Abstract
Background
Chronic usage of morphine elicits the production of inflammatory factors by glial cells and induces neuroinflammation. Ginger (Zingiber Officinale Roscoe) is a medicinal herb that has anti-inflammatory properties. It has been reported that ginger shows anti-addictive effects against chronic usage of morphine; however, its influence on morphine-induced neuroinflammation has not yet been clarified.
Methods
Morphine (12 mg/kg) was administrated intraperitoneally for 6 consecutive days. To evaluate the effect of ginger on morphine-induced neuroinflammation, ginger extract (100 mg/kg) was given orally 30 minutes before morphine. Glial fibrillary acidic protein (GFAP) and p38 mitogen-activated protein kinase (p38 MAPK) levels were assayed by immunoblotting in the rat nucleus accumbens (NAcc).
Findings
The injection of chronic morphine increased the levels of proteins involved in neuroinflammation (p38 MAPK and GFAP) in NAcc. Furthermore, the levels of p38 MAPK and GFAP significantly returned to the control levels by ginger extract.
Conclusion
The results suggest that the ginger extract can reduce morphine-induced neuroinflammation in NAcc.
Keywords: Morphine, Ginger, p38 Mitogen-activated protein kinases, Glial fibrillary acidic protein, Nucleus accumbens, Rats
Introduction
Opioids drugs, such as morphine, are used to manage pain over the past decades. However, long-term usage of these drugs induces side effects including addiction, tolerance, and physical dependence.1 In addition, it has been documented that chronic usage of morphine leads to neuroinflammation in central nervous system (CNS), which is associated with the enhancement of morphine-induced antinociceptive tolerance, and physical and psychological dependence.2 Morphine-induced neuroinflammation is commonly accompanied with the activation of glia, and production of inflammatory factors such as cytokines, chemokines, and surface antigens, which enhance immune response in the CNS.2,3
P38 mitogen-activated protein kinase (p38 MAPK) is one of the signal transduction pathways that regulate the production and release of inflammatory cytokines including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). In addition, inflammatory cytokines induce further p38 MAPK activation in neuronal cells.4 Previous studies have shown that chronic morphine induces glial activation through the p38 MAPK, which this signaling pathway leads to the release of several proinflammatory cytokines (such as TNF-a by astrocytes and microglia), and decreases subsequent morphine analgesia.5,6
It is clear that upregulation of glial fibrillary acidic protein (GFAP) is observed in the astrocytes after CNS injury, and it is known as biomarker of astrocytes activation.7-9 It has been demonstrated that upregulation GFAP is significantly expressed in the CNS of morphine-treated animals, and such upregulation can be involved in the development of opioid side effects.3,10
Recently, herbal products have attracted the attention of scientists in herbal medicine.11 Ginger (Zingiber officinale Roscoe, Zingiberacae) has been used in foods as a spice, and as a medicinal plant in traditional herbal medicine since ancient times. Ginger has been prescribed traditionally for the treatment of various diseases including fever, headache, diarrhea, nausea, infection diseases, arthritis, and rheumatic disorders.12,13 Numerous studies have shown that ginger has various biological activities including anti-inflammatory, anti-oxidative, antimicrobial and anticancer activities.12,14,15
It is well demonstrated that ginger and its bioactive components have analgesic and anti-inflammatory properties. Ginger and its bioactive constituents directly suppress the proinflammatory cytokines and chemokines at the sites of inflammation. It has been reported that ginger anti-inflammatory activity can be performed through the modulation biochemical pathways in chronic inflammation.16,17
We have recently reported that ginger has an anti-addictive property in morphine-treated rats.18 However, the effect of ginger on morphine-induced neuroinflammation has not been determined. The present study was performed to evaluate the effect of ginger on proteins involved in neuroinflammation (p38 MAPK and GFAP) in the nucleus accumbens (NAcc) of rats.
Methods
Animals: Male Wistar rats (weight of 230-250 g) were housed in a photo period controlled room (12-hour light/dark cycle) with temperature of 22 ± 2 °C. The animals had free access to food and water. The experimental protocol was firstly approved by the Animal Research Ethics Committee of the Kerman Neuroscience Research Center, Kerman, Iran (EC/KNRC/90).
Drugs and injections: Fresh ginger was prepared from the local market in Kerman City, Iran. The rhizome of herb was dried in air and ground into fine powder, and then extracted with 1 liter of ethyl alcohol 80%. A little of the plant extract were weighed and dissolved in physiological saline. The rats received 100 mg/kg of ginger extract intragastricly (i.g) 30 minutes before 12 mg/kg morphine which was injected intraperitoneally (once daily for 6 days). This dose was chosen according our previous study18 as effective anti-addictive dose. Morphine hydrochloride was prepared by TEMAD CO. (Tehran, Iran) and dissolved in physiological saline.
Western blot analysis: Tissue samples from NAcc were homogenized in radioimmunoprecipitation assay (RIPA) buffer, and centrifuged at 14,000 rpm for 20 minutes at 4 °C. Equal amounts (40 μg) of protein were electrophoresed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then gently transferred to polyvinylidene difluoride (PVDF) membrane (Roche, Germany). After blocking (for 2 hours) with 5% non-fat dried milk in Tris-buffered saline (TBS) with Tween 20 (blocking buffer, TBS-T, 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 0.1% Tween 20), the membranes were incubated with primary p38 and GFAP antibodies (1:1000) overnight at 4 °C. After washing in TBS-T (three times for 5 min), the membranes were incubated for 1 hour with matched horseradish peroxidase-conjugated secondary antibody (1:10000, Santa Cruz Biotechnology, CA, USA). The antibody-antigen reactions were luminated using the enhanced chemiluminescence (ECL) system, and detected on Lumi-Film chemiluminescent film (Roche, Germany). Lab Works software (UVP, UK) was used to assess the blotting bands density. β-actin (1:10000) was also blotted as loading control. The values were reported as tested proteins/β-actin ratio for each rat. Immunoblotting was performed on the days 9 and 13 after injections.
Data were analyzed using SPSS software (version 16, SPSS Inc., Chicago, IL, USA). One-way analysis of variance followed by post-hoc Tukey’s test were recruited. The P-value < 0.05 was considered statistically significant. The results were shown as mean ± standard error of mean (SEM).
Results
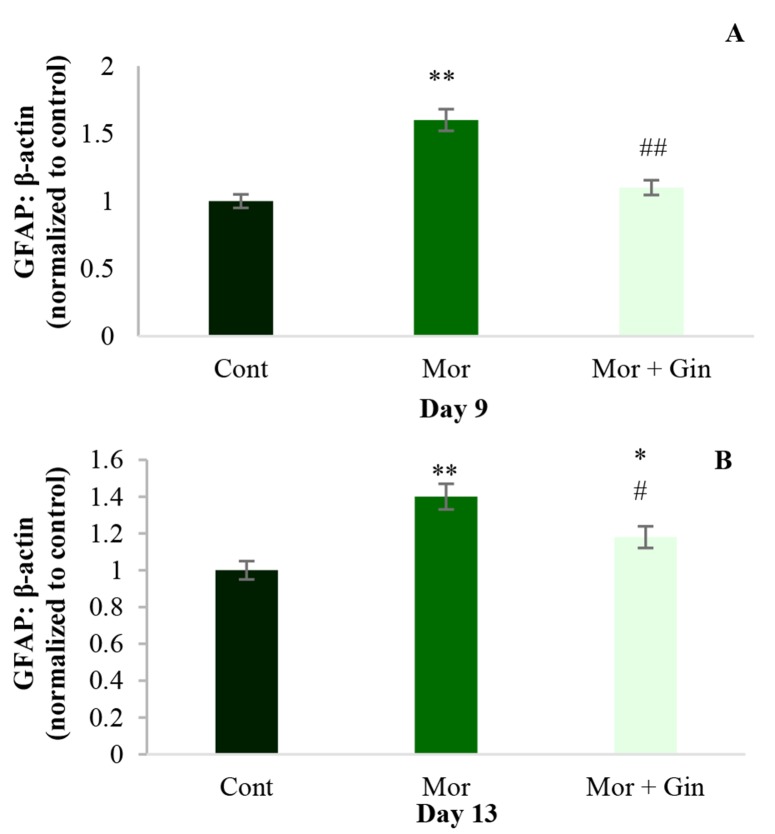
As shown in figure 1, following chronic administration of morphine, GFAP level significantly increased in NAcc (P < 0.01) on the days 9 and 13 after injections (Figure 1-A and B, respectively). Increase in GFAP level induced by morphine was significantly reversed by ginger extract on day 9 (P < 0.01) (Figure 1-A). In addition, ginger extract could significantly (P < 0.05) suppressed GFAP protein upregulation on 13th day (Figure 1-B).
Figure 1.
The levels of glial fibrillary acidic protein (GFAP) on 9th and 13th days following morphine injection in nucleus accumbens (NAcc) of control, chronic morphine-treated (12 mg/kg, intraperitoneally for 6 day) and morphine + ginger (12 mg/kg, intraperitoneally + 100 mg/kg, intragastricly) treated rats; beta-actin was used as an internal control. Values represent mean ± standard error of mean (SEM). Cont: Control; Mor: Morphine; Gin: Ginger *P < 0.05 and **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus morphine-treated group
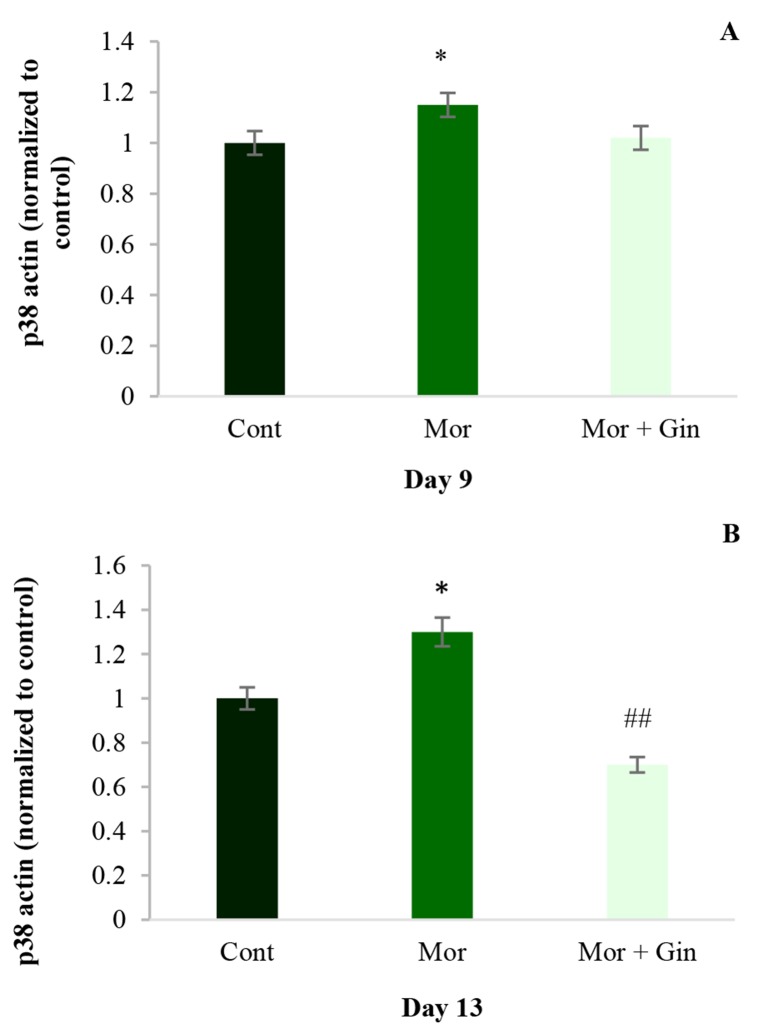
Immunoblot analysis showed that chronic morphine resulted in an elevation of p38 on 9th and 13th days after injections in rats’ NAcc (P < 0.05). The administration of an effective anti-tolerance and anti-addictive dose of ginger extract (100 mg/kg, i.g.) inhibited chronic morphine-induced p38 activation on day 13 (P < 0.01) (Figure 2).
Figure 2.
Immunoblot analysis of p38 on days 9 and 13 in nucleus accumbens (NAcc) of rats that received morphine (12 mg/kg, intraperitoneally) alone and 100 mg/kg ginger intragastricly plus morphine; beta-actin was used as an internal control. Values represent mean ± standard error of mean (SEM). Cont: Control; Mor: Morphine; Gin: Ginger *P < 0.05 versus control group; ##P < 0.01 versus morphinetreated group
Discussion
We have previously reported that ginger produces anti-addictive and anti-tolerance effects in morphine-injected rats.18
The present study examined the effect of ginger extract on neuroinflammation by assessing p38 and GFAP proteins expression in NAcc of rats. The data showed that ginger could decrease morphine-induced GFAP and p38 upregulation on 9th and 13th days after injections in the NAcc.
Morphine is a powerful opioid drug for treating pain but its long-term or chronic use activates astrocytes and microglia. Such activated glia can increase the release and promote gene expression of cytokines and chemokines, as well as elevate reactive oxygen species (ROS), which finally induce neuroinflammation in neural tissues. Neuroinflammation is involved in the intensification of antinociceptive tolerance and physical dependence induced by morphine.2,3,19 Although it has been demonstrated that neuroinflammation leads to the release of neuroexcitatory and inflammatory cytokines; the underlying mechanisms involved in the induction of morphine-induced neuroinflammation have not yet been fully clarified.
It has been demonstrated that morphine administration induces astrocytes activation in some areas of the brain such as NAcc, lateral septal, locus coeruleus, caudate nucleus, and prefrontal cortex.20 In addition, it has been reported that morphine leads to the activation of glia within the NAcc and subsequent upregulation of the expression of cytokines and chemokines, which oppose morphine-induced analgesia, and increase tolerance, respiratory depression, withdrawal, and reward to morphine.21 Cooper et al. have reported that drugs such as opioids activate glial cells and the modulators of glial cells reduce opioid-induced tolerance and dependence, and conditioned place preference.22
In recent years, numerous documents have focused on the property of medicinal plants and their active components in treating illnesses. Ginger is a safe herbal medicine widely used in around the world for the treatment of various disease such as arthritis, rheumatism, muscular aches, pains, sore throats, cramps, constipation, indigestion, vomiting, hypertension, dementia, fever, and infectious diseases. In addition, it has been reported that ginger shows beneficial effects as analgesic, neuroprotective, and anti-inflammatory agent.16
It is thought that ginger can modulate these activities through its main components (gingerols, shogaols, paradols, and zingerone). Previous studies have reported that ginger and its bioactive components play a fundamental role in the reduction of pain and inflammation. It has been documented that ginger inhibits proinflammatory cytokines, and decreases synthesis of proinflammatory prostaglandins and leukotrienes. It has been shown that ginger can suppress the phosphorylation of mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases 1 and 2 (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase (JNK) pathways.16,17,23
It has been reported that ginger extract can inhibit morphine side effects such as addictive seeking behavior in rats.18 Dedov et al. have been reported that ginger components function as agonists at vanilloid receptors, where present on pain afferents mediating joint pain.24 It has been well reviewed that ginger shows analgesic and anti-inflammatory properties in clinical studies.17 Therefore, it is possible that ginger exerts its biological effects through modulating the inflammation in the neural tissues.
It is well known that p38 MAPK pathway contributes to the overproduction, and release proinflammatory cytokines in CNS.4,25 In addition, it has been reported that long-term usage of morphine activates p38 MAP cascade to promote morphine tolerance, which leads to opposing morphine analgesia.6,26 We have previously reported that ginger extract has analgesic27 and anti-tolerance28 effects against morphine in rats. Jung et al. reported that ginger anti-neuroinflammatory effect is related to the suppression of p38 MAPK signaling pathway.27 In addition, it has been shown that 6-gingerol suppresses the production of proinflammatory cytokines through the downregulating of p38 MAP kinase activity and nuclear factor kappa B (NF-kB) expression.28
It is well documented that opioids activate astrocytes and microglia in animal brain.29-31 Chronic administration of morphine leads to astroglial hypertrophy and upregulation of GFAP levels in astrocyte cells.7 GFAP is expressed in the CNS where known as a specific marker of astrocytes activity.
Morphine exposure enhances GFAP levels in different brain areas including ventral tegmental area, NAcc, and striatum as well as frontal cortex.22 Studies have demonstrated that GFAP upregulation has a critical role in the induction of opioid tolerance and dependence.3,10 In addition, it has been reported that a specific inhibitor of glial cells (fluorocitrate) reduces morphine-induced tolerance and analgesia, and also inhibits GFAP enhancement in spinal cord.7
Conclusion
This study shows that ginger extract can attenuate NAcc protein levels involved in neuroinflammation induced by chronic usage of morphine. Therefore, it is possible that ginger anti-addictive effects is performed through the reducing glial activation and neuroinflammation.
Acknowledgments
The entire infrastructure required has been provided by the Payame Noor University, Tehran, Iran, and Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Footnotes
Conflicts of Interest
The Authors have no conflict of interest.
REFERENCES
- 1.Zhu H, Zhou W. Discharge activities of neurons in the nucleus paragigantocellularis during the development of morphine tolerance and dependence: A single unit study in chronically implanted rats. Eur J Pharmacol. 2010;636(1-3):65–72. doi: 10.1016/j.ejphar.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA. 2012;109(16):6325–30. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 4.Bachstetter AD, Van Eldik LJ. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1(3):199–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22(8):1178–89. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169(2):843–54. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39(3):281–6. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 8.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–7. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 9.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93(3):421–43. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Esmaeili-Mahani S, Ebrahimi B, Abbasnejad M, Rasoulian B, Sheibani V. Satureja khuzestanica prevents the development of morphine analgesic tolerance through suppression of spinal glial cell activation in rats. J Nat Med. 2015;69(2):165–70. doi: 10.1007/s11418-013-0796-6. [DOI] [PubMed] [Google Scholar]
- 11.Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW. Herbal medicines for the management of opioid addiction: Safe and effective alternatives to conventional pharmacotherapy? CNS Drugs. 2011;25(12):999–1007. doi: 10.2165/11596830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: An ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact. 2001;18(3-4):159–90. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen P. Ginger--Zingiber officinale Roscoe, Zingiberaceae. J Prim Health Care. 2011;3(3):235–6. [PubMed] [Google Scholar]
- 14.Rahmani AH, Shabrmi FM, Aly SM. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol. 2014;6(2):125–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Wohlmuth H. Phytochemistry and pharmacology of plants from the ginger family, Zingiberaceae [PhD Thesis]. Lismore, NSW, Australia: Southern Cross University; 2008. [Google Scholar]
- 16.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46(2):409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 17.Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–32. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 18.Torkzadeh-Mahani S, Nasri S, Esmaeili-Mahani S. Ginger (zingiber officinale roscoe) prevents morphine-induced addictive behaviors in conditioned place preference test in rats. Addict Health. 2014;6(1-2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 19.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazriev IL, Kiknadze GI, Kutateladze II, Nebieridze MI. Effect of morphine on the number and branching of astrocytes in various regions of rat brain. Bull Exp Biol Med. 2001;131(3):248–50. doi: 10.1023/a:1017699315355. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31(49):17835–47. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper ZD, Jones JD, Comer SD. Glial modulators: A novel pharmacological approach to altering the behavioral effects of abused substances. Expert Opin Investig Drugs. 2012;21(2):169–78. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules. 2011;16(2):1021–43. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedov VN, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, et al. Gingerols: A novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol. 2002;137(6):793–8. doi: 10.1038/sj.bjp.0704925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86(6):1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069(1):235–43. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 27.Jung HW, Yoon CH, Park KM, Han HS, Park YK. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem Toxicol. 2009;47(6):1190–7. doi: 10.1016/j.fct.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–86. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- 29.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30(11):581–91. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29(4):998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29(2):327–34. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]