Abstract
Throughout development GABAB receptors (GABABRs) are widely expressed in the mammalian brain. In mature auditory brainstem neurons, GABABRs are involved in the short-term regulation of the strength and dynamics of excitatory and inhibitory inputs, thus modulating sound analysis. During development, GABABRs also contribute to long-term changes in input strength. Using a combination of whole-cell patch-clamp recordings in acute brain slices and immunostainings in gerbils, we characterized developmental changes in GABABR-mediated regulation of synaptic inputs to neurons in the medial superior olive (MSO), an auditory brainstem nucleus that analyzes interaural time differences (ITDs). Here, we show that, before hearing onset, GABABR-mediated depression of transmitter release is much stronger for excitation than inhibition, whereas in mature animals GABABRs mainly control the inhibition. During the same developmental period, GABABR immunoreactivity shifts from the dendritic to the somatic region of the MSO. Furthermore, only before hearing onset (postnatal day 12), stimulation of the fibers originating in the medial and the lateral nucleus of the trapezoid body (MNTB and LNTB) activates GABABRs on both the inhibitory and the excitatory inputs. After hearing onset, GAD65-positive endings devoid of glycine transporter reactivity suggest GABA release from sources other than the MNTB and LNTB. At this age, pharmacological increase of spontaneous synaptic release activates GABABRs only on the inhibitory inputs. This indicates not only a profound inhibitory effect of GABABRs on the major inputs to MSO neurons in neonatal animals but also a direct modulatory role of GABABRs for ITD analysis in the MSO of adult animals.
Introduction
Neurons in the medial superior olive (MSO), a nucleus in the mammalian auditory brainstem, analyze sound direction based on interaural time differences (ITDs) (Goldberg and Brown, 1969; Yin and Chan, 1990; Spitzer and Semple, 1995; Brand et al., 2002). This is achieved using a coincidence detection mechanism, which compares the relative arrival times of the two excitatory inputs deriving from the contralateral and the ipsilateral anteroventral cochlear nucleus (AVCN) (Cant and Casseday, 1986; Skottun, 1998). In addition, MSO neurons receive major inhibitory projections originating from the medial and the lateral nucleus of the trapezoid body (MNTB and LNTB) (Cant and Hyson, 1992; Kuwabara and Zook, 1992; Grothe and Sanes, 1993) (see Fig. 1A). These inhibitory inputs adjust the output signal of MSO neurons such that large changes in the discharge rate are occurring within the physiological relevant range of ITDs an animal experiences (Brand et al., 2002; Pecka et al., 2008).
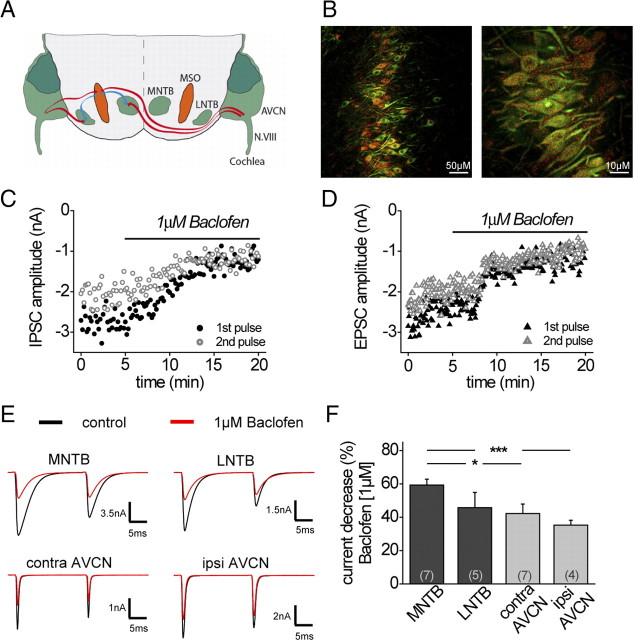
Figure 1.
Pharmacological activation of GABABRs depresses excitatory and inhibitory currents at all four major inputs to the MSO. A, The MSO circuit with its respective inputs from the MNTB, LNTB, and ipsilateral and contralateral AVCN (red afferents, excitatory; blue afferents, inhibitory). B, Fluorescent GABABR staining of the MSO in P19 gerbils (red, GABABR1; green, MAP2). Left, GABABRs were expressed along the entire dorsoventral extent of the MSO. Scale bar, 50 μm. Right, Cytosolic as well as marginal GABABR1 staining was detectable. Scale bar, 10 μm. C, Example time course of IPSC amplitude depression during bath application of baclofen (MNTB fiber stimulation). D, Example time course of EPSC amplitude depression during bath application of baclofen (contralateral AVCN fiber stimulation). E, Top, IPSCs under control and baclofen conditions. Bottom, EPSCs under control and baclofen conditions (examples display averaged evoked responses). F, Summary and statistics of the modulatory effect of baclofen on current amplitude for all major inputs to the MSO. The asterisks represent p values obtained by Student's two-tailed unpaired t test. Error bars indicate SEM.
In adult animals, this inhibition is mediated by glycine (Helfert et al., 1989; Smith et al., 2000). Yet, in neonatal animals up to postnatal day 12 (P12), GABA also represents an important inhibitory transmitter, as MNTB fiber stimulation activates GABAA receptor-mediated currents in neurons of the medial and lateral superior olive (Kotak et al., 1998; Smith et al., 2000; Kullmann et al., 2002; Nabekura et al., 2004). In most brain regions, GABA not only induces a chloride current via GABAA receptors but also activates the metabotropic GABAB receptor (GABABR). On the postsynaptic site, GABABR activation triggers a direct inhibitory action via the activation of potassium channels (Pitler and Alger, 1994; Lüscher et al., 1997; Nicoll, 2004). Presynaptically situated GABABRs modulate the release probability of inhibitory and excitatory neurotransmitters by depressing Ca2+ currents (Wojcik and Neff, 1984; Isaacson, 1998; Takahashi et al., 1998). Additionally, GABABRs can be indirectly involved in long-term plastic changes of synaptic efficacy (Kotak et al., 2001; Kamikubo et al., 2007). In the mature auditory brainstem, GABABRs primarily contribute to the dynamic regulation of transmitter release. We have previously shown that, in the lateral superior olive (LSO), GABABR activation by retrogradely released GABA regulates the balance of excitation and inhibition and thereby adjusts the sensitivity of these neurons to interaural intensity differences (Magnusson et al., 2008). Furthermore, presynaptic GABABRs have been implicated in decreasing short-term synaptic depression and might thereby improve faithful synaptic transmission for the representation of sound structure (Brenowitz et al., 1998; Mapelli et al., 2009). Preliminary immunostainings have revealed that in the auditory brainstem GABABRs are not only present before hearing onset, when the MNTB releases both GABA and glycine, but also in adult animals, when the MNTB input has become glycinergic (Heise et al., 2005; Hilbig et al., 2007). The primary goal of this study was to determine whether the GABABR-mediated regulation of the main excitatory and inhibitory inputs to the MSO changes during development. Moreover, we wanted to show to what extent endogenous GABABR activation in the MSO is altered during this developmental period.
Materials and Methods
Slice preparation.
All experiments were performed in conformity with the rules set by the European Community Council Directive (86/89/ECC) and German animal welfare legislation.
Acute transverse brain slices (140–190 μm) of the auditory brainstem containing MSO, MNTB, and LNTB were obtained from male and female gerbils (Meriones unguiculatus) aged P8–P32. After decapitation under isoflurane anesthesia, the brainstem was carefully removed and placed in an ice-cold slice solution (see below). Slices were cut in the rostral direction from the level of the facial nerve, with a vibratome (VT1000S; Leica), and incubated at 32°C in oxygenated artificial CSF (aCSF) (see below) for 5 min, after which they were allowed to cool down to room temperature (22 ± 2°C). Recordings were obtained within 4–5 h after the preparation.
Drugs and solutions.
To minimize potentially damaging Ca2+ influx into the neurons, a low-sodium, high-sucrose slice solution [containing the following (in mm): 85 NaCl, 2.5 KCl, 1.3 NaH2PO4, 2.5 NaHCO3, 75 sucrose, 25 glucose, 0.5 CaCl2, and 4 MgCl2] was used for the slicing procedure. The control aCSF, used for storage and recordings, was composed of the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 2 CaCl2, and 1 MgCl2. These external solutions were bubbled continuously with carbogen gas (95% O2, 5% CO2), generating a pH of 7.4.
Whole-cell voltage-clamp recordings were performed using a Cs-based internal solution [comprising the following (in mm): 70 CsMeSO4, 70 CsCl, 10 HEPES, 10 EGTA, 2 Na2-ATP, 2 Mg-ATP, 0.3 Na2-GTP, 1 CaCl2, and adjusted to pH 7.3 with CsOH]. Voltage-gated sodium and potassium currents were blocked by adding QX-314 (lidocaine N-ethyl bromide) (1 mm) and TEA-Cl (tetraethylammonium chloride) (5 mm) to the electrode solution before usage. For whole-cell current-clamp recordings and for the characterization of postsynaptic GABABR effects, we used an internal solution consisting of the following (in mm): 130 K-gluconate, 5 KCl, 10 HEPES, 1 EGTA, 2 Na2-ATP, 2 Mg-ATP, 0.3 Na2-GTP, 10 Na-phosphocreatine, adjusted to pH 7.3 with KOH.
Additionally, the following pharmacological agents were used: 6,7-dinitroquinoxaline-2,3-dione (DNQX), dl-2-amino-5-phosphonopentanoic acid (dl-APV), 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR95531), (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid (SCH50911), (R)-4-amino-3-(4-chlorophenyl)butanoic acid [(R)-baclofen], 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) (all Tocris Bioscience), strychnine, 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride hydrochloride (NO711 hydrochloride), and 4-aminopyridine (4-AP) (all Sigma-Aldrich). All drugs were dissolved in dH2O and stored at −20°C. Before the experiment, aliquots were thawed and added to the perfusate during the experiment.
Whole-cell recordings.
All recordings were performed at 32°C. After incubation, slices were transferred to a recording chamber perfused (1–2 ml/min) with oxygenated aCSF. MSO principal cells were viewed through an upright microscope (Zeiss Axioscope) using a 40× water-immersion objective (Achroplan; Zeiss) and infrared-differential interference optics equipped with an infrared-sensitive digital camera (KP-M2R; Hitachi Kokusai Electric). Whole-cell voltage- and current-clamp recordings were performed from the MSO with a Multiclamp 700A amplifier (Molecular Devices). Borosilicate glass microelectrodes (GC150F-10; Harvard Apparatus) were pulled on a DMZ Universal Puller (Zeitz Instruments), yielding a final tip resistance of 2–3.5 MΩ. The series resistance ranged from 5 to 13 MΩ and was compensated by 70–80% for voltage-clamp recordings. Furthermore, the series resistance was monitored throughout the duration of these experiments and was not allowed to vary by >20%. For current-clamp experiments, the bridge balance was applied.
Experimental procedure.
MSO principal neurons were optically identified through the bipolar fusiform shape of their somata with dendrites extending medially and laterally. In addition, only neurons with capacities >20 pF, as read from the compensation of the MultiClamp amplifier, were regarded as principal neurons and included in this study. Evoked synaptic responses were elicited with a glass microelectrode (tip opening, 1–2 μm) filled with NaCl (2 m), which was positioned in the ipsilateral MNTB or the ipsilateral LNTB fiber tract 100 to 150 μm away from the somatic region of the MSO. An analog isolated pulse generator (BSI 950; Dagan) at a rate of 0.2 Hz triggered a bipolar (+/−), paired stimulus pulse (from here on referred to as test pulse) with an interstimulus interval of 20 ms. The threshold stimulus strength was typically 20–80 V with pulse durations between 200 and 400 μs.
IPSCs were pharmacologically isolated by bath application of the AMPA receptor antagonist DNQX (10 μm). EPSCs were evoked in the presence of the glycine receptor antagonist strychnine (1 μm). In all experiments, dl-APV (50 μm) was applied to block NMDA receptors and SR95531 (10 μm) to block GABAA receptors. To determine GABABR-mediated effects, the corresponding agonist baclofen (1 μm) was applied to the perfusate. This baclofen concentration is slightly above the IC50 values of the dose–response curve for the excitatory ([IC50] = 0.62 μm) and inhibitory ([IC50] = 0.2 μm) inputs as measured in P14 animals (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Some of the train stimulation experiments were performed under the presence of the synaptosomal GABA uptake blocker NO711 (preferred GAT-1 selectivity) to increase the residual GABA concentration during MNTB or LNTB stimulation. Dependent on the hypothesis, baseline conditions consisted in all experiments of 5 min with or without the 100 Hz train preceding the test pulse. In some experiments, 4-AP (2 mm) was used to raise spontaneous synaptic activity levels in the slice (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Data analysis.
The signals were filtered with a low-pass four-pole Bessel filter at 10 kHz, sampled at 20–50 kHz, and digitized using a Digidata 1322A interface (Molecular Devices). Traces were digitally filtered at 2–5 kHz. Stimulus generation, data acquisition, and off-line analysis of data were performed using the pClamp Software (version 10.2; Molecular Devices). The paired-pulse ratio (PPR) was calculated as the mean amplitude of the synaptic response evoked by the second stimulus over that evoked by the first one. The coefficient of variation (CV) was calculated as the ratio between the SD of synaptic current amplitude and the mean amplitude (Faber and Korn, 1991). All data shown in percentage reflect values normalized to baseline conditions. Figures, which display averaged evoked responses, consist of at least 13 traces. Stimulation artifacts have been deleted for clarity in figures that show averaged traces. Results are expressed as mean ± SEM. Significant differences are marked as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. Values of p in this study were obtained by using Student's two-tailed paired or unpaired t test.
Immunohistochemistry.
Animals of different ages (P7, P18, P19, P30) were deeply anesthetized with isoflurane and then perfused with 0.9% Ringer's solution (5 min) followed by 4% paraformaldehyde (PFA) (30 min). Brains were removed and postfixated in PFA overnight at 4°C. The tissue was then sectioned at 40–60 μm using a vibratome. Immunohistochemistry was applied to free-floating sections. After extensive rinsing in PBS, sections were exposed to a blocking solution (BS) containing 1% bovine serum albumin, 0.5% Triton X-100, and 0.1% saponin in PBS (30 min). Subsequently, a double-immunofluorescence labeling was performed with the following primary antibody combinations: guinea pig anti-GABABR1 (1:2000; Millipore Bioscience Research Reagents)/chicken anti-microtubule-associated protein 2 (MAP2) (1:1000; Neuromics) and guinea pig anti-glycine transporter 2 (GlyT2) (1:1000; Millipore)/mouse anti-glutamate decarboxylase 65 (GAD65) (1:500; Millipore Bioscience Research Reagents) in PBS, containing the same BS overnight at 4°C. The immunoreactivity was visualized by incubating the sections with secondary antibodies raised in donkey and conjugated to either Cy3 (1:300; Millipore Bioscience Research Reagents), Alexa 488 (1:200; Invitrogen), or Cy5 (1:200; Dianova) in BS for 3 h at 37°C. Finally, the sections were rinsed, mounted, and coverslipped with Vectashield medium (Vector Laboratories). Confocal optical sections were acquired with a Leica TCS SP confocal laser-scanning microscope (Leica Microsystems) equipped with PL FLUOTAR 25×/0.75 numerical aperture (NA) and HXC PL APO 63×/1.32 NA oil-immersion objectives. Fluorochromes were visualized using an argon laser with excitation wavelengths of 488 nm (emission, 510–540 nm) for Alexa 488, a DPSS laser with a laser line of 561 nm (emission, 565–600 nm) for Cy3, and a helium–neon laser with an excitation wavelength of 633 nm (emission, 640–760 nm) for Cy5. Stacks of 8 bit grayscale images were obtained with axial distances of 300 or 1000 nm between optical sections and pixel sizes of 310–781 nm depending on the selected objective. After stack acquisition, Z chromatic shift between color channels was corrected. RGB stacks, montages of RGB optical sections, and average-intensity projections were created using ImageJ 1.37 k plug-ins.
Results
GABAB receptors modulate all four major inputs to MSO neurons
Neurons in the MSO show strong immunoreactivity against GABABRs (Fig. 1B, left). At higher magnification, this staining appears punctuated with a distribution over the entire cell body and along the proximal dendrites (Fig. 1B, right), which suggests a functional role of GABABRs in MSO neurons. Using whole-cell voltage-clamp recordings from MSO neurons in acute brain slices, we investigated whether pharmacological activation of GABABRs modulates the excitatory and inhibitory inputs to MSO neurons. These experiments, as well as the immunolabeling in Figure 1B, were performed in 19-d-old animals, an age when the properties of the excitatory and inhibitory inputs to the MSO are considered to be adult-like (Magnusson et al., 2005; Scott et al., 2005; Sonntag et al., 2009). In adult animals, MSO neurons receive two excitatory glutamatergic inputs from the ipsilateral and contralateral AVCN and two inhibitory glycinergic inputs from the ipsilateral MNTB and LNTB (Fig. 1A). IPSCs and EPSCs were evoked by either stimulation of the ipsilateral or contralateral fiber tract to MSO neurons. Since the excitatory and inhibitory inputs from each side run in the same fiber bundle (Cant and Hyson, 1992; Kuwabara and Zook, 1992; Grothe and Sanes, 1993; Smith et al., 1993), fiber stimulation usually resulted in a mixed excitatory/inhibitory response. For this reason, EPSCs and IPSCs had to be isolated pharmacologically by antagonizing either the excitatory or the inhibitory inputs, respectively (see Materials and Methods). To find out whether GABABR activation regulates MSO inputs, GABABRs were pharmacologically activated by bath application of baclofen (1 μm), a concentration that lies within the dynamic part of the dose–response curve for excitatory and inhibitory currents (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Both excitatory and inhibitory inputs projecting to the MSO profoundly decreased in amplitude on activation of GABABRs by baclofen application (Fig. 1C–E) (MNTB: control, −6.9 ± 1.2 nA; baclofen, −2.8 ± 0.5 nA; n = 7; LNTB: control, −1.7 ± 0.5 nA; baclofen, −0.8 ± 0.2 nA; n = 5; contra AVCN: control, −3.1 ± 0.6 nA; baclofen, −1.9 ± 0.6 nA; n = 7; ipsi AVCN: control, −3.3 ± 1.0 nA; baclofen, −2.1 ± 0.6 nA; n = 4). However, the relative decrease of current amplitude by baclofen application was strongest for MNTB inputs, whereas the excitatory inputs were significantly less affected (MNTB: 59.4 ± 3.5%, n = 7; contra AVCN: 42.2 ± 5.8%, n = 7; LNTB: 45.8 ± 9.2%, n = 5; ipsi AVCN: 35.3 ± 2.9%, n = 4; MNTB-contra AVCN: p ≤ 0.05; MNTB-ipsi AVCN: p ≤ 0.001) (Fig. 1F). On the contrary, the time course (decay time) of both the inhibitory or excitatory currents was not changed by pharmacological activation of GABABRs with baclofen (1 μm) (data not shown).
The relative effect of GABABR activation on inhibitory and excitatory currents changes during development
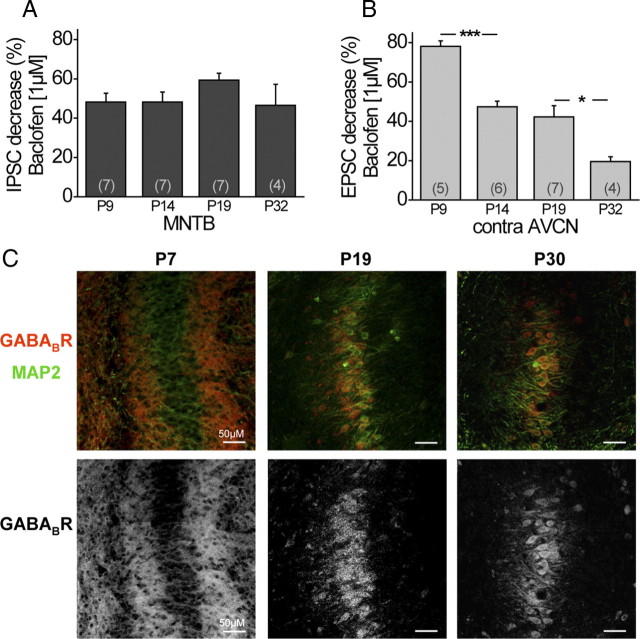
Before hearing onset, which occurs around P12, the MNTB projections to the LSO and the MSO undergo several structural and functional changes. Most importantly, these inputs change from a mixed GABA/glycinergic to a pure glycinergic transmitter phenotype (Kotak et al., 1998; Smith et al., 2000; Kullmann et al., 2002; Nabekura et al., 2004). In addition, this input switches from being depolarizing to hyperpolarizing caused by a significant decrease in the postsynaptic chloride concentration (Kandler and Friauf, 1995; Kakazu et al., 1999; Löhrke et al., 2005). Our next goal was to determine whether GABABRs might be involved in these functional changes. Therefore, we quantified GABABR-induced input modulation in MSO neurons considerably before hearing onset (P9), shortly after hearing onset (P14), at a more mature stage (P19), and from mature animals (P32) by application of baclofen (1 μm). In all age groups tested, baclofen depressed the inhibitory inputs evoked by MNTB fiber stimulation. Interestingly, this GABABR-mediated effect on the inhibitory inputs remained approximately constant during all developmental stages tested (P9: 48.2 ± 4.5%, n = 7; P32: 46.5 ± 10.7%, n = 4) (Fig. 2A). In contrast, GABABR-mediated depression of the excitatory, glutamatergic inputs decreased significantly after hearing onset (P9 contra AVCN: 78.1 ± 2.7%, n = 5; P14 contra AVCN: 47.4 ± 2.8%, n = 6; p ≤ 0.001) (Fig. 2B). This decrease of GABABR-mediated regulation of the excitatory inputs continued up to P32 when input properties are generally considered to be mature (P19 contra AVCN: 42.2 ± 5.7%, n = 7; P32 contra AVCN: 19.6 ± 2.5%, n = 4; p ≤ 0.05) (Fig. 2B). Similar developmental changes of GABABR-mediated modulation of inputs were observed for the projections from the LNTB (P9: 47.2 ± 4.2%, n = 4; P19: 45.8 ± 9.2%, n = 5) and the ipsilateral AVCN (P9: 71.8 ± 4.9%, n = 5; P19: 35.3 ± 2.9%, n = 4; p ≤ 0.001). Together, these data suggest that, before hearing onset, GABABRs more strongly regulate the excitation, whereas in the matured system their effect mainly remains in regulating the inhibitory input strength.
Figure 2.
Physiological efficiency and anatomical distribution of GABABRs changes during maturation for excitatory but not inhibitory inputs. A, Average decrease of normalized IPSC amplitudes by baclofen (1 μm) for different age groups (MNTB fiber stimulation). B, Average decrease of normalized EPSC amplitudes by baclofen (1 μm) for different age groups (contralateral AVCN fiber stimulation). C, Top, Antibody labeling in the MSO against GABABR1 (red) and MAP2 (green) at different developmental stages. Bottom, Isolated immunofluorescence of GABABR1. Scale bars, 50 μm. The asterisks represent p values obtained by Student's two-tailed unpaired t test. Error bars indicate SEM.
GABABR immunostaining changes from a predominantly dendritic to a mostly somatic location during development
This developmental decrease of baclofen effect on the excitatory MSO inputs was corroborated by immunostainings against the GABABR1 subunit in fixed tissue sections at different developmental stages (P7, P19, P30). In general, MSO neurons showed antibody reactivity for GABABRs at all age groups tested (Fig. 2C). Nevertheless, differences between the age groups became obvious by qualitatively comparing the distribution pattern of GABABR staining with the general dendritic MAP2 staining. At P7, GABABR staining was profound in the dendritic region medial and lateral to the MSO somata, whereas the somatic region was only slightly immunoreactive. In contrast, at P19 and even more pronounced later during development (at P30), GABABR immunoreactivity was considerably stronger at the MSO somata, whereas an obvious decrease of dendritic staining was detectable compared with gerbils before hearing onset. Physiological and anatomical data together strongly indicate a developmental change of the presynaptic GABABR distribution. During early developmental stages, GABABRs seem to be mainly located on the excitatory inputs, which mostly terminate on the dendrites (Stotler, 1953; Clark, 1969; Russell and Moore, 1999). In more mature animals, GABABRs seem to be predominantly located on the presynaptic inputs at the soma, which are mostly glycinergic and thus inhibitory (Kapfer et al., 2002).
At all developmental stages, GABABRs control transmitter release probability on the excitatory and inhibitory inputs to MSO principal neurons
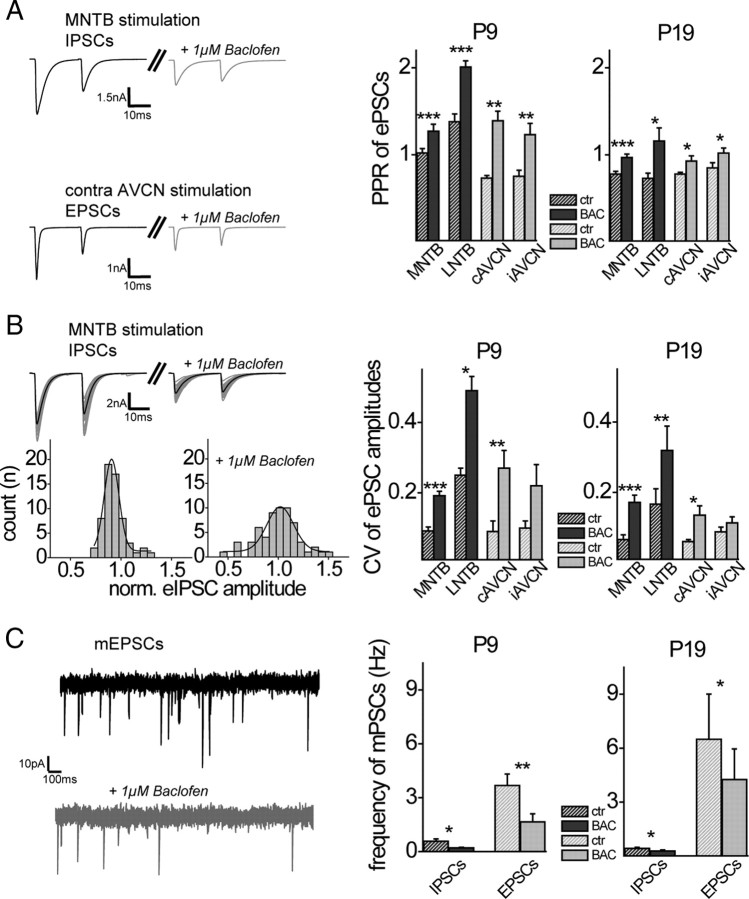
In most cases, modulation of input strength by GABABR activation is achieved either by presynaptic changes in release probability or by postsynaptic activation of K+-currents. Since in the previous experiments postsynaptic potassium channels were blocked pharmacologically (see Materials and Methods), the above-described decrease in synaptic strength by baclofen application is likely to be induced via activation of presynaptically situated GABABRs. To test this hypothesis, we analyzed the PPRs of evoked IPSCs and EPSCs before and during pharmacological activation of GABABRs. For both excitation and inhibition, baclofen significantly increased the PPR before (P9) and after hearing onset (P19), most likely reflecting a reduction in transmitter release probability at the given presynaptic input (Fig. 3A). We also analyzed the CV, an additional measurement to estimate changes in presynaptic release probability, for all major MSO inputs. Current amplitudes exhibited a stronger fluctuation in peak sizes after application of baclofen (Fig. 3B). To visualize this effect, we show a Gaussian function fitted to the IPSC amplitude distribution evoked by MNTB stimulation. As for the PPR, baclofen application increased the CV for excitatory and inhibitory inputs before and after hearing onset. Finally, the mean frequency of both, inhibitory and excitatory miniature postsynaptic currents (mIPSCs and mEPSCs) declined significantly during baclofen application for both prehearing and more mature animals (Fig. 3C). For mEPSCs, this decline was significantly larger for P9 compared with P19 animals (P9: 57 ± 6%, n = 5; P19: 37 ± 6%, n = 5; p ≤ 0.01), similar to the developmental changes in the GABABR-induced reduction of eEPSC amplitude. Consistent with our previous study (Magnusson et al., 2005), we observed a more than twofold increase in mIPSC amplitude between P9 (45 ± 2.1 pA; n = 7) and P19 (99 ± 10 pA; n = 5) (p ≤ 0.001), whereas mEPSC amplitudes did not change during the same developmental period (P9: 30 ± 2.4 pA, n = 5; P19: 31 ± 1.1, n = 5). Together, these data suggest that the observed reduction in input strength by GABABR activation is achieved by a decrease in transmitter release probability via presynaptic GABABRs before hearing onset as well as later during development.
Figure 3.
GABABRs are located presynaptically at inhibitory and excitatory inputs to the MSO before and after hearing onset. A, Left, PPR of IPSCs and EPSCs decreased during exposure to baclofen (examples display averaged evoked responses of P19 neurons). Right, Summary and statistics of baclofen (1 μm)-induced changes in PPR for all stimulation conditions in animals before (P9) and after (P19) hearing onset. B, Left top, Single IPSCs (gray) illustrate the scatter around the mean (black) under control conditions and during baclofen application in a P19 animal. Left bottom, Distribution of normalized IPSC amplitudes under control conditions and during baclofen application. Gaussian fit for the variance of IPSC amplitudes around the mean became broader during baclofen application. Right, Quantification of CVs of normalized evoked postsynaptic currents (ePSCs) for control conditions and with baclofen for the two age groups. C, Left, Example raw traces of mEPSCs under control conditions (black) and during baclofen exposure (gray). Right, Quantification of frequency changes of inhibitory and excitatory miniature postsynaptic currents induced by application of baclofen (1 μm). ctr, Control; BAC, baclofen. The asterisks represent p values obtained by Student's two-tailed paired t test. Error bars indicate SEM.
GABAB receptor activation has a postsynaptic effect in MSO neurons only before hearing onset
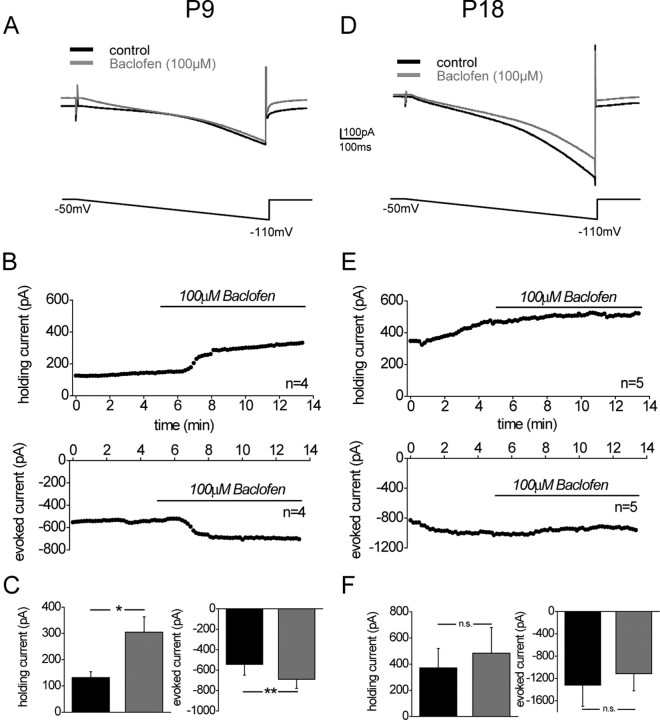
GABABRs not only control transmitter release at the presynaptic terminal but also change the conductance of postsynaptic ion channels, thereby exerting inhibition and altering the integrative properties of neurons. In the majority of neurons, postsynaptic GABABRs are directly coupled to G-protein inwardly rectifying K+ channels (GIRK channels). Activation of postsynaptic GABABRs leads to an opening of the GIRK channels and induces an increase in K+ current. We tested whether pharmacological activation of GABABRs activates a K+ current in MSO neurons, by analyzing the currents evoked by a hyperpolarizing voltage ramp (duration, 900 ms; voltage change, 0.14 mV/ms) before and during baclofen application (100 μm). In prehearing animals (P9), the hyperpolarizing current ramp induced a net inward current that, to a large part, consists of the hyperpolarization activated current (Ih) (Fig. 4A,B). At this age, the average amplitude of pharmacologically isolated Ih is 815 ± 91 pA (n = 11) (for −50 to −110 voltage steps) (U. Koch and B. Hassfurth, unpublished observation). Activation of GABABRs with baclofen (100 μm) in all neurons consistently and with a rapid onset changed the holding current to more positive values and increased the current evoked by the hyperpolarizing current ramp (Fig. 4B,C). This is consistent with an activation of a K+ conductance, presumably a GIRK conductance. In more mature animals (P18), the hyperpolarizing voltage ramp activated even larger Ih currents (Khurana et al., 2008). To avoid that the large Ih obscured activation of GIRK currents, Ih was partially blocked by the specific Ih antagonist ZD7288 (20 μm), which does not block baclofen-induced GIRK channel activation (Svoboda and Lupica, 1998; Takigawa and Alzheimer, 2002). At this developmental stage, no rapid change in holding current or evoked current was observed during baclofen application (Fig. 4D,E). On average, there was a slow but small increase in holding current and a decrease in evoked current, which is mostly consistent with a slow and gradual inactivation of the remaining Ih (Fig. 4F). We conclude that, only before hearing onset, activation of postsynaptic GABABRs induces a change in postsynaptic conductances, most likely an activation of GIRK. Several days after hearing onset, GABABR activation does not alter postsynaptic conductances. However, as indicated by the intracellular GABABR immunoreactivity, these receptors might be involved in long-term changes of synaptic efficacy (Kotak et al., 2001; Yevenes et al., 2003).
Figure 4.
Only before hearing onset baclofen activates a current in MSO neurons consistent with a K+ current. A, Example traces of a current evoked by a hyperpolarizing voltage ramp (voltage change, 0.14 mV/ms) under control conditions and during baclofen application (100 μm) in a P9 animal. B, Time course of averaged changes in holding current (top; n = 4) and evoked currents measured from the baseline of the holding current to the average current evoked during the last 100 ms of the voltage ramp (bottom; n = 4) induced by baclofen bath application in P9 animals. C, Averaged changes in holding current and control conditions (3 min before baclofen application, black bars) and during baclofen application (5 min after the start of baclofen application, gray bars) in P9 animals. D, Same as A, but in a P18 animal with additional ZD7288 (20 μm) applied to the bath to block the large Ih current. E, Same as B, but in P18 animals and with 20 μm ZD7288 applied to the bath. F, Same as in C, but in P18 animals. ctr, Control; BAC, baclofen. The asterisks represent p values obtained by Student's two-tailed paired t test. Error bars indicate SEM.
Before hearing onset, MNTB fiber stimulation activates presynaptic GABABRs
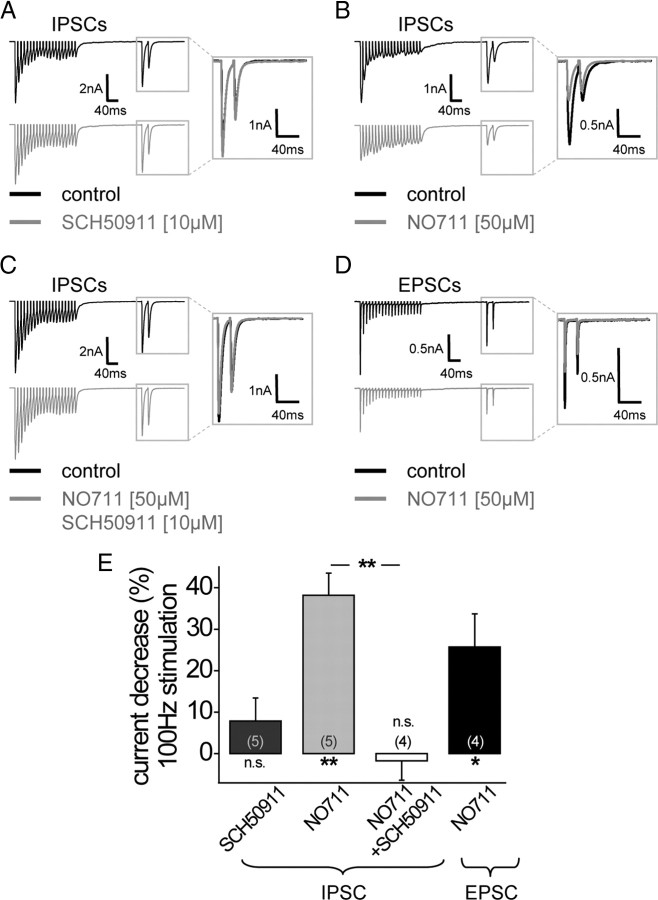
During early prehearing postnatal stages, LSO and MSO neurons receive a mixed GABAergic and glycinergic inhibition from inputs originating from the MNTB, whereas after hearing onset inhibitory transmission from the MNTB is predominantly glycinergic (Kotak et al., 1998; Smith et al., 2000). Hence, we next asked the question whether in young animals (P8) GABA release from MNTB fibers activates GABABRs and thereby modulates the glycinergic and/or glutamatergic inputs to MSO neurons. If, at this age, MNTB neurons indeed release GABA together with glycine from their terminals, this should activate GABABRs located presynaptically on the same synapse and thereby decrease the release probability of the glycinergic transmission. This was tested by analyzing the amplitude of the glycinergic inhibitory currents in response to a test stimulus 200 ms after a high-frequency train stimulation of MNTB fibers (100 Hz, 200 ms) (Sodickson and Bean, 1996). If GABABRs were activated during this high-frequency stimulation, the GABABR antagonist SCH50911 (10 μm) should block the GABABR-induced decrease in transmitter release probability of the glycinergic input. Surprisingly, pharmacological blockade of GABABRs did not induce a significant change in the amplitude of the inhibitory current at this developmental stage (7.8 ± 5.6%; n = 5) (Fig. 4A,E). It is possible that, in P8 animals, the concentration of released GABA is not sufficient to activate the presynaptic GABABRs without N0711 (Smith et al., 2000). However, even at P5, when stimulation of the MNTB–MSO projection still elicits a substantial GABAA receptor response (Smith et al., 2000), presynaptic GABABR activation in the absence of NO711 was negligible (change in IPSC amplitude with SCH50911: −5 ± 9%, n = 4). Also increasing the duration of the stimulus train (500 ms) in P8 animals did not yield a significant GABABR activation at the MNTB inputs, but instead caused a slow depression of synaptic currents even in the presence of GABABR receptor blockers, most likely induced by the long duration of fiber stimulation (change in IPSC amplitude with SCH50911: −29 ± 17%, n = 3). This suggested that high-frequency firing of the MNTB inputs alone was not sufficient to elevate the GABA concentration high enough to activate GABABRs effectively. However, performing the same experiment but applying the GABA uptake blocker NO711 (50 μm) to increase the overall GABA concentration, resulted in a significant decrease in the IPSC amplitude (38.2 ± 5.3%; n = 5; p ≤ 0.01) (Fig. 5B,E). This decrease in IPSC amplitude was completely abolished by the GABABR antagonist SCH50911 (10 μm) (−1.8 ± 4.7%; n = 4) (Fig. 5C,E) and differed significantly from application of NO711 alone (p ≤ 0.01).
Figure 5.
Before hearing onset (P8), high-frequency MNTB fiber stimulation activates GABABRs at the excitatory and inhibitory MSO inputs, but only under the prerequisite of a GABA uptake inhibitor. A, Example traces of averaged IPSCs evoked by MNTB fiber stimulation [stimulation protocol: high-frequency stimulation (100 Hz, 200 ms); 200 ms gap; test pulse with interstimulus interval of 20 ms] under control conditions and during GABABR blockade with SCH50911. B, Example traces of averaged IPSCs evoked by MNTB fiber stimulation (see A) under control conditions and during bath application of the GABA uptake inhibitor NO711. NO711 application decreased the response to the test pulse after high-frequency MNTB fiber stimulation. C, Blocking GABABRs abolished the NO711-induced amplitude change after high-frequency MNTB fiber stimulation. D, Example traces of averaged EPSCs evoked by AVCN fiber stimulation (stimulation protocol; see A) under control conditions and during bath application of the GABA uptake inhibitor NO711. NO711 application decreased also excitatory currents after high-frequency fiber stimulation. E, Quantification of the GABABR-mediated decrease in IPSC and EPSC amplitudes induced by high-frequency fiber stimulation during application of GABABR blocker and/or GABA uptake inhibitor (all at P8). The asterisks under columns represent p values obtained by Student's two-tailed paired t test; the asterisks between columns represent p values obtained by Student's two-tailed unpaired t test. Error bars indicate SEM.
Before hearing onset, MNTB fibers also terminate on the dendrites of MSO neurons (Kapfer et al., 2002; Werthat et al., 2008), which receive the majority of excitatory inputs from the ipsilateral and contralateral AVCN (Stotler, 1953; Clark, 1969; Russell and Moore, 1999). Therefore, GABA released from MNTB fibers should potentially also activate GABABRs located on the excitatory presynaptic terminals, which at this developmental stage show high sensitivity to very low concentrations of baclofen (Fig. 2B). Indeed, stimulating the fibers of the trapezoid body as in the previous experiment in combination with the application of the GABA uptake blocker NO711 resulted in a decrease of the excitatory currents similar to that observed for the inhibitory currents (25.7 ± 8.0%; n = 4; p ≤ 0.05) (Fig. 5E). This indicates that, before hearing onset, GABA release most likely originating from MNTB terminals controls transmitter release via GABABR activation of both the excitatory and the inhibitory inputs of MSO neurons.
Several studies show that, even after hearing onset, MNTB neurons release GABA from their synaptic terminals at their target sites (Kotak et al., 1998; Smith et al., 2000; Nabekura et al., 2004). Yet these results are based on the activation of GABAA receptors induced by MNTB fiber stimulation. Focusing on GABABR, we asked whether at later developmental stages potential GABA release from MNTB terminals could activate GABABRs on the presynaptic terminals of the MTNB inputs in animals just after hearing onset. However, at P14, high-frequency stimulation of trapezoid body fibers in combination with NO711 application had only marginal effects on glycinergic IPSCs (4.1 ± 3.3%; n = 5) (Fig. 6C). Also, increasing the stimulus duration and frequency to 500 ms and 200 Hz together with application of GABA uptake blockers did not result in GABABR activation on the inhibitory inputs after hearing onset (change in IPSC amplitude with SCH90511: −7.1 ± 5.7%, n = 5). This suggests that, after hearing onset, GABA release from MNTB neurons is not sufficient to activate GABABRs even in the presence of GABA uptake blockers.
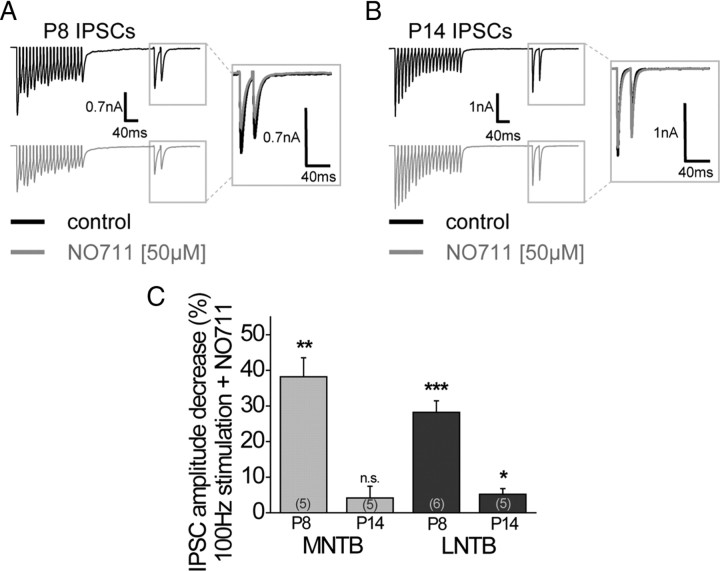
Figure 6.
High-frequency LNTB fiber stimulation only activates GABABRs before but not after hearing onset. Similar to the MNTB, GABABRs are only activated under the prerequisite of the GABA uptake inhibitor NO711. A, Example traces of averaged IPSCs evoked by LNTB fiber stimulation (stimulation protocol same as in Fig. 4A) under control conditions and together with the GABA uptake inhibitor NO711 in a prehearing animal (P8). B, Example traces of averaged IPSCs evoked by LNTB fiber stimulation (stimulation protocol same as in Fig. 4A) under control conditions and together with the GABA uptake inhibitor NO711 in animals after hearing onset (P14). C, Statistical analysis of GABABR-mediated changes in IPSC amplitude in response to the test pulse before and after hearing onset for MNTB and LNTB inputs to the MSO. The asterisks represent p values obtained by Student's two-tailed paired t test. Error bars indicate SEM.
The LNTB–MSO projection has no GABAergic component after hearing onset
Previous anatomical studies provide evidence that the ipsilateral inhibitory input to the MSO, the LNTB, comprises GABAergic neurons also in adult animals (Roberts and Ribak, 1987; Helfert et al., 1989; Spirou et al., 1998). Thus, we attempted to find out whether the LNTB could serve as a GABA source for the activation of GABABRs before hearing onset and later during development. Accordingly, we stimulated the ipsilateral fiber tract projecting from the LNTB to the MSO. At P8, the application of NO711 depressed glycinergic current amplitudes to a similar degree as already observed for MNTB fiber stimulation (28.2 ± 3.2%; n = 6; p ≤ 0.001) (Fig. 6A,C). At P14, however, high-frequency stimulation of the LNTB fibers in combination with NO711 application did not activate presynaptic GABABRs on the inhibitory inputs (NO711: 5.2 ± 1.6%, n = 5) (Fig. 6B,C). This suggests that, after hearing onset, despite the presence of GABA-immunopositive LNTB cells, LNTB inputs to the MSO do not provide enough GABA to induce GABABR-mediated control of MSO inputs.
Presynaptic GABABRs are not activated by retrograde GABA release in the MSO
We have previously demonstrated that neurons in the LSO retrogradely release GABA on spiking activity (Magnusson et al., 2008). This GABA activates presynaptic GABABRs on the respective inputs, thereby modulating transmitter release. Since neurons in the LSO and the MSO receive similar inputs, we tested whether this scenario might also hold for MSO principal cells. As in the LSO, this was tested by inducing high-frequency spiking activity (100–300 Hz; 500 ms) in MSO principal cells by short current step injections well above spiking threshold (1.5–2.5 nA) (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material). If spiking activity retrogradely released GABA and thereby activated presynaptic GABABRs, the amplitude of the postsynaptic potentials induced by fiber stimulation should decrease and the PPR should increase. However, neither the amplitude nor the PPR of EPSPs or IPSPs were changed by the preceding high-frequency spiking activity of the MSO neurons (supplemental Fig. 3B,C, available at www.jneurosci.org as supplemental material). This indicates that, unlike in the LSO, spiking activity of MSO neurons does not activate presynaptic GABABRs by presumed retrograde release of GABA. This is also consistent with the lack of GABA and GAD immunoreactivity in neurons of the MSO (Roberts and Ribak, 1987).
Anatomical evidence for other GABAergic input to MSO neurons
In the previous experiments, we found that, after hearing onset, despite clear evidence for pharmacological activation of presynaptic GABABRs in adult animals, neither the MNTB nor the LNTB input seems to release enough GABA to activate these receptors. We also did not observe GABABR activation by retrogradely released GABA from MSO principal neurons. To identify possible other sources of GABA on MSO neurons, we performed antibody stainings on paraformaldehyde-fixed brainstem sections containing the MSO (P18) against the GABA-synthesizing enzyme GAD65. To evaluate the distribution of GAD65-positive inputs, the dendrites of MSO neurons were visualized with a MAP2 antibody. Confocal microscopy at low magnification showed widely distributed GAD65 staining along the somata and dendrites of MSO neurons, indicating the presence of GABAergic inputs at the soma and the dendrites. We also tested whether possible GABAergic inputs colocalized with the presynaptic endings deriving from the MNTB or LNTB by immunostaining against GlyT2 (Fig. 7A). In contrast to the GAD65 distribution, the GlyT2 staining was very focused and dense only on the somata. At higher magnification, it became apparent that only little colocalization of GAD65- and GlyT2-positive synapses could be detected. Most of the GAD65-associated staining was not in close proximity to GlyT2-positive terminal endings. This indicates that MSO neurons at P18 receive GABAergic projections mainly from other sources than the glycinergic MNTB or LNTB neurons.
Figure 7.
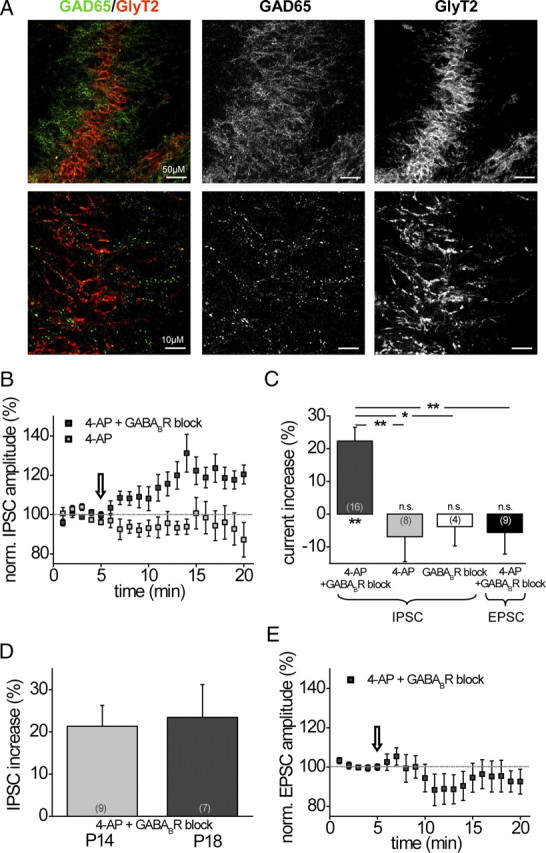
GABAergic inputs to the MSO after hearing onset are not colocalized with glycinergic inputs. Increasing spontaneous activity pharmacologically in slice preparations to in vivo-like levels activates GABABRs with a significant effect on inhibitory inputs. A, Top, Low-power (25×) magnification of antibody staining in P18 animals revealed a somatodendritic expression of GAD65 (green), whereas GlyT2 (red) was concentrated at the somatic region. Scale bar, 50 μm. Bottom, At high-power magnification (63×), colocalization of GAD65 and GlyT2 was scarce. Scale bar, 10 μm. B, Time course of averaged IPSC amplitudes (n = 16) evoked by MNTB fiber stimulation under in vivo-like spontaneous synaptic activity levels before and during GABABR blocker application (the arrow indicates the start of GABABR blocker application). During the entire time course, spontaneous activity levels had been raised by 4-AP (2 mm) in the bath, which had no effect on IPSC amplitudes (n = 8). C, Summary and statistics on the effect of in vivo-like spontaneous activity levels on GABABR activation for IPSCs and EPSCs in P14–P18 animals. D, IPSC amplitudes of P14 and P18 animals are equally affected by GABABR activation. E, Time course of averaged EPSC amplitudes (n = 9) evoked by AVCN fiber stimulation under in vivo-like spontaneous activity levels before and during GABABR blocker application (the arrow indicates the start of GABABR blocker application). During the entire time course, spontaneous activity levels had been raised by 4-AP (2 mm) in the bath. The asterisks under columns represent p values obtained by Student's two-tailed paired t test; the asterisks between columns represent p values obtained by Student's two-tailed unpaired t test. Error bars indicate SEM.
Raising spontaneous activity levels induces GABABRs activation even later during development
We next asked the question whether these GABAergic terminals could serve as a possible source for GABA release that activates the GABABRs located on the MSO inputs. To test this, spontaneous synaptic transmitter release was increased by applying 4-AP (2 mm) to the perfusate. 4-AP is a nonselective blocker of low-threshold potassium channels (KLT) and has been shown to depolarize neurons, thereby lowering the action potential threshold and increasing the spontaneous firing rate in, for example, the hippocampus and mouse inner hair cells (Avoli et al., 1996; Marcotti et al., 2003). In the present experiment, 4-AP elevated the spontaneous frequency of IPSCs in MSO neurons (55.8 ± 2.9 Hz; n = 23) to values that resemble in vivo-like spontaneous firing frequencies of MNTB neurons (Kopp-Scheinpflug et al., 2003, 2008; Hermann et al., 2007; McLaughlin et al., 2008). The influence of possible GABA release through elevated spontaneous release was now tested by application of the GABABR inhibitors SCH50911 (10 μm) or CGP55845 (2 μm). The results from these recordings were pooled since the data displayed no differences among drugs. MNTB fibers were stimulated to investigate GABABR-induced changes of MNTB input currents. As depicted in Figure 7B, IPSC amplitudes significantly increased when GABABR activity was blocked compared with baseline conditions (4-AP alone). This implies that, under baseline conditions, when spontaneous activity is raised by 4-AP, presynaptic GABABRs were endogenously activated, which resulted in a reduction of IPSC amplitudes. Application of the GABABR antagonists then abolished this GABABR-mediated depression of the current, resulting in an increase of current amplitude (22.3 ± 4.2%; n = 16; p ≤ 0.01) (Fig. 7C). Since neither 4-AP (−6.9 ± 7.6%; n = 8) nor GABABR inhibitors alone (−3.8 ± 5.9%; n = 4) increased peak current amplitudes (Fig. 7C), this effect could be ascribed directly to the activation of GABABRs. No difference was observed between P14 and P18 gerbils, which indicates that this mechanism persists in more mature animals (P14: 21.3 ± 5.0%, n = 9; P18: 23.5 ± 7.8%, n = 7) (Fig. 7D). Interestingly, excitatory input currents did not display an increase in amplitude during GABABR blockade which suggests that the GABA released by spontaneous activity did not activate the GABABRs on the excitatory inputs (Fig. 7E) (P14–P18: −5.6 ± 6.6%, n = 9) (Fig. 7C). This is in line with the observed downregulation of GABABRs on the dendrites later during development. Together, these results show that, even considerably beyond hearing onset, GABA is released onto MSO neurons, which selectively modulates the amplitude of the inhibitory inputs. The origin and thus the activation pattern of this GABAergic projection needs to be determined in future studies.
Discussion
The data presented here show that GABABRs differentially modulate the excitatory and inhibitory inputs of MSO neurons at all developmental stages. Around hearing onset, the GABABR-mediated depression of the excitatory inputs greatly decreases, whereas the GABABR-mediated depression of the inhibitory inputs remains constant throughout the same period. This developmental decay in GABABR-induced EPSC depression is paralleled by a progressive loss of GABABR expression in dendrites of MSO neurons after hearing onset. During the same developmental period, we also observe a loss of postsynaptic GABAB receptor-induced K+ current increase. Furthermore, we provide evidence that the source of GABA for GABABR activation changes during development. Before hearing onset, MNTB and LNTB fiber stimulation activates presynaptic GABABRs on the excitatory and inhibitory inputs to MSO neurons. After hearing onset, GAD65-positive endings often lack GlyT2 reactivity, suggesting GABA sources other than the MNTB or the LNTB.
Developmental changes in GABABRs distribution and function
We have found that, throughout all developmental stages, GABABRs regulate transmitter release at the major inhibitory and excitatory inputs to MSO neurons. Yet the relative effect of GABABRs on excitation and inhibition changes during the first postnatal weeks. Regulation of transmitter release by presynaptic GABABRs is a widespread mechanism in the brain and has been described in several auditory regions, such as the auditory brainstem (Isaacson, 1998; Takahashi et al., 1998; Lim et al., 2000; Yamauchi et al., 2000; Magnusson et al., 2008; Tang et al., 2009), midbrain (Sun et al., 2006), and the auditory cortex (Wehr and Zador, 2005). Furthermore, GABABRs have been implicated both in the regulation of circuit formation during development (Behar et al., 2000; Represa and Ben-Ari, 2005) and in the acute modulation of network properties in the mature animal (Scanziani, 2000; Magnusson et al., 2008; Oswald et al., 2009; Pan et al., 2009). Consistent with our data, anatomical and physiological studies in other brain structures suggest that presynaptic GABABRs are present and functional on excitatory and inhibitory inputs during early development and in adult animals (Gaiarsa et al., 1995; Varela et al., 1997; López-Bendito et al., 2002; Kirmse and Kirischuk, 2006). However, there is little information on changes in GABABR function during development. Here, we show that GABABR-mediated depression of the excitatory inputs declined significantly right after hearing onset with an additional decline over several weeks. In contrast, we observed that GABABR-mediated depression of inhibition remains constant during development similar to the CA3 region of the hippocampus (Caillard et al., 1998). In contrast, activation of K+-currents by postsynaptic GABAB receptors, which is abundant in hippocampal and cortical neurons in adult animals (Buonomano and Merzenich, 1998; Scanziani, 2000; Chen and Johnston, 2005; Oswald et al., 2009), disappears after hearing onset in neurons of the MSO. Thus, the integrative properties of mature MSO neurons seem to be unaffected by postsynaptic GABABRs, as previously observed for neurons in the lateral superior olive (Magnusson et al., 2008).
An important question arising from this is how developmental changes in GABABR distribution are regulated. One possibility is that the decline in GABABR number is genetically determined. However, it is more likely that overall activity levels regulate the availability of GABABRs. Indeed, excess excitation and augmented glutamate levels can downregulate GABABR expression and increase internalization of the receptor protein (Buhl et al., 1996; Haas et al., 1996; Vargas et al., 2008), which in both cases would result in an altered GABABR efficacy as observed for excitatory MSO inputs.
MNTB and LNTB fiber stimulation activates GABABRs in the MSO only before hearing onset
Our data indicate that, before hearing onset, high-frequency firing of MNTB neurons activates GABABRs on both the excitatory and inhibitory inputs to MSO neurons. Similarly, previous physiological data show that, before hearing onset, MNTB fiber stimulation also activates postsynaptic GABAA receptors on MSO neurons (Smith et al., 2000). This suggests that indeed GABA released from the MNTB fiber terminals activates presynaptic GABABRs. The fact that at the MNTB–LSO projection GABA and glycine are released from the same synaptic terminals or vesicles (Nabekura et al., 2004) indicates that GABABR-mediated regulation of glycine and GABA transmitter release is autosynaptic and should therefore be similar for both transmitter types. Consequently, during prolonged firing of MNTB neurons, GABA release should decrease and GABABR activation of the inputs should decline in a self-regulating process.
As GABA is released from inhibitory MNTB terminals and diffuses to excitatory presynaptic sites, GABABR-mediated depression of excitation is most likely heterosynaptic. Heterosynaptic activation of GABABRs has been shown to occur at many different sites in the brain (Lim et al., 2000; Mitchell and Silver, 2000; Lei and McBain, 2003; Guetg et al., 2009). In the MSO, the relatively large distance between the release sites and the excitatory presynaptic endings should result in much lower concentrations of GABA at the excitatory compared with the inhibitory terminals (Kapfer et al., 2002). Why then is the amplitude of GABABR-mediated depression similar for inhibition and excitation? One possible explanation is a differential sensitivity of the receptors to GABA, which might be the underlying cause for the larger effect of baclofen on excitation than inhibition observed before hearing onset. Such a mechanism operates in the hippocampus, in which heterosynaptic GABABRs located on the excitatory inputs are indeed more efficient in downregulating transmitter release compared with the GABABRs on the inhibitory inputs because of a different subunit composition (Guetg et al., 2009).
One unexpected result of our measurements was that, even several days before hearing onset, MNTB fiber stimulation only activated presynaptic GABABRs in the presence of the GABA uptake blocker NO711. In general, physiological activation of GABABRs usually requires strong stimulation intensities suggesting that the pooling of synaptically released GABA is required for their activation (Isaacson, 1998; Scanziani, 2000). This is consistent with ultrastructural data showing that most GABABRs are located perisynaptically or extrasynaptically (Fritschy et al., 2004; López-Bendito et al., 2004; Luján and Shigemoto, 2006). Indeed, in many studies using acute brain slice preparations GABA uptake blockers were required to activate presynaptic GABABRs (Mouginot et al., 1998; Mitchell and Silver, 2000; Lei and McBain, 2003). This also highlights the important role of GABA uptake mechanisms for the regulation of GABA concentration. An alternative mechanism for the lack of effect with low GABA concentrations might be a masking of the change in release probability by an increased readily releasable pool as observed for metabotropic glutamate receptor (mGluR) activation in the MNTB (Billups et al., 2005). In this case, mGluR effects became apparent through a decrease in recovery time constants. However, in our experiments, train stimulation without NO711 did not seem to change the recovery time constant, when we analyzed PSC amplitudes 200 ms after the termination of the train, a time interval usually well within the range of recovery from synaptic depression.
Endogenous GABABR activation in the MSO after hearing onset
All previous studies so far had focused on the developmental reduction in the activation of ionotropic GABAA receptors by MNTB fiber activity (Kotak et al., 1998; Smith et al., 2000). Our study indicates that presynaptic GABABRs cannot be activated by either MNTB or LNTB fiber stimulation after hearing onset. Furthermore, unlike in the LSO, spiking activity of MSO neurons does not retrogradely activate GABABRs on the presynaptic terminals. Under which physiological conditions are the GABABRs in the MSO then activated? In agreement with previous anatomical studies (Roberts and Ribak, 1987; Helfert et al., 1989; Adams and Mugnaini, 1990), our antibody labeling against the GABA-synthesizing enzyme GAD65 revealed a large number of GABAergic fibers contacting the soma and the proximal dendrites. We therefore propose that GABA released from these GAD65-positive terminals mediates the GABABR-induced depression of inhibitory inputs. But where do these GAD65-positive fibers originate, and under which physiological circumstances are they activated? Several possibilities have been discussed in the literature, including the ventral nucleus of the trapezoid body, the superior paraolivary nucleus, and descending fibers from the inferior colliculus (Roberts and Ribak, 1987). The experimental evidence for any of these projections is sparse (Schwartz and Wittebort, 1976; Kiss and Majorossy, 1983), and future experiments are required to determine the origin of the GABAergic terminals to MSO neurons in adult animals.
Possible functional significance of GABABRs in the MSO before and after hearing onset
The observed shift in the effect of GABABR-mediated depression of excitation and inhibition suggests a change of GABABR function before and after hearing onset. Before hearing onset, we found a profound depression of the excitatory inputs by GABABR activation, which indicates a block of activity by GABABR activation. At this age, also inhibitory inputs are affected by GABABR activity. The functional interpretation of the GABABR-mediated depression of inhibitory inputs before hearing onset is more complex. In neonatal animals, the chloride reversal is positive to the resting membrane potential, which results in a depolarization of neurons during activation of the GABA/glycinergic inputs (Kandler and Friauf, 1995; Kakazu et al., 1999; Löhrke et al., 2005). Hence, during this period, the main inputs to the MSO are excitatory. Accordingly, GABABRs might be the main source of inhibition in the MSO by decreasing transmitter release from both the glutamatergic excitatory and the depolarizing GABA/glycinergic inputs during periods of high spontaneous spiking activity. This scenario has previously been suggested for the neonatal hippocampal network (Gaiarsa et al., 1995; McLean et al., 1996) and could represent an effective mechanism to prevent overexcitation and apoptosis of neurons in the neonatal brain. In this case, the GABABR-mediated inhibition of excitation should remain elevated in deafened animals in which the chloride reversal potential remains depolarizing in neurons of the LSO (Shibata et al., 2004).
Our findings indicate that the function of presynaptic GABABRs changes after hearing onset. First, at this developmental stage, GABABR activation mostly modulates the strength of the inhibitory inputs to the MSO. Second, whereas in neonatal animals GABA is released during high-frequency firing of MNTB and LNTB neurons, our data suggest that, after hearing onset, fibers other than the MNTB or LNTB release GABA. Whether these fibers are driven by sound or whether they are associated with an attention-driven descending system is unclear at the moment, but in both cases activation of these fibers should result in a tonic downregulation of the inhibitory inputs to the MSO. We do know from previous experiments that the inhibitory inputs from the MNTB and LNTB to the MSO modulate ITD processing by shifting the steepest slope of the ITD function into the physiologically relevant range (Brand et al., 2002; Pecka et al., 2008). Activation of GABABRs on the excitatory and inhibitory inputs to the MSO could fine-tune ITD analysis in several ways. A reduction in EPSC amplitude could sharpen the coincidence detection window in response to binaural stimuli (Kuba et al., 2002). However, the main effect of GABABRs in adult animals clearly lies in the downregulation of the inhibitory inputs. Given the presumptive role of inhibition for ITD coding, a reduction in IPSC amplitude would move the peak of the ITD function closer to the midline and thereby decrease spike frequency changes within the physiologically relevant range. Moreover, for both the excitatory and the inhibitory inputs, constituent GABABR activation attenuates short-term synaptic depression (Brenowitz et al., 1998) and might be one of the underlying causes for the observed lack of synaptic short-term depression in the in vivo preparation, as observed in MNTB neurons (Lorteije et al., 2009). In general, GABABR activation on the inputs to the MSO would provide a mechanism to dynamically adjust ITD analysis in less than a second.
Footnotes
This work was supported by the Bernstein Center for Computational Neuroscience (B.H.), Deutsche Forschungsgemeinschaft Grant GRK1091 (B.H.), and the Graduate School of Systemic Neurosciences (B.H., U.K.). We thank Claudia Aerdker and Olga Alexandrova for technical support and Anna Magnusson, Felix Felmy, and Thomas Park for helpful comments on this manuscript.
References
- Adams JC, Mugnaini E. Immunocytochemical evidence for inhibitory and disinhibitory circuits in the superior olive. Hear Res. 1990;49:281–298. doi: 10.1016/0378-5955(90)90109-3. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Kurcewicz I, Pumain R, Barbarosie M. Extracellular free potassium and calcium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. J Physiol. 1996;493:707–717. doi: 10.1113/jphysiol.1996.sp021416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Billups B, Graham BP, Wong AY, Forsythe ID. Unmasking group III metabotropic glutamate autoreceptor function at excitatory synapses in the rat CNS. J Physiol. 2005;565:885–896. doi: 10.1113/jphysiol.2005.086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABAB receptors. Neuron. 1998;20:135–141. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Net interaction between different forms of short-term synaptic plasticity and slow-IPSPs in the hippocampus and auditory cortex. J Neurophysiol. 1998;80:1765–1774. doi: 10.1152/jn.1998.80.4.1765. [DOI] [PubMed] [Google Scholar]
- Caillard O, McLean HA, Ben-Ari Y, Gaïarsa JL. Ontogenesis of presynaptic GABAB receptor-mediated inhibition in the CA3 region of the rat hippocampus. J Neurophysiol. 1998;79:1341–1348. doi: 10.1152/jn.1998.79.3.1341. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Cant NB, Hyson RL. Projections from the lateral nucleus of the trapezoid body to the medial superior olivary nucleus in the gerbil. Hear Res. 1992;58:26–34. doi: 10.1016/0378-5955(92)90005-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GM. The ultrastructure of nerve endings in the medial superior olive of the cat. Brain Res. 1969;14:293–305. doi: 10.1016/0006-8993(69)90111-5. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Sidler C, Parpan F, Gassmann M, Kaupmann K, Bettler B, Benke D. Independent maturation of the GABAB receptor subunits GABAB1 and GABAB2 during postnatal development in rodent brain. J Comp Neurol. 2004;477:235–252. doi: 10.1002/cne.20188. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Tseeb V, Ben-Ari Y. Postnatal development of pre- and postsynaptic GABAB-mediated inhibitions in the CA3 hippocampal region of the rat. J Neurophysiol. 1995;73:246–255. doi: 10.1152/jn.1995.73.1.246. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Grothe B, Sanes DH. Bilateral inhibition by glycinergic afferents in the medial superior olive. J Neurophysiol. 1993;69:1192–1196. doi: 10.1152/jn.1993.69.4.1192. [DOI] [PubMed] [Google Scholar]
- Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, Bräuner-Osborne H, Shigemoto R, Kretz O, Frotscher M, Kulik A, Bettler B. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Moshé SL, Stanton PK. Kainic acid-induced seizures enhance dentate gyrus inhibition by downregulation of GABAB receptors. J Neurosci. 1996;16:4250–4260. doi: 10.1523/JNEUROSCI.16-13-04250.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise I, Magnusson AK, Grothe B, Koch U. Development of GABAB receptor distribution in the gerbil medial superior olive. Soc Neurosci Abstr. 2005;31:44–9. [Google Scholar]
- Helfert RH, Bonneau JM, Wenthold RJ, Altschuler RA. GABA and glycine immunoreactivity in the guinea pig superior olivary complex. Brain Res. 1989;501:269–286. doi: 10.1016/0006-8993(89)90644-6. [DOI] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol. 2007;98:807–820. doi: 10.1152/jn.00355.2007. [DOI] [PubMed] [Google Scholar]
- Hilbig H, Nowack S, Boeckler K, Bidmon HJ, Zilles K. Characterization of neuronal subsets surrounded by perineuronal nets in the rhesus auditory brainstem. J Anat. 2007;210:507–517. doi: 10.1111/j.1469-7580.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. GABAB receptor-mediated modulation of presynaptic currents and excitatory transmission at a fast central synapse. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Akaike N, Komiyama S, Nabekura J. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikubo Y, Tabata T, Kakizawa S, Kawakami D, Watanabe M, Ogura A, Iino M, Kano M. Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J Physiol. 2007;585:549–563. doi: 10.1113/jphysiol.2007.141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci. 2002;5:247–253. doi: 10.1038/nn810. [DOI] [PubMed] [Google Scholar]
- Khurana S, Rosa K, Golding NL. Development and modulation of h-channels in the principal neurons of the medial superior olive. Soc Neurosci Abstr. 2008;34:664–16. [Google Scholar]
- Kirmse K, Kirischuk S. Ambient GABA constrains the strength of GABAergic synapses at Cajal-Retzius cells in the developing visual cortex. J Neurosci. 2006;26:4216–4227. doi: 10.1523/JNEUROSCI.0589-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Majorossy K. Neuron morphology and synaptic architecture in the medial superior olivary nucleus. Light- and electron microscope studies in the cat. Exp Brain Res. 1983;52:315–327. doi: 10.1007/BF00238026. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dörrscheidt GJ, Rübsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2003;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tolnai S, Malmierca MS, Rübsamen R. The medial nucleus of the trapezoid body: comparative physiology. Neuroscience. 2008;154:160–170. doi: 10.1016/j.neuroscience.2008.01.088. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, DiMattina C, Sanes DH. GABAB and Trk receptor signaling mediates long-lasting inhibitory synaptic depression. J Neurophysiol. 2001;86:536–540. doi: 10.1152/jn.2001.86.1.536. [DOI] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Synaptic depression improves coincidence detection in the nucleus laminaris in brainstem slices of the chick embryo. Eur J Neurosci. 2002;15:984–990. doi: 10.1046/j.1460-9568.2002.01933.x. [DOI] [PubMed] [Google Scholar]
- Kullmann PH, Ene FA, Kandler K. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur J Neurosci. 2002;15:1093–1104. doi: 10.1046/j.1460-9568.2002.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, Zook JM. Projections to the medial superior olive from the medial and lateral nuclei of the trapezoid body in rodents and bats. J Comp Neurol. 1992;324:522–538. doi: 10.1002/cne.903240406. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Alvarez FJ, Walmsley B. GABA mediates presynaptic inhibition at glycinergic synapses in a rat auditory brainstem nucleus. J Physiol. 2000;525:447–459. doi: 10.1111/j.1469-7793.2000.t01-1-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhrke S, Srinivasan G, Oberhofer M, Doncheva E, Friauf E. Shift from depolarizing to hyperpolarizing glycine action occurs at different perinatal ages in superior olivary complex nuclei. Eur J Neurosci. 2005;22:2708–2722. doi: 10.1111/j.1460-9568.2005.04465.x. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairén A, Luján R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur J Neurosci. 2002;15:1766–1778. doi: 10.1046/j.1460-9568.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Kulik A, Vida I, Fairén A, Luján R. Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus. 2004;14:836–848. doi: 10.1002/hipo.10221. [DOI] [PubMed] [Google Scholar]
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci. 2009;29:13770–13784. doi: 10.1523/JNEUROSCI.3285-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci. 2006;23:1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Kapfer C, Grothe B, Koch U. Maturation of glycinergic inhibition in the gerbil medial superior olive after hearing onset. J Physiol. 2005;568:497–512. doi: 10.1113/jphysiol.2005.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson AK, Park TJ, Pecka M, Grothe B, Koch U. Retrograde GABA signaling adjusts sound localization by balancing excitation and inhibition in the brainstem. Neuron. 2008;59:125–137. doi: 10.1016/j.neuron.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Mapelli L, Rossi P, Nieus T, D'Angelo E. Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus. J Neurophysiol. 2009;101:3089–3099. doi: 10.1152/jn.91190.2008. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M, van der Heijden M, Joris PX. How secure is in vivo synaptic transmission at the calyx of Held? J Neurosci. 2008;28:10206–10219. doi: 10.1523/JNEUROSCI.2735-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean HA, Caillard O, Khazipov R, Ben-Ari Y, Gaiarsa JL. Spontaneous release of GABA activates GABAB receptors and controls network activity in the neonatal rat hippocampus. J Neurophysiol. 1996;76:1036–1046. doi: 10.1152/jn.1996.76.2.1036. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouginot D, Kombian SB, Pittman QJ. Activation of presynaptic GABAB receptors inhibits evoked IPSCs in rat magnocellular neurons in vitro. J Neurophysiol. 1998;79:1508–1517. doi: 10.1152/jn.1998.79.3.1508. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. My close encounter with GABAB receptors. Biochem Pharmacol. 2004;68:1667–1674. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Doiron B, Rinzel J, Reyes AD. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J Neurosci. 2009;29:10321–10334. doi: 10.1523/JNEUROSCI.1703-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BX, Dong Y, Ito W, Yanagawa Y, Shigemoto R, Morozov A. Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron. 2009;61:917–929. doi: 10.1016/j.neuron.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. J Neurophysiol. 1994;72:2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol. 1987;258:267–280. doi: 10.1002/cne.902580207. [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR. Effects of unilateral cochlear removal on dendrites in the gerbil medial superior olivary nucleus. Eur J Neurosci. 1999;11:1379–1390. doi: 10.1046/j.1460-9568.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Schwartz IR, Wittebort AZ. Axon terminals in the cat medial superior olivary nucleus. Anat Rec. 1976;184:525. [Google Scholar]
- Scott LL, Mathews PJ, Golding NL. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J Neurosci. 2005;25:7887–7895. doi: 10.1523/JNEUROSCI.1016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Kakazu Y, Okabe A, Fukuda A, Nabekura J. Experience-dependent changes in intracellular Cl− regulation in developing auditory neurons. Neurosci Res. 2004;48:211–220. doi: 10.1016/j.neures.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Skottun BC. Sound localization and neurons. Nature. 1998;393:531. doi: 10.1038/31134. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol. 1993;331:245–260. doi: 10.1002/cne.903310208. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag M, Englitz B, Kopp-Scheinpflug C, Rübsamen R. Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: an in vivo study in mice. J Neurosci. 2009;29:9510–9520. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirou GA, Rowland KC, Berrebi AS. Ultrastructure of neurons and large synaptic terminals in the lateral nucleus of the trapezoid body of the cat. J Comp Neurol. 1998;398:257–272. doi: 10.1002/(sici)1096-9861(19980824)398:2<257::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Spitzer MW, Semple MN. Neurons sensitive to interaural phase disparity in gerbil superior olive: diverse monaural and temporal response properties. J Neurophysiol. 1995;73:1668–1690. doi: 10.1152/jn.1995.73.4.1668. [DOI] [PubMed] [Google Scholar]
- Stotler WA. An experimental study of the cells and connections of the superior olivary complex of the cat. J Comp Neurol. 1953;98:401–431. doi: 10.1002/cne.900980303. [DOI] [PubMed] [Google Scholar]
- Sun H, Ma CL, Kelly JB, Wu SH. GABAB receptor-mediated presynaptic inhibition of glutamatergic transmission in the inferior colliculus. Neurosci Lett. 2006;399:151–156. doi: 10.1016/j.neulet.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol. 2002;539:67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZQ, Gao H, Lu Y. Control of a depolarizing GABAergic input in an auditory coincidence detection circuit. J Neurophysiol. 2009;102:1672–1683. doi: 10.1152/jn.00419.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas KJ, Terunuma M, Tello JA, Pangalos MN, Moss SJ, Couve A. The availability of surface GABAB receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J Biol Chem. 2008;283:24641–24648. doi: 10.1074/jbc.M802419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Werthat F, Alexandrova O, Grothe B, Koch U. Experience-dependent refinement of the inhibitory axons projecting to the medial superior olive. Dev Neurobiol. 2008;68:1454–1462. doi: 10.1002/dneu.20660. [DOI] [PubMed] [Google Scholar]
- Wojcik WJ, Neff NH. gamma-Aminobutyric acid B receptors are negatively coupled to adenylate cyclase in brain, and in the cerebellum these receptors may be associated with granule cells. Mol Pharmacol. 1984;25:24–28. [PubMed] [Google Scholar]
- Yamauchi T, Hori T, Takahashi T. Presynaptic inhibition by muscimol through GABAB receptors. Eur J Neurosci. 2000;12:3433–3436. doi: 10.1046/j.1460-9568.2000.00248.x. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nat Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]