Abstract
Water sensation is a specific taste modality in the fruit fly. Water-induced hypoosmolarity activates specific gustatory receptor neurons; however, the molecular identity of the putative osmolarity sensor in these neurons remains unknown. We found that amiloride and its analogs specifically antagonized the response of water gustatory receptor neurons and the behavior of flies toward water stimulation. Deletion of the gene that encodes the amiloride-sensitive PPK28 channel, a DEG/eNaC (degenerin/epithelial sodium channel) family member, abolished the water-induced activity of water gustatory receptor neurons and greatly diminished the behavioral response of flies to water. Ectopic expression of the PPK28 channel in the bitter cells within the intermediate-type sensilla renders these sensilla responsive to water stimuli. Thus, the amiloride-sensitive PPK28 channel may serve as the osmolarity sensor for gustatory water reception in the fruit fly.
Introduction
Water is so critical for life that diverse mechanisms have evolved in different species for the regulation of water intake. In Drosophila melanogaster, two unique systems are used to sense environmental water availability—the antennal hygrosensation for the detection of variation in air humidity, and the gustatory reception for the detection of water in liquids (Inoshita and Tanimura, 2006; Liu et al., 2007). Drosophila gustatory reception is mediated by taste sensilla, which are located at the labellum, legs, and wing margins (Stocker, 1994). Each taste sensillum encompasses one mechanosensory neuron and 2–4 gustatory receptor neurons (GRNs) (Stocker, 1994). Electrophysiological recordings of taste sensilla revealed the presence of four kinds of GRNs that exhibit tuned responses to four taste modalities (sugar, water, salt, and bitter): S and W cells respond to sugar and water, respectively, whereas L1 and L2 cells respond to low and high concentrations of salts, respectively, with L2 cells also being responsive to bitter compounds (Ishimoto and Tanimura, 2004). Molecular understanding of sugar and bitter reception was greatly promoted by the identification of the gustatory receptor (Gr) gene family (Clyne et al., 2000; Dunipace et al., 2001; Scott et al., 2001). Gr genes are expressed in two distinct groups of GRNs—the Gr5a and Gr64 gene cluster is expressed in S cells to mediate sugar reception (Dahanukar et al., 2001, 2007; Jiao et al., 2007, 2008; Slone et al., 2007), whereas other Gr genes are mostly expressed in L2 cells (Thorne et al., 2004; Wang et al., 2004), which mediate bitter reception (Moon et al., 2006, 2009; Lee et al., 2009). Salt reception may require ppk11 and ppk19 (Liu et al., 2003). In contrast, the molecular basis of gustatory water reception remains unknown. Gustatory water reception may be a special kind of osmosensation, and an “osmometer” hypothesis has been proposed, suggesting that the water cells could be activated by hypotonic force (Evans and Mellon, 1962).
Several types of ion channels have been implied in osmosensation, including members of the transient receptor potential (TRP) superfamily [e.g., TRPV members osm-9 (Colbert et al., 1997) and VR-OAC (Liedtke et al., 2000) and TRPM member TRPM3 (Fleig and Penner, 2004)], as well as chloride channels (Strange et al., 1996) and potassium channels (Vanoye and Reuss, 1999). It is possible that other channels are also involved in osmosensation. In this study, we screened the effect of various ion channel antagonists on water responses of GRNs in Drosophila. We discovered that water-evoked responses can be specifically blocked by amiloride and its analogs. Amiloride-sensitive DEG/eNaC (degenerin/epithelial sodium channel) channels (Kellenberger and Schild, 2002), which are known as pickpocket (PPK) channels in Drosophila (Adams et al., 1998; Liu et al., 2003), were tested as candidates for the Drosophila gustatory water receptor. PPK28 was identified as being essential for water taste, which suggests that PPK28 may be a potential gustatory water receptor in Drosophila.
Materials and Methods
Fly stocks.
Flies were cultured on standard cornmeal-agar-molasses medium at 25°C with a relative humidity of 70%. W1118 flies were used as the wild-type control. The PoxnM22;redistal2 mutant was provided by Markus Noll (University of Zurich, Zurich, Switzerland) (Boll and Noll, 2002). Trpl302 (Niemeyer et al., 1996), Nan36a (Kim et al., 2003), and Pyx3 (Lee et al., 2005) were kindly provided by Charles Zuker (University of California San Diego, La Jolla, CA), Changsoo Kim (Hanwha Chemical Company, Daejeon, Korea), and Jaeseob Kim (Korea Advanced Institute of Science and Technology, Daejeon, Korea), respectively. Other strains were from the Bloomington Drosophila Stock Center (Bloomington, IN) and the Harvard Exelixis Stock Center (Boston, MA).
Generation of ppk deletions and transgenic flies.
Flippase recombinase/flippase recombinase target recombination (Exelixis) was used to generate deletions of ppk28 and ppk23. The ppk28 deletion was generated from PBac insertion lines f05788 and e02329, and the ppk23 deletion was generated from lines f02390 and e03639. To generate the construct for ppk28 genomic rescue, a 4 kb genomic DNA fragment of ppk28 was PCR amplified from W1118 and was then cloned into the pattB vector. The transgenic line for ppk28 genomic rescue was generated using phiC31-integrase-mediated recombination at ZH-51C (Bischof et al., 2007). The coding sequence for ppk28 was inserted into the pUAST vector. UAS-ppk28 flies were generated through P-element-mediated transformation.
Chemicals and antagonists.
The following chemicals and antagonists were from Sigma–Aldrich: dimethylsulfoxide (DMSO), KCl, tricholine citrate (TCC), NaCl, sucrose, and berberine chloride; nimodipine, SKF-96365, niflumic acid, and 5-nitro-2-(3-phenylpropyl-amino)benzoic acid (NPPB); amiloride hydrochloride hydrate (amiloride), 5-(N-ethyl-N-isopropyl)amiloride (EIPA), 5-(N,N-dimethyl)amiloride hydrochloride (DMA), 5-(N,N-hexamethylene)amiloride (HMA), 5-(N-methyl-N-isobutyl)amiloride (MIA), 3,4-dichlorobenzamil hydrochloride (DCB), and 6-chloro-3,5-diamino-2-pyrazinecarboxamide (CDPC). Tetrodotoxin with citrate was from KangTe in China; 2-aminoethoxydiphenylborane (2-APB) was from Tocris Bioscience; ω-agatoxin TK, ω-conotoxin GVIA, and PLTX-II were from the Alomone Labs. Nimodipine, niflumic acid, NPPB, EIPA, DMA, HMA, MIA, DCB, and CDPC were dissolved in DMSO to prepare stock solutions.
Electrophysiology.
Tip-recording was performed according to previous reports (Dahanukar et al., 2001; Hiroi et al., 2002). The recording electrode (tip diameter, 15–25 μm) was filled with the following solutions to elicit firing of specialized GRNs: 1 mm KCl for W cells; 10 mm sucrose plus 30 mm TCC for S cells; 50 mm NaCl for L1 cells; and 1 mm berberine plus 30 mm TCC for L2 cells. The recording electrode was filled with the indicated concentrations of antagonists plus 1 mm KCl to analyze their antagonizing effect on water reception. Amiloride and EIPA were added to the respective tastant solutions to analyze their effect on S, L1, and L2 cells. Labial long-type (L5 or L7) sensilla were stimulated to analyze the firing patterns of W, S, and L1 cells (Hiroi et al., 2002). Labial short-type (S6) sensilla were stimulated to analyze L2 cell firing (Hiroi et al., 2002), as there was no robust response to berberine in long-type sensilla. To avoid adaptation, sensilla were stimulated for <10 s each time and allowed to recover for >2 min before applying another stimulus. Signals were acquired using an Axon-700B multiclamp amplifier and digitized with a 1322A D-A converter (sampling rate, 10 kHz, filtered at 2 kHz). Data were analyzed using the Clampfit 9 software (Molecular Devices). Spikes between 0.5 and 2 s after initiation of stimuli were counted for the estimation of the firing frequency evoked by the tastant. The effect of the antagonists was determined by the normalized response, which was defined as 2 × Nantagonist/(Nbefore + Nafter), where Nbefore, Nantagonist, and Nafter represented the number of spikes elicited by the tastant before, during, and after the application of the antagonist, respectively. For certain drugs dissolved by DMSO (DMSO final concentration is 0.2%), the effect of such drugs on water response was normalized to 1 mm KCl plus 0.2% DMSO.
Proboscis extension reflex assay.
Proboscis extension reflex (PER) was performed as described previously (Wang et al., 2004; Dahanukar et al., 2007), with slight modifications. To measure water response, fixed flies were first incubated for 2 h in a desiccating container with P2O5 and then transferred to the assay condition (23–25°C; relative humidity, 60–80%). Each fly was tested with pure water stimuli three times. For sugar and bitter responses, fixed flies were first incubated for 2 h in a wet chamber and further saturated with three rounds of water application. Flies were then tested with sucrose or berberine (with 100 mm sucrose) three times with spaced water applications.
The scores of the responses were defined as follows: 1, strong proboscis extension; 0.5, partial or delayed proboscis extension; and 0, no proboscis extension. The percentage of PER was calculated as the sum of scores divided by the number of total tests (×100%). For each genotype, 3–4 batches of 20–35 flies were tested on different days as independent duplicates.
Results
Pharmacological studies of gustatory water reception by ion channel antagonists
As a first step, we rechecked the existence of water taste using the PER test and the tip-recording method. Water was sufficient to induce a robust PER in thirsty flies, which was largely blocked in taste-deprived flies of the poxnM22 mutant (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Robust spiking of a specific neuron to water stimuli can be recorded in long-type and short-type sensilla, which can be suppressed by high-osmolarity solution. We then tested TRP channels as gustatory water receptor candidates. However, several TRP channel mutants (Trpl302, Nan36a, Pyx3, PKD21, Pain1, and TrpA1) were still highly responsive to water, as assessed by the tip-recording test (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The structure of the taste sensillum allows local exposure of tastants exclusively to its tip (Mitchell et al., 1999), which consists of the distal dendritic endings of W cell, where the putative ion channels that are responsible for gustatory water reception may be located. To screen for these ion channels at sensillar tips, we examined the effect of various ion channel antagonists on spiking of W cells using the tip-recording method. We found that water-elicited responses were not dramatically affected by pharmacological antagonists of most channels tested (Fig. 1A), which included voltage-dependent Na+ channels (tetrodotoxin), Ca2+ channels (nimodipine, ω-agatoxin TK, ω-conotoxin GVIA, and PLTX-II), Cl− channels (niflumic acid and NPPB), and TRP channels (2-APB and SKF-96365). Interestingly, amiloride partially reduced water-elicited spiking at 10 μm and largely suppressed the responses at 100 μm in both long- and short-type sensilla (Fig. 1A,B); this suppression was reversible (Fig. 1B).
Figure 1.
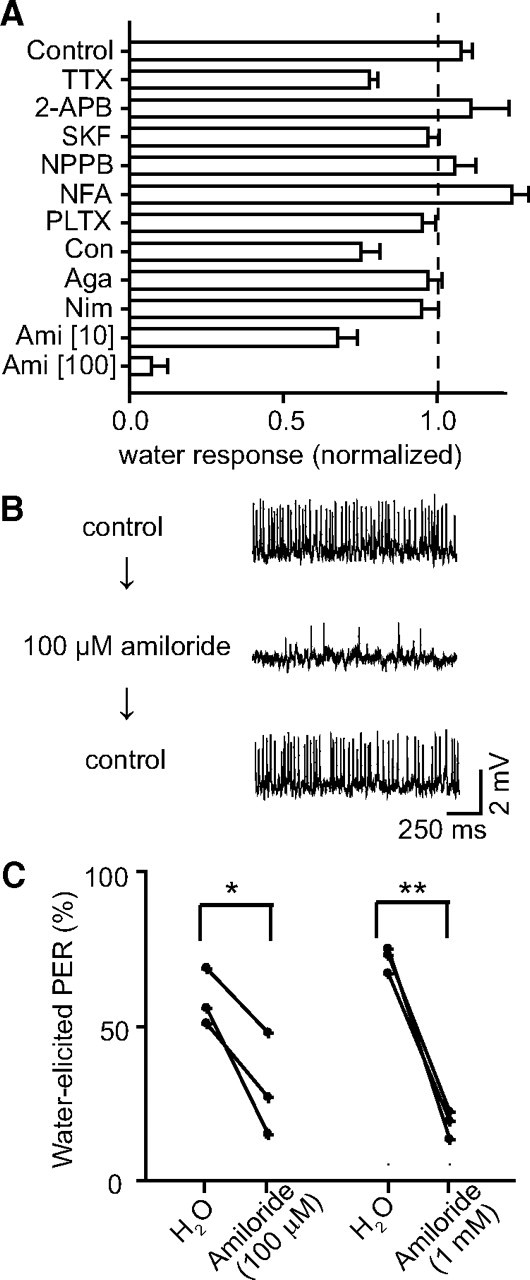
Amiloride antagonized gustatory water reception in Drosophila. A, Effects of ion channel antagonists on water-elicited spiking in taste sensilla. Data represent gustatory water responses in the presence of various antagonists, which were normalized to that elicited by the control stimulus (see Materials and Methods). Error bars, SEM (n = 5–19 sensilla for each treatment). Control was KCl (1 mm) alone (used as the water stimulus). KCl (1 mm) was included as an electrolyte for all antagonists tested: tetrodotoxin (TTX; 1 μm); 2-APB (100 μm); SKF96365 (SKF; 10 μm); NPPB, (100 μM); niflumic acid (NFA; 400 μm); PLTX-II (PLTX; 1 μm); ω-conotoxin GVIA (Con; 10 μm); ω-agatoxin TK (Aga; 1 μm); nimodipine (Nim; 10 μm); and amiloride (Ami; 10 or 100 μm). All drugs were tested in long-type (L5 or L7) sensilla of W1118 flies (statistics are shown in supplemental Table 1, available at www.jneurosci.org as supplemental material). B, Sample traces showing reversible blockade of amiloride to water-elicited spiking. Spiking of the same short-type (S6) sensillum in three sequential recordings (spaced by 2 min) to water stimuli, with amiloride added in the second recording, is shown. C, The PERs elicited by gustatory water stimuli were inhibited by amiloride. Data points represent percentage of PER responses in the absence or presence of amiloride (100 μm or 1 mm; *p < 0.05, **p < 0.01, paired t test).
We next assessed whether amiloride application was also sufficient to inhibit water-elicited behavioral responses. We found that, under our experimental conditions, normal water-induced PER was 60%; in contrast, PER was significantly reduced to 30 and 10% after addition of 100 μm and 1 mm amiloride, respectively, to the water stimulant (Fig. 1C). Thus, PER assays also confirmed the antagonistic action of amiloride on gustatory water reception.
Inhibitory efficacy and modality specificity of amiloride and its analogs
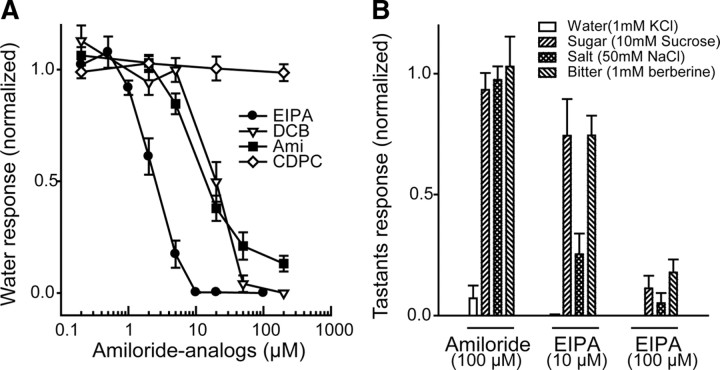
The examination of several amiloride analogs for their effect in blocking water-evoked responses revealed that that their efficacy, as defined by the half-maximal inhibitory concentration (IC50), was ranked in the following order: EIPA/DMA/MIA/HMA > DCB/amiloride ≫ CDPC (Fig. 2A; supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Regarding the most effective analog, EIPA, concentrations as low as 10 μm blocked water-elicited spiking completely (Fig. 2). The IC50 for amiloride (∼20 μm) was ∼5- to 10-fold higher than that observed for EIPA (Fig. 2A), which suggests that modification of the 5-amino group at the pyrazine ring enhanced the antagonizing effect (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). In addition, the amidino group in amiloride was critical for the inhibition, as the amiloride analog CDPC, which lacks the amidino group, failed to affect water-elicited firing, even at a concentration of 200 μm (Fig. 2A; supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These findings suggest that the amidino group is essential for the inhibitory effect on gustatory water responses and that a specific receptor for amiloride is involved in primary gustatory water reception.
Figure 2.
Relative specificity of amiloride analogs in the inhibition of gustatory water responses. A, Dose-dependent curves of the four analogs EIPA, DCB, amiloride (Ami), and CDPC (supplemental Fig. 3, available at www.jneurosci.org as supplemental Table material for compound structure). Data are presented as the average ± SEM (n = 12–21 sensilla for each data point). Statistics are shown in supplemental Table 2, available at www.jneurosci.org as supplemental material. B, Effects of amiloride and EIPA on the sensillar responses to other tastants, with the taste stimuli indicated: for water, KCl (1 mm); for sugar and bitter taste stimuli, 30 mm tricholine citrate was added as the electrolyte. Water/sugar/salt responses were tested in long-type sensilla, whereas the bitter response was tested in short-type (S6) sensilla (average ± SEM; n = 7–19 sensilla). Statistics are shown in supplemental Table 3, available at www.jneurosci.org as supplemental material.
We next examined the effects of amiloride on the spiking elicited by tastants of different modalities. In contrast with the marked inhibition of water-elicited spiking, tip-recordings from taste sensilla showed that amiloride at 100 μm had no significant effect on the spiking elicited by sucrose at 10 mm (sugar), NaCl at 50 mm (low salt), and berberine at 1 mm (bitter) (Fig. 2B). The more effective water response inhibitor EIPA (at 10 μm) had only a moderate effect on sucrose and berberine responses (Fig. 2B), whereas 10 μm EIPA inhibited the low-salt response strongly. EIPA at 100 μm inhibited spiking elicited by all four types of tastants (Fig. 2B). This lack of specificity at high concentrations suggests that EIPA may also act on other taste modalities. Together, these results indicate that low concentrations of amiloride and its analogs are relatively specific in inhibiting water-elicited spiking.
Deletion of ppk28 reduced behavioral response to water taste
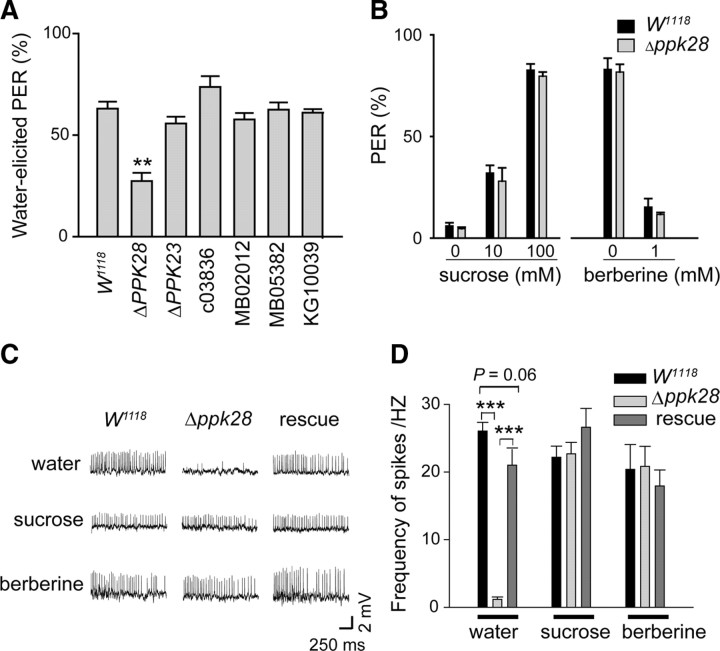
The inhibitory effect of amiloride and its analogs on water-elicited responses (described above) suggests that an amiloride-sensitive membrane protein is likely to be the specific receptor for gustatory water reception. We thus screened known amiloride targets in Drosophila. In particular, we focused on PPK channels, which are the Drosophila DEG/eNaC homologs, as some of these channels are specifically expressed in the labellum and tarsi (Liu et al., 2003). Flies with mutations in ppk genes were tested using PER behavioral assays. We found that disruption of several ppk genes did not have any significant effects on the water-elicited PER response (Fig. 3A). Interestingly, Δppk28 flies exhibited markedly reduced water-elicited PER responses (from ∼60 to ∼30%, Fig. 3A). Moreover, deletion of ppk28 did not affect the PER response of flies to sugar and bitter substances (Fig. 3B), which suggests the specific involvement of ppk28 in gustatory water reception.
Figure 3.
Deletion of the ppk28 gene resulted in behavioral defects and abolishment of water-elicited spiking. A, Water-elicited PER responses in flies mutant for several ppk genes (Δppk28, Δppk23, ppk23c03836, ppk11MB02012, ppk19MB05382, and CG32792KG10039). Only Δppk28 mutants displayed a significant reduction in PER (**p < 0.01, compared with W1118; t test). B, PER responses to sugar and bitter substances in W1118 and Δppk28 flies. C, Example traces showing recording of the responses of sensilla to water, sucrose, and berberine stimuli in W1118, Δppk28, and rescue flies. D, Summary of results of experiments similar to those described in C. Significant differences are marked (***p < 1 × 10−7, t test) for water (1 mm KCl), sucrose (10 mm), and berberine (1 mm). Rescue was Δppk28/Y;[ppk28]ZH-51C/+. Data are presented as the average ± SEM (n = 20–23 sensilla for water and sucrose; n = 10–12 sensilla for berberine).
PPK28 was required for gustatory water reception
We analyzed Δppk28 flies using the tip-recording method. There were robust spiking responses recorded from the sensilla of these Δppk28 flies toward sucrose and berberine stimulation, whereas almost no response from W cells was elicited by water stimulation (Fig. 3C,D). However, a robust water spiking response was found in these Δppk28 flies after reintroduction of the ppk28 gene (Fig. 3D) using a transgenic genomic rescue construct. In addition, the rescued water-elicited spiking responses remained sensitive to amiloride (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). In contrast, mutants of nine other ppk genes still exhibited robust spiking responses in their sensilla (supplemental Fig. 4B,C, available at www.jneurosci.org as supplemental material). Thus, PPK28 was specifically essential for gustatory water reception.
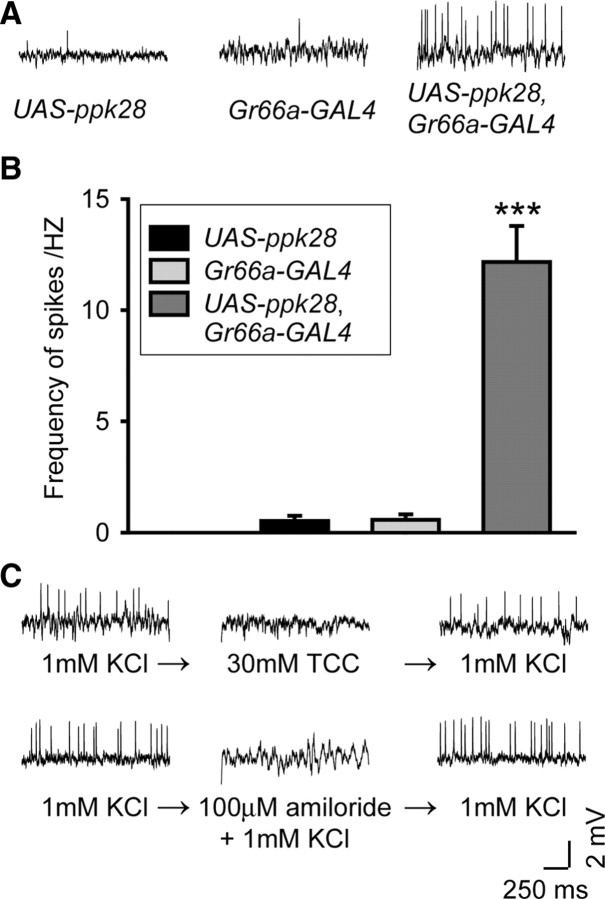
PPK28 was sufficient to confer water-elicited activity
To test whether it is sufficient to confer water sensitivity, PPK28 was overexpressed in the bitter taste neurons under control of Gr66a-GAL4 (Thorne et al., 2004; Wang et al., 2004). We then examined water-elicited spiking responses in the intermediate-type sensilla (I7 or I8), which themselves do not have water receptor cells (Hiroi et al., 2002). As shown in Figure 4A and B, the intermediate-type sensilla of UAS-ppk28 or Gr66a-GAL4 control flies showed little response to water stimuli, whereas the intermediate-type sensilla of Gr66a-GAL4>UAS-ppk28 flies displayed robust water-elicited spike activities. Furthermore, these water-elicited spike activities were sensitive to amiloride or high osmolarity (30 mm TCC) (Fig. 4C). These results suggest that PPK28 could serve as an osmosensor.
Figure 4.
PPK28 is sufficient to confer water sensitivity. A, Water-elicited spiking (1 mm KCl) in the intermediate-type sensilla (I7 or I8) of UAS-ppk28, Gr66a-GAL4, and Gr66a-GAL4>UAS-ppk28 flies. B, Quantification and statistics of results in A (***p < 1 × 10−5, t test, n = 9–21 sensilla). C, Inhibition of water-elicited spiking in Gr66a-GAL4>UAS-ppk28 flies by 30 mm TCC or 100 μm amiloride.
Discussion
Using pharmacological screening of a wide spectrum of ion channel antagonists, we found that low concentrations of amiloride inhibited the spiking of water gustatory receptor neurons in Drosophila. Interestingly, several previous studies also demonstrated the effect of amiloride on the spiking of these receptor neurons in the flesh fly and blowfly (Liscia et al., 1997; Sadakata et al., 2002). Using physiological recordings, behavioral assays, and mutational analyses, we identified PPK28 as the putative amiloride-sensitive receptor for gustatory water reception. Further gain-of-function studies showed that PPK28 is sufficient to confer hypoosmotic activity. These results suggest that PPK28 is a good candidate for the Drosophila gustatory water receptor.
The IC50 of amiloride necessary to inhibit the gustatory water response (∼20 μm) was about two orders of magnitude higher than that observed for mammalian ENaC; however, it was comparable to that used for the Drosophila RPK channel (Adams et al., 1998). We found that the efficacy ranking of amiloride analogs in inhibiting gustatory water response was EIPA/DMA/MIA/HMA > DCB/amiloride ≫ CDPC (Fig. 2). It is traditionally considered that EIPA/DMA/MIA/HMA are more specific in inhibiting Na+/Ca2+ or Na+/H+ exchangers compared with eNaC channels (Kleyman and Cragoe, 1988). We found that Drosophila mutants for Na+/Ca2+ or Na+/H+ exchangers (CalxA or CalxB, Nhe2f01515, and Nhe3KG08307) retained robust water-elicited spiking in taste sensilla (supplemental Fig. 5, available at www.jneurosci.org as supplemental material), which was in sharp contrast to that observed for the ppk28 deletion mutant. This result further supports the idea that PPK28 is the specific amiloride target involved in gustatory water reception.
While ppk28 deletion affected only water reception in both physiological and behavioral assays, we found that higher concentrations of EIPA also suppressed other taste modalities. It is possible that other ppk channels and other amiloride targets, e.g., Na+/Ca2+ or Na+/H+ exchangers, are involved in modulating the reception and transduction of salt, sugar, or bitter signals in their respective receptor neurons. The identification of these EIPA targets in other GRNs may provide new insights into the understanding of other taste modalities. Although deletion of ppk28 resulted in a strong behavioral phenotype in water-induced PER responses, Δppk28 mutants displayed ∼30% of PER response. This may be due to the following reasons. First, it is possible that the residual PER response may be contributed by taste-independent mechanisms, such as central regulatory mechanisms or other sensory signals (such as hygrosensation, vision, or mechanosensation), as the taste-deprived poxnM22 mutants still displayed ∼10% PER response to water under our assay conditions (Fig S1, available at www.jneurosci.org as supplemental material). In addition, although the water-elicited spikes from water cells can be almost completely abolished in Δppk28 mutants, sporadic spikes of small amplitude were recorded in Δppk28 mutant and wild-type flies (Fig. 3C). These small spikes were also seen in other work [Dahanukar et al. (2007), their Fig. 7A (in the trace for KCl)]. Such small spikes could be activities from GRNs other than canonical water cells and could also contribute to the residual PER responses in Δppk28 mutants.
The mechanisms via which PPK28 is involved in Drosophila gustatory water reception remain unknown. The PPK28 channel itself may be mechanosensitive and gated in the same manner as that shown for several members of the DEG/eNaC channel family, e.g., MEC-4 in Caenorhabditis elegans, under the regulation of cytoskeleton or extracellular matrix proteins (Chalfie, 2009). Additional structure–function studies of PPK28 in Drosophila water GRNs may shed light on the molecular mechanisms that underlie osmosensation and mechanosensation in general.
Footnotes
This work was supported by 973-2006CB806604, NSF30625020, Chinese Academy of Sciences Bai Ren Project, and KSCX1-YW-R-31 to Z.W. We thank Drs. M. Noll, T. Tanimura, C. Zuker, C. Kim, J. Kim, L. Liu, and M. Welsh for providing fly strains and Dr. K. Basler for pattB vector. We thank Dr. J. Carlson for help in tip-recording. We thank Dr. M.M. Poo for critical reading and comments on the manuscript.
References
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phi C31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129:5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van Naters W, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Evans DR, Mellon D., Jr Electrophysiological studies of a water receptor associated with the taste sensilla of the blowfly. J Gen Physiol. 1962;45:487–500. doi: 10.1085/jgp.45.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci U S A. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Tanimura T. Molecular neurophysiology of taste in Drosophila. Cell Mol Life Sci. 2004;61:10–18. doi: 10.1007/s00018-003-3182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi SK, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, Kernan MJ, Kim C. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion-transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscia A, Solari P, Majone R, Barbarossa IT, Crnjar R. Taste reception mechanisms in the blowfly: evidence of amiloride-sensitive and insensitive receptor sites. Physiol Behav. 1997;62:875–879. doi: 10.1016/s0031-9384(97)00257-6. [DOI] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Mitchell BK, Itagaki H, Rivet MP. Peripheral and central structures involved in insect gustation. Micros Res Tech. 1999;47:401–415. doi: 10.1002/(SICI)1097-0029(19991215)47:6<401::AID-JEMT4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Kottgen M, Jiao YC, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Hatano H, Koseki T, Koganezawa M, Shimada I. The effects of amiloride on the labellar taste receptor cells of the fleshfly Boettcherisca peregrina. J Insect Physiol. 2002;48:565–570. doi: 10.1016/s0022-1910(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Scott K, Brady R, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Reuss L. Stretch-activated single K+ channels account for whole-cell currents elicited by swelling. Proc Natl Acad Sci U S A. 1999;96:6511–6516. doi: 10.1073/pnas.96.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZR, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]