Introduction
Transcranial magnetic stimulation (TMS), first successfully demonstrated in 1985 (Barker et al., 1985), is a very safe, when following current safety guidelines (Rossi et al., 2009), and noninvasive method for affecting brain function. It relies upon the properties of electromagnetic induction; a rapidly changing magnetic field is generated when a high-voltage current is passed through a coil. When this coil is held in close proximity to any electrically conducting medium, such as the brain, this time-varying magnetic field induces current in a direction opposite to the original current in the coil (Fig. 1).
Figure 1.
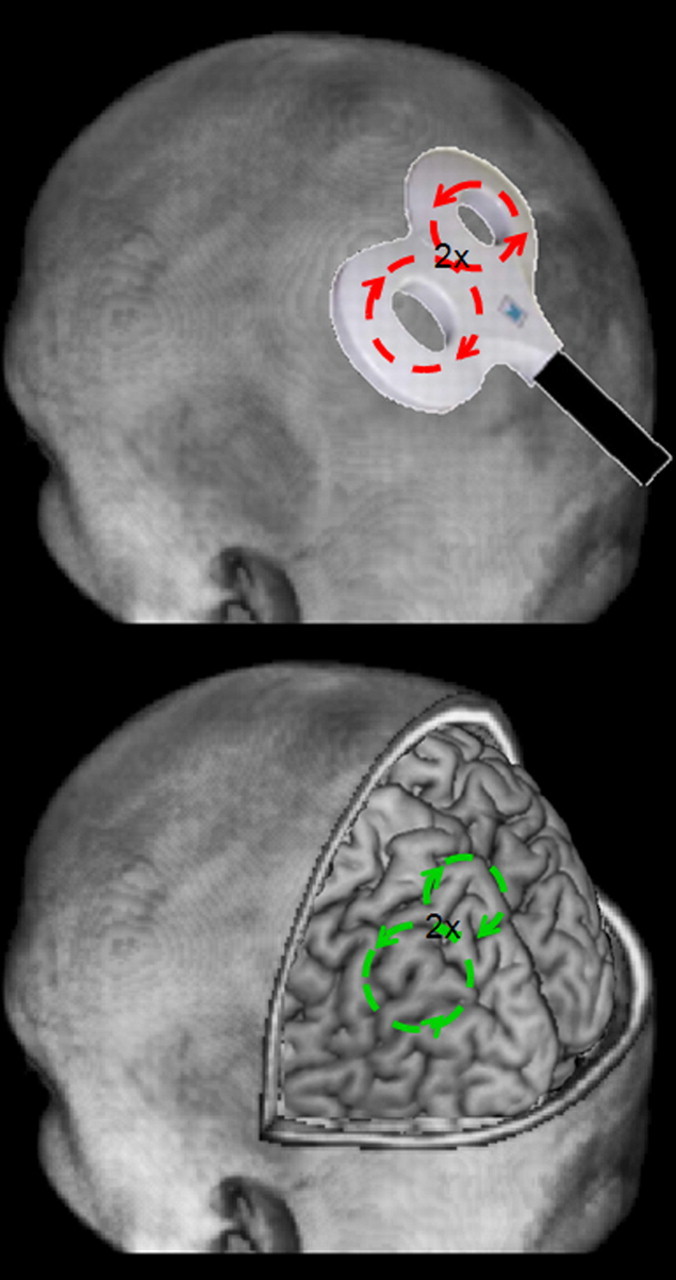
When a strong, rapid current is passed through a stimulating coil (top), a rapidly changing magnetic field is produced, which induces current into the brain (bottom).
As a result of this ion flow, action potentials are triggered in neurons that are within the induced current field, along with a subsequent period of deactivation, presumably through prolonged IPSPs. Because normal ongoing brain activity is disrupted by this induced current, TMS provides a way for investigators to produce a transient and reversible period of brain disruption or “virtual lesion.” Thus, unlike other experimental techniques [e.g., functional magnetic resonance imaging (fMRI), electroencephalography (EEG)/event-related potentials (ERPs)], TMS can assess whether a given brain area is necessary for a given function rather than simply correlated with it.
Spatial and temporal resolution
The spatial resolution of TMS is highly dependent upon the shape of the stimulating coil, but can be on the order of a few millimeters with certain coil types (e.g., figure-eight coils with 45 mm circular diameter components) and focal enough to stimulate regions as small as individual finger representations of the primary motor cortex (Ro et al., 1999). Several different coil designs are commercially available, including larger (e.g., 90 mm diameter) circular coils and the most commonly used 70 mm figure-eight coils, through which a focal point of stimulation is produced at the intersection of the two 70 mm diameter circular components comprising the figure-eight (Fig. 1).
The stimulating coil can be positioned over the brain either functionally or anatomically (Fig. 2a). Functional localization involves positioning the coil until some function is elicited or disrupted. For example, the primary motor and visual cortices can be localized by searching for an optimal coil position to produce activation of the contralateral hand muscles (Rossini et al., 1994) or visual phosphenes/suppression (Ro et al., 2003, 2004; Boyer et al., 2005), respectively. Similarly, the frontal eye fields, a cortical structure important for generating saccadic eye movements, can be localized by positioning the coil ∼1.5 cm anterior to the motor hand area and then demonstrating TMS-induced delays in saccadic eye movements (Müri et al., 1991; Priori et al., 1993; Ro et al., 1997; Olk et al., 2006). When anatomically localizing certain brain structures, a structural or functional MRI scan, which can be coregistered with a neuronavigation system (Fig. 2a), is required for MRI/fMRI-guided TMS. Alternatively, a set of standardized coordinates or scalp positions can be used to estimate the appropriate corresponding location in a given subject's head.
Figure 2.
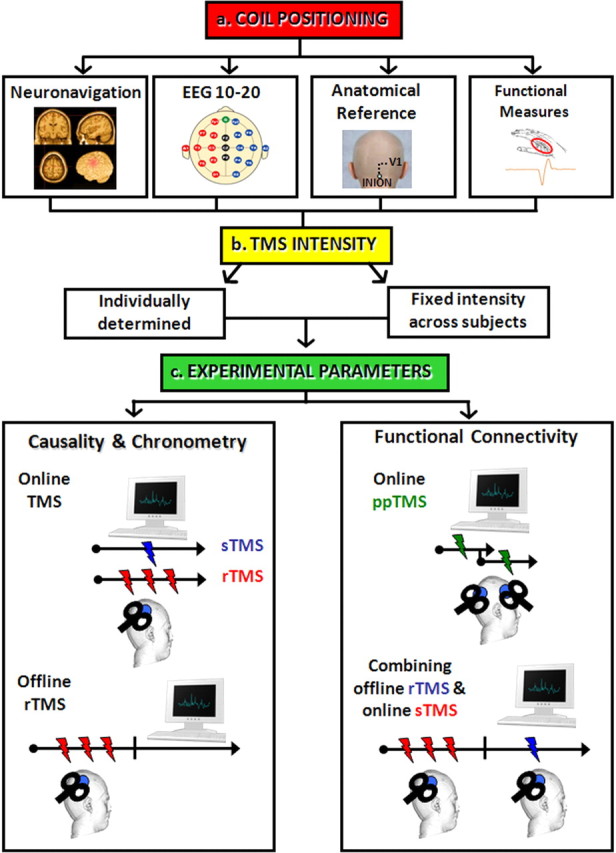
A schematic of the procedures and different types of TMS protocols that one can employ when using TMS to study brain function (see text for details).
The temporal resolution of TMS is also variable and dependent upon the stimulation parameters that one employs. When single-pulse TMS is used, the temporal resolution can be very high and can provide information about brain function on the order of milliseconds. For example, a single pulse of TMS delivered to the visual cortex will disrupt visual perception when delivered between ∼70 and 140 ms after a briefly flashed visual stimulus. This brief temporal window of disruption suggests that the effects of TMS on visual cortex are very transient, and, because of the transient nature of the TMS pulse, one can assess the timing and extent of visual cortical processing under varying experimental conditions (Amassian et al., 1989; Miller et al., 1996; Corthout et al., 1999a,b; Ro et al., 2003).
In motor cortex, a single TMS pulse can cause disruption in the contralateral hand muscles for ∼200 ms. These different temporal windows of disruption across different brain regions suggest that TMS differentially affects different cortical tissue, which may depend on overall neuronal size and orientation. Such differences in susceptibility to TMS across cortical regions, as well as between subjects, should be taken into account when attempting to set the intensity of TMS (Fig. 2b). The use of the same intensity across subjects and brain areas will likely introduce a substantial degree of variability into the data and would lead to too little or too much stimulation for most of the subjects. Therefore, when possible, intensity should be individually adjusted using functional measures, such as visual suppression/phosphene threshold, motor cortex threshold, or the threshold for disrupting the targeted process.
TMS parameters
If ones does not have a temporal hypothesis about when to deliver a single TMS pulse, one approach would be to apply single TMS pulses at varying time intervals across conditions/trials or to use repetitive TMS (rTMS), which consists of the application of rhythmic trains of multiple TMS pulses (Fig. 2c). When using rTMS, stimulation frequency seems to be the key parameter that determines the direction of the effects, although other variables should be taken into account when planning an rTMS experiment (e.g., duration of the train of stimulation, interval between trains, and total number of trains and of stimuli in a given session or to a given brain area). From a physiological point of view, when the temporal rate of rTMS is slow (<1 Hz), this tends to accentuate the inhibitory effects of TMS, whereas at faster rates of repetition (>1 Hz) the facilitatory effects come to the fore (Fitzgerald et al., 2006; Ziemann et al., 2008). The cutoff at 1 Hz is not entirely arbitrary and may be related to long-term depression and potentiation following tetanic stimulation of neurons; there is evidence that slow and high-frequency rTMS produce relatively distinct effects both on direct measures of brain activity and behavior (Chen et al., 1997). Typically, high-frequency rTMS protocols are applied either as a single short train of pulses or several trains with different intertrain intervals, while low-frequency rTMS is typically given as a prolonged continuous stimulation.
There are two main ways of administering rTMS. During the “online” approach, subjects perform the task, and, at a specific time just before or during the task, a train of TMS pulses is given to a particular area of the brain. Usually, in the online paradigm high-frequency rTMS is used, and the overall effect can be an improvement or an impairment of performance. For instance, 5 Hz rTMS delivered over the cortical representation of the right index finger of the primary somatosensory cortex can induce a lowering of tactile discrimination threshold of the right index finger, which is associated with an enlargement of the right index finger representation in primary somatosensory cortex, as measured by fMRI (Tegenthoff et al., 2005). However, high-frequency rTMS can also interfere with the undergoing process. For instance, Harris and coworkers (2008) applied short trains of 12 Hz rTMS to inferior parietal lobe, a region belonging to the dorsal visual stream, while subjects performing an object identification task (picture-word verification and categorizing objects) or an object orientation judgment task (picture-arrow verification and deciding the rotation of an object). This protocol induced an improvement of performance in object identification, but it also produced an impairment of orientation judgments, consequently showing that the right parietal lobe is critical for processing both the spatial attributes of objects and object recognition. Some have speculated that these opposite effects of rTMS (i.e., facilitation vs inhibition) may be a consequence of state-dependent effects of the rTMS on neural processing (Silvanto and Pascual-Leone, 2008; Miniussi et al., 2009).
Another popular approach for the use of rTMS is to stimulate at a site of interest for some minutes before starting a task (i.e., “offline” rTMS). Indeed, a crucial feature of rTMS is that it seems capable of changing the activity in a brain area even beyond the duration of the rTMS application itself. Given these long-lasting effects of rTMS, this technique is a potential tool to promote neuroplastic changes in neurological populations (Fregni and Pascual-Leone, 2007; Bolognini et al., 2009a). Offline low-frequency stimulation rTMS can be applied with the aim of inducing a longer lasting suppression of neural activity (Ridding and Rothwell, 2007). This approach has the advantage of not requiring rTMS at the same time as task performance or an assumption of when the brain processes the task, therefore removing many of the nonspecific concurrent effects of online TMS, such as nonspecific behavioral and attentional effects. Applied in earlier studies to investigate motor cortex excitability (Chen et al., 1997) and visual imagery (Kosslyn et al., 1999), this technique has been used across a variety of other cognitive tasks. For example, inhibiting the activity of the posterior parietal cortex with 20 min of 1 Hz rTMS impairs spatial orienting to modality-specific visual and auditory stimuli during a reaction time task given at the end of the rTMS (Bolognini et al., 2009b).
Alternative protocols of rTMS have also recently been developed, such as “theta burst TMS,” in which short bursts of 50 Hz rTMS are repeated at a rate in the theta range (5 Hz) as a continuous or intermittent train. The excitatory and inhibitory effects of this type of stimulation can be manipulated either by the continuous or intermittent delivery of these theta bursts over time. Continuous theta burst stimulation, like low-frequency rTMS, can induce inhibitory neural effects that outlast the duration of the stimulation (Huang et al., 2005).
Assessing connectivity with TMS
TMS not only changes neural activity at the site of stimulation, but it may be possible to probe the functional connectivity of different cortical areas in the human cortex using paired-pulse TMS (ppTMS). In this method, two TMS pulses are delivered to two different brain regions using two different coils: a conditioning stimulus and a test stimulus. What is measured is the effect of the conditioning stimulus on the response to the test stimulus, and, depending on the intensity of the conditioning stimulus and the interstimulus interval, both facilitation and inhibition may be detected. Since changes in the effectiveness of the conditioning pulse give an indication of how the excitability of the connection changes over time and during a specific task, this approach can uncover the functional interplay between different cortical areas. For instance, ppTMS has been used to explore the timing of interactions in visual cortex. The perception of a TMS-induced moving phosphene after stimulation of motion area MT/V5 can be significantly suppressed by a second TMS pulse applied to the primary visual cortex (V1) 10 to 40 ms later. This finding reflects the time window of the backprojections from V5 to V1, and indicates that such projections are necessary for visual awareness of motion (Pascual-Leone and Walsh, 2001). The double-pulse paradigm has been also successfully applied to study the time course of intra- and interhemispheric corticocortical interactions during preparation and execution of complex movement plans (Koch and Rothwell, 2009).
Functional connectivity within complex interconnected networks can also be explored by combining offline rTMS over a network related area and online single-pulse TMS over a different area. For instance, priming the posterior parietal cortex by 20 min of 1 Hz rTMS suppresses the cross-modal spatial influences on visual cortical excitability, as assessed by phosphene induction via single-pulse occipital TMS assessed after the end of the rTMS stimulation (Bolognini and Maravita, 2007). Similarly, the virtual lesion created by 1 Hz rTMS of the ventral premotor cortex or of the primary somatosensory cortex reduces the mirror motor facilitation contingent upon action observation, as measured by motor-evoked potentials to single-pulse TMS over the primary motor cortex during the offline window of the inhibitory rTMS effects (Avenanti et al., 2007).
TMS can be combined with other techniques for measuring brain function, such as EEG, positron emission tomography (PET), fMRI, and optical imaging. The combination of TMS with such other techniques can also assess cortical connectivity and interactions between different brain areas. For example, TMS of the human frontal eye fields has been shown to not only produce activation of this region underneath a stimulating coil, but has also been shown to produce remote activation in parietal and visual cortices (Paus et al., 1997; Ruff et al., 2009). Furthermore, although TMS cannot directly target subcortical structures, a recent study has shown that activity in the thalamus can be modulated by stimulation of parietal cortex (Blankenburg et al., 2008). This thalamic activation from parietal cortex TMS also produces disinhibition of the contralateral parietal cortex (Blankenburg et al., 2008) and may underlie subsequent hyperorienting/hypersensitivity responses to ipsilateral stimuli (Seyal et al., 1995; Hilgetag et al., 2001).
Limitations
Because the extent or spread of the induced current in the brain may be variable and impossible to assess without in vivo stimulation and recording studies, it is impossible to precisely determine which cortical neurons and how much cortical area is affected with each TMS pulse. Even if it were possible to determine which neurons were affected with each TMS pulse, the effects of the TMS on these neurons could be excitatory, inhibitory, or state dependent (Ziemann, 2010). Another limitation of TMS is that only surface structures of the brain (i.e., most of cortex and some of cerebellum) can be targeted. Although certain subcortical regions might be affected by using higher intensities of TMS or special types of coils (Zangen et al., 2005), especially in human subjects and animals with small heads, it is not possible to specifically target subcortical regions without affecting cortical ones because the strength of the time-varying magnetic field follows the inverse-square law. It may be possible to trans-synaptically affect subcortical structures by stimulating connected cortical areas (Strafella et al., 2003; Blankenburg et al., 2008); however, these indirect effects also make it difficult to determine which brain areas may be causally involved with some function or behavior. Finally, the loud click associated with the high current flowing through the metallic stimulating coil, and scalp sensations and head and neck muscle contractions, require careful control conditions to be included in any experimental design to rule out nonspecific effects of the TMS.
Conclusions
TMS is rapidly becoming an essential tool available to neuroscientists for assessing brain function and to clinicians for treating brain dysfunctions (e.g., stroke and depression). By using TMS to determine whether a brain area is causing some function, it nicely complements the limitations associated with correlation techniques, such as ERPs and fMRI, while also being able to provide several new insights into the operations of the brain. With its already exponentially increasing usage, future work using TMS in isolation, as well as in conjunction with other techniques, will allow for a deeper and more complete understanding of the human brain.
Footnotes
Editor's Note: Toolboxes are intended to describe and evaluate methods that are becoming widely relevant to the neuroscience community or to provide a critical analysis of established techniques. For more information, see http://www.jneurosci.org/misc/ifa_minireviews.dtl.
This work was supported in part by National Science Foundation Grants 0642801 and 0719969 to T.R.
References
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Maravita A. Proprioceptive alignment of visual and somatosensory maps in the posterior parietal cortex. Curr Biol. 2007;17:1890–1895. doi: 10.1016/j.cub.2007.09.057. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009a;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Miniussi C, Savazzi S, Bricolo E, Maravita A. TMS modulation of visual and auditory processing in the posterior parietal cortex. Exp Brain Res. 2009b;195:509–517. doi: 10.1007/s00221-009-1820-7. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Harrison S, Ro T. Unconscious processing of orientation and color without primary visual cortex. Proc Natl Acad Sci U S A. 2005;102:16875–16879. doi: 10.1073/pnas.0505332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Corthout E, Uttl B, Ziemann U, Cowey A, Hallett M. Two periods of processing in the (circum)striate visual cortex as revealed by transcranial magnetic stimulation. Neuropsychologia. 1999a;37:137–145. doi: 10.1016/s0028-3932(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Corthout E, Uttl B, Walsh V, Hallett M, Cowey A. Timing of activity in early visual cortex as revealed by transcranial magnetic stimulation. Neuroreport. 1999b;10:2631–2634. doi: 10.1097/00001756-199908200-00035. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Harris IM, Benito CT, Ruzzoli M, Miniussi C. Effects of right parietal transcranial magnetic stimulation on object identification and orientation judgments. J Cogn Neurosci. 2008;20:916–926. doi: 10.1162/jocn.2008.20513. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009;202:147–152. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284:167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- Miller MB, Fendrich R, Eliassen JC, Demirel S, Gazzaniga MS. Transcranial magnetic stimulation: delays in visual suppression due to luminance changes. Neuroreport. 1996;7:1740–1744. [PubMed] [Google Scholar]
- Miniussi C, Ruzzoli M, Walsh V. The mechanism of transcranial magnetic stimulation in cognition. Cortex. 2010;46:128–130. doi: 10.1016/j.cortex.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Müri RM, Hess CW, Meienberg O. Transcranial stimulation of the human frontal eye field by magnetic pulses. Exp Brain Res. 1991;86:219–223. doi: 10.1007/BF00231057. [DOI] [PubMed] [Google Scholar]
- Olk B, Chang E, Kingstone A, Ro T. Modulation of antisaccades by transcranial magnetic stimulation of the human frontal eye field. Cereb Cortex. 2006;16:76–82. doi: 10.1093/cercor/bhi085. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Rothwell JC, Day BL, Marsden CD. Some saccadic eye movements can be delayed by transcranial magnetic stimulation of the cerebral cortex in man. Brain. 1993;116:355–367. doi: 10.1093/brain/116.2.355. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future of therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Ro T, Henik A, Machado L, Rafal R. Transcranial magnetic stimulation of the prefrontal cortex delays contralateral endogenous saccades. J Cogn Neurosci. 1997;9:433–440. doi: 10.1162/jocn.1997.9.4.433. [DOI] [PubMed] [Google Scholar]
- Ro T, Cheifet S, Ingle H, Shoup R, Rafal R. Localization of the human frontal eye fields and motor hand area with transcranial magnetic stimulation and magnetic resonance imaging. Neuropsychologia. 1999;37:225–231. doi: 10.1016/s0028-3932(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Ro T, Breitmeyer B, Burton P, Singhal NS, Lane D. Feedback contributions to visual awareness in human occipital cortex. Curr Biol. 2003;13:1038–1041. doi: 10.1016/s0960-9822(03)00337-3. [DOI] [PubMed] [Google Scholar]
- Ro T, Shelton D, Lee OL, Chang E. Extrageniculate mediation of unconscious vision in transcranial magnetic stimulation-induced blindsight. Proc Natl Acad Sci U S A. 2004;101:9933–9935. doi: 10.1073/pnas.0403061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout A, Marsden C, Murray N, Rothwell J, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical applications: report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from “virtual lesions” to functional-network accounts of cognition. Cortex. 2009;45:1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Förster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS in cognitive neuroscience: virtual lesion and beyond. Cortex. 2010;46:124–127. doi: 10.1016/j.cortex.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC. Consensus: motor cortex plasticity protocols. Brain Stimulat. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]


