Memory is one of the core cognitive abilities, allowing us to encode and retrieve episodes of our daily life. Since the seminal work of Scoville and Milner (1957), the hippocampal formation has been implicated in the acquisition of new memories. Based on findings from a vast number of studies over the last 50 years using diverse methods including lesion and neuroimaging experiments in animals and humans, there is now consensus that the hippocampus plays a vital role for episodic memory (O'Keefe and Nadel, 1978; Eichenbaum, 2000).
In contrast to these systems-level and whole-brain techniques, it has proved harder to identify a correlate of episodic memory at the level of individual hippocampal neurons. The defining quality of an episode is the conjunction of an item or event and aspects of its context such as spatial location and time. However, since O'Keefe and Dostrovsky (1971) discovered place cells, the bulk of single-unit data from freely moving animals has suggested a chiefly spatial role for hippocampal neurons (including representations of where it has been and where it is going, as well as the current position). Place cells, pyramidal cells from regions CA3 and CA1 of the rodent hippocampus, exhibit the striking quality that they seem to provide a representation of an animal's position in its environment: as the rat moves around, a cell will typically be silent, only emitting spikes when the animal's head enters the receptive field (place field) (Fig. 1). In the open environments that many experimenters prefer, and in the absence of particular task demands, activity is independent of the animal's orientation, stable between visits to the same position even across days, and robust to the removal of individual spatial cues (O'Keefe, 1976). Transportation of the animal to a different and sufficiently distinct enclosure typically results in a new representation being established: place fields change position relative to one another and radically alter firing rates. Remapping, as this effect is known, has been understood as a process by which the hippocampus generates independent codes to represent distinct spatial contexts (Wills et al., 2005). Far from being a uniquely rodent curiosity, place cells seem to be important to the wider function of the hippocampus, because cells with similar properties have been identified in a range of animals as disparate as birds, monkeys, and humans (Ekstrom et al., 2003).
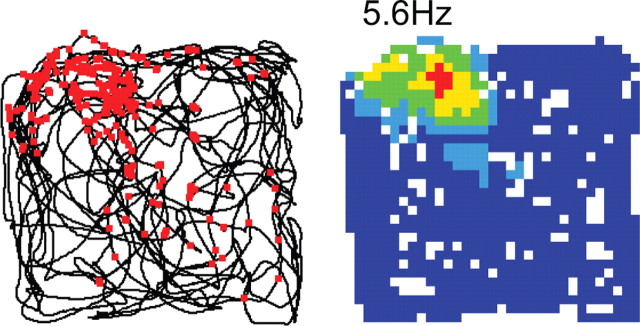
Figure 1.
A CA1 place cell (recorded by C.B.). From left to right, Rat's path is denoted by the continuous black line with action potentials superimposed (red dots); the recording was made in a 70 cm square enclosure. Rate map constructed from raw data. Peak rate is indicated above the map with bins of decreasing rate from “hot” to “cold.” White indicates unvisited bins.
While position is the most distinct correlate of place cell activity, a parallel body of work suggests how other factors might be encoded in addition to, or possibly in place of, the spatial code. Two examples are provided by Wood et al. (1999) and more recently Manns and Eichenbaum (2009). In the former case, rats moved around an open enclosure to perform a delayed-non-match-to-sample task, and in the latter, animals circled an annular maze encountering objects placed onto the track. Under these conditions, many hippocampal pyramidal cells encode nonspatial cues (e.g., presence of a specific odor) in addition to a primary spatial correlate (Manns and Eichenbaum, 2009). These cells exhibit conjunctive properties, for example, responding optimally to a particular combination of position and odor. Similar results have been observed when auditory fear conditioning was conducted while rats freely perambulated: place cells retained their spatial firing but at the same time firing became synchronized to the audible conditioned stimulus (Moita et al., 2003). Also, hippocampal recordings made from humans (patients with pharmacologically intractable epilepsy were asked to navigate in virtual reality) revealed that approximately a quarter of the cells characterized as place cells had conjunctive representations and were modulated by the subject's destination (Ekstrom et al., 2003).
Despite these indications that place cells can encode more than just spatial information, it has remained unclear how such conjunctive or alternative codes develop over time and how exactly they are related to behavior. Now an article by Komorowski et al. (2009) in The Journal of Neuroscience addresses this empirical gap and provides evidence that hippocampal cells that initially exhibit a purely spatial response ultimately represent an item in place. Furthermore, the development of that conjunctive representation is strongly correlated with the animals' ability to perform a task requiring assessment of item–position pairings.
The authors trained rats to move between two connected square environments (contexts). Within each environment were placed two pots distinguished both by odor and the digging media they contained (items). To earn a food reward, animals were required to dig in one pot and ignore the other. Both types of pot were present in each environment, but the rewarded pot varied between environments. Thus to perform correctly, the animal must discriminate based on the combination of pot and environment. The positions of the pots within each environment were counterbalanced but were irrelevant to the task.
Examining the periods when rats approached the pots, the authors distinguished two cell types from their CA3 and CA1 recordings: item–position cells, which responded optimally to one of the pots at one or both positions within a single context, and position-only cells, which responded optimally at one or both positions within a single context regardless of the pot type. Initially, rats performed at chance and the proportion of item–position cells was low (6.4%), but after training, performance exceeded 80% and the proportion of item–position cells reached 31.3%. In parallel, the proportion of position-cells did not change significantly, remaining ∼40%. During further training, the proportions of both cell types remained broadly stable.
Inspection of individual cells revealed that the additional item–position cells were drawn from the position-only population, which in turn was replenished by recruiting cells that had previously been silent. The implication that the increase in conjunctive coding is part of the neural representation required to solve the task is supported by strong positive correlations between measures of each rat's performance and the preponderance of item–position coding. More convincing is the observation that on error trials, when the animal dug in the wrong pot, position–item cells lost their specificity, effectively behaving as position-only cells. So, the activity of these cells effectively reflects the forthcoming behavioral response. The authors conclude that their data suggest that hippocampal neurons encode episodes in the form of item–context conjunctions.
Given the extensive literature regarding the spatial role of the hippocampus and the general desire to know whether and how place cells can support episodic-like memory, it would be interesting to examine the activity of these cells in a more obviously spatial setting. First, the environments used by Komorowski et al. (2009) were small (37 cm × 37 cm), and at the point at which pots were presented, the animal was constrained to be within half of this area. The effective environment then is only a little larger than the rat and on a similar scale to many place fields (Fig. 1). Second, the formal analysis of neuronal activity was limited to 1 s time windows initiated when the animals' noses entered the pots. This strategy is effective in standardizing behavior (the authors reliably discount behavioral changes as the source of the observed effect), but for the same reason it excludes perambulatory activity both near and away from the pots, which is typically associated with spatial responses. Example rate maps were provided in Figure 3 of Komorowski et al. (2009), but these were also limited to periods in which animals sampled the pots. Third, the statistical analysis treats context and position equivalently. Hence, neural activity limited to a single position within one environment is not distinguished from activity present across the entirety of a single environment. While these issues do not necessarily detract from the authors' conclusions, they do limit the scope of the findings. Further investigations are required to understand how the population of place cells representing an extensive environment are modified when an animal learns about discrete items within that environment.
One direction for future research would be to further disentangle spatial and nonspatial features in the environment. This paper asserts that the conjunctive responses of hippocampal neurons represent items in context: what and where. Plausibly though, the pots might be construed as purely spatial cues (where but not what). For example, the increasingly specific neural responses observed by the authors could be akin to the gradual divergence in place cell representations observed when rats are repeatedly exposed to subtly different environments (Lever et al., 2002). In this case, the hippocampus would be representing each combination of pot position and environment as a distinct space. A further parallel is evident with work conducted by Leutgeb et al. (2005), who showed that changes in the relative firing rates of cells can result from changes made to an animal's local environment; in contrast, gross changes to distal cues result in remapping.
Many systems-level experiments in humans indicate that the representation of an event's rich spatial and temporal context is the core feature of hippocampus-dependent memory. The findings by Komorowski et al. (2009) now provide evidence that single cells in the hippocampus represent the spatial context in conjunction with the items encountered and that this representation supports behavior. Furthermore, that the conjunctive code apparently develops from a purely spatial code. The results are entirely consistent with the theory that during first exposure to an environment a spatial map-like representation is formed in the hippocampus and items and events are then encoded onto that in their spatial context (O'Keefe and Nadel, 1978).
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. Oxford UP: 1978. The hippocampus as a cognitive map. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]