voxels). Slices were acquired with a 20° axial-to-coronal rotation relative to the anterior commissure-posterior commissure line (anterior downward) and the phase-encoding direction reversed to maximize coverage while reducing signal dropout in the medial temporal lobe (Weiskopf et al., 2006). The first four volumes were discarded to allow for signal T1 equilibrium. The same scanning protocol was used in both sessions. Anatomical images were acquired with a T1-weighted 3D FSPGR sequence [TR/TE/inversion time = 7.8/3.0/450 ms, flip angle = 20°, FOV = 256 × 192 × 172 mm, 1 mm isotropic resolution, 8 min acquisition time].
To minimize signal dropout in EPI data, high-order shimming was used and magnetic-field (B0) maps were acquired to correct regional image distortions caused by residual magnetic field inhomogeneities (Jezzard and Balaban, 1995). B0 field maps were calculated from the phase information from two 3D FSPGR images (TR = 20 ms, TE = 7 ms and 9 ms, aligned coplanar with the fMRI slices). Phase images at each TE were calculated and unwrapped before subtraction using Prelude (Jenkinson, 2003). The resulting phase difference images were converted to a field map and image unwarping was performed using FUGUE (www.fmrib.ox.ac.uk/fsl/fugue/index.html).
Data preprocessing and analysis.
Data preprocessing and analysis were performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (www.fmrib.ox.ac.uk/fsl). Spatial smoothing was applied using a full-width half-maximum Gaussian kernel of 5 mm. Preprocessing and analysis was performed using FEAT (fMRI Expert Analysis Tool, version 5.63). To remove low-frequency artifacts, each functional run was temporally filtered using a high-pass cutoff of 60 s. For each EPI run, nonbrain data were removed using the FMRIB Brain Extraction Tool (Jenkinson et al., 2002) and motion correction was applied using 3-dimensional coregistration of each image to the middle image of the time series with motion correction using the FMRIB Linear Image Registration Tool (Jenkinson et al., 2002). Registration of the functional data followed a two-stage process using linear registration with the same FMRIB tool: each functional run was first registered to a higher resolution T1-weighted FSPGR image (7 degrees of freedom), and then registered to the Montreal Neurological Institute (MNI) 152 standard template anatomical image (12 degrees of freedom).
The blood oxygenation level-dependent signal was modeled by convolving the predictor function of event timing with a standard model of the hemodynamic response function for each condition (i.e., regressors). These conditions were items attracting accurate “old” responses that were then separated according to the subsequent lag judgment (see above, Procedure). Comparisons of interest were tested using linear contrasts. Statistical analysis was first performed on participant's individual functional runs using the FMRIB Improved Linear Model. The second step combined the two functional runs for each participant using a fixed-effects model. For the third step, group-level analysis was performed using the FMRIB Local Analysis of Mixed Effects tool (Beckmann et al., 2003; Woolrich et al., 2004). Resulting Z statistic images were thresholded using a cluster-forming threshold of Z > 2.1, unless stated otherwise, and a family-wise error corrected cluster extent threshold of p = 0.05, based on the theory of Gaussian random fields.
Regions of interest (ROIs) were defined by 10 mm spheres centered around peak voxels that were revealed in the whole-brain analysis. Parameter estimates were extracted for each participant by averaging across ROI voxels and analysis was performed using the FMRIB Featquery tool, which applies the inverse of the transformation matrix from individual to standard space (generated during the initial registration) to warp the ROIs back into each participant's individual space for statistical analysis. The motion-corrected, smoothed, and filtered data across each entire ROI were probed for percentage signal change from baseline. ROIs were moved into MNI space for regional localization.
Results
Behavioral data
The probability of a correct new judgment was 0.90 (SD = 0.15) and the probability of a correct old judgment was superior for lag 10 than for lag 20 words (0.84 vs 0.79; t(16) = 4.7, p < 0.001). Only data from lag 10 and lag 20 words are reported because of the small number of filler words presented at lags 5, 15, and 25 (eight per participant, see Materials and Methods, Procedure).
Table 1 shows the probabilities and reaction times for each lag judgment for correctly identified old words that were repeated after lags 10 and 20. Bold values highlight the probabilities of correct lag judgments, which were above chance (0.20, both t(16) > 4.0, p < 0.001). The likelihood of correct lag 10 and 20 judgments was statistically equivalent, and the likelihood of incorrect lag judgments fell off with distance: lag 15 judgments were more likely than lag 10 judgments to lag 20 words (t(16) = 3.0, p < 0.01), and lag 15 judgments were more likely than lag 20 judgment to lag 10 words (t(16) = 4.9, p < 0.001). A 5 × 2 ANOVA contrasting the reaction times for each lag judgment to lag 10 and 20 words revealed no significant effects.
Table 1.
Probabilities for each lag judgment following correct ″old″ responses to words re-presented after 10 or 20 intervening words. Also shown are reaction times for the correct old judgments
| Judgment | Actual lag |
|||
|---|---|---|---|---|
| Lag 10 |
Lag 20 |
|||
| Response probability | RT (ms) | Response probability | RT (ms) | |
| Lag 5 | 0.17 (0.10) | 552.24 (24.64) | 0.04 (0.03) | 653.37 (61.79) |
| Lag 10 | 0.28 (0.09) | 638.19 (44.62) | 0.18 (0.09) | 699.12 (51.29) |
| Lag 15 | 0.29 (0.07) | 626.12 (39.72) | 0.30 (0.08) | 654.51 (48.59) |
| Lag 20 | 0.21 (0.11) | 616.04 (41.31) | 0.32 (0.10) | 568.73 (30.66) |
| Lag 25 | 0.06 (0.06) | 527.34 (58.79) | 0.17 (0.08) | 545.24 (34.20) |
Probabilities of correct lag judgments are in bold; SDs are in parentheses. RT, Reaction time.
Imaging analyses
The analyses were restricted to words that were presented at lags 10 and 20 and that were judged correctly to be old. Two initial whole-brain level analyses comprised contrasts between lag 10 and lag 15 judgments to lag 10 words. Although no brain region was reliably more active for lag 15 than lag 10 judgments, a number of frontal, parietal, and occipital areas (Table 2) exhibited greater activity for correct lag 10 than for incorrect (lag 15) judgments to lag 10 words.
Table 2.
Activation peaks for regions showing significantly greater activity for correct lag 10 judgments than for incorrect lag 15 judgments to lag 10 words
| Anatomical label | Hemisphere | Approximate BA | Voxel | Z max | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Occipital lobe | |||||||
| Lingual/fusiform gyrus | L | BA 18/17 | 3165 | 3.38 | −12 | −76 | 2 |
| Lingual gyrus | L | 3.25 | −12 | −84 | −8 | ||
| Lingual gyrus/intracalcarine cortex | L | 3.20 | −8 | −84 | 6 | ||
| Lingual gyrus supracalcarine cortex | L | 3.12 | −2 | −82 | 10 | ||
| Precuneus | R | 3.19 | 16 | −62 | 42 | ||
| Parietal lobe | |||||||
| Angular gyrus | L | BA 40 | 1047 | 3.03 | −48 | −56 | 50 |
| Inferior parietal lobe | L | 3.00 | −42 | −52 | 32 | ||
| Angular/supramarginal gyrus | L | 2.91 | −48 | −46 | 52 | ||
| Lateral occipital cortex | L | 2.85 | −32 | −60 | 46 | ||
| Frontal lobe | |||||||
| Inferior frontal gyrus | R | BA 6/45 | 1153 | 3.32 | 54 | 22 | 24 |
| Superior/middle frontal gyrus | R | 2.98 | 32 | 22 | 54 | ||
| Inferior/middle frontal gyrus | R | 2.93 | 46 | 18 | 28 | ||
| Precentral/middle frontal gyrus | L | BA 6/44 | 1103 | 3.33 | −50 | 6 | 40 |
| Middle frontal gyrus | L | 3.02 | −38 | 28 | 24 | ||
| Inferior/middle frontal gyrus | L | 2.96 | −46 | 12 | 32 | ||
BA, Brodmann area.
The pattern of activation in these regions is equally interpretable in terms of distance- or location-based processes, because greater activation accompanies the shorter as well as the correct lag judgment. The second set of analyses was designed to determine, via an orthogonal contrast, which regions behave in ways that would align them with distance- or location-based processes.
Parameter estimates for correct lag 20 and incorrect (lag 15) judgments to lag 20 words were extracted from 10 mm spheres that were centered around peak voxels taken from the clusters identified in the contrast between lag 10 and lag 15 judgments to lag 10 words (Table 2). Regions supporting distance-based processes will track the lag judgment (greater activation for the lag 15 than the lag 20 response), whereas regions supporting location-based processes will track accuracy [greater activation for the correct (lag 20) than the incorrect (lag 15) response].
The network of regions shown in Table 2 contained two regions that demonstrated reliable activation differences between correct and incorrect judgments to lag 20 words. One region, located in inferior parietal lobe (coordinates: −42, −52, 32), showed significantly greater activation for lag 15 than for lag 20 judgments (t(16) = 2.3, p < 0.05). A second region, located in the lingual gyrus (−2, −82, 10), showed the opposite pattern: greater activity for correct (lag 20) than for incorrect (lag 15) judgments to lag 20 words (t(16) = 2.2, p < 0.05).
In keeping with this set of outcomes, as well as the impression given in Figure 2, a 2 × 2 ANOVA on extracted parameter estimates for lag 20 words, including the factors of region (inferior parietal lobe vs lingual gyrus) and accuracy (correct vs incorrect), revealed a reliable interaction (F(1,16) = 13.0, p < 0.01). In a final set of analyses, parameter estimates did not differ in either region for incorrect (lag 15) judgments to lag 10 or lag 20 words. Moreover, as suggested by Figure 2, activation was greater for correct lag l0 than for correct lag 20 words (t(16) = 3.0, p < 0.01) in inferior parietal lobe, but not in the lingual gyrus.
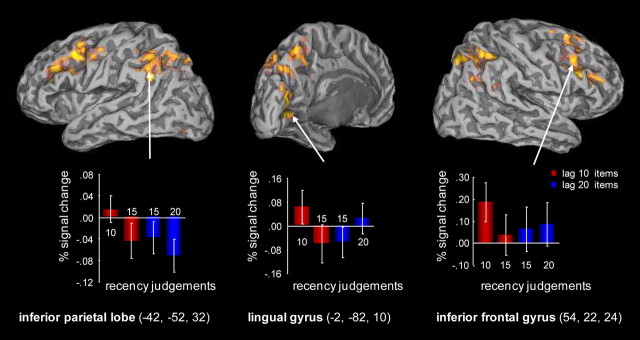
Figure 2.
Regions showing differences between blood oxygenation level-dependent activity for correct (lag 10) and incorrect (lag 15) judgments for lag 10 words. Parameter estimates for these response categories, as well as for correct (lag 20) and incorrect (lag 15) judgments to lag 20 words were extracted from 10 mm spheres centered around peak voxels in the inferior parietal lobe (left), the lingual gyrus (middle), and the inferior frontal gyrus (right). The activation profiles for the inferior parietal lobe and the lingual gyrus link these regions with distance- and location-based means of supporting lag judgments, respectively. Modulations in inferior frontal gyrus predict the accuracy of lag judgments for lag 10 words only.
For regions shown in Table 2 that did not respond differentially to lag 15 and lag 20 judgments to lag 20 words, the predominant pattern in the extracted parameter estimates was a level of activation that was equivalent for three conditions: correct judgments to lag 20 words, incorrect judgments to lag 20 words, and incorrect judgments to lag 10 words, all of which had lower levels of activation than correct judgments to lag 10 words. This pattern is shown for one region—the inferior frontal gyrus—on the right-hand side of Figure 2. The extracted parameter estimates for a further four regions are shown in the supplemental Figure (available at www.jneurosci.org as supplemental material).
Discussion
Two brain regions associated with functionally distinct memory operations were identified during a task requiring judgments of how recently events had occurred. In the inferior parietal lobe, activity increased as the lag judgments became more recent. In lingual gyrus, by contrast, there was greater activity for correct than for incorrect lag judgments at two different lags. These findings provide strong support for the separation between location- and distance-based means of making judgments concerning when events occurred (Fig. 2). In addition, previous studies have linked activity in both of these regions to the process of recollection (Gilboa et al., 2004; Wheeler and Buckner, 2004; Wagner et al., 2005; Woodruff et al., 2005; Viard et al., 2007; Vilberg and Rugg, 2008; Spaniol et al., 2009), which is recovery of qualitative information about a prior occurrence (Mandler, 1980; Yonelinas, 2002). The findings suggest, therefore, that recollected content is used in at least two ways to support judgments about when events occurred.
The fact that activity in the inferior parietal lobe tracked the lag judgment rather than the actual lag (10 or 20) suggests that this region supports distance-based processes (Fig. 2, left). The key outcomes supporting this claim are as follows: (1) activity is greater for correct lag 10 than lag 20 judgments, (2) incorrect (lag 15) judgments to lag 10 as well as lag 20 words are associated with equivalent levels of activity, and (3) this level of activity falls between that associated with correct lag 10 and lag 20 judgments.
Activity in this region of the inferior parietal lobe has been linked with recollection in numerous experiments (Rugg and Henson, 2002; Wheeler and Buckner, 2004; Wagner et al., 2005; Yonelinas et al., 2005; Vilberg and Rugg, 2008) and has been shown to index the volume or quality of information that is recovered (Vilberg and Rugg, 2007, 2009; Donaldson et al., 2010). These findings converge on the view that recollected content can be used heuristically to make judgments about when events occurred. This finding is important because it emphasizes that the separation between distance- and location-based processes does not have a one-to-one mapping with the processes of familiarity and recollection. Familiarity is commonly regarded as a graded strength signal that decays over time (Yonelinas, 2002), and these characteristics makes it a strong candidate for an information type that can support distance-based judgments. The link, however, between inferior parietal lobe and recollection means that the findings described here also align recollection with a distance-based means of making judgments about when events occurred.
A similar claim was made by Grove and Wilding (2009), who acquired event-related potentials (ERPs) in a task similar to that described here. They reported that the magnitude of an ERP index of recollection—the left parietal old/new effect—tracked the lag judgment rather than the actual lag. This finding is consistent with the behavior of the inferior parietal lobe in the experiment described here and provides further support for the view that recollected content is used in a distance-based manner to support recency judgments. Moreover, the inferior parietal lobe has been identified as a brain region that may generate the left parietal old/new effect (Vilberg and Rugg, 2007).
Grove and Wilding (2009) also reported that an ERP index of familiarity—the mid-frontal old/new effect—tracked the lag judgment that was made, a finding consistent with the view that familiarity can also support “when” judgments in a distance-based manner. This ERP finding begs the question, why were brain regions previously linked to familiarity (Rugg and Henson, 2002), not identified via the key contrasts in this experiment? One possibility is that ERPs provide a more sensitive index of familiarity than fMRI, although it should be noted that the lag intervals in this study were not the same as in the study reported by Grove and Wilding (2009). Also of relevance is behavioral work by Hintzman (2001), who used a very similar paradigm to that described here, the principal difference being that participants made “remember”/“know” judgments as well as lag judgments. With repetition lags below 40 intervening items, accurate lag judgments were accompanied primarily by “remember” responses. The reverse was true at longer lags (up to 80 intervening items). In so far as “know” judgments are linked to the process of familiarity, these findings suggest that regions supporting familiarity-based memory judgments might well be identified using fMRI in tasks with longer lags between first and second item presentations than were used here. In addition, given that this paradigm has not been used in fMRI studies before, the analyses were conducted at the whole-brain level. It may well be that a priori selection of regions that have been linked to the processes of recollection and familiarity (notably within the medial temporal lobe) will yield additional insights into the ways in which different neural substrates support lag judgments.
The fact that activity in the lingual gyrus predicted the accuracy of recency judgments for lag 10 as well as for lag 20 words links this region to location-based means of making task judgments (Fig. 2, middle). Activity in this region has been reported for successful recollection in some studies of autobiographical memory (Gilboa et al., 2004; Viard et al., 2007), and in one of these the level of activity in lingual gyrus was equivalent for remote and for recent memories (Gilboa et al., 2004). This finding parallels, albeit over different time scales, the equivalent levels of activation that were observed in this study for correct judgments at short (lag 10) and at longer lags (lag 20).
Gilboa et al. (2004) proposed, however, that the lingual gyrus activation they observed was a consequence of their use of complex photographic stimuli, and in keeping with this account, Woodruff et al. (2005) reported greater activity in lingual gyrus when information about studied pictures rather than studied words was recollected. One way to accommodate these findings with the lingual gyrus activation and the use of verbal stimuli in the current experiment is to propose that: (1) memories for associations that are formed between proximal list items upon initial presentation can contribute to location-based lag judgments, (2) the images that are generated by list words are one kind of content that can promote the formation of these associations, and (3) lingual gyrus activation in this study is linked to recovery of image-related content. This is a tentative proposal, but it is also notable that the greater activation for recollected pictures than for recollected words reported by Woodruff et al. (2005) occurred in a task where all test stimuli were words. Thus, the lingual gyrus activation they ascribed to recollection of pictures presumably reflects image content that is accessed via verbal materials. This account is conceptually similar to the proposal we have made for the link between recovery of image-related information and the kinds of content that can encourage formation of associations between proximal list items.
Finally, a number of regions in prefrontal cortex that were identified in the initial contrast between correct (lag 10) and incorrect (lag 15) judgments to lag 10 words did not show activation differences for the orthogonal contrast between incorrect (lag 15) and correct (lag 20) judgments to lag 20 words. The common pattern across these regions (greater activation for correct lag 10 judgments than the other three response categories) is illustrated in Figure 2, right, and in the supplemental Results (available at www.jneurosci.org as supplemental material). Using ERPs in a somewhat different paradigm to the one used here, Curran and Friedman (2003) linked frontally distributed right-lateralized scalp activity to location-based rather than distance-based processes, arguing that processes supported by the prefrontal cortex that are involved in the reconstruction of prior episodes would be required to a greater degree for location- than for distance-based recency judgments. It is not possible to make the same claim for the data in this experiment, because regions that predict the accuracy of lag judgments at a single lag only cannot be aligned unambiguously with either distance- or location-based processes. Moreover, the possibility remains that other kinds of task-relevant cognitive operations are reflected by activity in these regions. For example, the inferior frontal gyrus has been linked to cognitive control operations, including the selection of task-relevant material (Dobbins et al., 2002; Simons and Spiers, 2003; Ranganath and Blumenfeld, 2008). A greater selection demand may arise for items associated with a larger amount of recovered information, which in this case is most likely to be lag 10 words attracting correct lag judgments. Regardless of the accuracy of this account, however, the activation pattern for the middle, inferior, and superior frontal gyri is consistent with behavioral (Hintzman, 2001) as well as fMRI data (Konishi et al., 2002, 2006; St Jacques et al., 2008), suggesting that the processes contributing to judgments about when events occurred are not engaged equivalently across all time intervals.
Conclusions
The lingual gyrus and inferior parietal lobe play distinct functional roles in tasks requiring judgments about relative recency. These regions support location- and distance-based processes, respectively. These findings are the first fMRI data showing that recollected information is used in two fundamentally different ways to make judgments about when events occurred.
Footnotes
This work was funded by the Wales Institute of Cognitive Neuroscience and the UK Biotechnology and Biological Sciences Research Council.
References
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Curran T, Friedman WJ. Differentiating location- and distance-based processes in memory for time: an ERP study. Psychon Bull Rev. 2003;10:711–717. doi: 10.3758/bf03196536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE. Remember the source: dissociating frontal and parietal contributions to episodic memory. J Cogn Neurosci. 2010;22:377–391. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- Dudukovic NM, Wagner AD. Goal-dependent modulation of declarative memory: neural correlates of temporal recency decisions and novelty detection. Neuropsychologia. 2007;45:2608–2620. doi: 10.1016/j.neuropsychologia.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Memory for the time of past events. Psychol Bull. 1993;113:44–66. [Google Scholar]
- Friedman WJ. Distance and location processes in memory for the times of past events. In: Medin DL, editor. The psychology of learning and motivation. San Diego: Academic; 1996. pp. 1–41. [Google Scholar]
- Friedman WJ. Memory processes underlying humans' chronological sense of the past. In: Hoerl C, McCormack T, editors. Time and memory: issues in philosophy and psychology. Oxford: Clarendon; 2001. pp. 139–167. [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Grove KL, Wilding EL. Retrieval processes supporting judgements of recency. J Cogn Neurosci. 2009;21:461–473. doi: 10.1162/jocn.2009.21040. [DOI] [PubMed] [Google Scholar]
- Hinrichs JV. A two-process memory-strength theory for judgment of recency. Psychol Rev. 1970;77:223–233. [Google Scholar]
- Hinrichs JV, Buschke H. Judgment of recency under steady-state conditions. J Exp Psychol. 1968;78:574–579. doi: 10.1037/h0026615. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Judgements of frequency and recency: how they relate to reports of subjective awareness. J Exp Psychol Learn Mem Cogn. 2001;27:1347–1358. [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Kimura HM, Hirose S, Kunimatsu A, Chikazoe J, Jimura K, Watanabe T, Abe O, Ohtomo K, Miyashita Y, Konishi S. Differential temporo-parietal cortical networks that support relational and item-based recency judgments. Neuroimage. 2010;49:3474–3480. doi: 10.1016/j.neuroimage.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Konishi S, Uchida I, Okuaki T, Machida T, Shirouzu I, Miyashita Y. Neural correlates of recency judgment. J Neurosci. 2002;22:9549–9555. doi: 10.1523/JNEUROSCI.22-21-09549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Asari T, Jimura K, Chikazoe J, Miyashita Y. Activation shift from medial to lateral temporal cortex associated with recency judgements following impoverished encoding. Cereb Cortex. 2006;16:469–474. doi: 10.1093/cercor/bhi126. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychol Rev. 1980;87:252–271. [Google Scholar]
- Rajah MN, McIntosh AR. Dissociating prefrontal contributions during a recency memory task. Neuropsychologia. 2006;44:350–364. doi: 10.1016/j.neuropsychologia.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 2010;46:535–549. doi: 10.1016/j.cortex.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Prefrontal cortex and memory. In: John HB, editor. Learning and memory: a comprehensive reference. Oxford: Academic; 2008. pp. 261–279. [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker A, Wilding EL, Bussey TJ, editors. The cognitive neuroscience of memory encoding and retrieval. Hove, UK: Psychology; 2002. pp. 3–37. [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta- analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Rubin DC, LaBar KS, Cabeza R. The short and long of it: neural correlates of temporal-order memory for autobiographical events. J Cogn Neurosci. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Ecphoric processes in episodic memory. Philos Trans R Soc Lond B Biol Sci. 1983;302:361–370. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chételat G, Lebreton K, Landeau B, Young A, De La Sayette V, Eustache F. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cereb Cortex. 2007;17:2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. Neuroimage. 2009;45:562–571. doi: 10.1016/j.neuroimage.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. Neuroimage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla LT, Aguirre GK, Zarahn E, Cannon TD, D'Esposito M. Activation of the prefrontal cortex during judgments of recency: a functional MRI study. Neuroreport. 1996;7:2803–2806. doi: 10.1097/00001756-199611040-00079. [DOI] [PubMed] [Google Scholar]