Abstract
Decision making refers to the process by which subjects choose between competing courses of action based on the expected costs and benefits of their consequences. Lesion studies in rats suggest that the anterior cingulate cortex and the nucleus accumbens are key structures of a neural system that subserves effort-based decision making. Little is known about brain activation associated with effort-based decisions in intact rats. Using an open hypothesis approach, we used 2-deoxy-2[18F]fluoro-d-glucose positron emission tomography (FDG-PET) to assess regional metabolic changes in two conditions of an effort-based decision making task. In the “same effort” condition, male rats could choose between two response options associated with the same effort but different reward sizes, i.e., decision making was simply a function of reward size. By contrast, in the “different effort” condition, an integration of different efforts and reward sizes associated with the two response options was necessary before making a decision. Separate PET scans were performed from each condition. Subtractive analysis revealed that metabolic activity was increased in the different effort relative to the same effort condition in the left anterior cingulate, left orbitofrontal and prelimbic cortex region. Metabolic activity was decreased in the infralimbic cortex and septum region. Our findings suggest that making decisions on how much effort to invest to obtain greater rewards evokes changes of metabolic activity in multiple brain areas associated with cognitive, limbic, motor and autonomic functions. This study demonstrates that FDG-PET provides a tool to determine in rats regional brain metabolic activity in cognitive tasks.
Introduction
Decision making refers to the process by which subjects choose between competing courses of action based on the expected costs and benefits of their consequences. For example, starlings are weighing the metabolic costs of distinct foraging modes and their benefits in terms of reward rate or magnitude before deciding which course of action to choose (Bautista et al., 2001). Likewise, rats make effort-based decisions involving cost-benefit assessments in T-maze (Salamone, 1994; Cousins and Salamone, 1996) or instrumental tasks (Salamone et al., 2007), e.g., their preference for pressing a lever to obtain preferred food over approaching freely available, less-preferred food decreased with increasing energetic requirements of the operant response.
Recent studies suggest that the anterior cingulate cortex (ACC) and the nucleus accumbens (NAc) are key structures of a neural system that subserves effort-based decision making. For instance, in rats tested in a T-maze cost-benefit task, excitotoxic lesions of the ACC and NAc caused a bias away from the response option requiring more effort to obtain greater reward (Walton et al., 2003; Schweimer and Hauber, 2005; Rudebeck et al., 2006; Hauber and Sommer, 2009).
Although lesion studies provided increasing knowledge about the neural network basis of decision making, little is known about regional brain activation associated with effort-based decisions in intact rats. Therefore, the goal of our study was to examine brain activation during effort-related decision making using functional imaging. We used 2-deoxy-2[18F]fluoro-d-glucose (FDG) micro positron emission tomography (μPET) in rats to assess FDG uptake, a measure of brain metabolic activity (Sokoloff et al., 1977; Phelps et al., 1979). Changes in brain metabolic activity were assessed in rats exposed on separate days to two conditions of an effort-based decision making task in an operant box. In one condition (“different effort”), trained animals could choose between a high reward lever requiring high effort (8 lever presses), and a low reward lever requiring low effort (4 lever presses). In the other condition (“same effort”), the efforts were equated, i.e., trained animals could choose between a high and low reward lever both demanding high effort (8 lever presses). Thus, in the same effort condition, decision making was simply a function of reward magnitude, while in the different effort condition, an integration of effort and reward magnitudes of the available response options was necessary before making a decision. Lesion studies implicated the ACC and NAc in effort-based decision making in the different effort, but not in the same effort condition (Walton et al., 2003; Hauber and Sommer, 2009; Walton et al., 2009). Therefore, we predicted that decision making in the different effort relative to the same effort condition should selectively engage the ACC and NAc. As PET allows an assessment of metabolic activity in the whole brain, we used an open hypothesis approach and did not limit our analysis to the ACC and NAc. Our study shows for the first time that making effort-based decisions in intact rats results in alterations of metabolic activation of multiple brain areas including various subregions of the prefrontal cortex.
Materials and Methods
Subjects and apparatus
Subjects were 8 naive male Lister Hooded rats (Harlan-Winkelmann). The rats were housed in groups of four in transparent plastic cages (55 × 39 × 27 cm; Ferplast) in a temperature- and humidity-controlled room (20 ± 2°C, 50–60%) on a 12:12 h light-dark cycle (lights on at 7:00 A.M.). Throughout the experiment the rats had ad libitum access to water. Standard laboratory maintenance chow (Altromin) was given ad libitum for 2 d after arrival, after which food was restricted to 15 g per animal per day to maintain them at ∼85% of their free-feeding weight. All animal experiments were conducted according the German law on animal protection and approved by the governmental authorities.
Training and testing took place in operant chambers (24 × 21 × 30 cm; Med Associates) housed within sound-attenuating cubicles. The operant chamber was equipped with a pellet dispenser that delivered 45 mg of Noyes Pellets (formula A/I; Sandown Scientific) into a magazine which was positioned in the middle of the right wall. The chamber also contained two retractable levers located on either side of the magazine. A 24 V/3 W house light mounted on the top-center of the opposite wall illuminated the chambers and an electric fan integrated into the cubicle provided a constant background noise (∼70 dB). A computer with the PC program MED-PC IV controlled the equipment and recorded the data.
Lever press training
On all training and testing days, each rat received a single session per day. On the first day, rats underwent a 15 min magazine training session using a fixed interval schedule during which two pellets were delivered every 180 s along with simultaneous magazine light illumination. The house lights were illuminated throughout. On the following 2 d, rats were trained to lever press for a single pellet delivery using a fixed ratio-1 schedule (FR1, i.e., one lever press per reward). Both levers were extended for the entire session and the house light was continuously illuminated. An animal had to reach a criterion of making at least 20 presses for each lever per session.
For all subsequent training and testing sessions one of the two levers was designated as being the high reward (HR) lever and the other as the low reward (LR) lever. The assignment of the HR and LR lever remained constant for each animal throughout the study and was counterbalanced across rats. On the next 2 d, selection of the HR lever resulted in the delivery of two pellets, selection of the LR lever of one pellet using a FR1 schedule, respectively. An animal had to reach a criterion of making at least 25 presses for each lever per session. On the next 2 d, selection of the HR lever resulted in the delivery of four pellets, selection of the LR lever in two pellets using a FR2 schedule, respectively. Again, an animal had to reach a criterion of making at least 25 presses for each lever per session.
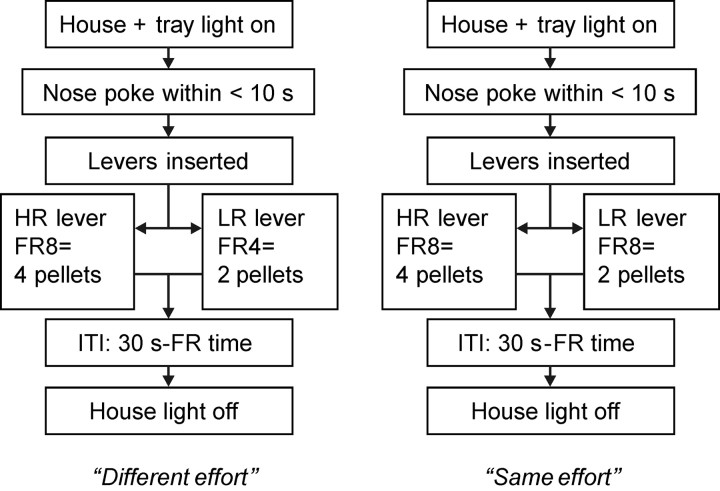
On the following days, the rats underwent a training regime on the full behavioral task. The design largely corresponds with a task used by Walton et al. (2009) and comprised two conditions. In the different effort condition, the HR lever was associated with high effort (FR8), the LR lever with low effort (FR4). In the same effort condition, both the HR and LR lever were associated with high effort (FR8) (Fig. 1). Regardless of the condition, a session consisted of 48 trials. Two types of trials were given. In “forced trials” only one lever, either the HR or LR, was available, in “choice trials” both the HR and LR lever were available. Each training trial started with house and magazine light illumination, and with the levers retracted. Once a nose-poke response in the magazine had been made, the magazine light extinguished and, in a forced trial, one of the levers was extended. Two forced trials were always given consecutively, one with HR, the other with LR lever presentation (counterbalanced across animals and trials). In choice trials, the HR and LR lever were extended at the same time and the lever not selected by an animal was immediately retracted. If the rat responded to a lever in forced or choice trials, the magazine light was illuminated and two or four pellets were delivered to the magazine depending on which lever had been introduced. After 6 s, both the magazine and house light extinguished, marking the end of a trial. Initially, the intertrial interval (ITI) was set at 5 s. Across subsequent sessions the ITI was increased further. To equate frequency of reward delivery on the two levers, in the final training sessions the ITI was calculated as being 30 s minus the amount of time taken to complete the FR schedule (i.e., if 10 s were required to complete the FR4 response, then the ITI would be 20 s). Furthermore, the delays to food delivery after responding on a FR4 and FR8 ratio were equalized, i.e., a delay of 2 s to receive 2 pellets after completing the FR4 ratio was implemented. One session comprised 48 trials with repetitive blocks of 6 trials always starting with 2 forced trials followed by 4 choice trials. A total of 30 training sessions was given, 15 in the different effort condition, 15 in the same effort condition in an alternating order. The duration of the final two training sessions and the subsequent test sessions (in combination with μPET) was set at 30 min.
Figure 1.
Schematics of the order of events in the effort-based decision making task. The task comprised two conditions (different vs same effort). A test session always started with 2 forced trials in which, in a random order, the HR or LR was available followed by 4 choice trials in which both the HR and LR lever were available. In choice trials, the lever not selected was retracted immediately after an animal's choice. Blocks of 2 forced and 4 subsequent choice trials were repeated until 30 min elapsed.
Experiment 1: effort-based decision making
Test design and imaging.
On test day 1, all animals (mean body weight 338 ± 9 g; range: 284–365 g) received intraperitoneal injections of FDG (1.7–2.1 mCi in 500 μl of Na-phosphate buffer, 228 mm). Five minutes later, animals were examined for effort-based decision making for 30 min in a counterbalanced manner with half of the rats tested in the different effort condition, the other half in the same effort condition. Then, animals were returned to their home cages. Fifty minutes after FDG administration, animals were anesthetized using inhalation anesthesia with isoflurane (5%, delivered in 70% N2O and 30% O2), and fixed in the animal holder equipped with a tooth bar and a respiratory mask.
PET scans were performed over 30 min starting 60 min after FDG injection. Schiffer et al. (2007) demonstrated that FDG concentration in blood is highest 5–35 min after intraperitoneal FDG injection, i.e., the time window we performed behavioral testing. Therefore, PET images mainly reflect FDG uptake during behavioral testing. The blood FDG/glucose ratio estimated from specific FDG activity was lower than 1:10,000. Thus, it is unlikely that injected FDG interfered with glucose metabolism.
PET scans were obtained under isoflurane anesthesia using a Focus 220 micro PET scanner (CTI-Siemens) with a resolution at center of field of view (FOV) of 1.4 mm. Breathing rate was kept at 50–70 per minute by adjusting isoflurane concentration (1.5–2.5%). Body temperature was held at 37°C using feedback-controlled warm water flow through the animal holder. Following Fourier rebinning, data were reconstructed using OSEM3D/MAP reconstruction (Qi et al., 1998), resulting in voxel sizes of 0.38 × 0.38 × 0.82 mm.
On day 2, T2-weighted structural MRI of each subject's brain was acquired under isoflurane anesthesia. MRI scans were performed in a 4.7-T BioSpec animal scanner (Bruker BioSpin) using a quadrature transmit/receive birdcage coil (Rapid Biomedical) with an inner diameter of 38 mm. A T2-weighted sequence, rapid acquisition with relaxation enhancement (RARE) was used: RARE factor = 8, repetition time/effective echo time = 5000/56.0 ms, averages = 2, matrix size = 256 × 256, FOV = 4.6 × 4.6 cm2, 21 slices, slice thickness = 1.3 mm, interslice spacing = 1.8 mm. Inhalation anesthesia procedures were similar to those used during μPET scans.
On day 3, the FDG-PET procedures and behavioral testing as described for day 1 were repeated with a reversed group–task condition assignment.
MRI and PET data analysis and statistics.
MRI and PET data were analyzed using the imaging software tool VINCI (Vollmar et al., 2007). First, MRI images were manually matched on a master brain derived from the atlas of Swanson (2003) and examined for gross structural anomalies, e.g., ventricular enlargement. As no such anomalies were detected all animals were included in this study. Next, the MRI images were used as individual templates for manual matching of the μPET images. As the overall brain activities in both task conditions were likely to be very similar, we used intensity normalization with the whole brain as reference dividing every image by the respective mean value of a whole brain volume-of-interest (VOI), a technique referred to as ratio normalization (Arndt et al., 1996).
We used an open hypothesis approach, i.e., we did not limit our analysis to the ACC and NAc, but analyzed changes of FDG uptake in the whole brain. However, as the rat brain comprises about 19,000 voxels, voxel-based statistical calculations included multiple comparisons associated with a considerable increase in the type I error rate. Matched voxels from the different effort and same effort conditions were compared using the paired t test, and p-values were corrected for multiple comparisons using the Benjamini-Hochberg control of false discovery rate (Benjamini and Hochberg, 1995). However, as in previous PET studies with low degrees of freedom (Nichols and Hayasaka, 2003; Rocke et al., 2005), all individual voxel comparisons missed significance if using the false discovery rate procedure. As proposed by Genovese et al. (2002), we therefore set a threshold of p = 0.01 (corresponding to t = 3.48, df = 7) for uncorrected p-values.
Behavioral data analysis and statistics.
In choice trials the preference for the HR lever was measured, in forced trials the latency to respond to the inserted lever [reaction time (RT)]. Forced trials with prolonged RT (>9000 ms) in the same and different effort condition (n = 2 in each condition) were excluded from analysis. t tests were used to analyze choice behavior; correlation analysis (Pearson product moment correlation) was used to examine relationships between different performance measures. Data were given as means ± SEM. These statistical computations were performed with STATISTICA (version 7.1, StatSoft). The level of statistical significance (α-level) was set at p < 0.05.
Experiment 2: effort-based decision making after prefeeding
In an additional experiment, we investigated whether responding in the effort-based decision making task was reward-directed or habit-like. Therefore, using a separate group of male Lister Hooded rats (n = 8), we examined the effects of prefeeding on effort-based decision making in the same effort and the different effort condition. No FDG-PET imaging was performed in these rats. The general procedures for this experiment were identical to those described for the previous experiment unless otherwise noted. The final two training sessions and the subsequent two test sessions comprised 48 trials, no session time limit was used. There was no time window for a response to be made. Thereafter, on test day 1 animals were examined in the different effort condition without prefeeding, on day 2 animals were examined in the different effort condition after prefeeding, i.e., immediately before testing animals had for 20 min free access to pellets (45 mg of Noyes Pellets; formula A/I; Sandown Scientific, Hampton, Middlesex, UK) used as reward in the decision making task. A test session comprised 48 trials with repetitive blocks of 6 trials always starting with 2 forced trials followed by 4 choice trials.
Thereafter, animals received two further training sessions. Subsequently, two test sessions were run. On test day 1 animals were examined in the same effort condition without prefeeding, on day 2 animals were examined in the same effort condition with prefeeding as described above. In the first test session (same effort condition without prefeeding) one animal did not respond to the levers and was therefore excluded from further analysis.
Results
Experiment 1: effort-based decision making
Effort-based decision making on test days
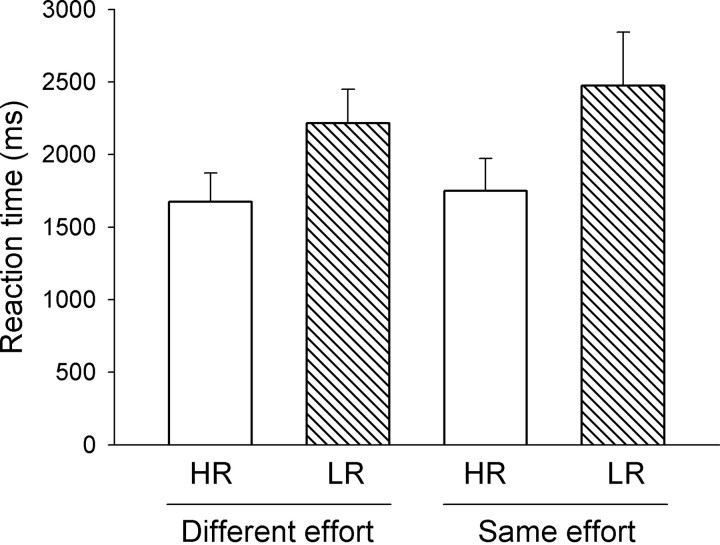
In choice trials, animals had a high preference for the HR lever in the different effort (mean HR choice: 88.5 ± 4.5%) and same effort condition (mean HR choice: 89.7 ± 4.1%) that did not differ significantly (t(7) = −0.38, p = 0.71; t test). Furthermore, as shown in Figure 2, in forced trials of the different effort condition, RT for the HR lever was clearly, though not significantly, shorter than for the LR lever (t(7)= −2.07, p = 0.078, t test). An exploratory analysis of RT based on all forced trials of all animals rather than of the averaged individual means showed that RT for the HR and LR lever were significantly different (t(47)= −2.25, p = 0.027).
Figure 2.
Effort-based decision making. Mean RT in forced trials in the different effort and same effort condition of the decision making task. In the different effort condition, the HR lever (4 pellets) was associated with high effort (FR8), the LR lever (2 pellets) with low effort (FR4). In the same effort condition, both the HR and LR lever were associated with high effort (FR8).
In addition, in forced trials of the same effort condition, RT for the HR lever was markedly, albeit not significantly, shorter than for the LR lever (t(7)= −1.82, p = 0.11, t test) (Fig. 2). A further analysis revealed that one animal showed a strong bias for fast responding for the LR lever. After exclusion of this animal, RT for the HR and LR lever in forced trials were significantly different (t(6)= −2.74, p = 0.034). Correspondingly, an additional analysis of RT based on all forced trials of all animals rather than of the averaged individual means showed that RT for the HR and LR lever were significantly different (t(48)= −3.1, p = 0.0032).
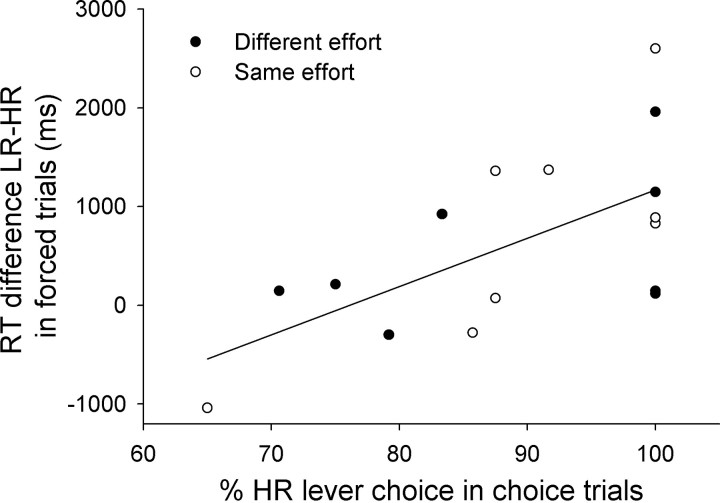
The number of lever presses as well as the number of pellets received in both conditions did not differ significantly (Table 1). Notably, choice behavior in choice trials and response latencies in forced trials were related as shown in Figure 3. A comparison of choice performance and RT of individual animals revealed a significant overall correlation (Pearson correlation coefficient: r = 0.62, p = 0.01). A separate analysis of the correspondence of choice behavior and response latencies in forced trials revealed a significant correlation between HR choice and RT in the same effort condition (r = 0.689, p = 0.009), but not in the different effort condition (r = 0.274, NS).
Table 1.
Number of lever presses and pellets received in choice and forced trials in the different effort and same effort condition (duration 30 min, respectively)
| Number of lever presses | Number of food pellets | |
|---|---|---|
| Different effort | 259.5 ± 15.9 | 129.8 ± 8.0 |
| Same effort | 288.0 ± 12.7* | 127.7 ± 6.3* |
NS (paired t test).
Figure 3.
Correlation between the percentage of HR lever choices in choice trials and the differences between RT on forced HR and LR lever trials for each individual animal. Individual rats are shown by symbols separately for the different effort and same effort condition. (Pearson correlation coefficient r = 0.62, p < 0.01).
Effort-based decision making on final training days
Choice behavior on the final two training days was similar as in subsequent test days with FDG administration and PET. HR preference was 89.0 ± 5.7% (different effort condition) versus 91.2 ± 2.6% (same effort condition) (t(7) = 0.39, p = 0.71). In the different effort condition, RT for the HR lever was 1703.3 ± 217.8 ms, RT for the LR lever 2299.4 ± 217.9 ms (t(7)= −2.14 p = 0.07). In the same effort condition, RT for the HR lever was 1529.2 ± 122.0 ms, RT for the LR lever 2198.12 ± 320.9 ms (t(7)= −3.9, p = 0.012) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Also, a comparison of choice performance and RT differences of HR and LR responses in forced trials of individual animals revealed a significant correlation (Pearson correlation coefficient: r = 0.56, p = 0.023). A separate analysis of the correspondence of choice behavior and response latencies in forced trials revealed a near significant correlation between HR choice and RT in the same effort condition (r = 0.68, p = 0.065), but not in the different effort condition (r = 0.27, NS) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
Experiment 2: effort-based decision making after prefeeding
Effects of prefeeding in the different effort condition
In this condition, prefeeding significantly reduced the number of completed choice trials (test day 1; outcome valued: 32.0 ± 0.0; test day 2, outcome devalued by prefeeding: 24.7 ± 3.8, p < 0.05, Wilcoxon paired rank test). Furthermore, the number of completed forced trials to the HR and LR was significantly decreased (test day 1; outcome valued: 8.0 ± 0.0 for the HR and LR, respectively; test day 2, outcome devalued by prefeeding: HR: 6.14 ± 0.94; p < 0.05 vs test day 1, Wilcoxon paired rank test; LR: 6.14 ± 0.92, p < 0.05, vs test day 1; Wilcoxon paired rank test) and the time to complete the session was significantly increased (test day 1; outcome valued: 53.1 ± 0.4 min; test day 2, outcome devalued by prefeeding: 65.7 ± 4.2 min, t(7)= −3.06, p < 0.023, t test). Prefeeding reduced the preference for the HR in choice trials (t(7) = 2.20, p = 0.063, t test) and increased the overall RT in forced trials (t(15)= −2.5, p = 0.0243, t test) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). On test day 1 (outcome valued) RT for LR was 1405.2 ± 212.9 ms, for HR 1564.5 ± 244.8 ms (t(7)= −0.23), p = 0.44, t test), on test day 2 (outcome devalued by prefeeding) RT for LR was 2185.1 ± 405.6 ms, RT for HR 2324.0 ± 319.3 ms (t(7)= −3.06, p = 0.73, t test).
Effects of prefeeding in the same effort condition
In this condition, prefeeding significantly reduced the number of completed choice trials (test day 1; outcome valued: 32.0 ± 0.0; test day 2, outcome devalued by prefeeding: 21.2 ± 4.0, p < 0.01, Wilcoxon paired rank test). The number of completed forced trials to the HR and LR was moderately reduced (test day 1; outcome valued: 8.0 ± 0.0 both for the HR and LR, respectively; test day 2, outcome devalued by prefeeding: HR: 7.0 ± 0.9; NS, vs test day 1, Wilcoxon paired rank test; LR: 6.4 ± 0.8, NS, vs test day 1; Wilcoxon paired rank test) and the time to complete the session was increased to some extent (test day 1; outcome valued: 52.8 ± 0.3 min; test day 2, outcome devalued by prefeeding: 59.0 ± 3.4 min, t(6)= −1.8, NS, t test). Prefeeding did not reduce the preference for the HR in choice trials (t(6) = 1.09, NS, t test), but increased the overall RT in forced trials (t(13)= −2.2, p = 0.046, t test) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). On test day 1 (outcome valued) RT for LR was 1664.7 ± 194.95 ms, for HR 1302.2 ± 344.6 ms (t(6)= −1.66, p = 0.15, t test), on test day 2 (outcome devalued by prefeeding) RT for LR was 2101.6 ± 318.5 ms, RT for HR 1868.1 ± 489.3 ms (t(7)= −0.43, p = 0.68, t test). Thus, after prefeeding, overall RT in forced trials became longer and HR preferences in the different, but not in the same, effort condition were reduced considerably.
FDG-PET imaging
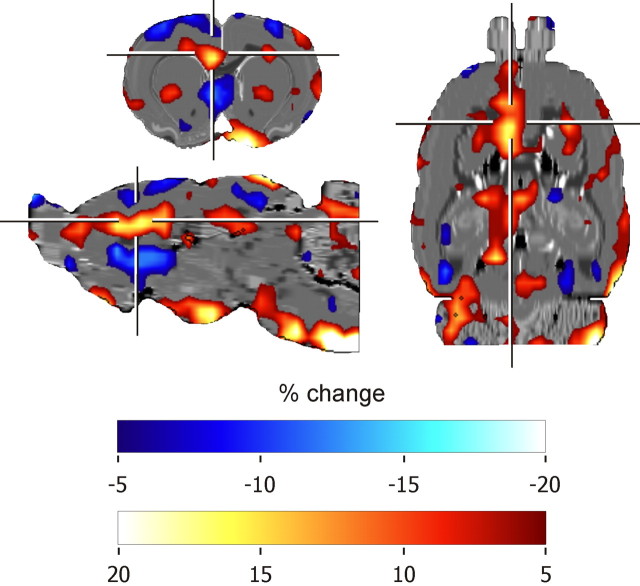
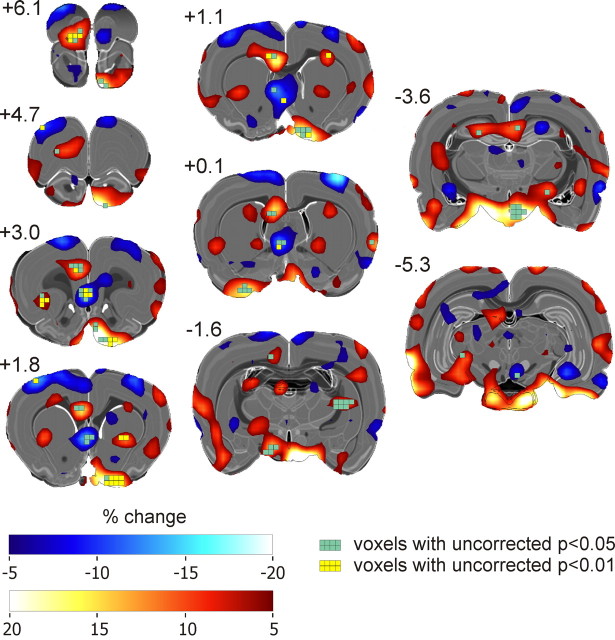
Regional brain metabolic changes were investigated only in experiment 1. FDG uptake associated with the different effort condition compared with the same effort condition was significantly enhanced in a number of brain regions as shown in Figure 4 and Table 2. A prominent increase in FDG uptake was observed in the left prefrontal cortex region including the rostral part of the left ventral and lateral orbitofrontal cortex (OFC), the left prelimbic cortex (PL), and the left cingulate area 2 (Cg2) (Fig. 5). An increased FDG uptake was also found in the rostral part of the right ventral pallidum/olfactory tubercle complex and bilaterally in the hypothalamus region (Fig. 5). Smaller patches of increased FDG uptake were present in the caudal part of the left lateral OFC, in the striatum and the hippocampus. By contrast, FDG uptake was decreased in the septal region and rostrally in the infralimbic cortex (IL) region. No change of metabolic activity was found in the NAc.
Figure 4.
Changes in prefrontal FDG uptake from rats tested in two conditions of an effort-based decision making task. Separate PET scans were performed for each condition. The different effort condition involved a choice between a HR lever requiring high effort and a LR lever requiring low effort, the same effort condition a choice between a HR and LR lever both demanding high effort. Percentage of changes in FDG uptake in the different effort relative to the same effort condition (control) was given in a transverse, a sagittal, and a horizontal section. Calculation was as follows: for each animal (n = 8) metabolic activity in the different effort condition) × 100/metabolic activity in the same effort condition was calculated, then, grand average image over all animals.
Table 2.
Relative metabolic activity in clusters with significant changes in the different effort compared to the same effort condition
| Cluster | Same effort | Different effort | % change |
|---|---|---|---|
| Anterior cingulate and prelimbic cortex (left) | 1.03 ± 0.09 | 1.12 ± 0.05 | 8.6 |
| Septum and infralimbic cortex (left) | 0.93 ± 0.03 | 0.81 ± 0.06 | −12.7 |
| Striatum (right) | 1.28 ± 0.08 | 1.40 ± 0.10 | 9.4 |
| Orbitofrontal (left) | 1.42 ± 0.08 | 1.54 ± 0.08 | 8.7 |
| Hippocampus (left) | 0.91 ± 0.05 | 0.99 ± 0.04 | 8.9 |
| Ventral pallidum and olfactory tubercle (right) | 0.83 ± 0.13 | 0.96 ± 0.10 | 15.3 |
| Piriform cortex (left) | 0.73 ± 0.09 | 0.84 ± 0.11 | 14.2 |
| Hypothalamus | 0.47 ± 0.07 | 0.53 ± 0.10 | 12.9 |
Figure 5.
Changes in brain FDG uptake from rats tested in two conditions of an effort-based decision making task. Separate PET scans were performed for each condition. The “different effort condition involved a choice between a HR lever requiring high effort and a LR lever requiring low effort, the same effort condition a choice between a HR and LR lever both demanding high effort. Percentage of changes in FDG uptake in the different effort relative to the same effort condition (control) was given in transverse sections. Calculation was as follows: For each animal (n = 8) metabolic activity in the different effort condition × 100/metabolic activity in the same effort condition was calculated, then, grand average image over all animals. Percentage of changes in FDG uptake was superimposed with significant voxels.
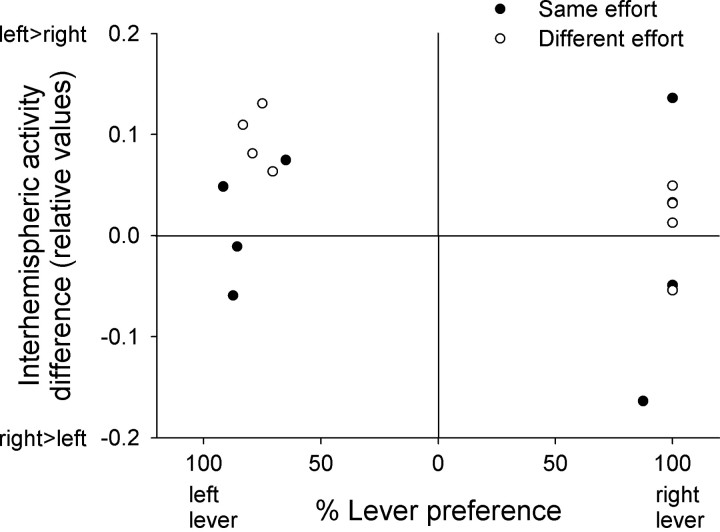
The pronounced unilateral increase of FDG uptake does not simply reflect a spatial response bias to the HR lever as the assignment of the left versus right as being the HR lever was counterbalanced and remained constant throughout the task for each rat. Accordingly, the hemispheric activity dominance calculated as the difference between the FDG uptake values, e.g., for the left and right Cg2, did not correlate with the spatial position of the preferred lever (Fig. 6).
Figure 6.
Correlation between the percentage of left or right lever choices in choice trials and the differences between the normalized metabolic activities in the left versus right Cg2 regions. Individual rats are shown by symbols separately for the different effort and same effort condition.
Further analyses failed to find any significant association between FDG uptake and performance, e.g., the percentage of HR lever choices in choice trials, neither in the different nor in the same effort condition (data not shown).
Discussion
Effort-based decision making
Animals showed a strong preference for the high effort-HR option when having the choice to obtain a LR with little effort as observed in earlier studies (Cousins and Salamone, 1994; Hauber and Sommer, 2009). For example, using a similar operant task, Walton et al. (2009) demonstrated that intact rats preferred the HR lever (4 pellets) set at FR 12 over the LR lever (2 pellets) set at FR 4. Consistent with previous studies (Hauber and Sommer, 2009; Walton et al., 2009), we further observed that if the effort was equated on both response options animals still had a strong preference for the HR option. Not surprisingly, in some earlier studies the preference for the HR response option was markedly higher in the same versus different effort condition (Hauber and Sommer, 2009; Walton et al., 2009). However, in our present study, the high preference (∼90%) for the HR lever in the different effort condition might have prevented a further increase in the same effort condition.
In the different effort condition, RT on forced trials were moderately shorter in HR than in LR trials. By contrast, in the same effort condition, the differences between RT in forced HR and LR trials became considerably higher. Correspondingly, Walton et al. (2006) revealed that the differences between RT on forced HR and LR responses are a function of the disparity of the FR schedules on both levers. Also, we observed a significant correlation between the HR lever preference in choice trials and RT differences between HR and LR responses in forced trials. Thus, the degree of HR lever preference in choice trials was mirrored by the RT differences on forced trials. In forced trials, RT of HR and LR responses depended on the expected efforts and reward magnitudes suggesting that responding was not habit-like. Furthermore, after prefeeding overall RT were longer and HR preferences in the different, but not in the same, effort condition considerably lower, also indicating that responding in our task was reward-directed rather than habit-like. In line with this account, animals tested in a similar task using a similar training protocol were sensitive to daily shifts of the cost-benefit ratios of the available response options (Walton et al., 2006).
FDG-PET imaging
Our study shows that high resolution FDG-PET can be used in rats to determine regional metabolic correlates of cognitive functions. Limitations are a low temporal resolution and spatial registration errors of up to 0.7 mm (Rowland et al., 2005).
During decision making in the different relative to the same effort condition the metabolic activity was markedly altered in a number of brain regions. The number of lever presses, rewards obtained and forced trials were similar in both task conditions, so that differences, e.g., in the sensorimotor demands or available rewards in both task conditions, are unlikely to account for observed changes in FDG uptake. Furthermore, results show that unilateral increases of the metabolic activity in various brain areas did not simply reflect a spatial response bias to the HR lever. Rather, it appears that the changes in FDG uptake were primarily specific to the different effort condition that required an integration of both the costs and benefits of the available response options before making a decision, as opposed to the same effort condition where choice was simply a function of the reward size associated with both response options.
FDG uptake in the different relative to the same effort condition was significantly increased in the left OFC and PL region as well as in the left ACC region, in particular in the Cg2 field. This latter finding is consistent with lesion studies in rats that implicated the ACC in effort-related decision making in the different effort, but not in the same effort condition (Walton et al., 2003; Hauber and Sommer, 2009; Walton et al., 2009). The relative increase of brain activity in the different effort condition might primarily reflect the specific cognitive demands of this task condition. In line with this notion, a close relationship between ACC activation and task difficulty or effort has been observed in the human brain as highlighted in a review that included >100 PET studies (Paus et al., 1998). Thus, functional imaging in intact rats as used here provides further support to the notion that ACC might be an important neural substrate of effort-based decision making (Rushworth, 2008; Botvinick et al., 2009).
Whether the unilateral activation of the ACC and adjacent prefrontal regions reflects a hemispheric specialization remains to be investigated. Lesions studies revealed that the rat brain shows a substantial hemispheric specialization (Denenberg, 1981; Sullivan, 2004). Furthermore, PET and fMRI imaging studies in humans demonstrated unilateral activations of prefrontal subregions while performing cost-benefit-related decisions making tasks (Rogers et al., 1999; Ernst et al., 2002; Knoch et al., 2006; Rao et al., 2008). Notably, in effort-based cost-benefit valuation, an activation of the dorsal ACC in the left hemisphere was observed (Croxson et al., 2009).
Lesion studies in rats suggest that the OFC is involved in delay-based but not in effort-based decision making (Rudebeck et al., 2006; Walton et al., 2003). Furthermore, the PL may not contribute to effort and delay-based decision making (Cardinal et al., 2001; Walton et al., 2003). By contrast, our present results demonstrate that, at least in intact rats, not only the ACC, but also the OFC and PL, were activated during effort-based decision making. However, the OFC and PL subserve cognitive functions such as representing expected values of different response options (Murray et al., 2007) and action-outcome contingencies (Corbit and Balleine, 2003), respectively. This suggests that in the intact rat brain, the OFC and PL may process information related to effort-based decisions, although these regions do not appear to be essential in guiding choice in these situations.
Lesion studies suggest that the PL-IL are not necessary for the rats' ability to make effort-based decisions (Walton et al., 2003). For unknown reasons, the FDG uptake during effort-based decision making was markedly decreased in the IL region and the septal region bilaterally. In turn, the prominent projections from the IL to the septum (Vertes, 2004, 2006) may lead to reduced activation of the septal region observed here.
Furthermore, a bilateral increase of FDG uptake in the ventrolateral caudate-putamen was observed, which might be related to the role of this striatal subregion in mediating lever pressing (Cousins and Salamone, 1996). Likewise, our results show a strong activation of the hypothalamic region. The ACC sends major projections to areas associated with autonomic control such as the hypothalamus (Floyd et al., 2000; Gabbott et al., 2005). In view of these connections, the ACC might play an important role in generating autonomic arousal during effortful cognitive and motor behavior. Also, we observed an increased FDG uptake within the dorsal hippocampal region that could possibly be related to the suggested role of the hippocampus as key component of a circuitry important in the use of predictions to guide decisions (Buckner, 2010).
At variance with predictions from lesion studies (Hauber and Sommer, 2009), metabolic activity was not altered in the NAc during effort-based decision making, or changes were too small to be detected. However, it is conceivable that a possible involvement of the NAc did not lead to a change in NAc FDG uptake. For instance, the NAc might have enabling functions for effort-related assessments (Salamone et al., 2007). Therefore, task-related changes, e.g., of the level of effort, may not lead directly to significant alterations in NAc FDG uptake. Furthermore, it is also possible that if there is a slightly increased brain glucose demand in the NAc, this does not necessarily involve an increased FDG uptake. For instance, in activated tissue a shift of the ratio of FDG and glucose net influx rate constants (“local lumped constant”) can occur (Holden et al., 1991) indicating a relative increase in the uptake of glucose over FDG.
Conclusions
This study demonstrates that FDG-PET can be used in intact rats to determine brain metabolic activation in cognitive tasks. Our findings suggest that making decisions on how much effort to invest to obtain greater rewards evokes changes of metabolic activity in multiple brain areas associated with cognitive, limbic, motor and autonomic functions. For instance, effort-based decision making changes metabolic activity in a number of prefrontal subregions, i.e., the ACC, OFC and PL region, while in the IL region metabolic activity was reduced. By contrast, lesion studies implicated the ACC, but not the OFC and PL-Il in cost-benefit decisions. Lesion and imaging methods are complementary and have their own potentials and limitations. Lesion data can identify areas that are necessary for controlling a cognitive function, while techniques that monitor brain metabolic activity can reveal brain areas involved with a cognitive task. PET data as measured here provide a unique window into brain function of intact rats and demonstrate that decisions that require an assessment on how much effort to invest for a reward involve an activation of multiple brain areas.
Footnotes
W.H. was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG HA2340/9-1).
References
- Arndt S, Cizadlo T, O'Leary D, Gold S, Andreasen NC. Normalizing counts and cerebral blood flow intensity in functional imaging studies of the human brain. Neuroimage. 1996;3:175–184. doi: 10.1006/nimg.1996.0019. [DOI] [PubMed] [Google Scholar]
- Bautista LM, Tinbergen J, Kacelnik A. To walk or to fly? How birds choose among foraging modes. Proc Natl Acad Sci U S A. 2001;98:1089–1094. doi: 10.1073/pnas.98.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Skilled motor deficits in rats induced by ventrolateral striatal dopamine depletions: behavioral and pharmacological characterization. Brain Res. 1996;732:186–194. doi: 10.1016/0006-8993(96)00519-7. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH. Hemispheric laterality in animals and the effects of early experience. Behav Brain Sci. 1981;4:1–21. [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19:2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- Holden JE, Mori K, Dienel GA, Cruz NF, Nelson T, Sokoloff L. Modeling the dependence of hexose distribution volumes in brain on plasma glucose concentration: implications for estimation of the local 2-deoxyglucose lumped constant. J Cereb Blood Flow Metab. 1991;11:171–182. doi: 10.1038/jcbfm.1991.50. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Qi J, Leahy RM, Cherry SR, Chatziioannou A, Farquhar TH. High-resolution 3D Bayesian image reconstruction using the microPET small-animal scanner. Phys Med Biol. 1998;43:1001–1013. doi: 10.1088/0031-9155/43/4/027. [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke DM, Goldberg Z, Schweitert C, Santana A. A method for detection of differential gene expression in the presence of inter-individual variability in response. Bioinformatics. 2005;21:3990–3992. doi: 10.1093/bioinformatics/bti667. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DJ, Garbow JR, Laforest R, Snyder AZ. Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol. 2005;32:567–572. doi: 10.1016/j.nucmedbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med. 2007;48:277–287. [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 2005;12:334–342. doi: 10.1101/lm.90605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Ed 3. San Diego: Academic; 2003. [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vollmar S, Hampl J, Kracht L, Herholz K. Integration of functional data (PET) into brain surgery planning and neuronavigation. Adv Med Eng. 2007;114:98–103. [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Groves J, Jennings KA, Croxson PL, Sharp T, Rushworth MF, Bannerman DM. Comparing the role of the anterior cingulate cortex and 6-hydroxydopamine nucleus accumbens lesions on operant effort-based decision making. Eur J Neurosci. 2009;29:1678–1691. doi: 10.1111/j.1460-9568.2009.06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]