Abstract
We have recently demonstrated that systemic administration of 5-HT2C and 5-HT2A receptor antagonists significantly enhanced and impaired spatial reversal learning, respectively. In this study, the role of 5-HT2C and 5-HT2A receptor subtypes in the mediation of these opposing effects was further investigated with respect to neuroanatomical specificity. The roles of 5-HT2C and 5-HT2A receptors were examined within some of the brain regions implicated in cognitive flexibility, namely the orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), and nucleus accumbens (NAc), by means of targeted infusions of selective 5-HT2C and 5-HT2A receptor antagonists (SB 242084 and M100907, respectively). Intra-OFC 5-HT2C receptor antagonism produced dose-dependent effects similar to those of systemic administration, i.e., improved spatial reversal learning by reducing the number of trials (all doses: 0.1, 0.3, and 1.0 μg) and perseverative errors to criterion (0.3 and 1.0 μg) compared with controls. However, the highest dose (1.0 μg) showed a nonselective effect, as it also affected retention preceding the reversal phase and decreased learning errors. Intracerebral infusions of SB 242084 into the mPFC or NAc produced no significant effects on any behavioral measures. Similarly, no significant differences were observed with intra-OFC, -mPFC, or -NAc infusions of M100907. These data suggest that the improved performance in reversal learning observed after 5-HT2C receptor antagonism is mediated within the OFC. These data also bear on the issue of whether 5-HT2C receptor antagonism within the OFC might help elucidate the biological substrate of obsessive-compulsive disorder, offering the potential for therapeutic application.
Introduction
Prefrontal cortex (PFC)-mediated executive functions support the ability spontaneously to adapt behavior in response to changing situational demands, thus promoting behavioral flexibility. Without this top-down cognitive control, behavior controlled by other cortical and subcortical regions is more likely to be inflexible, dependent on simple sensory motor associations or habits (Miller and Cohen, 2001). Such inflexible behavior constitutes a common feature of various psychiatric afflictions, including schizophrenia, depression, and obsessive-compulsive disorder (OCD).
On the behavioral level, discrimination reversal learning is one of the laboratory tasks used to investigate translationally aspects of executive control and behavioral flexibility. Neuroanatomically speaking, converging evidence from a plethora of studies has implicated the orbitofrontal cortex (OFC) in efficient reversal learning, as damage to this region produces selective reversal deficits [human (Rolls et al., 1994; Hornak et al., 2004), monkey (Iversen and Mishkin, 1970; Jones and Mishkin, 1972; Dias et al., 1996; Clarke et al., 2008), rat (Schoenbaum et al., 2002; McAlonan and Brown, 2003; Boulougouris et al., 2007)]. The medial region of the PFC (mPFC) has been shown to mediate behavioral flexibility in attentional set-shifting tasks, requiring the updating of attentional biases or rules (Owen et al., 1991; Dias et al., 1996; Birrell and Brown, 2000; Ragozzino and Kesner, 2001). The striatum is also implicated in reversal learning: electrolytic lesions of the ventrolateral head of the caudate nucleus in macaques (Divac et al., 1967), excitotoxic lesions of the medial striatum in marmosets (Clarke et al., 2008), and nucleus accumbens (NAc) lesions in monkeys (Stern and Passingham, 1995) have been shown to produce reversal impairments. However, studies in rats have produced equivocal results that seem related to lesion location [e.g., NAc (Schoenbaum and Setlow, 2003) vs ventral or medial striatum (Ferry et al., 2000)] and the nature of the behavioral task [e.g., discrimination go/no-go (Ferry et al., 2000) vs rule reversal (Block et al., 2007)]. Finally, although amygdala lesions do not seem to disrupt reversal learning (Izquierdo and Murray, 2007; Clarke et al., 2008), a recent study demonstrated that basolateral amygdala lesions abolished OFC-induced reversal learning impairments (Stalnaker et al., 2007).
In neurochemical terms, preclinical and clinical research suggest that a dysregulation of serotonergic systems (5-HT) is involved in behavioral flexibility and response inhibition (for review, see Boulougouris and Tsaltas, 2008). Furthermore, 5-HT projections to the mPFC and NAc as well as indirect actions via 5-HT receptors regulating the ventral tegmental area, may be involved in executive processes including response inhibition (Robbins, 2000; Chudasama and Robbins, 2004; Winstanley et al., 2006).
We have recently demonstrated that systemic administration of 5-HT2C and 5-HT2A receptor antagonists showed significant effects, respectively, enhancement and impairment of spatial reversal learning (Boulougouris et al., 2008). These effects were observed in the early phase of reversal learning (i.e., perseveration stage). In this study, the role of 5-HT2C and 5-HT2A receptor subtypes in the mediation of these opposing effects on perseverative responding during spatial reversal learning are also investigated with respect to their neuroanatomical specificity. The role of 5-HT2C and 5-HT2A receptors were examined within the brain regions implicated in cognitive flexibility, namely the OFC, mPFC, and NAc, by means of targeted infusions of selective 5-HT2C and 5-HT2A receptor antagonists (SB 242084 and M100907, respectively).
Materials and Methods
Subjects
One hundred sixty-eight experimentally naive adult male Lister Hooded rats (Charles River) weighting 280–320 g at the start of the experiments were pair-housed under a reversed light cycle (lights on from 7:00 P.M. to 7:00 A.M.). Before the beginning of training, rats were handled for ≈5 min daily for 5 d and were put onto a food-restriction schedule (18 g of Purina laboratory chow per day). Water was available ad libitum, and testing took place between 1:00 P.M. and 4:00 P.M. 7 d per week. Table 1 presents the number of rats allocated to each experiment, the number of rats that were excluded from each experiment, the doses used, and the final number of rats in each group.
Table 1.
Summary of animals used in each experiment
| Experiment | Drug | Dose (μg) | Numberof rats | Number of rats excluded | Final n |
|---|---|---|---|---|---|
| 1 | SB 242084, OFC | Vehicle | 8 | 1, died on surgery | 7 |
| 0.1 | 7 | 7 | |||
| 0.3 | 8 | 1, cannula misplacement | 7 | ||
| 1.0 | 9 | 1, died on surgery | 8 | ||
| 2 | SB 242084, mPFC | Vehicle | 9 | 3, sickness | 6 |
| 0.3 | 8 | 2, sickness | 6 | ||
| 1.0 | 9 | 4, sickness | 5 | ||
| 3 | SB 242084, NAc | Vehicle | 9 | 9 | |
| 0.3 | 10 | 1, sickness | 9 | ||
| 1.0 | 9 | 9 | |||
| 4 | M100907, OFC | Vehicle | 8 | 8 | |
| 0.1 | 8 | 1, sickness | 7 | ||
| 0.3 | 8 | 2, sickness | 6 | ||
| 1.0 | 8 | 8 | |||
| 5 | M100907, mPFC | Vehicle | 8 | 2, died on surgery | 6 |
| 0.3 | 8 | 2, sickness | 6 | ||
| 1.0 | 8 | 8 | |||
| 6 | M100907, NAc | Vehicle | 9 | 9 | |
| 0.3 | 10 | 2, dysfunctional | 8 | ||
| 1.0 | 9 | 9 | |||
| Total | 164 | Total animals for analyses | 148 |
Surgery
Rats were anesthetized using isofluorane in oxygen and secured in a stereotaxic frame fitted with atraumatic earbars. Bilateral stainless-steel guide cannulae (22 gauge; Plastics One) were aimed dorsal to the target brain structure using standard stereotaxic techniques. The coordinates used were as follows (Paxinos and Watson, 1998): OFC: anteroposterior (AP), +3.2 from bregma; lateral (L), ±2.5 from bregma; dorsoventral (DV), −2.2 from dura; mPFC: AP, +3.0 from bregma; L, ±0.7 from bregma; DV, −2.0 from dura; NAc: AP, +1.7 from bregma; L, ±1.9 from bregma; DV, −2.2 from the skull. The incisor bar was set at −3.3 mm relative to the interaural line for a flat skull position. Three small screws and cranioplastic cement secured the guide cannulae to the skull. Removable stylets (Plastics One) were placed into the guide cannulae to prevent occlusion and were held in place with a screw-on dust cap. After surgery, the animals were housed individually and allowed 7–9 d of recovery.
Intracranial drug infusions
Infusions took place before each experimental session. The rats were held gently and habituated to the infusion procedure 1 d before behavioral training, where they were lightly restrained and the stylet was removed and then replaced. The following day, the behavioral task was introduced: rats received a drug or vehicle infusion (PBS) immediately before behavioral testing.
During intracranial infusions, rats were gently restrained while stainless-steel injectors (28 gauge; Plastics One) extending 2, 1.5, or 5 mm below the length of the guide cannulae were inserted into the OFC, mPFC or NAc, respectively. The injectiors were attached by polyethylene tubing (Portex) to 10 μl Hamilton syringes that were mounted on an infusion pump (Harvard Apparatus). One minute elapsed after inserting the injectors to the relevant brain structure before beginning the infusions of vehicle or drug (0.5 μl), after which the injector was left in place for 1 min to allow diffusion of the drug into the tissue surrounding the injector. The injector was then slowly removed, and the stylet was replaced.
Drugs
6-Chloro-5-methyl-1-[2(2methylpyridyl-3-oxy)-pyrid-5-yl carbamoyl] (SB 242084; Solvay) and R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (M100907) were tested in six different experiments. Before drug infusions, animals were divided in four (OFC infusions) or three (mPFC and NAc infusions) groups, matched for their performance during the discrimination phase before surgery. Each group received infusions of either SB 242084 (experiment 1: 0, 0.1, 0.3, 1.0 μg; experiments 2 and 3: 0, 0.3, 1.0 μg) or M100907 (experiment 4: 0, 0.1, 0.3, 1.0 μg; experiments 5 and 6: 0, 0.3, 1.0 μg). All drugs were infused daily immediately before the start of the behavioral task.
SB 242084 was dissolved in 25 mm citric acid and 8% cyclodextrine in 0.9% saline, and the pH was adjusted to 6.4 using 0.1 m NaOH. M100907 was dissolved in 0.01 m PBS and 0.1 m HCl, and the pH was adjusted to 6.4 using 0.1 m NaOH.
Histology
After the completion of behavioral testing, animals were given a lethal dose of sodium pentobarbitone (1.5 ml per rat; Euthatal, 200 mg/ml; Genus Express) and perfused transcardially with 0.01 m PBS followed by 4% paraformaldehyde. The brains were removed, postfixed in 4% paraformaldehyde for 24 h, and dehydrated in 20% sucrose in 0.01 m PBS overnight. Coronal sections of 60 μm were cut on a freezing microtome and mounted on double-subbed glass slides. They were then stained with cresyl violet and coverslipped with DePeX mounting medium (BDH). The sections were then used to verify cannulae placement. The location of the cannulae was mapped onto standardized sections of the rat brain, the cytoarchitectonic borders and nomenclature of which were taken from Paxinos and Watson (1998).
Behavioral procedure
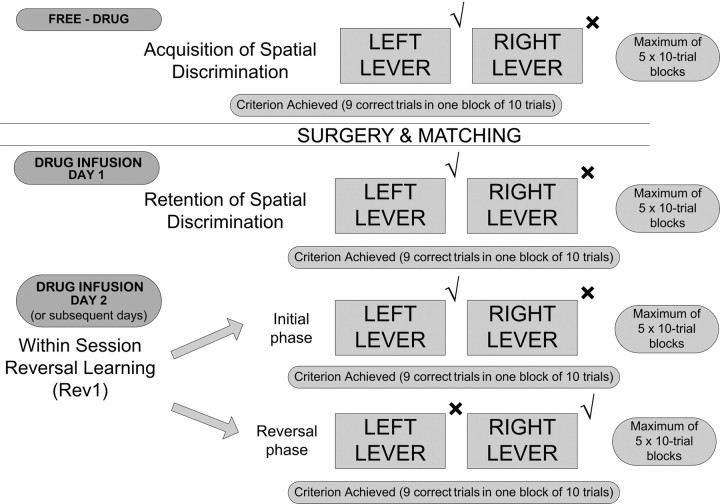
Rats were trained on the instrumental two-lever spatial discrimination and serial reversal learning task as described and illustrated previously (Boulougouris et al., 2007, 2008, 2009) (see Fig. 1). Briefly, rats were initially trained to nose-poke in the central magazine to trigger presentation of the retractable levers and to respond on them under a fixed ratio 3 (FR-3) schedule for food delivery (pretraining). The FR-3 schedule was used to preclude the possibility of reinforcing single accidental presses on the correct lever and to render the reversal task more difficult, as the change in reversal contingencies cannot be detected from a single lever press.
Figure 1.
Flow diagram of the behavioral procedure. Rats responded to levers under a FR-3 schedule to obtain a pellet reward. After surgery, drug infusions were given before each experimental session. The √ and × symbols indicate which lever was correct and incorrect at each stage. The correct lever was counterbalanced across rats.
Acquisition of spatial discrimination.
Training continued with the acquisition of a two-lever discrimination task. Now both levers were presented at trial onset, and the rat had to learn that three lever presses on only one of these levers would result in reward. Each session lasted 20 min and consisted of a maximum of five 10-trial blocks. Each trial began with the presentation of both levers and a visual stimulus [a lit light-emitting diode (LED)]. The lit LED was used as a distractor, and its location (left/right) varied from trial to trial according to a pseudo-random schedule so that the light was presented an equal number of times on each side for the session. Thus, the only stimulus with informational value for the discrimination at this phase was the spatial position of the retractable levers. Throughout the session, three lever presses on one lever (lever A) would produce a single pellet reward (correct responses) and the retraction of both levers, whereas three responses on lever B would result in lever retraction without reward delivery (incorrect responses). The position of the reinforced lever (left or right) was kept constant for each rat, but was counterbalanced between subjects.
Each rat had one training session per day and was trained to a criterion of nine correct trials in one block of 10 trials (binomial distribution p < 0.01, likelihood of attaining criterion in a 10-trial block). Once this criterion was reached, this initial discrimination phase was considered complete, and the animal was returned to the home cage. If the criterion was not achieved, this phase was repeated the next day until criterion attainment. Animals needed 1–3 d for criterion attainment during this phase.
Within-session serial reversal learning task.
In the next training session, reversal learning was introduced. By definition, reversal learning presupposes retention of a previously acquired discrimination. Accordingly, in the reversal session, animals were again exposed to the initial discrimination task described above (with the same lever rewarded as before: discrimination retention). This initial retention phase preceding reversal also comprised a maximum of five 10-trial blocks. Once the criterion of nine correct trials in a 10-trial block was achieved, the position of the reinforced lever was reversed (reversal phase). The reversal phase also consisted of a maximum of five 10-trial blocks. The learning criterion was the same as in the initial phase (nine correct trials in a 10-trial block). Animals always required more than one session to reach criterion on the reversal phase. Thus, they received multiple separate training sessions, the data of which were summed together to produce the final results. During these sessions, the initial contingency was determined by retention performance: for example, day 1: A+, B− (retention without reversal-criterion achieved); day 2: A+, B− (retention preceding reversal-criterion achieved), A−, B+ (reversal phase-criterion NOT achieved); day 3: A+, B− (retention preceding reversal-criterion achieved), A−, B+ (reversal phase-criterion achieved). Trials and incorrect responses to criterion would be added for days 2 and 3 in the example.
On day 3 of the example, the session began with the original A+, B− stimulus. This is because pilot studies had shown that if the session on day 3 began with the reversal contingency (A−, B+) of day 2, animals started responding to the lever A (having the A+, B− contingency in mind). After very few lever presses on the (nonreinforced) lever led the animals to extinguish lever pressing, the session resulted in omissions only. For this reason, we decided to use the previously acquired contingency (A+, B−) as an initial phase for reinforcing lever pressing before reversal contingency was introduced. In terms of analyses, we have three different phases: retention without reversal phase (A+, B−: animals have not experienced the reversal contingency), retention preceding reversal phase [animals have experienced the reversal contingency (A−, B+) the day before, but they need to respond according to the initial retention contingency (A+, B−) to get food], and reversal phase [after the previous phase, animals have achieved the criterion on the retention contingency (A+, B−) and they are required to adapt their responding to the new reversed contingency (A−, B+) to get food].
Given that the opposing effects of 5-HT2C and 5-HT2A receptors were observed in the first reversal only (Boulougouris et al., 2008), one reversal was given.
Statistical analysis
The main measures of the animals' ability to learn the discrimination and reversals were as follows: (1) the number of trials to criterion; (2) the total number of incorrect responses to criterion on completed (correct and incorrect) trials (i.e., one incorrect trial equals three incorrect responses); and (3) the total number of errors (i.e., incorrect trials) to criterion. The type of errors was also analyzed: six or more consecutive errors during the entire session were termed “perseverative errors” (i.e., reversal performance significantly worse than chance), whereas all other errors were termed “learning errors” (i.e., reversal performance above chance; an example is provided in the supplemental material, available at www.jneurosci.org). Additional secondary measures recorded for each trial were (4) the latency to respond, (5) the latency to collect the reward, and (6) the number of omissions.
Data for each variable were subjected to a repeated-measures ANOVA. Where significant interactions were detected, they were further explored through planned comparisons (contrast testing) to establish simple effects. For all comparisons, significant difference was assumed at p < 0.05. The between-subject factor was group (experiments 1 and 4, four levels: three different doses of each drug plus vehicle; experiments 2, 3, 5, and 6, three levels: two different doses of each drug plus vehicle), and the within-subject factors were initial retention phase without reversal, subsequent retention phase preceding reversal, or reversal phase. Perseverative and learning errors were subjected to one-way ANOVAs followed by contrast testing. The between-subject factor was group as described above.
It should be noted here that the analyses and figures provided in Results below are for the measures of trials to criterion and type of errors. Additional analyses and figures for the measure of incorrect responses to criterion are provided in the supplemental material (available at www.jneurosci.org).
Results
Histological results: experiments 1–6
Figure 2 shows a schematic reconstruction of the actual position of injector tips in the OFC, mPFC, and NAc. Animals were excluded from data analyses if the cannula position was not correct or if the cannula revealed tissue damage beyond local physical damage around the injector tract. Figure 3 presents photomicrographs of coronal sections taken from representative rats.
Figure 2.
Schematic diagrams showing the location of the injector tips in the OFC (experiments 1 and 4), mPFC (experiments 2 and 5), and NAc (experiments 3 and 6). Data are reconstructed from Paxinos and Watson (1998).
Figure 3.
Photomicrographs of coronal sections taken from representative rats: A, OFC; B, mPFC; C, NAc.
Behavioral results: performance before drug infusions
Before intracerebral drug infusions, the groups did not differ in the number of incorrect responses to reach performance criterion in the acquisition of spatial discrimination (F < 1; NS; data not shown).
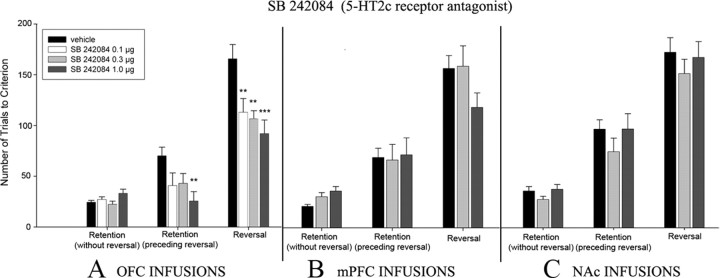
Experiment 1: effects of intra-OFC infusions of SB 242084 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA yielded significant main effects of group and phase (F (3,25) = 6.24, p = 0.0026 and F (2,50) = 128.89, p < 0.001, respectively) and a significant group × phase interaction (F (6,50) = 4.30; p = 0.0014) (Fig. 4 A). After planned comparisons, there were no significant differences between drug groups on the initial retention (without reversal) of the spatial discrimination acquired before drug administration. Although no significant differences between drug groups were noted on the subsequent retention preceding reversal phase, the highest dose of SB 242084 (1.0 μg) did significantly decrease trials to criterion (vehicle vs 1.0 μg: F (1,25) = 10.04; **p = 0.004) (Fig. 4 A). In the reversal learning phase, animals infused with SB 242084 exhibited a highly significant improvement of performance. Specifically, SB 242024 significantly reduced trials to criterion at all doses used compared with vehicle controls (vehicle vs 0.1 μg: F (1,25) = 8.42, **p = 0.008; vehicle vs 0.3 μg: F (1,25) = 10.6, **p = 0.003; vehicle vs 1.0 μg: F (1,25) = 17.56, ***p = 0.0003) (Fig. 4 A).
Figure 4.
A–C, Experiments 1–3. Number of trials to criterion through the retention phase (without reversal) (A), retention (initial) phase (preceding reversal) (B), and reversal phase (C). Data are presented as mean values ± SEM. Asterisks denote significant differences (ANOVA; ***p < 0.001; **p < 0.01) from vehicle controls.
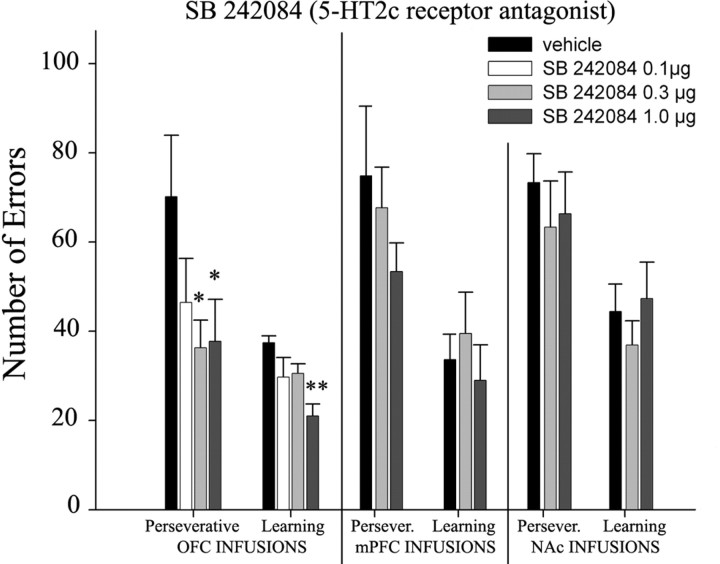
Perseverative versus learning errors
One-way ANOVAs showed that there was a marginally significant main effect of group in the perseveration stage (F (3,25) = 2.33; p = 0.098) and a significant main effect of group in the learning stage (F (3,25) = 5.62; p < 0.001). Animals infused with the two highest doses of SB 242084 (0.3 and 1.0 μg) made significantly fewer perseverative errors than controls in reversal phase (vehicle vs 0.3 μg: F (1,25) = 5.36, p = 0.029; vehicle vs 1.0 μg: F (1,25) = 5.23, p = 0.03) (Fig. 5). The highest dose (1.0 μg) also reduced the number of learning errors (vehicle vs 1.0 μg: F (1,25) = 16.56; p = 0.0004) (Fig. 5).
Figure 5.
Mean error score ± SEM of all groups (experiments 1–3) during each learning stage of reversal performance (perseveration and learning). Asterisks denote significant differences (ANOVA; *p < 0.05; **p < 0.001) from vehicle controls.
Experiment 2: effects of intra-mPFC infusions of SB242084 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA showed that there was no significant main effect of group (F (2,14) = 0.29; p = 0.756), but there was a significant main effect of phase (F (2,28) = 98.76; p < 0.001). No significant group × phase interaction was noted (F (4,28) = 2.20; p = 0.084) (Fig. 4 B). No significant differences between drug groups were noted on any experimental phase. However, it is noteworthy that the highest dose (1.0 μg) reduced the number of trials compared with controls, but this difference was nonsignificant (vehicle vs 1.0 μg: F (1,14) = 2.68; p = 0.124).
Perseverative versus learning errors
One-way ANOVAs showed that there was no main effect of group in either the perseverative stage (F (2,14) = 0.84; p = 0.45) or learning stage (F (2,14) = 0.44; p = 0.65) (Fig. 5).
Experiment 3: effects of intra-nAcc infusions of SB 242084 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA showed that there was no significant main effect of group (F (2,24) = 1.1; p = 0.348), but there was a significant main effect of phase (F (2,48) = 178.8; p < 0.001). No significant group × phase interaction was noted (F (4,48) = 0.3; p = 0.905) (Fig. 4 C). No significant differences between drug groups were noted on any experimental phase.
Perseverative versus learning errors
One-way ANOVAs showed that there was no main effect of group in either the perseverative stage (F (2,24) = 0.33; p = 0.72) or learning stage (F (2,24) = 0.65; p = 0.53) (Fig. 5).
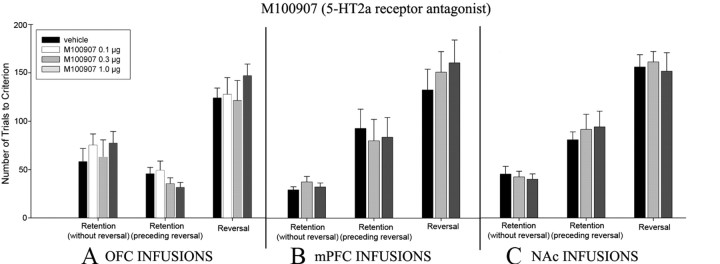
Experiment 4: effects of intra-OFC infusions of M100907 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA yielded that there was no significant main effect of group (F (3,25) = 0.46; p = 0.712), but there was a significant main effect of phase (F (2,50) = 71.79; p < 0.001). No significant group × phase interaction was noted (F (6,50) = 0.87; p = 0.53) (Fig. 6 A). No significant differences between drug groups were noted on any experimental phase.
Figure 6.
A–C, Experiments 4–6. Number of trials to criterion through the retention phase (without reversal) (A), retention (initial) phase (preceding reversal) (B), and reversal phase (C). Data are presented as mean values ± SEM.
Perseverative versus learning errors
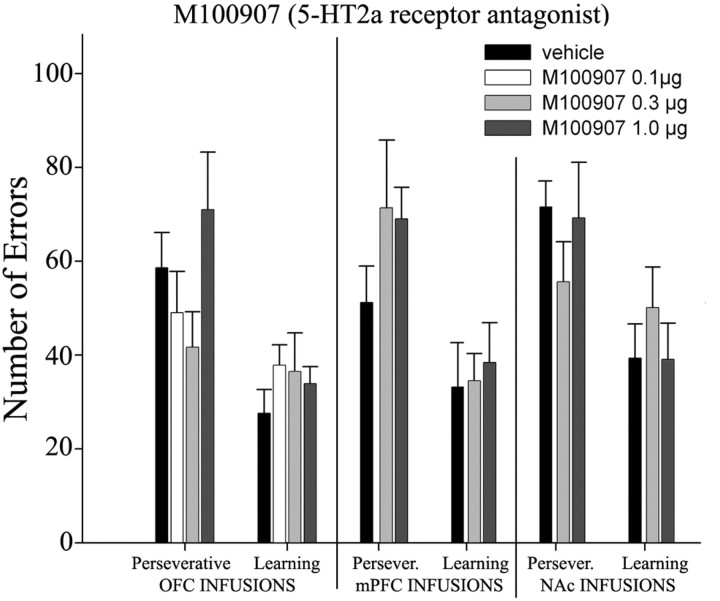
One-way ANOVAs showed that there was no main effect of group in either the perseverative stage (F (3,25) = 1.73; p = 0.19) or learning stage (F (3,25) = 0.774; p = 0.52) (Fig. 7).
Figure 7.
Mean error score ± SEM of all groups (experiments 4–6) during each learning stage of reversal performance (perseveration and learning).
Experiment 5: effects of intra-mPFC infusions of M100907 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA showed that there was no significant main effect of group (F (2,17) = 0.07; p = 0.935), but there was a significant main effect of phase (F (2,34) = 53.60; p < 0.001). No significant group × phase interaction was observed (F (4,34) = 0.59; p = 0.67) (Fig. 6 B). No significant differences between drug groups were noted on any experimental phase.
Perseverative versus learning errors
One-way ANOVAs showed that there was no main effect of group in either the perseverative stage (F (2,17) = 1.25; p = 0.31) or learning stage (F (2,17) = 0.114; p = 0.89) (Fig. 7).
Experiment 6: effects of intra-NAc infusions of M100907 in reversal learning
Number of trials to criterion
A repeated-measures ANOVA yielded that there was no significant main effect of group (F (2,23) = 0.07; p = 0.93), but there was a significant main effect of phase (F (2,46) = 79.85; p < 0.001). No significant group × phase interaction was observed (F (4,46) = 0.27; p = 0.89) (Fig. 6 C). No significant differences between drug groups were noted on any experimental phase.
Perseverative versus learning errors
One-way ANOVAs showed that there was no main effect of group in either the perseverative stage (F (2,23) = 0.86; p = 0.43) or learning stage (F (2,23) = 0.62; p = 0.55) (Fig. 7).
Discussion
Local blockade of the 5-HT2C receptors in the OFC produced dose-dependent effects similar to those of systemic administration (Boulougouris et al., 2008). Specifically, intra-OFC infusions of the 5-HT2C receptor antagonist SB 242084 improved spatial reversal learning by reducing the number of trials compared with vehicle controls. This improvement occurred in the early phases of reversal learning (i.e., perseverative phase) without affecting the late learning phase (apart from the highest dose), suggesting that it is a circumscribed effect, rather than generalized improvement. This facilitatory effect of 5-HT2C receptor blockade in the OFC was observed in reversal learning after all doses (0.1, 0.3, and 1.0 μg) of the 5-HT2C receptor antagonist in the number of trials to criterion. Although no significant differences were noted during the initial retention (without reversal) phase, the highest dose (1.0 μg), apart from its facilitatory effects on reversal learning by reducing both perseverative and learning errors, also appeared to promote the ability to retain associative relationships between stimuli and rewards once the reversal phase had been experienced (i.e., retention preceding reversal phase). These nonselective effects may be attributable to the improved reversal learning performance (i.e., increased flexibility) of this group, which in turn led to improved retention and less reversal trials. Another possibility might be that the 1.0 μg dose of the 5-HT2C receptor antagonist SB 242084 has been reported to offer decreased pharmacological specificity, or a preferential activation of a subpopulation of 5-HT2C receptors in different cellular compartments and/or phenotypes (Marek et al., 2005).
In contrast to intra-OFC infusions, intra-mPFC and intra-NAc infusions of SB 242084 did not produce any significant effects on any behavioral measure. This suggests that reversal learning facilitation after the 5-HT2C receptor antagonist is mediated by sites within the OFC (but not the mPFC or NAc).
As for SB 242084, the 5-HT2A receptor antagonist M100907 also failed to affect reversal when infused in mPFC and NAc. However, in contrast to SB 242084, intra-OFC infusion of M100907 also failed to affect significantly any behavioral measure at any stage of the experiment. This supports the hypothesis of a specific involvement of 5-HT2C receptors within the OFC in certain aspects of cognitive flexibility and response inhibition, particularly in those pertaining to compulsive responding. The absence of M100907 effects on this behavioral paradigm does not necessarily exclude the possibility that 5-HT2A receptor blockade participates in the control of behavioral flexibility (even at the doses used here). However, it may modulate aspects beyond the scope of this study such as impulsive responding (Winstanley et al., 2004).
Another issue that needs to be considered is the contribution of other brain structures in the mediation of behavioral flexibility and response inhibition. Pilot studies have shown that lesions of the dorsomedial striatum (but not the NAc) impair performance in the same spatial reversal learning paradigm (Castane et al., in press). Moreover, it has been reported that NMDA and cholinergic receptor blockade in the dorsomedial striatum also disrupts reversal learning (Palencia and Ragozzino, 2004; Ragozzino et al., 2009). In light of this evidence, the involvement of the 5-HT2C and 5-HT2A receptors in the dorsomedial striatum needs to be investigated further.
There are relatively few studies investigating the behavioral effects of serotonergic manipulations within the OFC. Roberts and colleagues have shown that OFC 5-HT depletion in marmosets impairs behavioral flexibility. In line with this, OFC 5-HT depletion, produced by 5,7-dihydroxytryptamine, resulted in a perseverative deficit in a detour-reaching (Walker et al., 2006) and a visual discrimination reversal learning task [marmosets (Clarke et al., 2004, 2005, 2007, 2008), rats (O. Lehmann, D. M. Eagle, D. H. Theobald, J. W. Dalley, T. W. Robbins, unpublished observations)]. Additionally, OFC 5-HT depletion has also been shown to produce stimulus-bound responding on other tests assessing flexible behavior such as conditioned reinforcement and discrimination extinction (Walker et al., 2009).
The results reported in the present study, namely that 5-HT2C receptor blockade reduced perseverative responding by promoting spatial reversal learning, is apparently at odds with the lesion studies mentioned above. However, they are in line with the findings of another study that assessed the effects of intra-OFC infusions of a 5-HT2C receptor antagonist in an animal model of OCD. In that study intra-OFC infusions of the 5-HT2C receptor antagonist RS 102221 decreased “surplus” lever pressing in the signal attenuation model, whereas a 5-HT2A receptor antagonist did not produce any significant effects (Flaisher-Grinberg et al., 2008).
This is not the only instance in which contrasting effects between 5-HT depletion and 5-HT receptor antagonism have been reported. For example, recent studies showed no effect of 5-HT depletion on the delayed discounting task (Winstanley et al., 2003), whereas the 5-HT1A receptor agonist 8-OH-DPAT (shown to turn off 5-HT release at autoreceptors) produces impulsive choice (Winstanley et al., 2005). Therefore, although the discrepancy could be attributed to task differences between lesion and antagonist studies (e.g., differences in the modalities of the reversal learning task used here and in Roberts and colleagues (Walker et al., 2006; Clarke et al., 2004, 2005, 2007, 2008): object vs spatial response reversal), such explanations would appear rather superficial. A more interesting hypothesis could be that incomplete 5-HT depletion from OFC may result in 5-HT2C receptor supersensitivity [as may occur in OCD (Graf et al., 2003; Yamauchi et al., 2004)], a possibility that could perhaps be investigated through infusions of 5-HT2C and 5-HT2A receptor antagonists in 5-HT-depleted animals. However, the result may well depend on a more complex interaction among 5-HT receptors that may leave the antagonist to boost 5-HT neurotransmission in the OFC. One way of addressing this speculative possibility is to examine the regional distribution and function of 5-HT receptor subtypes in this structure, which, to our knowledge, has not yet been done. It seems very likely, based on other functional studies of OFC, that this structure contains many 5-HT receptor subtypes (e.g., 5-HT1 postsynaptic, 5-HT1B, 5-HT2A and 5-HT2C, 5-HT4, 5-HT6 etc.). Unfortunately, there are no studies of ultrastructural neuronal localization in OFC, although there are in the mPFC; for example, Liu et al. (2007) showed how the 5-HT2A and 5-HT2C receptors are localized on different classes of GABA-inhibitory and glutamatergic neurons. An important new study by Calcagno et al. (2009) showed that there is a balance of 5-HT2A and 5-HT2C receptor activity in this region, which regulates the impulsivity response to glutamate manipulations, presumably via opposite effects on pyramidal cells. Unfortunately, we cannot find any evidence of an opposite effect of the 5-HT2A receptor antagonist M100907 intra-OFC, although we have shown that such an effect occurs with systemic administration (Boulougouris et al., 2008).
An understanding of how the 5-HT2C antagonist benefits reversal learning will ultimately depend on a closer understanding of how these receptors affect neuronal function within the OFC. As mentioned above, Liu et al. (2007) showed that these receptors definitely occur in certain parts of the rat PFC and have characteristic neuronal locations: this may suggest a similar pattern in the OFC and remains an issue to be addressed in future. One possibility is an involvement of 5-HT6 receptors, as it has been shown that 5-HT6 receptor antagonists also enhance reversal learning, although they appear to do so by affecting monoamines and acetylcholine release in the cortex (Hatcher et al., 2005). Another possibility worth considering arises from the finding that 5-HT2C antagonists enhance neuronal activity in the dorsal raphé, although this has so far been demonstrated to depend on the descending influence of the habenula rather than the OFC (Sharp et al., 2007). If this regulation also results from descending influences from mPFC (Amat et al., 2005), then this may imply a descending modulation of the dorsal raphé by OFC 5-HT2C receptors. Such an influence could actually enhance dorsal raphé activity, which would help to explain the apparent paradox between our findings and the established effects of OFC 5-HT depletion on reversal learning.
Reversal learning and OCD
The present findings may be relevant to various neuropsychiatric disorders in which inflexible behavior is a feature. Although OCD patients are not markedly impaired on simple reversal learning that has been associated in animal studies with damage to the OFC and to 5-HT-modulated mechanisms there, they have impairments in other tasks sensitive to OFC function such as alternation learning, a task related to reversal learning (Freedman et al., 1998). They also show impairments on laboratory tests of frontal lobe function involving response shifting and inhibitory processing that correlate with the severity of their symptoms (Veale et al., 1996; Rosenberg et al., 1997; Schmidtke et al., 1998; Hollander and Rosen, 2000).
The serotonergic system is heavily implicated in OCD, for example, via the therapeutic effects of the selective serotonin reuptake inhibitors (SSRIs) (Baumgarten and Grozdanovic, 1998; El Mansari and Blier, 2006) and the 5-HT2 receptor families in the pathophysiology of OCD as well as in the mediation of the antiobsessive effects of SSRIs (for discussion, see Boulougouris et al., 2008). Neuroanatomically speaking, OFC is also implicated in reversal learning and OCD, since abnormally reduced activation of the lateral OFC was recently reported during reversal learning in OCD patients and their clinically unaffected close relatives, supporting the existence of an underlying previously undiscovered endophenotype for this disorder (Chamberlain et al., 2008). Under these lines of evidence, the present finding that the anticompulsive effect of 5-HT2C receptor blockade is neuroanatomically specific and mediated within the OFC is critical for the clinical setting, as it bears on the issue of whether 5-HT2C receptor antagonism within the OFC might help understand the biological substrate of a number of neuropsychiatric disorders in which cognitive deficits are a feature (including schizophrenia, depression, and OCD) and offer the potential for additional therapeutic advances. The suggestion that blockade of 5-HT2C receptors in the OFC might alleviate obsessive-compulsive symptomatology is in accordance with the hypersensitivity of the 5-HT2C receptors noted in OCD patients (de Leeuw and Westenberg, 2008) and the exacerbation of their symptoms after activation of the same receptors (for discussion, see Boulougouris et al., 2008). However, a recent study assessing the effects of chronic SSRI administration in rodents has suggested that the anticompulsive effects of the SSRIs is mediated by enhanced 5-HT release in the OFC that activated normosensitive postsynaptic 5-HT2 receptors (El Mansari and Blier, 2006).
Compulsivity versus impulsivity
The orderly effects described in this study with respect to persistent behavior are of great interest in terms of the pharmacology of the 5-HT2 receptors. Furthermore, they acquire additional behavioral and clinical importance because they define a pattern that is quite opposite to that observed in corresponding studies of measures of impulsive responding. Specifically, it has been shown previously that it is the 5-HT2A receptor antagonist that reduces impulsive responding in the 5CSRTT, whereas the 5-HT2C receptor antagonist exacerbates it (Winstanley et al., 2004). These opposing effects on impulsivity have been shown to be mediated in the NAc, but not in the OFC, prelimbic cortex, or infralimbic cortex (Robinson et al., 2008). Moreover, intra-mPFC M100907 mimicked the effects of its systemic administration in opposing changes in extracellular glutamate induced by the NMDA antagonist CPP [3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid] and attentional performance deficits in the 5CSRTT, indicating that 5-HT2A receptors of the mPFC are mainly involved (Ceglia et al., 2004; Carli et al., 2006). These reports, combined with our finding that the enhancing effects of 5-HT2C receptor antagonism in reversal learning is mediated in the OFC, suggest that it is possible to dissociate, neuroanatomically and neurochemically, impulsive from compulsive behavior in the relationship of which sustains much debate in the psychiatric literature relevant to OCD and attention-deficit hyperactivity disorder.
Footnotes
This work was supported by a programme grant from the Wellcome Trust (076274/4/Z/04/Z) to T.W.R. The Behavioural and Clinical Neuroscience Institute was supported by a joint award from the Medical Research Council and the Wellcome Trust. V.B. was supported by the Domestic Research Studentship, the Cambridge European Trusts, the Bakalas Foundation Scholarship, and the Oon Khye Beng Ch'ia Tsio Studentship from Downing College. We thank Jeffrey C. Glennon for providing us the M100907 and David Theobald for his assistance.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z. Role of serotonin in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998:13–20. [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Calcagno E, Carli M, Baviera M, Invernizzi RW. Endogenous serotonin and serotonin2C receptors are involved in the ability of M100907 to suppress cortical glutamate release induced by NMDA receptor blockade. J Neurochem. 2009;108:521–532. doi: 10.1111/j.1471-4159.2008.05789.x. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DH, Robbins TW. Selective lesions of the dorsomedial striatum impair spatial reversal learning in rat. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.02.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW. The 5-HT receptor antagonist M100,907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem. 2004;91:189–199. doi: 10.1111/j.1471-4159.2004.02704.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw AS, Westenberg HG. Hypersensitivity of 5-HT2 receptors in OCD patients. An increased prolactin response after a challenge with meta-chlorophenylpiperazine and pre-treatment with ritanserin and placebo. J Psychiatr Res. 2008;42:894–901. doi: 10.1016/j.jpsychires.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Blier P. Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:362–373. doi: 10.1016/j.pnpbp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal–thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Klavir O, Joel D. The role of 5-HT2A and 5-HT2C receptors in the signal attenuation rat model of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2008;11:811–825. doi: 10.1017/S146114570800847X. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cereb Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Graf M, Kantor S, Anheuer ZE, Modos EA, Bagdy G. m-CPP-induced self-grooming is mediated by 5-HT2C receptors. Behav Brain Res. 2003;142:175–179. doi: 10.1016/s0166-4328(02)00404-7. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DN. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology (Berl) 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Hollander E, Rosen J. Impulsivity. J Psychopharmacol. 2000;14:S39–S44. doi: 10.1177/02698811000142S106. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–78. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus—reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Ed 2. Sydney: Academic; 1998. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Kesner RP. The role of rat dorsomedial prefrontal cortex in working memory for egocentric responses. Neurosci Lett. 2001;308:145–148. doi: 10.1016/s0304-3940(01)02020-1. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Dick EL, O'Hearn KM, Sweeney JA. Response-inhibition deficits in obsessive-compulsive disorder: an indicator of dysfunction in frontostriatal circuits. J Psychiatry Neurosci. 1997;22:29–38. [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K, Schorb A, Winkelmann G, Hohagen F. Cognitive frontal lobe dysfunction in obsessive-compulsive disorder. Biol Psychiatry. 1998;43:666–673. doi: 10.1016/s0006-3223(97)00355-7. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the “post”: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Stern CE, Passingham RE. The nucleus accumbens in monkeys (Macaca fascicularis). III. Reversal learning. Exp Brain Res. 1995;106:239–247. doi: 10.1007/BF00241119. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. Eur J Neurosci. 2006;23:3119–3123. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex. 2009;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Tatebayashi T, Nagase K, Kojima M, Imanishi T. Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacol Biochem Behav. 2004;78:683–689. doi: 10.1016/j.pbb.2004.05.003. [DOI] [PubMed] [Google Scholar]