Abstract
Microglia were recently shown to play unexpected roles in normal brain development and adult physiology. This has begun to dramatically change our view of these resident “immune” cells. Here, we briefly review topics covered in our 2011 Society for Neuroscience minisymposium “The Role of Microglia in the Healthy Brain.” This summary is not meant to be a comprehensive review of microglia physiology, but rather to share new results and stimulate further research into the cellular and molecular mechanisms by which microglia influence postnatal development, adult neuronal plasticity, and circuit function.
Introduction
Microglia are key players in brain injury and disease (for review, see Kreutzberg, 1996; Ransohoff and Perry, 2009; Kettenmann et al., 2011). However, their role in the intact postnatal brain has remained elusive. “Resting” microglia are extremely dynamic in vivo, perpetually changing their morphology by extending and retracting highly motile processes on a time scale of minutes (Davalos et al., 2005; Nimmerjahn et al., 2005). This unexpected finding led to a series of discoveries suggesting potential roles of microglia in postnatal development, adult neuronal plasticity, and circuit function. Here, we review these findings and discuss their implications for normal brain function (see Fig. 1 for a schematic overview of microglial behavior in the healthy brain).
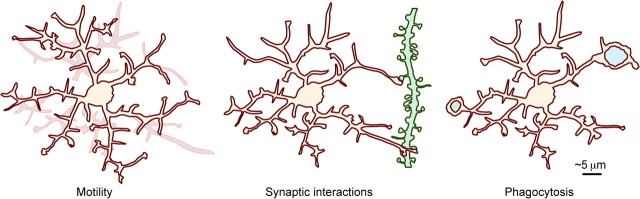
Figure 1.
Overview of microglial behavior in the healthy brain. Highly motile microglial processes continuously remodel their local environment (left), structurally and functionally interact with synaptic elements (middle; dendritic branch and spines, green) through direct contacts and exchanges of molecular signals, and contribute to restructuring of neuronal circuits by phagocytosing synaptic elements and newborn cells (right; cellular inclusions, blue and green). Microglial morphology and behavior display variability across CNS regions and stages of the lifespan.
Postnatal development: microglial phagocytosis of extranumerary synapses
The first 2 weeks of postnatal development are a period of remarkable plasticity with new synapses being actively formed and remodeled. Initially, neurons make far more synaptic connections than are maintained in the mature brain. During an activity-dependent developmental process termed synaptic pruning, a large number of these immature synapses are permanently eliminated while a subset of synapses are maintained and strengthened (Katz and Shatz, 1996; Hua and Smith, 2004; Huberman et al., 2008). Surprisingly, the cellular and molecular mechanisms by which specific synapses are eliminated have remained elusive.
Emerging evidence implicates microglia as key players in developmental synaptic pruning. Process-bearing “activated” microglia have been observed in several brain regions during postnatal synaptic remodeling including thalamus, cerebellum, olfactory bulb, and hippocampus (Perry et al., 1985; Dalmau et al., 1998; Fiske and Brunjes, 2000). Moreover, recent in vivo imaging and high-resolution electron microscopy (EM) studies in healthy mouse cortex revealed that fine processes of microglia actively associate with dendritic spines in an experience-dependent manner, suggesting that microglia play an active role in remodeling synaptic circuits (Wake et al., 2009; Tremblay et al., 2010). In addition, recent research revealed that synapse development is impaired in mice deficient in CX3CR1, a chemokine receptor specific to microglia that binds to the chemokine fractalkine (CX3CL1) expressed by neurons (Harrison et al., 1998; Jung et al., 2000; Ransohoff et al., 2009). CX3CR1 knock-out mice have a significant reduction in the density of microglia during the postnatal period and exhibit transient defects in synaptic connectivity and plasticity in the postnatal hippocampus (Paolicelli et al., 2011). Although it is unclear whether or how microglia-specific CX3CR1 mediates synaptic pruning, these findings suggest that disruptions in microglia number and/or function during the early postnatal period can impair synapse development and plasticity.
Are microglia required for the permanent elimination of extranumerary synapses? And if so, what are the underlying molecular mechanisms? These questions were directly investigated in the developing mouse retinogeniculate system, a classic model for studying developmental synapse elimination. During the first week of postnatal development, retinogeniculate synapses undergo a dramatic remodeling process called eye-specific segregation, which involves the pruning or elimination of inappropriate binocular retinal ganglion cell (RGC) inputs and elaboration and strengthening of the remaining synapses in the correct eye-specific region of the dorsal lateral geniculate nucleus (dLGN) (Campbell and Shatz, 1992; Jaubert-Miazza et al., 2005; Hooks and Chen, 2006; Stevens et al., 2007; Huberman et al., 2008). Using a combination of high-resolution imaging and immuno-EM, microglia were found to engulf presynaptic RGC inputs during the peak pruning period in dLGN. Moreover, genetic or pharmacological disruptions in microglia-mediated engulfment resulted in sustained functional deficits in synaptic pruning and synaptic connectivity (Schafer et al., 2010, 2011). Recent research suggests that microglia-mediated engulfment of developing synapses is mediated by an interaction between the phagocytic complement receptor expressed on the surface of microglia and its ligand C3, a complement protein that is enriched at developing synapses (Schafer et al., 2010, 2011). Indeed, the classical complement cascade was recently implicated in the developmental elimination of CNS synapses (Stevens et al., 2007), but the mechanism by which the complement eliminates specific synapses has remained unknown. In the immune system, C3 opsonizes the surface of cells/debris and “tags” them for elimination by phagocytic macrophages that express C3 receptors (i.e., C3R/cd11b) (Carroll, 2004; van Lookeren Campagne et al., 2007). Given that C3 expression and synaptic localization are tightly correlated with the peak of synaptic remodeling in the dLGN (Stevens et al., 2007), these new findings suggest a model in which C3 tags extranumerary synaptic inputs for elimination by microglia via C3-mediated phagocytosis (Schafer and Stevens, 2010). Interestingly, engulment of dendritic spines by microglia has also been observed in other brain regions during synaptic remodeling (Tremblay et al., 2010; Paolicelli et al., 2011), suggesting that complement-dependent synaptic pruning by microglia may be a common mechanism by which synaptic connections are physically eliminated during development. Together, this work along with future studies will be important for understanding mechanisms by which microglial cells control normal brain wiring with consequences for identifying molecular and cellular targets involved in diseases of the CNS.
Adult circuit plasticity and function
Microglial phagocytosis of apoptotic newborn neurons
Phagocytosis is the process of terminal removal of cellular debris by phagocytes. In vertebrates, phagocytosis is performed mostly by macrophages and other specialized innate immune cells by engulfing the cellular debris in phagosomes, membrane protrusions that then fuse with lysosomes for terminal degradation. Phagocytosis of bacteria during infections, or phagocytosis of necrotic and apoptotic cells during brain development, neurodegenerative diseases, and senescence, is essential for the maintenance of tissue homeostasis throughout the lifespan. Contrary to phagocytosis of necrotic cells, phagocytosis of apoptotic cells is highly efficient, prevents the spillover of cellular contents, and elicits anti-inflammatory responses (Savill et al., 2002; Gregory and Pound, 2011).
In the adult brain, phagocytosis of apoptotic debris is performed by microglia, the resident macrophages (for review, see Neumann et al., 2009). Although extensively studied, the overall contribution of microglia to brain diseases has been difficult to pinpoint. While inflammation is detrimental, and proinflammatory cytokines are excitotoxic (Pickering et al., 2005), phagocytosis of apoptotic debris is beneficial because it reduces the secretion of proinflammatory cytokines (Magnus et al., 2001), chemoattractants, and the migration of T lymphocytes (Chan et al., 2006). However, inflammation itself activates microglial phagocytosis, which then induces apoptotic neuronal death, at least in vitro (Neher et al., 2011). To further investigate microglia's role in this context, we resorted to investigating microglial phagocytosis in the adult hippocampal neurogenic cascade, in which apoptosis occurs in the absence of inflammation (Monje et al., 2003).
In the adult hippocampus, stem and progenitor cells persist in the subgranular zone (SGZ) and give rise to newborn granule cells that maturate and integrate into the hippocampal circuitry over a period of a month (Kempermann et al., 2004). While these newborn cells participate in some forms of learning and memory, mood regulation, and fear conditioning (Kempermann, 2008; Ming and Song, 2011), the majority are pruned early during their development and undergo apoptosis in the first few days of cell life. In this basal condition, phagocytosis is performed by ramified, unchallenged microglia through terminal or en passant branches forming “ball-and-chain” structures (Sierra et al., 2010), in contrast to the phagocytosis by amoeboid microglia observed during neurodegeneration (Kettenmann, 2007). Furthermore, phagocytosis by unchallenged microglia is highly efficient, as shown by the high proportion of apoptotic cells completely engulfed (phagocytic index, >90%), the proportion of microglia engaged in engulfing (phagocytic capacity, >35%), and the time to completely eliminate apoptotic cells (clearing time, 1.2–1.5 h) (Sierra et al., 2010). The consistent proportion of apoptotic cells not engulfed by microglia (<10%) is compatible with a delay between the onset of the apoptotic program and the release and exposure of the “find me” and “eat me” signals that prime the local unchallenged microglia to execute phagocytosis. These results also suggest that prior activation of microglia via inflammatory challenge is not a prerequisite for phagocytosis. Moreover, although bacterial lipopolisaccharides (LPS) induced apoptosis in the SGZ (Sierra et al., 2010), it remains unknown whether the proapoptotic effect of LPS is due to excitotoxic properties of proinflammatory cytokines (Pickering et al., 2005) or to direct activation of microglial phagocytosis (Neher et al., 2011). However, LPS does not alter the phagocytic index (Sierra et al., 2010), as would have been expected if microglial phagocytosis were responsible for apoptosis. More importantly, LPS doubles the phagocytic capacity (>80%) by rising the number of phagocytic pouches per microglia (Sierra et al., 2010). Overall, these data suggest a high phagocytic potential of unchallenged microglia that can be even further enhanced during neurodegeneration.
Regulation of microglia–synapse interactions by neuronal activity
Microglia–synapse interactions in the mature CNS were first described under pathological conditions. The work of Blinzinger and Kreutzberg (1968) revealed that after facial nerve axotomy in the rat, activated microglia can participate in “synaptic stripping,” the separation of presynaptic axon terminals from postsynaptic neuronal cell bodies or proximal dendrites by intervening glial processes. However, another study showed that inhibiting the proliferation of activated microglia had no effect on synaptic stripping after facial nerve axotomy (Kalla et al., 2001). More recently, MeCP2-deficient microglia from a Rett syndrome mouse model were shown to release high levels of excitotoxic glutamate in vitro, which induced abnormal changes in dendritic elements such as stunted and beaded morphologies, disrupted microtubules, and reduced numbers of postsynaptic densities (Maezawa and Jin, 2010). Given this contradictory evidence, the precise role of microglia in synaptic interactions has remained unclear (Perry and O'Connor, 2010).
To address this issue, we recently examined dynamic interactions between highly motile microglial processes and synaptic structures under nonpathological conditions in adult mouse visual cortex using two-photon in vivo imaging (Wake et al., 2009). In this study, microglial processes were found to interact with axon terminals and dendritic spines in a transient manner, for an average of ∼5 min and at a rate of approximately one microglial contact per hour. Reducing neural activity by enucleating both eyes induced retraction of microglial processes over 6 to 8 h. Lowering body temperature to 32°C or injecting the sodium channel blocker tetrodotoxin binocularly produced milder effects, reducing the frequency of microglial contacts with axon terminals to a rate of ∼0.5 microglial contact per hour while leaving the basal motility of microglial processes (∼0.3 μm/min) unchanged. Direct contacts between microglial processes and excitatory synapses were confirmed with immuno-EM. These experiments revealed that both presynaptic and postsynaptic elements could simultaneously receive microglial contact (Wake et al., 2009). During ischemia, induced by photochemical occlusion of the middle cerebral artery, microglial contacts with axon terminals were prolonged, lasting up to 120 min, and sometimes resulted in the disappearance of contacted terminals. At the ultrastructural level, microglial contacts with excitatory synapses appeared more extended after ischemia (Wake et al., 2009).
Together, these observations suggest that periodical interactions between microglia and synapses exist in the absence of pathological insult and may contribute to synaptic elimination and/or stripping during brain injury and disease. The mechanisms underlying the structural interactions between microglia and synapses, however, are still unknown and could involve, for example, major histocompatibility complex class I molecules, as is the case in classical immune recognition (Cullheim and Thams, 2007; Shatz, 2009).
Regulation of neuronal activity by microglia–astrocyte–synapse interactions
Until the discovery that astrocytes can respond to synaptic glutamate by releasing gliotransmitters that modulate synaptic transmission, fine-tuning of neuronal activity was thought to be purely based on neuronal interactions (for review, see Halassa and Haydon, 2010; Perea and Araque, 2010). Whether microglia influence synaptic transmission in similar ways is unclear. Under physiological conditions, microglial cells are present in all regions of the adult brain (Lawson et al., 1990) and are closely associated with both neurons and astrocytes (Nimmerjahn et al., 2005; Wake et al., 2009; Tremblay et al., 2010). Microglial processes are highly dynamic, reacting rapidly to modifications of their environment (Davalos et al., 2005; Nimmerjahn et al., 2005). Receptors expressed on microglial membranes are thought to allow sensing of neuronal activity and/or communication with astrocytes (Pocock and Kettenmann, 2007). Thus, microglia display functional features of synaptic partners.
We found that activation of microglia achieved by application of LPS onto acute mouse hippocampal slices enhanced the frequency of the spontaneous EPSCs in few minutes. The synaptic effect of this microglial activation was prevented by NBQX-mediated blockade of AMPA transmission, but not by NMDA or GABAA receptor antagonists (Ben Achour et al., 2010). This indicates that microglial activation increases the frequency of AMPAergic synapses and that microglial cells are capable of modulating neuronal activity.
We next investigated the intermediates between microglial activation and modulation of neuronal activity. We found that the effect of microglial activation was still present in mice deficient for TNFα or NOS2, but abolished by application of purinergic antagonists, specifically P2Y1 receptor antagonist (Ben Achour et al., 2010). This indicates that microglial activation likely acts via ATP and P2Y1 receptor. In the hippocampus, P2Y1 receptors are only expressed by interneurons and astrocytes. To functionally test the involvement of astrocytes in this process, we impaired their function using fluoroacetate (FAC), a compound commonly used to block the astrocytic glutamine cycle (Panatier et al., 2006; Henneberger et al., 2010). FAC treatment blocked the LPS-induced increase in EPSC frequency without significantly changing basal neuronal activity. Stimulation of astrocytes by a specific P2Y1 receptor agonist enhanced the frequency of EPSC and therefore mimicked the effect of microglial activation, even in brain slices from Pu1 mice devoid of microglia. This indicates that purinergic signaling onto astrocytes acts downstream of microglial activation to regulate EPSCs. Because activation of astrocytic P2Y1 receptor is known to trigger the release of glutamate (Domercq et al., 2006; Zeng et al., 2008), which binds to neuronal receptors such as mGluR receptors to modulate AMPAergic synapses (Fiacco and McCarthy, 2004; Perea and Araque, 2007), we applied the mGluR5 antagonist MPEP. MPEP abolished the effect of microglial activation and prevented the P2Y1-induced EPSC frequency increase (O. Pascual, S. Ben Achour, P. Rostaing, A. Triller, and A. Bessis, unpublished observations). This suggests that mGluR signaling acts downstream of P2Y1 receptors, probably influencing neurons as shown previously.
Together, these data support a model in which activation of microglia induces a rapid production of ATP. Microglial ATP then recruits astrocytes to amplify ATP production and release glutamate. Astrocytic glutamate increases EPSC frequency through neuronal mGluR5. Thus, microglia might act as a genuine regulator of neurotransmission and upstream partner of astrocytes.
Microglial reorganization of neuronal circuits
Beyond mediating fast changes in neuronal activity, a recent study also suggested that microglia participate in slower, experience-dependent remodeling and elimination of synapses in the mature healthy brain (Tremblay et al., 2010). Specifically, it was found at the ultrastructural level that the vast majority of microglial processes (∼94%) in adolescent mouse visual cortex directly juxtaposed neuronal and astrocytic excitatory synaptic elements, including synaptic clefts in situ. These interactions generally occurred en passant, without any morphological evidence of specialization. Nevertheless, coated pits on either side of microglia–synapse interfaces were occasionally observed, and a three-dimensional reconstructed microglial terminal process displayed finger-like protrusions that wrapped around a dendritic spine contacted by an axon terminal (Tremblay et al., 2010).
Furthermore, in vivo two-photon imaging revealed that microglia appeared to preferentially contact small dendritic spines. During microglial interaction, these spines transiently expanded, similar to synaptically active spines in vitro (Alvarez and Sabatini, 2007), with small spines showing the most pronounced changes. However, over the course of 2 d contacted spines disappeared (∼24%) more often than noncontacted spines (∼7%). Only small spines became eliminated (Tremblay et al., 2010). Therefore, despite an extensive seemingly random sampling of the brain environment, microglial processes seem to preferentially target a subset of structurally dynamic and transient dendritic spines.
This surprising specificity suggested that microglia might perceive the functional state of synapses (for review, see Pocock and Kettenmann, 2007) and contribute to plastic changes, potentially through remodeling of extracellular spaces and phagocytic elimination of synaptic elements (Tremblay and Majewska, 2011). Indeed, microglial processes were found to be surrounded by pockets of extracellular space of various sizes and shapes. Additionally, ∼30% of microglial processes were found to show phagocytic specializations and cellular inclusions containing material that in some cases resembled axon terminals and dendritic spines (Tremblay et al., 2010). Microglia remodeling of the extracellular matrix is thought to be mediated by secretion of metalloproteases and tissue-type plasminogen activator (for review, see Nakanishi, 2003). Both factors have been shown to promote dendritic spine motility and pruning, as well as experience-dependent plasticity in vitro and in vivo (Pizzorusso et al., 2002; Mataga et al., 2004; Oray et al., 2006). Thus, these findings suggest that a direct functional interaction exists between microglia and a subset of neuronal synapses (Le Roy and Wrana, 2005).
To further test this hypothesis, mice were deprived of visual experience for 1 week, a manipulation that results in a reduction of synaptic strength (Maffei et al., 2006; Goel and Lee, 2007). Following this manipulation, microglial processes were found to preferentially contact subsets of persistently shrinking dendritic spines and to be associated with expanded extracellular spaces. Additionally, microglial processes more often (>60% of processes) displayed phagocytic structures. During reexposure to daylight for 2 d, these effects reversed. Microglial phagocytosis, however, remained enhanced (Tremblay et al., 2010). Together, these observations suggest that microglia might influence synaptic structures in an experience-dependent manner.
Finally, during adulthood and normal aging, a progressive accumulation of phagocytic inclusions was observed in both mouse visual and auditory cortices. This accumulation was accelerated by age-related loss of vision and hearing, respectively. Additionally, microglial processes showed more frequent contact with synaptic clefts and interacted with synaptic element in ways reminiscent of synaptic stripping (Zettel et al., 2010). This suggests that microglia may influence neuronal circuit organization not only during postnatal development, but also throughout the animal's lifespan.
Regulation of microglial distribution, morphology, and motility
In the healthy adult brain, microglial distribution is influenced by local cytoarchitecture (Lawson et al., 1990). For a given brain region, parenchymal microglia are generally arranged in a regular fashion showing little cell layer or blood vessel pattern dependency. However, a tendency to be excluded from cell dense fields such as granule cell regions has been described. Across brain regions, microglia density varies considerably. While up to 12% of substantia nigra cells are microglia, this applies to only ∼5% of cells in corpus callosum. Similarly, microglia morphology varies considerably. While white matter microglia show elongated somata and processes preferentially oriented along fiber tracts, microglia in the circumventricular organs, a region characterized by a leaky blood–brain barrier, exhibit compact morphology with few short processes. In contrast, gray matter microglia exhibit many elaborate radially oriented arbors (Lawson et al., 1990). The molecular determinants of this anatomical diversity are largely unknown.
Microglia dynamics have been investigated in both mice and zebrafish. In both model systems, microglia show largely invariant soma positions but continually and rapidly moving processes (average velocity, ∼2.5 μm/min; range, 0.2–6.5 μm/min, depending on branch order) (Davalos et al., 2005; Nimmerjahn et al., 2005; Kim and Dustin, 2006; Wu et al., 2007; Peri and Nusslein-Volhard, 2008; Liang et al., 2009; Wake et al., 2009; Dibaj et al., 2010b; Nimmerjahn, 2011). Some regional differences have been found: for example, microglia processes in mouse spinal cord show average velocities of 1.0–2.1 μm/min in vivo and ∼5.4 μm/min in mouse retina ex vivo (Liang et al., 2009; Dibaj et al., 2010b). To what extent these differences in structural dynamics may be attributed to different recording conditions and/or analysis methods is presently unclear.
Process movement generally occurs in a way that maintains overall size and symmetry of individual microglia's territory. At the same time, contact between microglial processes is avoided (Nimmerjahn et al., 2005). Direct physical contact with neighboring cellular elements, however, is observed and may be necessary for maintaining the structural and functional integrity of the CNS (for review, see Hanisch and Kettenmann, 2007). The high degree of motility, which depends on actin polymerization (Nimmerjahn et al., 2005; Hines et al., 2009), facilitates efficient immune surveillance of the brain but also represents considerable energy expenditure.
Microglia's functional state and process dynamics are collectively maintained or altered by a multitude of soluble and membrane-bound factors originating from both neuronal and nonneuronal cells (Hanisch and Kettenmann, 2007; Kettenmann et al., 2011). The precise receptor composition on microglia membranes in the healthy brain is unknown. However, given the differences in transmitter environment, myelin content, and blood–brain barrier properties across brain regions, it likely differs regionally. Additionally, microglia may retain features acquired during previous immune challenges, resulting in a receptor composition that may also differ locally and temporally, entailing varying signaling outcomes (Kettenmann et al., 2011). Factors known to have a pronounced, mostly stimulating but not necessarily “activating” influence over microglia dynamics include ATP and other nucleotides (Davalos et al., 2005; Dibaj et al., 2010a; Fontainhas et al., 2011). For example, increases in the level of extracellular ATP potentially released through astrocytic hemichannels lead to an increased overall microglial process length and surveillance frequency. Tonic activation of microglial purinoreceptors and volume change facilitating potassium channels likely mediate this effect (Wu et al., 2007). However, the precise set of purinoreceptors and ion channels involved in regulating baseline motility and actin reorganization are still unknown. P2Y12 receptors and chloride channels have been ruled out (Haynes et al., 2006; Hines et al., 2009). Factors thought to exert a calming influence on microglia phenotype include CX3CL1, CD200, and CD47, which act on microglial CX3CR1, CD200R, and SIRPα receptors, respectively (Hanisch and Kettenmann, 2007). While the effects of CD200 and CD47 signaling on microglial baseline motility are unknown, disrupted CX3CR1 signaling results in ∼30% reduced process speed ex vivo (Liang et al., 2009). Other chemokines, neurotransmitters, adhesion molecules, and peptides may also influence microglia baseline dynamics (Hanisch and Kettenmann, 2007; Kettenmann et al., 2011). The role of glutamate and GABA in regulating baseline motility remains controversial (Nimmerjahn et al., 2005; Wu and Zhuo, 2008; Wake et al., 2009; Fontainhas et al., 2011). Because microglia in the healthy brain likely lack the corresponding receptors and electrophysiological responses to synaptic events, indirect routes of action, particularly through astrocytes, appear plausible (Wu and Zhuo, 2008; Fontainhas et al., 2011).
Summary
Together, these findings indicate that microglia in the healthy brain habitually interact with neuronal and nonneuronal elements, both structurally and functionally. Forms of interactions include phagocytosis of synaptic structures during postnatal development, phagocytosis of newborn neurons during adult neurogenesis, and active remodeling of the perisynaptic environment and release of soluble factors in the mature and aging brain. These interactions can influence neuronal plasticity and function directly or indirectly, and may be regulated by sensory experience. Therefore, beyond their decisive role in pathological conditions, microglia are emerging as important contributors to normal brain physiology.
Footnotes
This work was supported by Fonds de la Recherche en Santé du Québec and Canadian Institutes of Health Research postdoctoral fellowships (M.-E.T.), the Smith Family Foundation and the Dana Foundation (B.S.), the seventh European Framework Program Moodinflame (A.B.), and the Rita Allen Foundation Scholars Program and the Whitehall Foundation (A.N.). We apologize to all colleagues whose important work was not directly cited due to space limitations. A.S. is grateful to Juan M. Encinas for critical reading and Mirjana Maletic-Savatic and the Farish Foundation for funding support. H.W. is grateful to R. Douglas Fields and Philip R. Lee for critical reading.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O, Bessis A. Microglial contribution to astrocytic gliotransmission. Abstract Viewer/Itinerary Planner. Soc Neurosci Abstr. 2010;36:554–12. [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Chan A, Hummel V, Weilbach FX, Kieseier BC, Gold R. Phagocytosis of apoptotic inflammatory cells downregulates microglial chemoattractive function and migration of encephalitogenic T cells. J Neurosci Res. 2006;84:1217–1224. doi: 10.1002/jnr.21029. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Thams S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007;55:89–96. doi: 10.1016/j.brainresrev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Steffens H, Nadrigny F, Neusch C, Kirchhoff F, Schomburg ED. Long-lasting post-mortem activity of spinal microglia in situ in mice. J Neurosci Res. 2010a;88:2431–2440. doi: 10.1002/jnr.22402. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, Neusch C, Kirchhoff F. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010b;58:1133–1144. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96:807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang MH, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:14. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223:177–194. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y(12) receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia. 2009;57:1610–1618. doi: 10.1002/glia.20874. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R, Liu Z, Xu S, Koppius A, Imai Y, Kloss CU, Kohsaka S, Gschwendtner A, Moller JC, Werner A, Raivich G. Microglia and the early phase of immune surveillance in the axotomized facial motor nucleus: impaired microglial activation and lymphocyte recruitment but no effect on neuronal survival or axonal regeneration in macrophage-colony stimulating factor-deficient mice. J Comp Neurol. 2001;436:182–201. [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kettenmann H. Neuroscience: the brain's garbage men. Nature. 2007;446:987–989. doi: 10.1038/nature05713. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim JV, Dustin ML. Innate response to focal necrotic injury inside the blood-brain barrier. J Immunol. 2006;177:5269–5277. doi: 10.4049/jimmunol.177.8.5269. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YQD, Ma WX, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Grauer O, Toyka KV, Gold R. Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J Immunol. 2001;167:5004–5010. doi: 10.4049/jimmunol.167.9.5004. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–176. doi: 10.1385/MN:27:2:163. [DOI] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A. Two-photon imaging of microglia in the mouse cortex in vivo. In: Helmchen F, Konnerth A, editors. Imaging in neuroscience: a laboratory manual. Ed 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2011. pp. 961–979. [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Effects of synaptic activity on dendritic spine motility of developing cortical layer v pyramidal neurons. Cereb Cortex. 2006;16:730–741. doi: 10.1093/cercor/bhj019. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Alves Ferreira T, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2010;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2(5):e00047. doi: 10.1042/AN20100024. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Barres B, Stevens B. Synaptic pruning in the CNS: the role of microglia and the complement system. Soc Neurosci Abstr. 2010;36:554–5. [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AK, Koyama R, Mardinly AR, Barres BA, Stevens B. Microglia shape neural circuits in the developing brain. Soc Neurosci Abstr. 2011;37:663–03. [Google Scholar]
- Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–2032. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Zeng JW, Liu XH, He WJ, Du L, Zhang JH, Wu XG, Ruan HZ. Inhibition of ATP-induced glutamate release by MRS2179 in cultured dorsal spinal cord astrocytes. Pharmacology. 2008;82:257–263. doi: 10.1159/000161063. [DOI] [PubMed] [Google Scholar]
- Zettel M, Tremblay ME, Ison JR, Allen PD, Morrison WZ, Inglis A, Stanley HE, Rosene DL, Majewska AK. Age-related loss of hearing or sight in mice yields greater apparent aging effects in corresponding sensory cortices. Soc Neurosci Abstr. 2010;36:765–3. [Google Scholar]