Abstract
Insulin signaling plays a prominent role in regulation of dauer formation and longevity in Caenorhabditis elegans. Here, we show that insulin signaling also is required in benzaldehyde–starvation associative plasticity, in which worms pre-exposed to the odor attractant benzaldehyde in the absence of food subsequently demonstrate a conditioned aversion response toward the odorant. Animals with mutations in insulin-related 1 (ins-1), abnormal dauer formation 2 (daf-2), and aging alteration 1 (age-1), which encode the homolog of human insulin, insulin/IGF-1 receptor, and PIP3 kinase, respectively, demonstrated significant deficits in benzaldehyde–starvation associative plasticity. Using a conditional allele, we show that the behavioral roles of DAF-2 signaling in associative plasticity can be dissociated, with DAF-2 signaling playing a more significant role in the memory retrieval than in memory acquisition. We propose DAF-2 signaling acts as a learning-specific starvation signal in the memory acquisition phase of benzaldehyde–starvation associative plasticity but functions to switch benzaldehyde-sensing amphid wing C neurons into an avoidance signaling mode during memory retrieval.
Introduction
With a 302-neuron nervous system, Caenorhabditis elegans emerges as an excellent invertebrate model for the molecular study of behavioral plasticity. The nematode, despite its simple neural wiring, exhibits behavioral plasticity in a variety of learning paradigms. For example, C. elegans is capable of associating temperature or NaCl with the absence of food as demonstrated in thermotaxis learning and salt chemotaxis learning paradigms (Wen et al., 1997; Kodama et al., 2006; Tomioka et al., 2006). C. elegans also has the ability to associate the odorant butanone or salt taste with the presence of food (Wen et al., 1997; Torayama et al., 2007). Furthermore, the nematode can associate sickness caused by ingesting pathogenic bacteria with odorants that are specific to only the pathogenic strain (Zhang et al., 2005). We also have shown that the nematode's dramatic change in odorant preference after exposure to a high concentration of benzaldehyde in the absence of food is a result of associative learning in which the nematode forms an association between benzaldehyde and starvation (Nuttley et al., 2002). We refer to this behavioral plasticity as benzaldehyde–starvation associative plasticity. While the molecular and cellular bases of this type of plasticity remain unclear, it presents a simple yet elegant platform from which to approach the greater question of how neural plasticity leads to emergent changes in animal behavior.
Recent work has shown that insulin signaling, well known for its role in regulating aging and growth in C. elegans, also mediates thermotaxis learning and salt chemotaxis learning in worms (Kodama et al., 2006; Tomioka et al., 2006). The components of insulin signaling, including the insulin homolog INS-1, its receptor DAF-2, and the PIP3-kinase homolog AGE-1, are highly conserved between C. elegans and mammals. In mammals, insulin signaling pathways also have been implicated in learning and memory. For example, in mice, administration of insulin into the brain improved memory retention, whereas blocking insulin's downstream signaling pathway by inhibition of PIP3-kinase activity disrupted learning (Park et al., 2000; Barros et al., 2001). Although previous studies have highlighted the importance of insulin signaling in C. elegans associative learning, its role in mediating the changes in neural plasticity that underlies learning and memory are not well understood. It also is unclear behaviorally whether insulin signaling functions in memory acquisition or retrieval.
In this report, we demonstrate that worms with mutations in components of the insulin signaling pathways are defective in benzaldehyde–starvation associative plasticity, and that insulin signaling plays a more significant role in the retrieval than the acquisition of memory. INS-1 can act from multiple neurons and AGE-1 acts in benzaldehyde-sensing amphid wing C (AWC) sensory neurons to direct benzaldehyde–starvation associative plasticity. Our findings dissociate the behavioral roles of insulin signaling in the regulation of learning versus memory recall and better elucidate the molecular mechanism involved in this associative plasticity in C. elegans.
Materials and Methods
Strains and general methods.
The mutant strains used in this study were as follows: ins-1(nr2091), age-1(hx546), age-1(mg305), daf-18(e1375), age-1(m333); daf-18(e1375), and daf-2(e1370). Wild-type Bristol N2, age-1(hx546), daf-2(e1370), and daf-18(e1375) strains were obtained from the Caenorhabditis Genetics Center at the University of Minnesota. ins-1(nr2091) and age-1(mg305) were a generous gift from Dr. Gary Ruvkun (Harvard/Massachusetts General Hospital, Boston, MA) All experiments used well fed adult animals cultivated at 20°C (except in experiments using daf-2 and age-1(mg305) mutants, in which animals including controls were cultivated at 15°C) on nematode growth medium (NGM: 50 mm NaCl, 15 g/L agar, 20 g/L peptone, 1 mm cholesterol, 1 mm CaCl2, 1 mm MgSO4, 1 mm KH2PO4, pH 6.0) seeded with Escherichia coli strain OP50 under standard conditions unless otherwise specified (Brenner, 1974).
Behavioral assays.
All the behavioral assays were performed at 20°C unless otherwise specified.
Odorant chemotaxis assay.
Chemotaxis assays were performed as previously described (Nuttley et al., 2002). Approximately 100 animals were transferred onto a 10 cm Petri dish containing 6 ml of NGM agar. A 1 μl drop of testing odorant diluted in ethanol was added to one end and 1 μl of control odorant ethanol was added on the opposite end of the agar. Ten minutes before the animals were transferred, 1 μl of 1 m NaN3 was applied to the ends of the agar plates, to which the odorants would later be added to immobilize the animals once they reached the odorant spot. The dilution (V/V) for testing odorants was 1:100 for benzaldehyde and 1:500 for butanone. A chemotaxis index (C.I.) was calculated 1 h (unless otherwise specified) after animals' introduction to assess their preference for the test odorant. C.I. is calculated by the number of animals within 2 cm of the test spot minus the number of animals within 2 cm of the control spot divided by the total number of animals on the plate.
Odorant–starvation plasticity assay.
The learning assays were performed as previously described (Nuttley et al., 2002). Note that in the plasticity assay, conditioned stimulus (CS) during training/conditioning (100% benzaldehyde) is different from CS during testing (1% benzaldehyde). Since animals will exhibit an unconditioned avoidance response to 100% benzaldehyde (Bernhard and van der Kooy, 2000), a lower concentration of benzaldehyde was used during testing to prevent the masking of the conditioned avoidance response by the unconditioned avoidance response triggered by 100% benzaldehyde. Naive animals refer to animals that are placed directly onto the assay plates from their growth plates. Since mock-conditioned animals (i.e., gone through the same manipulations as the test conditions) for all the strains used in this report behaved essentially similar to that of naive animals, we have omitted the mock-conditioned group in the graphs for simplicity. In this assay, ∼1000 worms are pre-exposed to the odorant of interest placed on the lid of the plate in the absence of food for 1 h (unless specified otherwise) before being tested to the chemotaxis assay. Animals' C.I. for the odorant after training is then measured using the chemotaxis assay as described above. In the benzaldehyde–starvation learning assay, animals were exposed to 2 μl of pure benzaldehyde placed on a piece of Parafilm on the lid of the dish for 1 h on a standard 10 cm Petri dish containing 6 ml of NGM agar and tested immediately to the chemotaxis assay after training. To train animals in the presence of serotonin, 400 μl of 100 μm serotonin solution was added to the conditioning plate and allowed to try before plating the worms. In the butanone–starvation learning assay, animals were exposed to 2 μl of pure butanone for 1 h before being tested in the chemotaxis assay. Animals were trained and tested at 20°C unless otherwise specified.
Benzotaxis assay.
The assay was performed as previously described (Bernhard and van der Kooy, 2000). Briefly, the benzotaxis assay was used to measure worms' response toward 100% benzaldehyde with respect to time. An NGM plate was placed on a grid that divided the plate into five regions (A–E) by four parallel lines that were 18 mm apart. Approximately 150 worms were placed at the center of the plate and subsequently 2 μl of benzaldehyde was placed on a small piece of Parafilm that was placed at the edge of the agar. The plate was capped and sealed with Parafilm. Animals were allowed to move freely for a given period of time before the plate was placed in −20°C for 1 min and moved to 4°C to immobilize all the moving animals. The numbers of animals in the five regions were counted and a weighted C.I. was calculated using the following formula: ([2 × no. in A + no. in B] − [2 × no. in E + no. in D])/(total number of animals on the plate). A maximum score of +2 indicated a strong attraction to the odorant as 100% of the worms were at region A. A minimum score of −2 indicated a strong aversion as 100% of the worms were at region E.
Butanone enhancement assay.
The butanone enhancement assay was performed as described previously (Torayama et al., 2007) with slight modifications. One thousand animals were placed on an NGM plate with a thick bacterial lawn and were exposed to 2 μl of butanone as described for the odorant–starvation assay. After 1 h of exposure, animals were washed off the bacterial lawn and tested for chemotaxis to butanone using the chemotaxis assay.
PMA treatment.
PMA treatment was performed as described previously (Okochi et al., 2005). Briefly, adult animals were placed in standard culture plates containing 1 μg/ml PMA (PMA+) or DMSO solvent (PMA−) in the agar for 2 h before being subject to chemotaxis assay to assess their chemotaxis to benzaldehyde.
Induction of heat shock.
Heat shock treatment was performed as described previously (Tomioka et al., 2006) with slight modifications. Animals were incubated at 33°C for 30 min in M9 buffer and then transferred to a NGM plate seeded with OP50 E. coli at 20°C for 30 min before their examination in the benzaldehyde–starvation learning assay.
Generation of rescue strains and plasmid constructions.
ins-1::Venus and age-1 expression constructs were generated using the GATEWAY system (Invitrogen) except str-1p::age-1 and gcy-5p::age-1, which were made by replacing the dpy-30 promoter of pCAW112 (dpy-30p::age-1 construct, a gift from C. A. Wolkow (NIH, Baltimore, MD) with 4 kb of str-1 promoter (Troemel et al., 1995) and 2 kb of gcy-5 promoter (Yu et al., 1997) amplified by PCR from C. elegans genomic DNA. To construct entry vectors with the promoter sequences, the 2.7 kb promoter region of odr-3 (Roayaie et al., 1998), the 3.1 kb promoter region daf-7 (Ren et al., 1996), the 1.3 kb promoter region including the third intron of lin-11 (Hobert et al., 1998), the 0.9 kb promoter region including the first intron into the third exon of ttx-3 (Wenick and Hobert, 2004), the 4 kb promoter region of sra-6 (Troemel et al., 1995), the 3 kb promoter region of sre-1(Troemel et al., 1997), the 5.3 kb promoter region of glr-1 (Brockie et al., 2001), the 4.7 kb promoter region of ser-2 (Tsalik et al., 2003), the 1.1 kb promoter region of odr-10 (Sengupta et al., 1996), and the 5.8 kb promoter region of ins-1 (Pierce et al., 2001) were amplified from C. elegans genomic DNA, and then incorporated into the pDONR201 vector via site-specific recombination. To create destination vectors with age-1 cDNA, age-1 cDNA were amplified by PCR from pCAW112, and linked with the Cmr-ccdB sequence by site-specific recombination with pDONR201. The Venus clone was inserted into the destination vector including ins-1 cDNA to create ins-1::Venus destination vector. Details of the GATEWAY system can be accessed at Yuchi Iino's laboratory website, http://park.itc.u-tokyo.ac.jp/mgrl/IINO_lab/Gateway/Gateway_overview1.html.
Germ-line transformation was performed by microinjection as described previously (Mello et al., 1991). pPD93.97(from A. Fire, Stanford University, Stanford, CA), which contained myo-3p::gfp or myo-3p::Venus were used as a coinjection marker for the rescue experiments. The DNA concentrations were 5 ng/μl for plasmid DNA carrying ins-1p::ins-1::Venus cDNA, along with 30 ng/μl myo-3p::GFP DNA and 65 ng/μl carrier DNA for ins-1 rescue experiments. The DNA concentrations were 30 ng/μl for plasmid DNA containing daf-7p::ins-1::Venus cDNA, glr-1p::ins-1::Venus cDNA, ser-2p::ins-1::Venus cDNA, odr-3p::ins-1::Venus cDNA, or lin-11p:: ins-1::Venus cDNA, along with 10 ng/μl myo-3p::Venus and 60 ng/ μl carrier DNA for ins-1 rescue experiments. The DNA concentrations were 70 ng/μl for plasmid DNA containing ins-1(s)p::ins-1::Venus cDNA, along with 30 ng/μl myo-3p::GFP for ins-1 rescue experiments. The DNA concentrations were 70 ng/μl for plasmid DNA containing ins-1(s)p::ins-1::Venus cDNA and 30 ng/μl for daf-7p::ins-1::Venus cDNA, along with 30 ng/μl myo-3p::GFP for rescue lines injected with both rescue constructs. The DNA concentrations were 50 ng/μl for plasmid DNA carrying age-1 cDNA under various promoters (except plasmid with gcy-5p::age-1 cDNA, for which 30 ng/μl was used), along with 50 ng/μl myo-3p::GFP DNA and carrier DNA for age-1 rescue experiments.
Statistical analysis.
Two-way or three-way ANOVAs were used to examine group differences in all experiments. Bonferroni's t tests were used for post hoc analyses. The level of significance for all comparisons was p < 0.05.
Results
Expression of ins-1 is required for benzaldehyde–starvation associative plasticity
Wild-type N2 naive (untrained) animals demonstrated a strong attraction response toward benzaldehyde that switched into an avoidance response after a 1 h exposure to a high concentration of benzaldehyde in the absence of food (Fig. 1A). The avoidance response was more prominent when the odorant exposure time was extended to 2 h (Fig. 1A). This switch in odorant preference results from associative learning where animals associate the CS benzaldehyde with the unconditioned stimulus (US) starvation, as the presence of food during benzaldehyde exposure attenuate this change in behavior (Nuttley et al., 2002).
Figure 1.
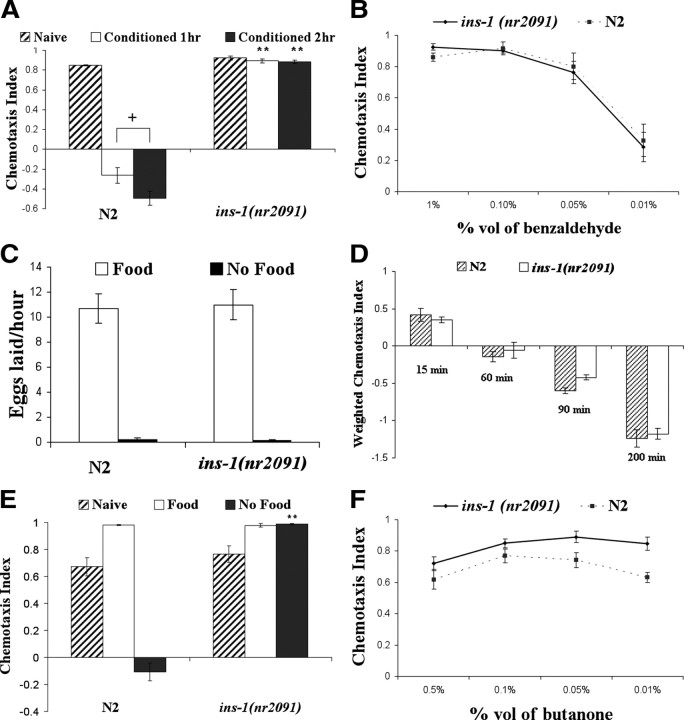
ins-1 mutants are defective in odorant–starvation associative plasticity. For all panels in the figure, double asterisks represent significant differences from N2 within the same experiment (**p < 0.05 by Bonferroni's t test). Crosses represent significant differences between the indicated data points (+p < 0.05 by Bonferroni's t test). Data represent means ± SEM. A, Benzaldehyde–starvation learning in wild-type N2 and ins-1(nr2091) worms. Animals were conditioned to 100% benzaldehyde in the absence of food for either 1 or 2 h and immediately following conditioning, their chemotaxis to 1% benzaldehyde was examined. ins-1 mutants demonstrated significant deficits in benzaldehyde–starvation associative plasticity both after 1 or 2 h of training. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 147.12, p < 0.05 (n = 6 plates for each data point). B, Chemotaxis of N2 and ins-1(nr2091) to various concentration of benzaldehyde. N2 and ins-1(nr2091) were comparable in their chemotaxis to 1, 0.1, 0.05, and 0.01% benzaldehyde, suggesting ins-1(nr2091) animals sense benzaldehyde normally. A two-way ANOVA revealed a main effect of benzaldehyde concentrations, F(3,31) = 37.97, p < 0.05, and no main effect of strain F(1,31) = 0.078, p < 0.05 (n = 4 plates for each data point). C, Number of eggs of N2 and ins-1(nr2091) laid in 1 h on and off food. N2 or ins-1(nr2091) animal was placed on a standard OP50 food lawn or blank NGM agar and the number of eggs laid in 1 h was scored. Starvation suppressed egg laying in both strains. A two-way ANOVA revealed a main effect of food availability, F(1,35) = 155.71, p < 0.05, and no main effect of strain F(1,35) = 0.017, p < 0.05 (n = 12 plates for each data point). D, Chemotaxis of N2 and ins-1(nr2091) to 100% of benzaldehyde at various time points. A two-way ANOVA revealed no main effect of strain F(1,31) = 1.427, p = 0.244 (n = 4 plates for each data point). E, Butanone enhancement and butanone–starvation associative plasticity in N2 and ins-1(nr2091) mutants. Animals were conditioned to 100% butanone in the presence or absence of food for 1 h and tested their chemotaxis to 0.5% butanone. ins-1(nr2091) mutants were normal in butanone enhancement but were defective in butanone–starvation associative plasticity. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 86.82, p < 0.05 (n = 6 plates for each data point). F, Chemotaxis of N2 and ins-1(nr2091) to various concentration of benzaldehyde. A two-way ANOVA revealed no interaction between strain and butanone concentrations, F(3,31) = 0.714, p = 0.53 (n = 4 plates for each data point).
To better understand learning and memory at a cellular and molecular level, we searched for mutants that demonstrate deficits in benzaldehyde–starvation associative plasticity. Given the role of insulin signaling in learning and memory in both C. elegans and mammals, we examined insulin mutants and found that a mutation in the ins-1 gene, a C. elegans homolog of mammalian insulin (Pierce et al., 2001), completely suppressed the emergence of benzaldehyde avoidance after benzaldehyde training in the absence of food (Fig. 1A).
This lack of benzaldehyde–starvation learning in ins-1(nr2091) animals might be attributed to defects in sensing benzaldehyde (CS) or starvation (US), rather than in associative learning. To test the possibility that the ins-1animals' plasticity deficit is a result of abnormal benzaldehyde sensation, we compared the naive chemotaxis of N2 and ins-1 animals toward 1, 0.1, 0.05, and 0.01% benzaldehyde and found no significant difference in naive benzaldehyde approach between the two strains (Fig. 1B). To determine whether ins-1 animals can sense starvation, we examined the number of eggs laid on and off in ins-1 mutants. Consistent with the data reported previously that ins-1 mutants have normal starvation-induced behavior (Kodama et al., 2006), we observed no difference in egg-laying behaviors on or off food between ins-1 and N2 (Fig. 1C). The data suggest that mutations in ins-1 did not disrupt the animals' ability to sense at least some aspects of starvation. To determine whether the lack of conditioned avoidance response in ins-1 mutants is due a lack of general innate avoidance response, ins-1 mutants were tested to high concentration of benzaldehyde in benzotaxis assay. ins-1 mutants exhibited avoidance response to high concentration of benzaldehyde comparable to that of wild-type animals (Fig. 1D), suggesting that INS-1 is not required for innate odorant avoidance.
ins-1 mutants are normal inbutanone–food associative plasticity but defective in butanone–starvation associative plasticity
As with benzaldehyde, after exposure to butanone (an AWC-sensed odorant) in the absence of food, N2 wild-type worms subsequently avoid butanone (Fig. 1E). However, after exposure to butanone in the presence of food, N2 wild-type animals were more strongly attracted toward the odorant compared with naive (untrained) animals (Fig. 1E). This latter form of butanone–food associative plasticity has been termed “butanone enhancement” (Torayama et al., 2007). To examine the extent of ins-1(nr2091)'s deficiency in odorant associative learning, we tested ins-1mutants in the butanone enhancement assay. ins-1 animals demonstrated normal butanone–food associative plasticity when they were trained to pair the presence of food with butanone (Fig. 1E). Surprisingly, when ins-1 animals were exposed to butanone in the absence of food, not only did ins-1 mutants not demonstrate an avoidance behavior seen in the wild type, they exhibited an enhanced approach toward butanone similar to that seen in the butanone–food associative plasticity (Fig. 1E). The data imply that absence of INS-1 perhaps activates pathways that signal a “well fed” state or the rewarding aspect of food. Therefore, even when exposed to butanone in the absence of food, ins-1 mutants are still capable of associating the aberrant rewarding signal with butanone, thereby leading to an enhanced attraction toward the odorant. Although not statistically significant, ins-1 animals exhibited a trend to be more sensitive to butanone compared with wild-type animals when tested to various concentration of butanone (Fig. 1F). Therefore, it cannot be ruled out completely that such a small difference in butanone sensitivity may contribute to the butanone plasticity deficit observed in ins-1 mutants.
Animals with mutations in the components of DAF-2 signaling are defective in benzaldehyde–starvation associative plasticity
Given that insulin can act as a neuropeptide to activate insulin/IGF-1 signaling in rodent neurons (Zhao et al., 2004), we examined mutants of daf-2, which encodes a homolog of the insulin/IGF-1 receptor, to determine whether a component of insulin signaling also regulates benzaldehyde–starvation associative plasticity. We found that daf-2(e1370) mutants demonstrated a strong defect in benzaldehyde–starvation associative plasticity at 23°C (Fig. 2A). Consistent with the fact that daf-2(e1370) is a temperature-sensitive allele, in which the deficit in DAF-2 function is only apparent at temperatures >20°C, we did not observe any plasticity defects when animals were trained and tested at 15°C (Fig. 2A). However, the plasticity defect in daf-2(e1370) became apparent when the animals were conditioned and tested at 20°C and the deficit was further exacerbated when the training and testing temperatures were raised to 23°C (Fig. 2A). The plasticity deficit observed in daf-2(e1370) likely is not attributable to sensory defects, as these mutants have normal benzaldehyde sensation and starvation-induced behaviors (Vellai et al., 2006).
Figure 2.
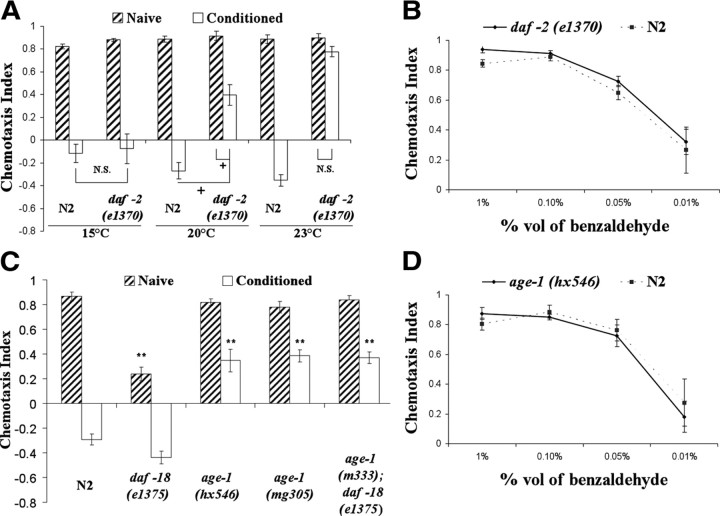
Animals with mutations in the components of DAF-2 signaling are defective in benzaldehyde–starvation associative plasticity. For all panels in the figure, double asterisks represent significant differences from N2 within the same group (**p < 0.05 by Bonferroni's t test). Crosses represent significant differences between the indicated data points (+p < 0.05 by Bonferroni's t test). N.S., Not significant. Data represent means ± SEM. A, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants trained and tested at various temperatures. Wild-type N2 and daf-2(e1370) mutants were conditioned and tested to benzaldehyde at 15°C, 20°C, and 23°C. daf-2 mutants exhibited defective benzaldehyde–starvation associative plasticity when trained and tested at 23°C (the temperature at which daf-2 is presumably defective in function), but had normal benzaldehyde–starvation associative plasticity when trained at tested at 15°C (the permissive temperature). A three-way ANOVA revealed a significant interaction between strain, conditioning, and temperature F(2,71) = 23.564, p < 0.05 (n = 6 plates for each data point). B, Chemotaxis of N2 and daf-2(e1370) to various concentration of benzaldehyde at 23°C. N2 and daf-2(e1370) were comparable in their chemotaxis to 1, 0.1, 0.05, and 0.01% benzaldehyde, suggesting ins-1(nr2091) animals sense benzaldehyde normally at 23°C. A two-way ANOVA revealed a main effect of benzaldehyde concentrations, F(3,31) = 35.37, p < 0.05, and no main effect of strain F(1,31) = 1.682, p = 0.21 (n = 4 plates for each data point). C, Benzaldehyde–starvation associative plasticity in mutants of the AGE-1 signaling pathway. age-1 mutants demonstrated partial associative plasticity defects. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(4,59) = 22.26, p < 0.05 (n = 6 plates for each data point). D, Chemotaxis of N2 and age-1(hx546) to various concentration of benzaldehyde. N2 and age-1(hx546) were comparable in their chemotaxis to various concentration of benzaldehyde, suggesting age-1(hx546) animals sense benzaldehyde normally. A two-way ANOVA revealed a main effect of benzaldehyde concentrations, F(3,31) = 27.42, p < 0.05, and no main effect of strain F(1,31) = 0.188, p = 0.66 (n = 4 plates for each data point).
Since AGE-1 (a homolog of PIP3-kinase) is a known downstream target of DAF-2 for the regulation of dauer formation and longevity in C. elegans (Baumeister et al., 2006), we sought to determine whether AGE-1 also plays a role in benzaldehyde–starvation associative plasticity. Two strains with hypomorphic alleles of age-1(hx546) and age-1(mg305) demonstrated a partial deficit in benzaldehyde–starvation associative plasticity (Fig. 2C). To examine whether the partial defects observed in age-1 mutants were as a result of the hypomorphic alleles, animals with a null age-1 mutation were investigated. Although null mutants of age-1 are lethal, the lethality can be suppressed by the absence of DAF-18 [a homolog of mammalian Phosphatase and Tensin Homolog (PTEN)]. In our learning paradigm, age-1(m333null); daf-18(e1375) double mutants exhibited a similar partial deficit in benzaldehyde–starvation associative plasticity to those observed in age-1(hx546) and age-1(mg305) mutants (Fig. 2C). The partial learning deficit seen in age-1 mutants cannot be attributed to sensory defects given that these animals have normal benzaldehyde sensation and starvation behaviors (Vellai et al., 2006). The data suggest that AGE-1 is likely a downstream target of DAF-2, but perhaps not the sole target activated by DAF-2 to mediate benzaldehyde–starvation associative plasticity.
daf-18 animals exhibit abnormal odorant chemotaxis and odorant associative plasticity
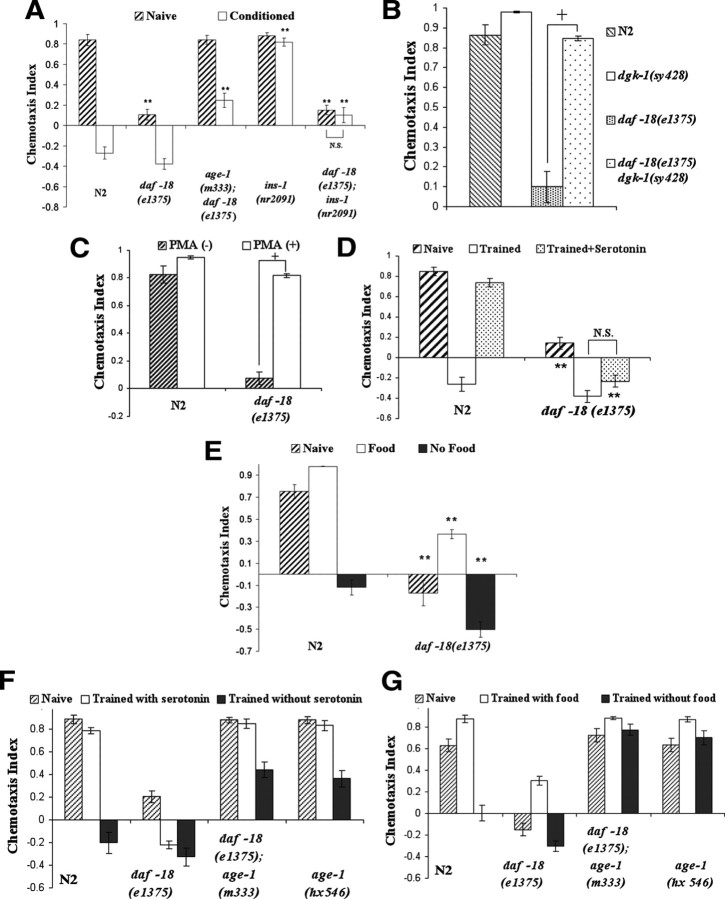
To gain more insight into how AGE-1 signaling is required for benzaldehyde–starvation associative plasticity, mutants with hyperactive AGE-1 signaling were examined. Activation of AGE-1 leads to the generation of 3-phoshoinositide, whereas DAF-18 dephosphorylates 3-phoshoinositides (Ogg and Ruvkun, 1998). Animals with defective daf-18 therefore have an elevated level of 3-phosphoinositides and presumably hyperactive AGE-1 signaling. When tested to the benzaldehyde plasticity paradigm, daf-18(e1375) animals showed a significant decreased naive attraction toward benzaldehyde, but demonstrated a normal conditioned benzaldehyde avoidance response that could be blocked by ins-1 mutation (Fig. 3A). Preventing the accumulation of 3-phospoinositide with the age-1(m333null) mutation fully reversed the decreased chemotaxis phenotype and the plasticity performance of daf-18 animals to those of age-1 mutants (Fig. 3A), suggesting that enhanced AGE-1 signaling is responsible for the daf-18 phenotype. Given that a decrease in odorant chemotaxis can be a result of reduced synaptic transmission (Tsunozaki et al., 2008), we crossed daf-18 animals to dgk-1(sy428) mutants that are known to have elevated diacylglycerol (DAG) signaling and enhanced neurotransmission (Lackner et al., 1999). The mutation in dgk-1 fully reversed the attenuated benzaldehyde attraction in daf-18 animals (Fig. 3B). Furthermore, treating daf-18 animals with β-phorbol ester phorbol 12-myristate 13-acetate (PMA), a pharmaceutical analog of DAG known to increase synaptic transmission through activation of DAG signaling (Betz et al., 1998; Lackner et al., 1999), for 2 h before testing significantly suppressed their attenuated naive attraction phenotype (Fig. 3C). Together, the data suggest that the level of AGE-1 signaling and perhaps the level of insulin signaling likely governs the extent and direction of benzaldehyde chemotaxis (i.e., attraction or avoidance) through modulation of synaptic release.
Figure 3.
daf-18 animals exhibit abnormal odorant chemotaxis and odorant associative plasticity. For all panels in the figure, double asterisks represent significant differences from N2 within the same group (**p < 0.05 by Bonferroni's t test). Crosses represent significant differences between the indicated data points (+p < 0.05 by Bonferroni's t test). N.S., Not significant. Data represent means ± SEM. A, Benzaldehyde–starvation associative plasticity in animals with the daf-18 mutation. daf-18 mutants demonstrated an attenuated naive benzaldehyde attraction but normal benzaldehyde–starvation associative plasticity. The abnormal benzaldehyde chemotaxis could be suppressed by the age-1 mutation, while the normal associative plasticity could be blocked by the ins-1 mutation in daf-18 mutants. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(4,59) = 32.06, p < 0.05 (n = 6 plates for each data point). B, Chemotaxis of dgk-1 and daf-18; dgk-1 mutants to 1% benzaldehyde. The dgk-1 mutation fully reversed the attenuated naive benzaldehyde attraction in daf-18 mutants. A Kruskal-Wallis one-way ANOVA revealed significance in groups, H = 19.7, p < 0.05 (n = 6 plates for each data point). C, Naive benzaldehyde chemotaxis of N2 and daf-18 animals after 2 h of PMA treatment. Treating daf-18 animals with PMA significantly reversed their decreased benzaldehyde chemotaxis. A two-way ANOVA revealed a significant interaction between strain and PMA treatment, F(1,19) = 63.236, p < 0.05 (n = 5 plates for each data point). D, Benzaldehyde–starvation associative plasticity in animals conditioned in the presence of serotonin. Serotonin treatment during conditioning did not significantly alter benzaldehyde–starvation associative plasticity in daf-18 mutants. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 31.86, p < 0.05 (n = 6 plates for each data point). E, Butanone–starvation associative plasticity and butanone–food associative plasticity in daf-18 mutants. daf-18 mutants exhibited naive butanone avoidance, enhanced butanone–starvation associative plasticity, and impaired butanone–food associative plasticity. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 7.83, p < 0.05 (n = 6 plates for each data point). F, Benzaldehyde–starvation associative plasticity in animals conditioned in the presence of serotonin. Serotonin treatment during conditioning did not significantly alter benzaldehyde–starvation associative plasticity in daf-18 mutants. daf-18; age-1 double mutants showed performance similar to that of age-1 mutants in the assay. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(6,47) = 13.34, p < 0.05 (n = 4 plates for each data point). G, Butanone–starvation associative plasticity and butanone–food associative plasticity in daf-18, daf-18; age-1, and age-1 mutants. daf-18 mutants exhibited naive butanone avoidance, enhanced butanone–starvation associative plasticity, and impaired butanone–food associative plasticity. daf-18; age-1 double mutants showed performance similar to that of age-1 mutants in the assay. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(6,47) = 13.76, p < 0.05 (n = 4 plates for each data point).
We have previously shown that serotonin likely mimics the effect of food in mediating benzaldehyde–starvation associative plasticity (Nuttley et al., 2002). To determine the relationship between insulin signaling and serotonin signaling in regulating this associative plasticity task, daf-18 animals were trained to associate benzaldehyde and starvation in the presence of serotonin. While the presence of serotonin during training significantly suppressed benzaldehyde associative plasticity in N2 wild-type animals, serotonin did not block the conditioned avoidance response observed in daf-18 mutants (Fig. 3D), suggesting that AGE-1 signaling may act downstream of serotonin signaling. Interestingly, daf-18 animals exhibited impaired butanone–food associative plasticity and enhanced butanone–starvation associative plasticity (Fig. 3E). Furthermore, daf-18; age-1 double mutants appeared identical to age-1 mutants when conditioned to benzaldehyde in the presence of serotonin or in butanone enhancement, consistent with the notion that the daf-18 phenotype is a consequence of unmitigated insulin signaling through AGE-1. (Fig. 3F,G) Together, the data imply that insulin signaling may function as a learning-specific starvation signal that antagonizes downstream food signals activated by serotonin in benzaldehyde–starvation associative plasticity.
INS-1 released from ASI and AIA that acts on benzaldehyde-sensing AWC sensory neurons is sufficient to mediate benzaldehyde–starvation associative plasticity
To better visualize and determine in which neurons INS-1 is required for benzaldehyde–starvation associative plasticity, we performed rescue experiments in ins-1(nr2091) by expressing INS-1 proteins fused with the reporter Venus. We found that the learning deficit was fully rescued when INS-1::VENUS was expressed from ins-1 promoter (Fig. 3A), confirming that INS-1:: VENUS was functional. Given that INS-1 has a widespread expression in the nervous system but the expression is strongest and most consistent in AIA interneurons (Tomioka et al., 2006), we hypothesized INS-1 is required in AIA interneurons for benzaldehyde–starvation associative plasticity. We constructed a short form of ins-1 promoter that drove INS-1::VENUS protein specifically in AIA (details will be published elsewhere; M. Tomioka and Y. Iino, unpublished data) and found that expression of INS-1::Venus in AIA only partially rescued the plasticity deficit observed in ins-1 mutants. Expression of INS-1::VENUS from different sets of neurons within the benzaldehyde-sensing AWC neural circuitry using glr-1, ser-2, daf-7, odr-3, and lin-11 promoters also partially rescued the learning deficit of ins-1 mutants (Fig. 4A,B). When ins-1 mutants were injected with both of the constructs that drive INS-1::VENUS under the ins-1(short) and daf-7 promoters, the expression of INS-1 in ASI and AIA was sufficient to fully rescue the learning deficit (Fig. 4A). Interestingly, close examination of ins-1p::ins-1::Venus expression and the rescue results revealed that expression of INS-1::VENUS from certain neurons that do not express INS-1 under physiological conditions could partially rescue the plasticity deficit in ins-1 mutants. Given that INS-1::VENUS is expressed in ASI and AIA under ins-1 promoter and that expression of INS-1 in ASI and AIA fully rescued the plasticity defect in ins-1 mutants (Fig. 4A), INS-1 likely acts non-cell autonomously from ASI and AIA either as a hormone or through synaptic delivery to mediate benzaldehyde–starvation associative plasticity under physiological conditions.
Figure 4.
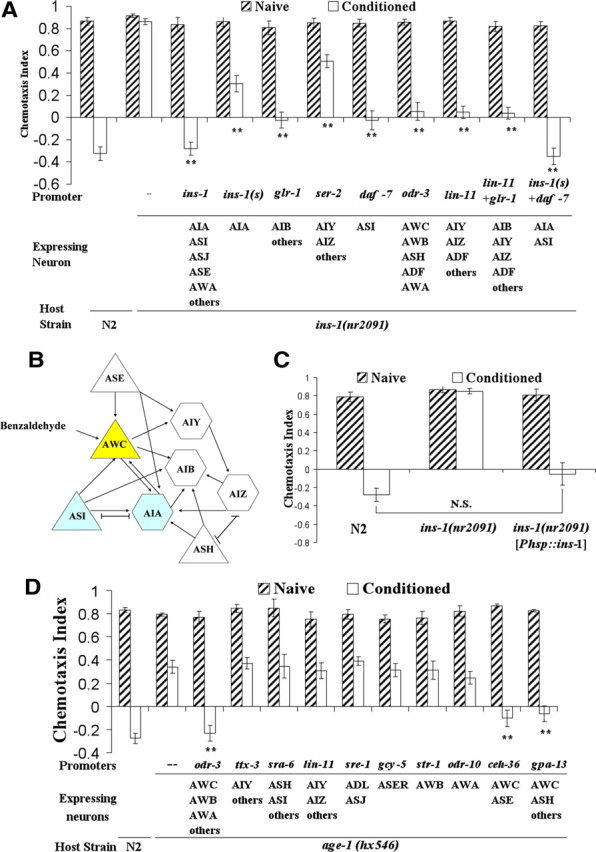
INS-1 can act from multiple neurons and AGE-1 acts in AWC to regulate benzaldehyde–starvation associative plasticity at the adult stage. For all panels in the figure, double asterisks represent significant differences from N2 within the same group (**p < 0.05 by Bonferroni's t test). N.S., Not significant. Data represent means ± SEM. A, Rescue effect of INS-1::VENUS expression under different promoters in ins-1 animals. Expression of INS-1::VENUS from multiple sets of neurons partially rescued the plasticity deficit in ins-1 mutants, and expression of INS-1::VENUS in ASI and AIA was sufficient to fully rescue the deficit. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(10,131) = 20.72, p < 0.05 (n = 6 plates for each data point). B, Part of the neural circuitry involved in benzaldehyde sensation (White et al., 1986). Arrows indicate synaptic connections; H shapes indicate gap junctions; triangles and hexagons represent sensory neurons and interneurons respectively. C, Rescue effect of ins-1 cDNA expression under a heat shock-inducible promoter in ins-1 animals. Animals were heat shocked at 33°C and allowed to recover before exposure to benzaldehyde in the absence of food. Expression of INS-1 under a heat shock promoter rescued the plasticity deficit in ins-1 mutants after heat shock. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 32.06, p < 0.05 (n = 6 plates for each data point). D, Rescue effect of AGE-1 expression using various promoters in age-1(hx546) animals. The learning defect is significantly rescued by the expression of AGE-1 in AWC neurons using the odr-3, ceh-36, and gpa-13 promoters. Expression of AGE-1 in AIY interneurons using ttx-3, in subsets of neurons including AIZ using lin-11 promoter, in ADL and ASJ neurons using the sre-1 promoter, in ASER neurons using the gcy-5 promoter, in AWB neurons using str-1 promoter, or in ASH and ASI neurons using promoter sra-6 did not rescue the plasticity defect in age-1(hx546) animals. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(11,143) = 12.18, p < 0.05 (n = 6 plates for each data point).
To test the possibility that expression of INS-1 is required for the normal development of neurons involved in benzaldehyde–starvation learning, we performed a heat shock rescue experiment. We generated rescue animals in which ins-1 cDNA is under the control of a heat shock promoter and then heat shocked the animals at the adult stage. Expression of INS-1 during adult stage in ins-1(nr2091) animals rescued the defect in benzaldehyde–starvation learning (Fig. 4C), suggesting that the learning deficit observed in ins-1(2091) did not result from defects in neuronal development.
Since it is difficult to express DAF-2 from an extra-chromosomal array (Tomioka and Iino, unpublished), we generated rescue lines of age-1(hx546) injected with constructs driving age-1 cDNA in different subsets of neurons to identify the INS-1 targeting neurons in benzaldehyde–starvation associative plasticity. Expression of AGE-1 from neurons including interneurons AIY and AIZ using ttx-3 and lin-11 promoters did not rescue the plasticity defect seen in age-1(hx546) animals (Fig. 4D). However, when age-1 cDNA was expressed in AWC sensory neurons using odr-3, gpa-13, and ceh-36 promoters, the plasticity deficit was rescued completely (Fig. 4D). In contrast, AGE-1 expression in other sensory neurons such as ASI, ASH, ADL, ASJ, AWB, AWA and ASER using sra-6, sre-1, str-1, odr-10, and gcy-5 promoters did not rescue the learning deficit (Fig. 4D). These results indicate that AGE-1 functions in AWC sensory neurons, and INS-1 released from ASI and AIA might act on benzaldehyde-sensing AWC sensory neurons [perhaps through direct synaptic connections to AWC (White et al., 1986)] to regulate benzaldehyde–starvation associative plasticity.
DAF-2 signaling plays a more significant role in the memory retrieval than in the memory acquisition of benzaldehyde–starvation associative plasticity
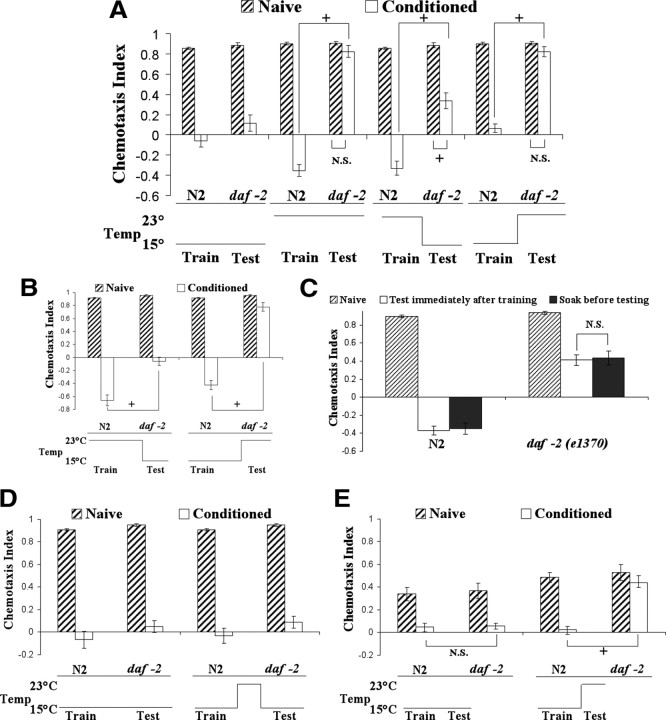
To determine whether the defective associative plasticity in insulin signaling mutants is a result of a deficit in memory acquisition or memory retrieval, we exploit the fact that daf-2(e1370) is a temperature-sensitive conditional allele, in which the mutation in daf-2(e1370) is within the tyrosine kinase domain and is predicted to affect substrate binding (Patel et al., 2008). Given that the mutation in not within the regulatory region, it is likely that shifting the temperature can disrupt or resume insulin signaling by affecting the protein conformation rather than the level DAF-2 expression. Since the daf-2(e1370) allele is impaired at 23°C but presumably normal at 15°C and also no plasticity deficit was observed when daf-2(e1370) animals were trained and tested at 15°C (Fig. 2A), we examined daf-2(e1370) animals' learning ability when conditioned at 15°C but tested at 23°C and vice versa. This temperature shift assay permitted us to examine whether daf-2 animals would exhibit a deficit in behavioral plasticity by disrupting insulin signaling specifically during the acquisition or the recall phase of benzaldehyde–starvation associative plasticity. We found that daf-2 animals trained at 15°C and tested at 23°C demonstrated a similar benzaldehyde attraction response compared with that of naive daf-2 animals (and thus no evidence of any memory performance), while daf-2 mutants conditioned at 23°C and tested at 15°C showed some degree of learning relative to that of the wild type (Fig. 5A). Even when the conditioning time was extended to 2 h to facilitate the acquisition of benzaldehyde–starvation associative memory, the same trend in memory performance by daf-2 mutants was observed, in which the plasticity deficit was more prominent when the mutants experienced a temperature up-shift during testing period (Fig. 5B). To determine whether the amount of functional DAF-2 restored during the interval of testing at 15°C may be insufficient, daf-2 animals were trained to benzaldehyde in the absence of food for 1 h at 23°C and soaked in 15°C M9 buffer for 30 min before testing their chemotaxis to benzaldehyde at 15°C. Compared with daf-2 mutants that were tested at 15°C immediately after training at 23°C, soaking animals for 30 min in 15°C M9 buffer did not significantly alter daf-2 mutants' partial learning defect (Fig. 5C), suggesting that the partial defect observed after temperature downshift during testing is not due a lack of restored functional DAF-2. These data suggest that DAF-2 signaling is only partially involved in memory acquisition, but is essential in memory retrieval.
Figure 5.
DAF-2 signaling is partially involved in memory acquisition but essential in memory retrieval. For all panels in the figure, double asterisks represent significant differences from N2 within the same group (**p < 0.05 by Bonferroni's t test). Crosses represent significant differences between the indicated data points (+p < 0.05 by Bonferroni's t test). N.S., Not significant. Data represent means ± SEM. A, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants when DAF-2 signaling is disrupted during memory acquisition or retrieval. Wild-type N2 and daf-2(e1370) mutants were grown at 15°C and then conditioned at 15 or 23°C and tested to benzaldehyde at 15 or 23°C. Disruption of DAF-2 signaling during training partially blocked benzaldehyde–starvation associative plasticity while disruption of DAF-2 signaling during testing fully blocked this plasticity in daf-2(e1370) mutants. A three-way ANOVA revealed a significant interaction between strain, conditioning, and temperature F(3,127) = 13.30, p < 0.05 (n = 8 plates for each data point). B, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants when the conditioning period is extended to 2 h at two different temperatures. Disruption of DAF-2 signaling during training partially blocked benzaldehyde–starvation associative plasticity while disruption of DAF-2 signaling during testing fully blocked this plasticity in daf-2(e1370) mutants. A three-way ANOVA revealed significant interaction between strain, conditioning, and temperature F(1,47) = 77.63, p < 0.05 (n = 6 plates for each data point). C, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants given more time for functional DAF-2 restoration. Extending the restoration time of functional DAF-2 did not significantly change daf-2 mutants' conditioned response toward benzaldehyde. A two-way ANOVA revealed a significant interaction between strain and conditioning, F(2,35) = 33.54, p < 0.05 (n = 6 plates for each data point). D, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants with transiently disruption of DAF-2 signaling after conditioning. Animals were conditioned at 15°C, washed and left in 23°C M9 for 10 min before testing at 15°C. Transient disruption of DAF-2 signaling did not block benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants. A two-way ANOVA revealed no significant interaction between strain and conditioning F(2,35) = 33.54, p < 0.05 (n = 6 plates for each data point). E, Benzaldehyde–starvation associative plasticity in daf-2(e1370) mutants when DAF-2 signaling is disrupted during memory retrieval. Animals were conditioned at 15°C and tested 15°C or 23°C for 10 min. daf-2 animals demonstrated a significant plasticity deficit when tested at 23°C for 10 min compared with that of wild-type N2. A three-way ANOVA revealed a significant interaction between strain, conditioning, and temperature F(1,47) = 7.08, p < 0.05 (n = 6 plates for each data point).
It is interesting to note that animals trained at a lower temperature exhibit a smaller degree of conditioned avoidance response compared with animals trained at a higher temperature. This effect is perhaps caused by the difference in the odor volatility or cellular metabolism rate at different temperatures. Alternatively, since animals were cultured at 15°C for the temperature shifting assay, their previous experience that food was present at 15°C may interfere with the association between starvation and benzaldehyde.
The memory retrieval deficit caused by a disruption of DAF-2 signaling can either reflect an erasure of benzaldehyde–starvation memory or a block of access to or output from the otherwise intact memory. To distinguish between these two possibilities, we devised a conditioning protocol in which daf-2(e1370) animals were trained at 15°C for 1 h, washed and left in 23°C M9 buffer for 10 min to transiently disrupt DAF-2 signaling, and subsequently tested at 15°C to restore insulin signaling. If the disruption of DAF-2 signaling blocks the access to or output from the intact memory, then daf-2 animals should show normal memory retrieval after insulin signaling is restored. On the other hand, if the retrieval deficit in daf-2 mutants is caused by an erasure of memory, then the animals should not demonstrate any change in odorant preference (i.e., memory performance) once DAF-2 signaling is blocked even if the disruption is only temporary. daf-2 animals demonstrated comparable benzaldehyde–starvation associative plasticity to that of wild-type animals when subjected to the temporary disruption of insulin signaling before testing (Fig. 5D), suggesting that the momentary disturbance in DAF-2 signaling did not impair memory performance of daf-2 animals. To determine whether 10 min of 23°C up-shift is sufficient to disrupt insulin signaling, daf-2 animals were trained at 15°C and tested to benzaldehyde at 23°C for 10 min rather than the standard 60 min testing period used in other experiments. daf-2 mutants tested at 23°C for 10 min demonstrated a significant plasticity deficit compared with that of N2 worms, while the mutants tested at 15°C for 10 min did not exhibit any significant defect (Fig. 5E), suggesting that 10 min of temperature up-shift is sufficient to inactivate insulin signaling. Together, the data suggest the retrieval deficit observed in daf-2 animals is not a consequence of memory erasure but rather of a block in the access to or output from the memory.
Discussion
In this study insulin signaling was shown to be crucial in regulating benzaldehyde–starvation associative plasticity in C. elegans. Animals lacking functional INS-1, which is the closest human insulin homolog in C. elegans (Pierce et al., 2001), exhibited a severe deficit in benzaldehyde–starvation associative plasticity compared with wild-type animals (Fig. 1A). Furthermore, animals with mutations in daf-2 and age-1 (which encode an insulin/IGF-1 receptor and a PI-3 kinase that are implicated in insulin signaling) are also defective in benzaldehyde–starvation associative plasticity (Fig. 2A,B). The rescue experiments suggest that INS-1 may act from ASI and AIA under physiological conditions and that DAF-2 signaling is required in the benzaldehyde-sensing AWC sensory neuron to mediate benzaldehyde–starvation associative plasticity (Fig. 4A–D). Given that ASI and AIA both synapse onto AWC (White et al., 1986), we speculate that INS-1 may act best through synaptic delivery to activate DAF-2 signaling in AWC for this associative plasticity. However, given that INS-1 expression from neurons within the benzaldehyde-sensing circuitry that have no synaptic connections with AWC also partially rescued the plasticity deficit in ins-1 mutants (Fig. 4A,B), it cannot be ruled that INS-1 may act non-cell autonomously in a hormone fashion.
Insulin signaling plays a partial role in the memory acquisition of benzaldehyde–starvation associative plasticity
It is of significant interest that the present data suggest that the behavioral roles of insulin signaling in benzaldehyde–starvation associative plasticity can be dissociated between memory acquisition and memory retrieval, with insulin signaling playing a partial role in memory acquisition but an essential role in memory retrieval (Fig. 5A–D). INS-1 appears to mediate associative learning tasks that involve starvation as the US in C. elegans. Supporting this, ins-1 mutants are defective in thermotaxis learning, salt chemotaxis learning, and benzaldehyde–starvation associative plasticity in which starvation is the US (Kodama et al., 2006; Tomioka et al., 2006), but are normal in other associative learning tasks such as butanone–food associative plasticity and odor-pathogenic food associative plasticity (Fig. 1E) (C. H. A. Lin and D. van der Kooy, unpublished data). Therefore, insulin signaling might function as one of the starvation signals necessary for the association process during the memory acquisition of benzaldehyde–starvation associative plasticity. This hypothesis is also consistent with our findings that daf-18 animals are resistant to the suppression of benzaldehyde–starvation associative plasticity mediated by serotonin signaling, and that daf-18 mutants have enhanced butanone–starvation associative plasticity but impaired butanone–food associative plasticity (Fig. 3D,E). Even though ins-1 animals exhibit normal starvation behaviors such as the enhanced slowing response and starvation-suppressed egg laying (Kodama et al., 2006), it remains possible that multiple pathways might regulate different starvation responses, and that INS-1 transmits a learning-specific starvation signal required for benzaldehyde–starvation associative plasticity. In this model, insulin signaling acts upstream of memory acquisition and perhaps functions as a part of the US pathways. However, it cannot be ruled out that INS-1 might be released from multiple neurons to maintain basal insulin signaling in neurons that are permissive for benzaldehyde–starvation associative plasticity, and that the disruption of this neuronal insulin signaling might have a detrimental effect that perturbs the machinery required for the memory acquisition.
Insulin signaling is essential in the memory retrieval of benzaldehyde–starvation associative plasticity
Using our temperature shift assay, we found that functional DAF-2 signaling is critical during the retrieval phase of benzaldehyde–starvation associative plasticity (Fig. 5A,B). Furthermore, the benzaldehyde–starvation associative memory cannot be degraded by temporary disruption of DAF-2 signaling between training and testing (Fig. 5D), suggesting that continuous DAF-2 signaling is not required for the maintenance of memory.
It is likely that the output of benzaldehyde–starvation associative memory may result from a switch in the signaling mode of AWC sensory neurons, depending on the level of insulin signaling in AWC. Animals with elevated AGE-1 signaling (i.e., daf-18 mutants) demonstrated an attenuated benzaldehyde naive attraction that could be reversed by increasing synaptic transmission through dgk-1 mutation and PMA treatment (Fig. 3B,C). Furthermore, AGE-1 is required in benzaldehyde-sensing AWC for benzaldehyde–starvation associative plasticity (Fig. 4D). We therefore propose that high levels of insulin signaling within AWC, a neuron associated with naive attractive responses toward benzaldehyde, perhaps switch the signaling mode of AWC from attraction to repulsion through decreasing DAG signaling and synaptic release. Indeed, it has recently been proposed that AWCON neuron possesses the capacity to switch from an attractive to repulsive signaling mode by decreasing excitability or synaptic release through downregulation of gcy-28 activity and DAG/PKC signaling, thereby suppressing animals' attraction toward benzaldehyde and generating an avoidance behavior toward the odorant butanone (Tsunozaki et al., 2008). Further supporting the hypothesis that such a mechanism exists as an output of benzaldehyde–starvation associative memory, it has been suggested that elevation of insulin signaling might lead to suppression of neurotransmitter release from ASER sensory neurons (Tomioka et al., 2006). Additionally, the PIP3-kinase pathway, which operates downstream of DAF-2 in the worm (Baumeister et al., 2006), has been shown to modulate the excitability of rodent olfactory receptor neurons (Spehr et al., 2002). The finding that daf-18 mutants avoided AWC sensed butanone under a naive condition (Fig. 3E) is also consistent with the hypothesis that high levels of insulin signaling switch the signaling mode of AWC. It will be interesting to determine whether high levels of insulin signaling in AWC might lead to a decrease in excitability or synaptic release through lowering gcy-28 activity, thereby switching the output signaling mode from attraction to repulsion.
Models for benzaldehyde–starvation associative plasticity
It has been proposed that a switch between attraction signaling and avoidance signaling in AWC contributes to the plasticity behavior after animals are exposed to the AWC sensed odorant either in the presence or the absence of food, and that odorant plasticity arises at the level of AWC synaptic release (Tsunozaki et al., 2008). To put insulin signaling in the context of this switch model, one possibility is that the exposure to benzaldehyde in the absence of food enhances the levels of insulin signaling in AWC and that high levels of insulin signaling promote repulsion signaling in AWC. In this scenario, INS-1 is released from AIA and ASI in the absence of food, and activates DAF-2 signaling in AWC to transmit the starvation signal, but the level of DAF-2 signaling in AWC is limited by the number or activity of DAF-2 or its downstream components, thereby maintaining the attraction signaling mode in benzaldehyde-sensing AWC under a naive condition. Sensory association of starvation signals and benzaldehyde sensed by AWC may result in a sustained increase in the synaptic integration of DAF-2 receptors or the activity of DAF-2 downstream components in AWC. Therefore, once the association processes have taken place, release of INS-1 can lead to high levels of DAF-2 signaling in AWC and switch the neuron into a repulsion signaling mode, thereby generating an avoidance behavior upon subsequent benzaldehyde exposure. However, such a model implies that AWC is in a predetermined repulsion mode after memory acquisition, and therefore fails to account for the context-dependent aspect of memory retrieval. Indeed, animals trained to associate benzaldehyde with starvation in the presence of salt lack benzaldehyde–starvation associative plasticity upon subsequent exposure to benzaldehyde in a salt-free environment, suggesting that the memory retrieval of benzaldehyde–starvation associative plasticity follows an occasion-setting mechanism, in which context cues (i.e., salt) function in a hierarchical fashion to modulate memory recall by defining an appropriate setting (Law et al., 2004).
To account for the occasion-setting effect in benzaldehyde–starvation associative plasticity, we propose that a switch in the AWC signaling of mode (i.e., the change in the level of insulin signaling) occurs after the associative plasticity memory is retrieved (Fig. 6). In this model, AWC senses benzaldehyde and receives context signals (i.e., salt) from ASE and starvation signals (INS-1 is one of the starvation signals) from ASI and AIA and other unknown neurons. The sensory signals are associated along with the context cues and leads to molecular changes that encode the associative memory within AWC. During the memory retrieval phase of benzaldehyde–starvation associative plasticity, the memory is recalled when AWC senses benzaldehyde under the appropriate context (i.e., salt). The retrieval of the associative memory subsequently leads to high levels of DAF-2 signaling in AWC either through an increase in synaptic integration of DAF-2 or activity of its downstream components, and switches AWC from an attractive to a repulsive signaling mode, thereby generating an avoidance behavioral output toward benzaldehyde. Indeed, not seeing an enhancement in memory performance in the rescue lines overexpressing INS-1 (Fig. 4A) is consistent with the notion that insulin signaling is regulated downstream of INS-1 (perhaps through the cell surface levels of daf-2 or daf-2's downstream effectors) rather than through a flux in INS-1 levels. Remarkably, such a model would suggest that a single sensory neuron in C. elegans may possess the capacity of sensory association, memory storage, and memory recall when such complex neural functions are believed to be mediated by multiple neurons in specific brain regions in vertebrate organisms.
Figure 6.
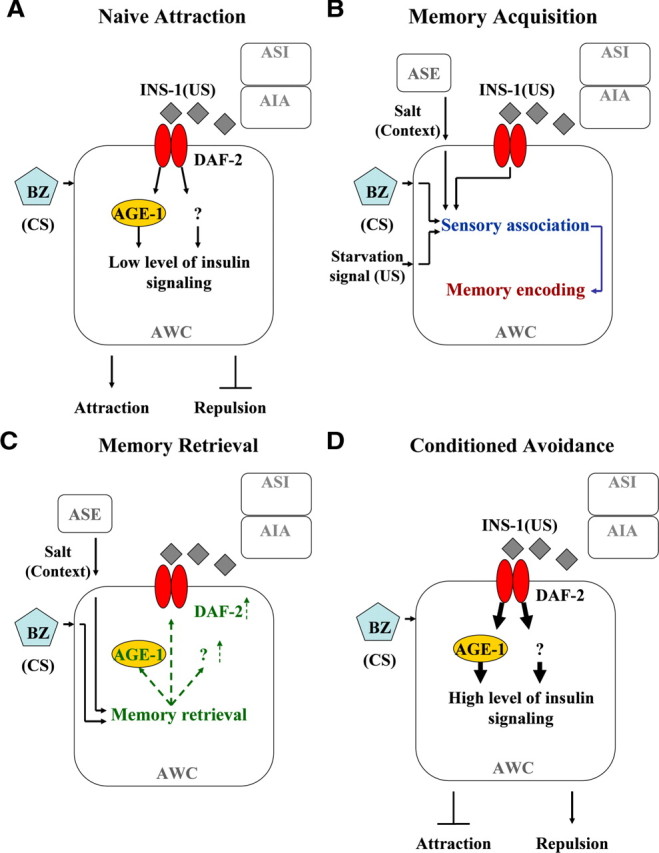
A working model for the regulation of benzaldehyde–starvation associative plasticity by insulin signaling in which memory is stored in the AWC. A, Benzaldehyde-sensing AWC neurons are in an attraction signaling mode under low levels of insulin signaling, thereby generating a naïve attraction response toward benzaldehyde (BZ). INS-1 is released from AIA and ASI from the synapse in the absence of food to activate insulin signaling in AWC, but the level of insulin signaling in AWC is limited either through the number of DAF-2 receptors or activity of its downstream effectors under the naive condition. B, During the memory acquisition phase, benzaldehyde-sensing AWC receives context information (i.e., presence of salt) from salt-sensing ASE and US starvation signals (i.e., INS-1 and other unknown proteins) from ASI, AIA, and other unknown neurons. Under CS benzaldehyde exposure, the CS pathways and US pathways are associated along with the context, thereby inducing molecular changes in AWC to store the associative memory. C, During the memory retrieval phase, sensation of benzaldehyde and reception of the appropriate context signal (i.e., presence of salt) in AWC leads active recall of the associative memory that subsequently leads to either an increase in the membrane integration of DAF-2 or the activity of its downstream components in AWC. D, Once the memory is retrieved, the increase in DAF-2 or the activity of its downstream effectors leads to high levels of insulin in AWC, which then suppresses the attraction signaling and promotes the repulsion signaling, thereby generating a conditioned avoidance response toward the odorant benzaldehyde.
Footnotes
This work was funded by the Natural Sciences and Engineering Research Council of Canada (D.v.d.K.) and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.I.). We thank G. Ruvkun and Patrick Hu for the ins-1(nr2091) and age-1(mg305) strains, C.A. Wolkow for the age-1 cDNA, O. Hobert for the ttx-3 promoter, members in the van der Kooy laboratory for comments on this manuscript, and Prateek Goyal, Karen Kok, and members of the Peter Roy laboratory for experimental assistance. All other nematode strains used in the study were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
References
- Barros DM, Mello e Souza T, de Souza MM, Choi H, DeDavid e Silva T, Lenz G, Medina JH, Izquierdo I. LY294002, an inhibitor of phosphoinositide 3-kinase given into rat hippocampus impairs acquisition, consolidation and retrieval of memory for one-trial step-down inhibitory avoidance. Behav Pharmacol. 2001;12:629–634. doi: 10.1097/00008877-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- Bernhard N, van der Kooy D. A behavioral and genetic dissection of two forms of olfactory plasticity in Caenorhabditis elegans: adaptation and habituation. Learn Mem. 2000;7:199–212. doi: 10.1101/lm.7.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001;21:1510–1522. doi: 10.1523/JNEUROSCI.21-05-01510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, D'Alberti T, Liu Y, Ruvkun G. Control of neural development and function in a thermoregulatory network by the LIM homeobox gene lin-11. J Neurosci. 1998;18:2084–2096. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, Iino Y, Mori I. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20:2955–2960. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Law E, Nuttley WM, van der Kooy D. Contextual taste cues modulate olfactory learning in C. elegans by an occasion-setting mechanism. Curr Biol. 2004;14:1303–1308. doi: 10.1016/j.cub.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–2137. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Patel DS, Garza-Garcia A, Nanji M, McElwee JJ, Ackerman D, Driscoll PC, Gems D. Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics. 2008;178:931–946. doi: 10.1534/genetics.107.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Spehr M, Wetzel CH, Hatt H, Ache BW. 3-Phosphoinositides modulate cyclic nucleotide signaling in olfactory receptor neurons. Neuron. 2002;33:731–739. doi: 10.1016/s0896-6273(02)00610-4. [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron. 2006;51:613–625. doi: 10.1016/j.neuron.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Torayama I, Ishihara T, Katsura I. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J Neurosci. 2007;27:741–750. doi: 10.1523/JNEUROSCI.4312-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunozaki M, Chalasani SH, Bargmann CI. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–971. doi: 10.1016/j.neuron.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, McCulloch D, Gems D, Kovács AL. Effects of sex and insulin/insulin-like growth factor-1 signaling on performance in an associative learning paradigm in Caenorhabditis elegans. Genetics. 2006;174:309–316. doi: 10.1534/genetics.106.061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JY, Kumar N, Morrison G, Rambaldini G, Runciman S, Rousseau J, van der Kooy D. Mutations that prevent associative learning in C. elegans. Behav Neurosci. 1997;111:354–368. doi: 10.1037//0735-7044.111.2.354. [DOI] [PubMed] [Google Scholar]
- Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci U S A. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]