Abstract
Morphine loses analgesic potency after repeated administration. The underlying mechanism is not fully understood. Glia are thought to be involved in morphine tolerance, and P2X7 purinergic receptor (P2X7R) has been implicated in neuron–glia communication and chronic pain. The present study demonstrated that P2X7R immunoreactivity was colocalized with the microglial marker OX42, but not the astrocytic marker GFAP, in the spinal cord. The protein level of spinal P2X7R was upregulated after chronic exposure to morphine. Intrathecal administration of Brilliant Blue G (BBG), a selective P2X7R inhibitor, significantly attenuated the loss of morphine analgesic potency, P2X7R upregulation, and microglial activation. Furthermore, RNA interference targeting the spinal P2X7R exhibited a similar tolerance-attenuating effect. Once morphine analgesic tolerance is established, it was no longer affected by intrathecal BBG. Together, our results suggest that spinal P2X7R is involved in the induction but not maintenance of morphine tolerance.
Introduction
Morphine is a highly potent analgesic for pain management. However, its analgesic potency fades rapidly during repetitive administration, so that progressively higher doses are required to achieve comparable analgesic levels. Considerable progress has been made concerning mechanisms underlying morphine tolerance; however, a clear understanding is still lacking regarding its complexity.
Lines of evidence have demonstrated that multiple factors are known to be involved in morphine tolerance, including desensitization of opioid receptors (Martini and Whistler, 2007) and functional changes in glutamate receptors (Mao et al., 1995; Mayer et al., 1999) and transporters (Mao et al., 2002; Tai et al., 2007). Noticeably, studies by us (Song and Zhao, 2001) and others suggest that spinal glia may contribute to the development of morphine tolerance (Watkins et al., 2005).
Various signaling molecules are expressed in glia (Watkins et al., 2007). Among them, P2X7 receptor (P2X7R) is an ATP-gated nonselective cation channel (Sperlágh et al., 2006). In the spinal cord, P2X7R is predominately present in microglia (Collo et al., 1997; Yu et al., 2008). There is mounting evidence that P2X7R plays an important role in the production of proinflammatory cytokines such as interleukin-1 β (IL-1β) (Takenouchi et al., 2009) and tumor necrosis factor-α (TNFα) (Suzuki et al., 2004). Furthermore, P2X7R is involved in the induction of neuropathic pain (Honore et al., 2006; Broom et al., 2008). Together, these lines of evidence point to a potential role for P2X7R in the development of morphine tolerance.
In the present study, we explored the effects of P2X7R on morphine tolerance using behavioral, immunohistochemical, pharmacological, and gene interfering methods. Changes in spinal P2X7R expression were detected during long-term morphine administration. Moreover, morphine tolerance was prevented with either blockade of spinal P2X7R by antagonist or targeting RNA interference.
Materials and Methods
Animals.
Adult male Sprague Dawley rats initially weighing 180–200 g (The Animal Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) were used. Animals were housed under a 12 h light/dark cycle at a room temperature of 22 ± 1°C with food and water available ad libitum. All experiments were performed with the approval of the Shanghai Animal Care and Use Committee and followed the policies issued by the International Association for the Study of Pain on the use of laboratory animals.
Drugs.
Morphine hydrochloride was purchased from Shengyang First Pharmaceutical Factory. Brilliant Blue G (BBG), a noncompetitive selective antagonist of P2X7R, was purchased from Sigma. Both drugs were diluted with saline to attain the final concentration.
Induction of morphine tolerance.
Morphine was given subcutaneously twice daily with 12 h intervals, from day 1 to at most day 9 at 10 mg/kg body weight, to establish systemic analgesic tolerance. To evaluate the development of morphine tolerance, morphine antinociception to mechanical and thermal stimuli were assessed 30 min after an acute dose (5 mg/kg) of morphine given intraperitoneally, and the analgesic effects before and after a defined period of tolerance induction were compared.
Behavioral tests.
Modified von Frey test was used to evaluate morphine analgesic effect to mechanical stimuli. A series of seven von Frey filaments (Stoelting) were applied on the plantar surface of a hindpaw in ascending order (2, 4, 6, 8, 10, 15, and 26 g). Each filament was applied five times with each time lasted for 2 s and a 30 s interval between applications. A positive response to a filament was when at least three of the five applications evoked flinching. Paw-withdrawal threshold was defined as the lowest filament bending force that elicited positive responses. Hargreaves' test and tail-flick test were used to evaluate morphine analgesic effect to thermal stimuli and were performed as described previously (Sun et al., 2008; Wang et al., 2009).
Small interfering RNAs.
Small interfering RNA (siRNA) targeting the rat P2X7R mRNA containing four pooled SMARTselected duplexes or a nontargeting control siRNA (Thermo Scientific Dharmacon). The sense sequences of the four duplexes were as follows: (1) GUACAGUGGCUUCAAGUAU; (2) GGAUGGACCCACAAAGUAA; (3) UUACAGAGGUGGCAGUUCA; (4) GAACGAUGUCUUUCAGUAU. siGENOME RISC-Free Control siRNA was used as nontargeting control. Branched polyethylenimine (PEI) was purchased from Sigma, and siRNA–PEI complex (1.8 μl of 10 mm PEI/μg RNA) was prepared according to a published method (Tan et al., 2005).
Drug delivery.
To minimize inflammatory responses from intrathecal surgery, drugs or siRNA–PEI complexes were delivered into the spinal space via lumbar puncture with a 30 gauge needle between L5 and L6 vertebrae as described previously (Xu et al., 2006). A brisk tail twitch was considered as an indicator of the accuracy of intrathecal injection.
Western blotting.
Animals were anesthetized by overdose urethane (SCR Co.), and the spinal cord tissue (L4–L6) was rapidly removed. Collected tissue samples were homogenized in a lysis buffer containing a mixture of protease inhibitors (Roche) and PMSF (Sigma). Protein samples were separated on SDS-PAGE (5–12% gels; Bio-Rad) and transferred onto polyvinylidene fluoride membranes (Millipore Corporation). The membranes were blocked and then incubated overnight at 4°C with a primary antibody [rabbit anti-P2X7R, 1:2000 (Alomone Labs); rabbit anti-Iba1, 1:5000 (Wako); rabbit anti-p-p38, 1:500 (Cell Signaling Technology; rabbit anti-p38, 1:1000 (Cell Signaling Technology)]. The membranes were then incubated with the goat anti-rabbit HRP-conjugated secondary antibody (1:1000; Santa Cruz Biotechnology) for 2 h at room temperature before the blots were visualized in ECL solution (Pierce) and exposed to x-ray films. The developed x-ray films were scanned for data analysis.
Immunohistochemistry.
Animals were anesthetized with overdose urethane and perfused transcardially. Lumbar spinal cord samples (L4–L6) were removed, postfixed for 4 h at 4°C, and immersed in 10–30% gradient sucrose in 0.01 m phosphate buffer. Transverse spinal sections (35 μm) were cut in a cryostat. Sections were blocked and then incubated with primary antibodies [rabbit anti-P2X7R, 1:400 (Alomone Labs); mouse anti-OX42, 1:100 (AbD Serotec); or mouse anti-GFAP, 1:1000 (Cell Signal Technology)] overnight at 4°C. The corresponding secondary antibodies were applied for 90 min at 4°C. Immunofluorescent sections were observed with a Leica fluorescence microscope, and images were captured with a CCD spot camera for data analysis.
Data analysis.
Data were expressed as means ± SEM. For behavioral tests, morphine analgesic effects were converted to the percentage of maximal possible effect (%MPE) using the following formula: %MPE = [(test − baseline)/(cutoff − baseline)] × 100. Student's t test was used to analyze the siRNA data. One-way repeated-measures ANOVA was used to analyze morphine tolerance time course. Two-way ANOVA was used to analyze the effect of BBG on the maintenance of morphine tolerance. For image data from Western blotting and immunohistochemistry, the intensity of immunoreactive bands or sections were measured using NIH ImageJ. Differences in intensity were compared using Student's t test.
Results
Colocalization of P2X7R and OX42 in the spinal cord
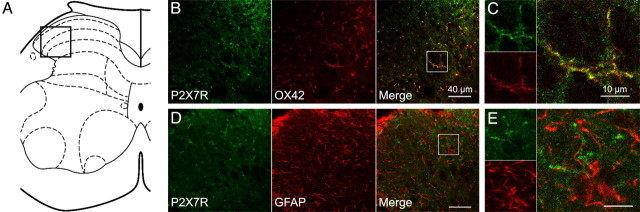
In the spinal dorsal horn of saline treated rats, P2X7R-immunoreactivity (IR) was predominantly colocalized with the microglial marker OX42 but not the astrocytic marker GFAP (data not shown). To investigate whether there are changes in distribution after chronic morphine treatment, we also examined P2X7R expression in morphine-tolerant rats (Fig. 1). After 5 d of morphine treatment (10 mg/kg, s.c., twice daily), P2X7R-IR remained exclusively colocalized with OX42 (Fig. 1B,C) but not GFAP (Fig. 1D,E).
Figure 1.
Double immunostaining of P2X7R and cell-specific markers in morphine-tolerated rats. A, Schematic diagram of the spinal cord. Black open square (A) marks the corresponding scope of confocal images (B, D) on the spinal cord section. White open squares (B, D) mark the corresponding scope of amplified images (C, E) on the confocal images. B, C, Double immunostaining of P2X7R and the microglial marker OX42. D, E, Double immunostaining of P2X7R and the astrocytic marker GFAP. Scale bars: B, D, 40 μm; C, E, 10 μm.
Upregulation of spinal microglial expression and P2X7Rs in morphine-tolerated rats
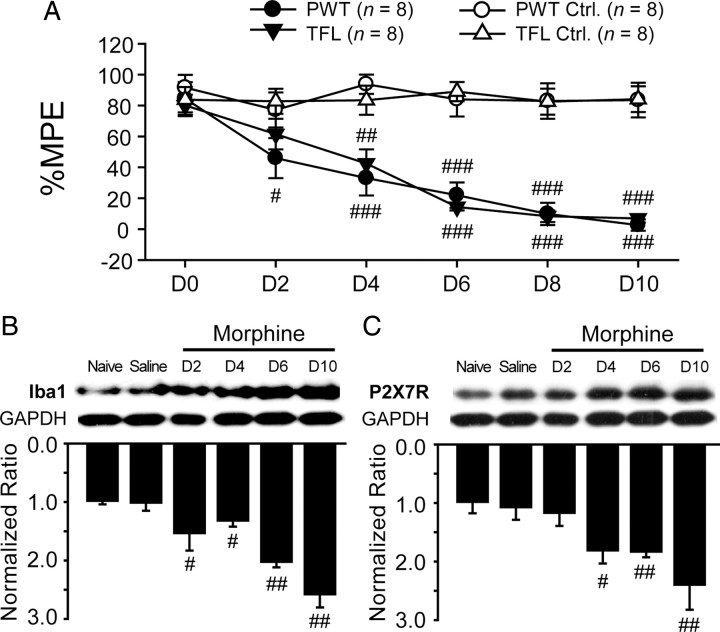
Chronic morphine treatment (10 mg/kg, s.c., twice daily) resulted in a significant loss of analgesic potency of acute morphine (5 mg/kg, i.p.) in both mechanical and thermal tests (Fig. 2A). We then investigated possible changes in both the expression of microglia marker Iba1 and P2X7R in morphine-tolerant rats. Parallel to behavioral changes, the protein levels of spinal microglia marker Iba1 were upregulated over time (Fig. 2B). Likewise, upregulation of P2X7R in the dorsal horn was observed in morphine-tolerant rats starting at day 4 (Fig. 2C), suggesting an involvement of the spinal P2X7R in the development of morphine tolerance.
Figure 2.
Upregulation of spinal expression of microglia and P2X7R in morphine-tolerant rats. Nine days of subcutaneous morphine (10 mg/kg, twice daily) were given to induce analgesic tolerance. In behavioral tests (A), analgesic efficacy of acute morphine (5 mg/kg, i.p.) was decreased significantly during repeated administration (#p < 0.05, ##p < 0.01, ###p < 0.001, compared with the corresponding saline group). Western bolt analysis showed that the protein levels of microglial marker Iba1 (B) and P2X7R (C) were upregulated significantly by chronic morphine treatment (B, C; #p < 0.05, ##p < 0.01, compared with saline group; n = 4). The spinal tissue samples of saline group were collected on day 10. PWT, Paw-withdrawal threshold; TFL, tail-flick latency.
P2X7R antagonist prevented morphine tolerance and suppressed P2X7R upregulation and microglial activation
The effects of BBG, a selective P2X7R antagonist, on morphine tolerance were examined using the von Frey test and tail-flick test. The baseline levels of pain threshold were not altered by repeated intrathecal administration of BBG (10 μm, 20 μl, twice daily for 5 d) in either test compared with the intrathecal saline group (20 μl, twice daily). Chronic morphine treatment (10 mg/kg, s.c., twice daily) after intrathecal injection of saline (20 μl, 30 min before subcutaneous morphine application) produced significant tolerance to acute morphine challenge (5 mg/kg, i.p.) on day 6. In contrast, intrathecal BBG (10 μm, 20 μl, 30 min before subcutaneous morphine application) significantly retained morphine antinociceptive effects to both mechanical and thermal stimuli (Fig. 3A,B), indicating a critical role for the spinal P2X7R in the development of morphine tolerance. Parallel to the behavioral changes, immunohistochemical data showed that BBG treatment inhibited the P2X7R upregulation and the microglial activation induced by chronic morphine on day 6 without altering their baseline levels (Fig. 3C–E). These results were confirmed by the Western blot experiments (Fig. 3F,G). Similar results were also observed in the spinal expression of phosphorylated p38 mitogen-activated protein kinase (MAPK) (Fig. 3H). Given that chronic morphine can activate microglial p38 MAPK in the spinal cord (Cui et al., 2006), our data suggest that BBG treatment attenuated morphine analgesic tolerance at least partly by inhibiting the activation of spinal microglia.
Figure 3.

Effects of P2X7R antagonist on the induction and maintenance of morphine analgesic tolerance. Chronic morphine (MOR)-induced analgesic tolerance was attenuated in von Frey test (A) and tail-flick test (B) by intrathecal BBG treatment (#p < 0.05, ###p < 0.001, compared with Saline + Saline; **p < 0.01, compared with MOR + Saline). Immunohistochemical analysis (C) showed that intrathecal BBG treatment abolished the immunoreactive upregulation of P2X7R (D) and the microglial marker OX42 (E) induced by chronic morphine (#p < 0.05, compared with Saline + Saline). Western blot analysis indicated that the protein levels of P2X7R (F), the microglial marker Iba1 (G), and phosphorylated p38 MAPK (H) with chronic morphine were also inhibited by BBG treatment (#p < 0.05, ##p < 0.01, compared with Saline + Saline; *p < 0.05, compared with MOR + Saline; n = 3). Five days of subcutaneous morphine (10 mg/kg, twice daily) produced analgesic tolerance to acute morphine challenge (5 mg/kg, i.p.) in von Frey test (I) and tail-flick test (J). After 3 d of BBG treatment (10 μm, 20 μl, i.t., 30 min before subcutaneous morphine, twice daily) did not affect the onset of morphine tolerance. Data represent means ± SEM (###p < 0.001, compared with day 0).
We also examined whether the spinal P2X7R is involved in the maintenance of morphine tolerance. After morphine tolerance was established on day 6, BBG (10 μm, 20 μl, twice daily) was applied via lumbar puncture 30 min before regular subcutaneous morphine (10 mg/kg, twice daily) from day 7 to day 9. BBG failed to restore morphine antinociceptive efficacy compared with the saline control group in behavioral tests on day 10 (Fig. 3I,J), implying that the spinal P2X7R contributes to the induction, but not the maintenance, of morphine tolerance.
Spinal siRNAs targeting P2X7R inhibited both induction of morphine tolerance and activation of spinal microglia
To confirm the pharmacological results, an RNA interference approach was used. Figure 4A illustrates the test schedule. Briefly, a single injection (17 μl) of siRNA targeting P2X7R mRNA was applied intrathecally. The control group received a single intrathecal injection (17 μl) of nontargeting control siRNA. Expressions of the spinal P2X7R on day 7 and day 16 post siRNA injection were assessed to determine the knockdown efficiency. At these two time points, P2X7R protein levels were significantly lower in P2X7 siRNA injected rats than those from control rats (Fig. 4B), indicating a substantial and stable P2X7R knockdown. Significant differences in behavioral baseline levels were not found between day 0 and day 7 within each group or between these two groups at day 0 or day 7 (data not shown). Therefore, we performed the morphine tolerance tests from day 8 to day 14. After morphine challenge (5 mg/kg, i.p.) analgesic tests on day 8, morphine (10 mg/kg, s.c., twice daily) was given from day 9 to day 13 to induce tolerance. As shown in Figure 4E–G, analgesic effect of acute morphine showed no difference between the groups on day 8. Although significant reductions in morphine antinociception were observed in Hargreaves' test (Fig. 4F) and tail-flick test (Fig. 4G) on day 14, it was still significantly higher in the P2X7 siRNA group compared with the control group. In addition, P2X7 siRNA injection also inhibited the upregulation of Iba1 (Fig. 4C) and phosphorylated p38 MAPK (Fig. 4D) by chronic morphine.
Figure 4.
Intrathecal P2X7 siRNA attenuated the induction of morphine analgesic tolerance. A, Schematic showing the experimental schedule. P2X7 siRNA and control siRNA were injected intrathecally on day 0. Acute morphine analgesic tests were performed on day 8 and day 14, respectively. Chronic morphine consisted of subcutaneous morphine injection (twice daily) from day 9 to day 13. Western blot analysis (B) showed that intrathecal P2X7 siRNA significantly reduced the protein level of P2X7R in the spinal cord on day 7 and day 16 after siRNA injection (##p < 0.01, compared with Naive). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. The protein levels of Iba1 (C) and phosphorylated p38 MAPK (D) in the spinal cord were significantly lower in the P2X7 siRNA groups after chronic morphine exposure (*p < 0.05, compared with control siRNA group; n = 4). P2X7 siRNA-treated rats exhibited a significantly higher morphine analgesic potency in von Frey test (E), Hargreaves' test (F), and tail-flick test (G) after chronic morphine treatment (#p < 0.05, ###p < 0.001, compared with day 8; *p < 0.05, compared with control siRNA group). PWL, Paw-withdrawal latency.
Discussion
Much progress has been made toward illustrating the mechanisms underlying morphine tolerance with studies prevailingly focused on neuronal involvement. In the present study, we examined microglial involvement and obtained two major findings. First, P2X7R was upregulated in the spinal cord along with microglia marker Iba1 by chronic morphine treatment. Second, antagonism of the spinal P2X7R by either the selective antagonist BBG or targeting siRNA not only attenuated morphine tolerance but also reduced the upregulation of the spinal P2X7R, Iba1, and phosphorylated p38 MAPK induced by chronic morphine. Our results highlight an intimate association between the spinal microglia-expressed P2X7R and the development of morphine tolerance.
Our previous study showed that functional interruption of astrocyte by glial inhibitor fluorocitrate reduced GFAP expression and blocked morphine tolerance, suggesting a possible involvement of astrocytic action (Song and Zhao, 2001). This has been confirmed and extended by other investigators, showing that several pain-facilitatory cytokines are released from activated glia (Raghavendra et al., 2002, 2004). It is suggested that pain-facilitatory cytokines counteract morphine analgesia, thus contributing to the morphine tolerance (Watkins et al., 2005). Expression and function of astrocytic P2X7R were described in in vitro studies (Duan et al., 2003; Narcisse et al., 2005). However, such expression of P2X7R was not detected in spinal astrocytes by us or others (Yu et al., 2008). Therefore, astrocyte contribution to morphine analgesic tolerance is unlikely to be directly mediated by P2X7R. Given that neuronal P2X7R was mostly observed in spinal motoneurons (Wang et al., 2004), it seems less likely that neuronal P2X7R is associated with morphine tolerance.
In the present study, neither pharmacological antagonism nor siRNA inhibition of spinal P2X7R produced a significant change in baseline response to nociceptive stimuli or acute morphine analgesia, suggesting that P2X7R is not involved in physiological pain. It has been reported that P2X7R is activated only by high concentration (>100 μm) of its endogenous ligand ATP (Roberts et al., 2006). It is, therefore, conceivable that P2X7R is inactive in physiological condition because of the insufficient ATP concentration in the spinal cord. Given that morphine application increased glutamate concentration in the CSF in morphine-tolerant rats (Wen et al., 2004), it might be that elevation of glutamate results in excess ATP release from spinal glia in an AMPA receptor-mediated calcium-dependent manner (Queiroz et al., 1999; Liu et al., 2006). This may create an ATP-rich local extracellular environment that could contribute to the activation of spinal P2X7R. Additionally, when activated, P2X7R can directly mediate both ATP and glutamate release (Ye et al., 2003; Suadicani et al., 2006). This positive feedback may also contribute to the sustained activation of P2X7R in morphine tolerance.
Our findings provided evidence of microglial P2X7R being a crucial molecule in the development of morphine tolerance. It has been reported that p38 MAPK, a transducer of various extracellular stimuli, regulates the release of inflammatory factors (Saklatvala, 2004) and is involved in the development of morphine analgesic tolerance associated with microglia (Cui et al., 2006, 2008). Therefore, we examined the effect of p38 MAPK on P2X7R-mediated morphine tolerance. In our experiments, both pharmacological antagonism and siRNA inhibition of spinal P2X7R suppressed the activation of spinal microglia and p38 MAPK, suggesting that p38 MAPK is a downstream effector of P2X7R activation in morphine tolerance, which is consistent with previous studies (Pfeiffer et al., 2004; Papp et al., 2007). Therefore, the involvement of spinal P2X7R in morphine tolerance is achieved at least partially through activation of microglia via the p38 MAPK signaling pathway. Previous studies have provided clues for interaction between P2X7R and other signal molecules. P2X7R has been shown to regulate the production and release of IL-1β and TNFα (Suzuki et al., 2004; Takenouchi et al., 2009). These two proinflammatory factors are potent pain mediators, and they are implicated in morphine tolerance (Shavit et al., 2005; Mika, 2008). Additionally, NMDA receptor is critically involved in the induction and maintenance of morphine tolerance (Mayer et al., 1999). P2X7R-mediated glutamate release may serve as an important component to initiate NMDA receptor activation. Furthermore, glutamate transporter is crucial to morphine antinociceptive effect. Chronic morphine downregulated glutamate transporter, and an activator of glutamate transporter attenuated morphine analgesic tolerance (Tai et al., 2007). Recent evidence suggested that activation of P2X7R decreased glutamate transport efficiency (Morioka et al., 2008). It is highly possible that P2X7R also contributes to morphine analgesic tolerance by disrupting the balance between glutamate release and uptake.
In summary, we found that P2X7R is predominantly expressed in microglia in the spinal cord. Activation of microglia-expressed P2X7R is involved in the generation of morphine tolerance. These findings highlight the possibility of a new clinical strategy to prevent morphine tolerance.
Footnotes
This work was supported by National Basic Research Program of China Grants 2006CB500807, 2007CB512303, and 2007CB512502 and National Natural Science Fund of China Grant 30830044. We are grateful to Prof. Lei Yu from Rutgers University for input about this manuscript.
References
- Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK, Yu W, Yuan J, Sun SH, Ochoa R, Krause JE, Wustrow DJ, Cortright DN. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-aminopyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino)methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Kalous A, Werry EL, Bennett MR. Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol Pharmacol. 2006;70:851–859. doi: 10.1124/mol.105.021436. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine-tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- Morioka N, Abdin MJ, Kitayama T, Morita K, Nakata Y, Dohi T. P2X7 receptor stimulation in primary cultures of rat spinal microglia induces downregulation of the activity for glutamate transport. Glia. 2008;56:528–538. doi: 10.1002/glia.20634. [DOI] [PubMed] [Google Scholar]
- Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1 beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp L, Vizi ES, Sperlágh B. P2X7 receptor mediated phosphorylation of p38MAP kinase in the hippocampus. Biochem Biophys Res Commun. 2007;355:568–574. doi: 10.1016/j.bbrc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kügelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Vial C, Digby HR, Agboh KC, Wen H, Atterbury-Thomas A, Evans RJ. Molecular properties of P2X receptors. Pflugers Arch. 2006;452:486–500. doi: 10.1007/s00424-006-0073-6. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol. 2004;4:372–377. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X(7) receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Cao H, Han M, Li TT, Zhao ZQ, Zhang YQ. Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res Bull. 2008;75:83–93. doi: 10.1016/j.brainresbull.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Tsai RY, Wang JJ, Tao PL, Liu TM, Wang YC, Wong CS. Amitriptyline preserves morphine's antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain. 2007;129:343–354. doi: 10.1016/j.pain.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-induced release and processing of IL-1 beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Therapy. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZH, Chang YC, Cherng CH, Wang JJ, Tao PL, Wong CS. Increasing of intrathecal CSF excitatory amino acids concentration following morphine challenge in morphine-tolerant rats. Brain Res. 2004;995:253–259. doi: 10.1016/j.brainres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: a novel method for the experimental study of opioid tolerance. Anesth Analg. 2006;103:714–720. doi: 10.1213/01.ane.0000226100.46866.ea. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]