FIGURE 2.
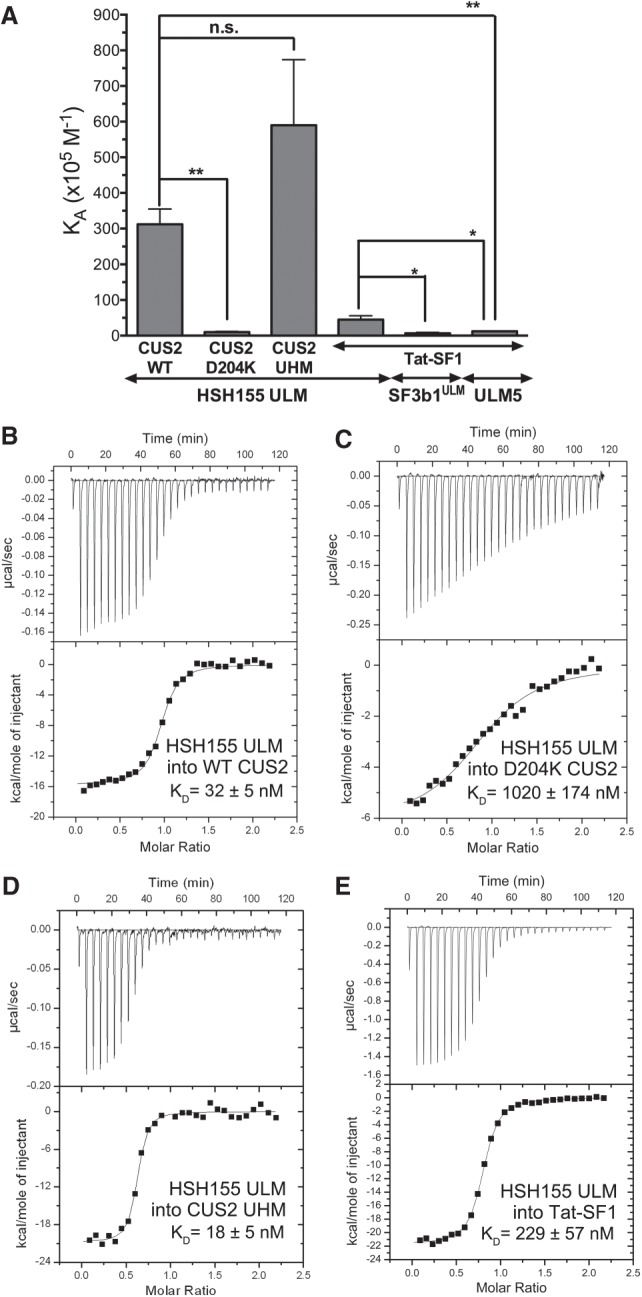
Cus2 and its UHM interact with the ULM of Hsh155 with high affinity in vitro. (A) Apparent equilibrium affinities (KA) of Hsh155 ULM (residues 95–109) binding to full-length Cus2 (isotherm B), D204K mutant Cus2 (isotherm C), Cus2 UHM (isotherm D, residues 177–269), or Tat-SF1 (isotherm E, residues 1–360). The KA’s of the ULM-containing SF3b1 region (SF3b1ULM, residues 190–344) or SF3b1 ULM5 (residues 333–351) binding Tat-SF1 were determined under matching conditions (Loerch et al. 2018) and are shown for comparison. Unpaired, two-tailed t-tests with Welch's correction: (*) P < 0.05, (**) P < 0.007. (B–E) Representative isotherms of three replicates for each of the indicated pairs of proteins or protein domains. The average fitted KA’s and SDs are plotted in A and given in Table 1.
