Abstract
Self-regulation of brain activity in humans based on real-time feedback of functional magnetic resonance imaging (fMRI) signal is emerging as a potentially powerful, new technique. Here, we assessed whether patients with Parkinson's disease (PD) are able to alter local brain activity to improve motor function. Five patients learned to increase activity in the supplementary motor complex over two fMRI sessions using motor imagery. They attained as much activation in this target brain region as during a localizer procedure with overt movements. Concomitantly, they showed an improvement in motor speed (finger tapping) and clinical ratings of motor symptoms (37% improvement of the motor scale of the Unified Parkinson's Disease Rating Scale). Activation during neurofeedback was also observed in other cortical motor areas and the basal ganglia, including the subthalamic nucleus and globus pallidus, which are connected to the supplementary motor area (SMA) and crucial nodes in the pathophysiology of PD. A PD control group of five patients, matched for clinical severity and medication, underwent the same procedure but did not receive feedback about their SMA activity. This group attained no control of SMA activation and showed no motor improvement. These findings demonstrate that self-modulation of cortico-subcortical motor circuits can be achieved by PD patients through neurofeedback and may result in clinical benefits that are not attainable by motor imagery alone.
Introduction
Neurofeedback training with real-time brain activation data obtained by functional magnetic resonance imaging (fMRI) is emerging as an effective way to self-regulate neural circuits in the human brain (Weiskopf et al., 2003; deCharms et al., 2004; deCharms, 2008). This method relies on rapid decoding of brain states from fMRI data to provide participants with feedback, on a moment-to-moment basis, about activity within predetermined brain regions. Thus, it has the potential to be useful in upregulating or downregulating neural activity in disease states. Self-regulation of emotion networks through fMRI-based neurofeedback (Caria et al., 2010; Johnston et al., 2011) has been used in schizophrenia (Ruiz et al., 2011), and fMRI has also been used to document effects of neurofeedback with electroencephalography (EEG) in attention deficit/hyperactivity disorder (Beauregard and Lévesque, 2006).
However, apart from a study on chronic pain (deCharms et al., 2005) and a preliminary report on tinnitus (Haller et al., 2010), the clinical potential of fMRI neurofeedback for neurological disorders has not been explored.
Parkinson's disease (PD) is a particularly suitable target for such a neurofeedback intervention because imbalance between cortical and subcortical motor circuits is at the heart of pathophysiological models (Albin et al., 1989; DeLong, 1990; Obeso et al., 2008). We chose the supplementary motor area (SMA) as target area for upregulation because it has direct connections with the basal ganglia pathways implicated in PD (Mink, 1996; Nambu et al., 1996) and can also show consistently reduced activity in PD patients during the performance of motor tasks (Playford et al., 1992; Jahanshahi et al., 1995; Haslinger et al., 2001; Nachev et al., 2008; Munzert et al., 2009). This study was intended as a proof of principle for the use of fMRI neurofeedback for patients with a neurodegenerative disorder. We furthermore wanted to assess whether there might be clinical benefits in patients with early-stage PD when adjunctive treatments are particularly important to help reduce the amount of dopaminergic medication, which can have important short- and long-term side effects.
It has been shown that healthy participants can learn self-control of motor areas through fMRI neurofeedback (deCharms et al., 2004). We hypothesized that PD patients without significant cognitive impairment would be able to learn upregulation of SMA activity. Furthermore, we expected that this upregulation would be reflected in altered activity patterns in the basal ganglia. A final prediction was that successful upregulation of the SMA and practice of the associated strategies would lead to improvements in motor function that would not be seen in a control group engaged in motor imagery without specific feedback about local brain activity.
Materials and Methods
Participants
Ten patients (six males; four females) with PD, aged 39–75 years and in an early stage of the disease (Hoehn and Yahr stage I–III), took part in the experiment. Our criteria for inclusion in the study was based on the Hoehn and Yahr stage, but we did not restrict the age range, apart from an upper age limit of 75 years. All were currently receiving treatment with l-DOPA or dopamine agonists and there was no change in medication regimen and dosage during the course of the study (except one patient who was not on any medication) (Table 1). There was no significant difference in l-DOPA equivalent dosage between the two groups of patients (t(8) = −0.152; p > 0.05). They had no history of psychiatric or other neurological problems and no family history of PD, except for one patient in the experimental group (early-onset PD) whose mother had been diagnosed with late-onset PD. The experiment was approved by the ethics committees of the School of Psychology, Bangor University, and the local National Health Service Trust, and all patients gave informed consent.
Table 1.
Demographic, clinical measures and medication of PD patients
| Patient | Sex | Age | Duration of illness | Hoen and Yahr stage | Total l-DOPA | Dopamine agonists: Pram (mg of salt); Rop (mg) | MAOB inhibitors: rasagiline (mg) | LEDD to the nearest mg (LeWitt et al., 2007) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | 4 years | 1 | 100 (50) | Pram 4.5 (1.5) | 1 | 550 |
| 2 | F | 39 | 1 year | 1 | Rop 20 | 1 | 400 | |
| 3 | M | 63 | 3 years | 1 | Rop 18 | 1 | 350 | |
| 4 | M | 75 | 3 years | 1 | 150 (50) | 1 | 150 | |
| 5 | M | 52 | 4 years | 1 | Pram 4.5 (1.5) | 1 | 450 | |
| 6 | M | 75 | 1 year | 1 | — | — | — | |
| 7 | F | 64 | 4 years | 1 | 500 | — | — | 500 |
| 8 | M | 67 | 3 years | 1 | 300 (100) | — | — | 300 |
| 9 | M | 71 | 4 years | 2 | 300 (100) | Pram 3 (1) | — | 600 |
| 10 | F | 71 | 11 years | 3 | 300 (100) | Pram 3 (1) | — | 600 |
Patients 1–5 belong to the experimental group, and patients 6–10 belong to the control group. Abbreviations: Pram, Pramipexole; Rop, ropinirole; MAOB, monoamine oxidase E inhibitor; LEDD, l-DOPA equivalent daily dosage.
Procedure
Of the 10 participants, 5 were in the experimental group (EG) (with feedback) and the other 5 were in the control group (CG) (without feedback). In the first session, all participants underwent an initial clinical and behavioral assessment, followed by a scan session and postscanning behavioral tests. The second session (2–6 months later, depending on participant availability) consisted of prescanning and postscanning behavioral tests and another scan session with neurofeedback/imagery. The final session (2 weeks after the second scanning session) only consisted of the behavioral test and the final clinical assessment. The study protocol is summarized in Table 2.
Table 2.
Study protocol
| Session | Prescan | Scan | Postscan |
|---|---|---|---|
| 1 | UPDRS | T1 anatomical | POMS |
| VMIQ-2 | Localizer run | Stroop test | |
| POMS | Neurofeedback run 1 | GoNoGo task | |
| Stroop test | Neurofeedback run 2 | Finger tapping | |
| GoNoGo Task | |||
| Finger-tapping test | |||
| 2–6 months: Practice of neurofeedback strategy at home (EG) | |||
| 2 months: Practice of motor imagery at home (CG) | |||
| 2 | VMIQ-2 | Localizer run | POMS |
| POMS | Neurofeedback run 1 | Stroop test | |
| Stroop test | Neurofeedback run 2 | GoNoGo task | |
| GoNoGo task | Finger tapping | ||
| Finger-tapping test | |||
| 2 weeks: Practice of neurofeedback strategy/motor imagery at home | |||
| 3 | UPDRS | No scan | |
| POMS | |||
| Stroop test | |||
| GoNoGo task | |||
| Finger-tapping test | |||
The study included assessments at three time points with fMRI scanning on the first two sessions, and intervening practice periods. See Materials and Methods. Abbreviations: POMS, Profile of Mood States; VMIQ-2, Vividness of Movement Imagery Questionnaire 2.
Each scan session started with a localizer run in which patients alternated between 20 s periods of left-hand movement and rest over 10 cycles to yield reliable estimates of the localization of higher motor areas, including the SMA. The localizer run was followed by two runs in which, instead of moving their hand, the participants had to increase activity in the target area, the SMA, through motor imagery alone.
Localizer run
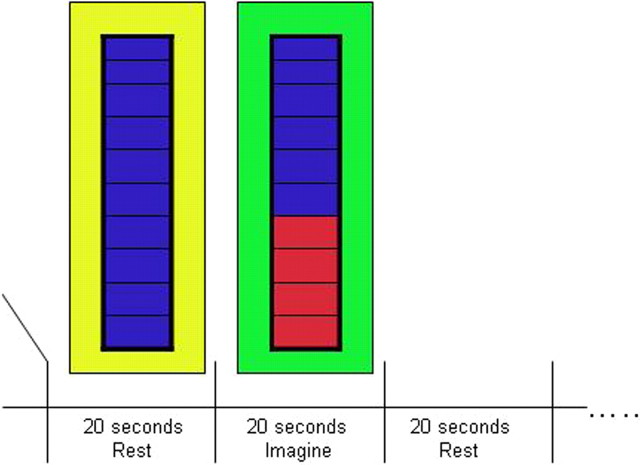
In EG and CG, the participants were presented with an image of a thermometer (which had 10 levels) in the center of a colored background at a visual angle of 4.76°. The image was backprojected onto a projector screen situated at the end of the bore of the magnet; the participant viewed the screen via a mirror mounted on the head coil, in front of the participant's eyes. When the background was green, their task was to continually squeeze and release the fingers of their left hand (for 20 s) until the background was yellow, at which point they had to rest (for 20 s). The thermometer, in the center of the screen, remained blue (i.e., no feedback) and did not change throughout the run. They performed 10 cycles of each move and rest (Fig. 1). The run lasted for ∼6.5 min. This simple motor task has been shown to reliably activate motor networks, including the SMA that we aimed to target (Gerloff et al., 1997).
Figure 1.
Experimental procedure. During fMRI scanning, participants saw a yellow or green screen with a central thermometer. During the localizer runs, patients were required to rest when a yellow background appeared (for 20 s) and squeeze their left hand when the background turned green (for 20 s). The thermometer display remained blue throughout the run. A run consisted of 10 cycles. The two neurofeedback runs were similar to the localizer run except here the patients had to increase brain activity when the background turned green without overt movement. As brain activity in the target area increased, the blue bars on the thermometer filled up with red, and as activity decreased, the red bars empty back to blue.
Neurofeedback/imagery runs
EG.
During the two neurofeedback runs (6.5 min each), the procedure was similar to the localizer run, but here, instead of moving their hand, the participants had to increase activity in the target area via motor imagery. They received continuous feedback about the height of activation through the thermometer display, where the number of red bars increased with increasing fMRI signal (Fig. 1). We informed them that this area was involved in motor control and that motor imagery might be an effective way of increasing its activity but did not prescribe any strategy. Participants were therefore free to think of any type of movement.
CG.
The procedure for the control group was similar to that of the experimental group, the difference being that they did not receive any feedback about activation in the target area during the imagery runs. They were asked to look at the thermometer display (which showed activation taken from a subject in the experimental group) but to ignore the changes that took place. Their instructions were to try and increase activity in the motor area of the brain by using motor imagery without actual movement. At debriefing sessions after the scanning, participants in both groups reported using complex imagery of complex motor tasks, including their favorite sports or manual tasks from their work.
In the time between the two scan sessions, participants in the EG were asked to practice the strategy they had found to be effective during the neurofeedback sessions in the laboratory at home on a daily basis, while the CG participants were also asked to practice the motor imagery they used in the scanner. Importantly, however, they were instructed to refrain from overt motor practice. Participants did not keep a record of how long they practiced motor imagery every day, but reported doing so on a regular basis. They also reported having no side effects from taking part in the experiment. All assessments were conducted during the “on” medication phase, ∼2–3 h after the last dose of medication (for medication details, see Table 1).
fMRI: data acquisition
Functional and anatomical scans were performed on all participants in a 3 Tesla Philips Achieva (Philips Healthcare) MRI scanner at the Wolfson Centre for Cognitive Neuroscience, Bangor University. A high-resolution T1-weighted anatomical scan (176 slices) covering the whole brain was acquired during the first scan session using a turbo field echo gradient echo pulse sequence. Functional data was obtained using a single-shot EPI sequence (TR, 2 s; TE, 30 ms; 30 slices; 3 mm thick; flip angle, 90°; in-plane resolution, 2 × 2 mm). The first four volumes were discarded to allow for T1 equilibration effects. A total of 204 volumes were acquired in each functional run for each participant.
Electromyography
We used MRI-compatible surface electromyography (EMG) to measure muscle activity (tremors) during the first scan session. Data were collected using an MR Plus amplifier and MRI-compatible electrodes and recorded on a Dell laptop using the Vision Recorder software (Brain Products). A sampling rate of 5000 Hz was used. The participants were fitted with two electrodes on the abductor pollicis brevis muscle of their affected hand (left side for all participants) along with a reference electrode at the base of the wrist and a ground electrode near the elbow before they went into the scanner.
fMRI data analysis (on-line)
Data from the localizer and neurofeedback runs were analyzed on-line. fMRI data were transferred as they were acquired from the reconstructor to an analysis computer through the Philips Direct Reconstructor Interface (DRIN) interface, which has been used in previous neurofeedback studies (Johnston et al., 2010). The data are read volume by volume by the real-time fMRI software package, Turbo-BrainVoyager (TBV) (Brain Innovation). The first four volumes were discarded to avoid T1 saturation effects. The data are corrected for angular and translational motion in the Cartesian coordinate system and analyzed with an incremental general linear model. For the localizer run, we used one predictor for the movement blocks (convolved with a canonical hemodynamic response function) to identify areas of movement-related activation. The t value for the contrast between movement and rest in the localizer map was set at 3.
We took the peak midline activation from the posterior part of the mesial aspect of the superior frontal gyrus within 2 cm of the paracentral sulcus during the localizer run as the target area for the neurofeedback runs. The region of interest was selected by choosing the whole of the extent in the x–y plane (on the axial slice) encompassing the activity in the SMA with an extent across three slices in the z direction.
In the neurofeedback runs, the “thermometer” displayed to participants (Fig. 1) the percentage signal change from baseline from the top third of most active voxels of the region of interest for an average of three time points. The baseline value was set to the average signal intensity value recorded from the last three time points during the preceding “fixation” period to the current upregulation block. There is a time lag and participants are informed of this. The thermometer display was continuously updated every 2 s with the third most active voxels. The stimulation interface was custom programmed in PsychoPy (Peirce, 2007, 2008) and presented from a Macintosh computer. This interface allows updating of feedback within ∼1 s of data acquisition.
fMRI data analysis (off-line)
Once the scan session was completed, the data were further preprocessed, off-line, using the Brain Voyager software package (version 2.1). Motion correction and temporal filtering were performed on the raw data to remove artifacts due to head movement and physiological noise. The functional data were coregistered to the T1 anatomical scan obtained. Finally, the data were transformed into Talairach space, spatially smoothed with a Gaussian filter FWHM of 6 mm, and temporally filtered (Gaussian FWHM, 3 s). We defined a general linear model (GLM) of the localizer experiment with one predictor for the “move” condition. For the neurofeedback runs, a single predictor for “upregulation” was used. In both cases, the regressors were convolved with a canonical hemodynamic reference function to account for the temporal delay and dispersion of the hemodynamic response. In both cases, we added the six motion confounds for each run to the GLM.
We used these models both for a group whole-brain analysis and for a ROI analysis. For the latter, we computed a GLM over the neurofeedback runs across the individually localized target areas. The aim of this procedure was to ascertain the significance of activation changes in the target area during neurofeedback. The group whole-brain analysis was aimed at investigating which other brain regions, in addition to the target area, were activated during the localizer and neurofeedback tasks. We computed a GLM for the 19 neurofeedback runs from the experimental group (four for four participants; three for one participant, due to scanner problems during his fourth session) and 20 neurofeedback runs from the control group separately and analyzed the effects of upregulation versus rest. We applied a threshold of p < 0.001 (fixed-effects analysis, Bonferroni corrected). Because of the low number of participants, we could not perform a random-effects analysis, and thus the validity of our results is confined to the sample detailed here. For areas with the highest activation during neurofeedback, we also compared activation levels (GLM β values) between neurofeedback and localizer runs with the Wilcoxon signed-rank test.
EMG analysis
During analysis, the data were downsampled to 1000 Hz. A 20 Hz low-pass filter (48 db/oct) and 1 Hz high-pass filter (12 db/oct) were applied to reduce scanner artifacts.
Each period of data corresponding to “movement”/“imagined movement” and “rest” was segmented into 4 s epochs. Fast Fourier transformation on the segmented data was performed using Vision Analyzer 2 software. This generated a profile for the movement and rest conditions using the localizer, which was comparable with the imagined movement and rest conditions.
Clinical and behavioral assessments and analysis
Unified Parkinson's Disease Rating Scale.
The Unified Parkinson's Disease Rating Scale (UPDRS) is a rating scale used to quantify the signs and symptoms of PD and is made up of different sections focusing on varying aspects of the impact of the disease on daily function: (1) mentation, behavior, and mood; (2) activities of daily living (the modified Schwab and England activities of daily living scale); and (3) motor examination. The scale is administered in the form of an interview and each subsection is given a score of 0–4. Higher scores denote higher impairment. In the present study, we only used the motor part of the scale because the procedure primarily targeted motor circuits. It was administered by coauthor J. V. Hindle, a consultant physician specializing in PD, at the beginning of the first and after the third session to yield estimates of changes in motor functions.
Finger-tapping test.
We used a standardized finger-tapping device. A wooden board with an electronic counter attached to it (to count the number of taps) on which the participants had to place their hand with the palm resting flat on the board and the index finger placed on a movable metal piece attached to the counter. The tap was valid only if the index finger reached a certain height before touching the surface of the board. Motor speed was measured as number of taps per minute for both hands. Each participant completed three 20 s trials, four 15 s trials, or six 10 s trials, although this was dependent on how soon participants experienced fatigue. These interval times were maintained for each participant across all sessions. The participants performed this test before and after both scan sessions and at the final assessment, after they had had time to familiarize themselves with the device.
We only report the UPDRS and finger tapping as these are our main outcome measures and since the main target is the motor circuits.
Statistical analysis
The behavioral and clinical measures from the first and the last session for the participants were compared by using the Wilcoxon signed-rank test (one-tailed) in SPSS (version 15; SPSS). This was done for both the experimental and control groups.
UPDRS.
We compared the total scores from the motor exam of the UPDRS from session 1 and session 3 for each of the two groups separately.
Finger-tapping test.
The total number of finger taps per minute for each hand was calculated for each participant and a comparison between session 1 and session 3 was made to see whether there was a significant increase in the number of finger taps attained as a result of the neurofeedback for both groups separately.
Results
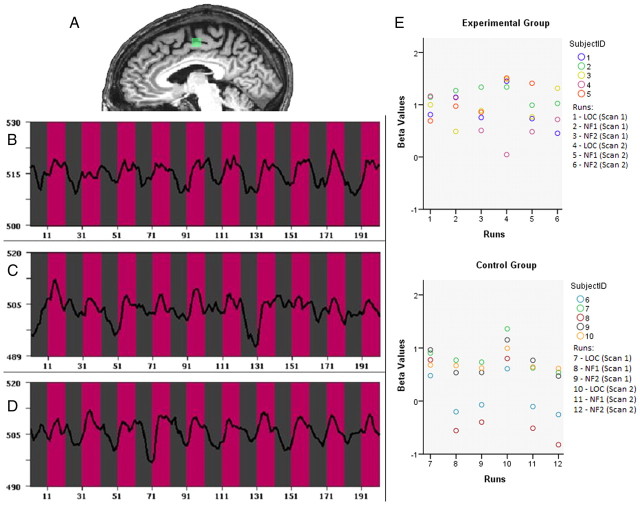
For the EG functional activation maps for the localizer task, thresholded at p = 0.001 (Bonferroni corrected), revealed a significantly activated cluster in the SMA for each participant. The target area used for neurofeedback was centered on the peak voxel of activation in the SMA, and we averaged the signal from all suprathreshold voxels in a 10 × 10 × 10 mm3 cubic region (Fig. 2A–D for example from one patient). We calculated the activation difference between upregulation and rest in the target area during neurofeedback runs at the group level with a one-sample t test on the β values. The changes were significant for the EG during both neurofeedback runs in session 1 (run 1: t(4) = 7.33, p = 0.002, two-tailed; run 2: t(4) = 6.45, p = 0.003, two-tailed) and session 2 (run 1: t(4) = 5.64, p = 0.005, two-tailed; run 2: t(3) = 4.705, p = 0.018, two-tailed).
Figure 2.
The region-of-interest analysis shows the success of the neurofeedback procedure for the experimental group. A, Example of target area for the neurofeedback run for one patient (x = −6). B–D, The mean time course during the localizer run (B), neurofeedback run 1 (C), and neurofeedback run 2 (D). The pink bars in B–D indicate activity in the target area during periods of upregulation, and the gray bars indicate rest periods. The patient was able to increase activity during upregulation and decrease activity during the rest periods for both neurofeedback runs. x-axis, Time (1TR = 2 s); y-axis, fMRI signal strength. E, Mean β values for the localizer runs and neurofeedback/imagery runs during both the scan sessions for all patients in the experimental and control group shown as scatter plots depicting the values for each individual person. The plots indicate that all patients in the experimental group were able to activate the target area during the neurofeedback (NF) session; this was not the case for all CG patients. Note: One patient in the EG did not show significant localizer (LOC) activation in scan 2, but this area was used to define the ROI for the neurofeedback runs based on the anatomical criteria, and then successfully upregulated.
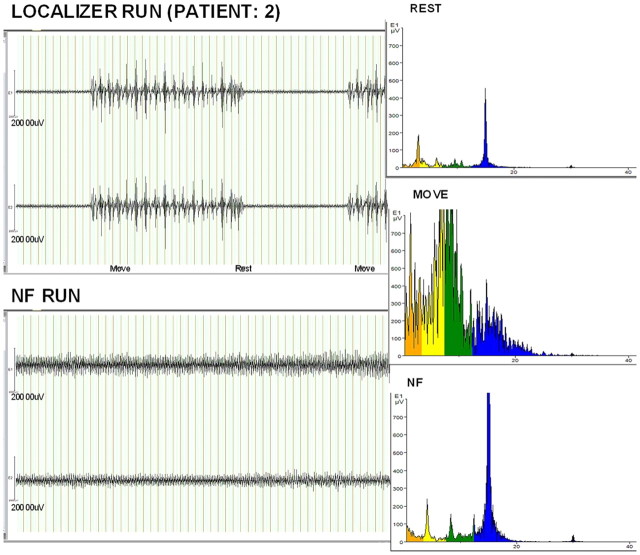
Activity in the SMA target area during the neurofeedback runs for the EG was similar to the activity during actual movement in the localizer runs (p > 0.05, Wilcoxon's signed-rank test). Thus, the analysis of activity in the target area showed that all participants were able to activate the SMA during the upregulation blocks without changes in overt movements, which was measured specifically for the abductor pollicis brevis muscle of the hand used for the localizer, by the EMG analysis (Fig. 3).
Figure 3.
EMG recordings ruled out the effect of overt movements. EMG data (for one patient) from two channels during the localizer and neurofeedback runs over a similar time period. The upper pair of traces shows the expected increased innervation of hand muscles during the movement periods. No such activity was observed during the corresponding (upregulation) periods of the neurofeedback run (bottom pair of traces). The right column shows that the frequency signature of the EMG was similar during neurofeedback (NF) to that recorded during rest and very different from that recorded during overt movements.
For the CG, we identified an area in the SMA with similar criteria to the target area of the EG. Activity of this area in the localizer blocks was not significantly different from the EG localizer blocks: t(18) = 1.158, p = 0.262. However, activity in this area during the imagery runs was lower than during the localizer blocks in the CG and this decrease was statistically significant in both scan sessions (session 1: run 1, p = 0.043; run 2, p = 0.043; session 2: run 1, p = 0.043; run 2, p = 0.043, Wilcoxon's signed-rank test). Indeed, activation in the imagery runs did not differ significantly from baseline (session 1: run 1, t(4) = 0.924, p = 0.408; run 2, t(4) = 1.296, p = 0.265; session 2: run 1, t(4) = 1.127, p = 0.323; run 2, t(4) = 0.389, p = 0.717). This difference in group effects—only the EG attained control over SMA activity—suggests that neurofeedback was helpful in learning to upregulate the target area.
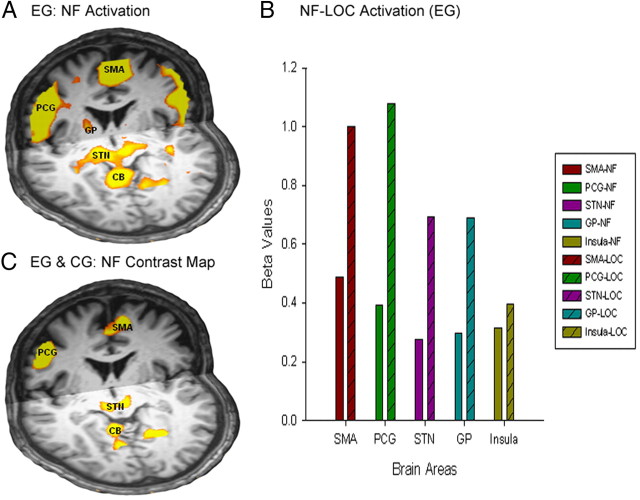
Whole-brain maps for the upregulation predictor for the EG showed bilateral activation in the SMA, precentral gyrus (PCG), subthalamic nucleus (STN), thalamus, globus pallidus internus (GPi), the insula, and the cerebellar vermis (Fig. 4A, Table 3). We found areas of deactivation in the posterior cingulate and the anterior medial frontal cortex. All the activated areas described above were significantly less active (p < 0.05) during the neurofeedback runs compared with the preceding localizer (Fig. 4B) except for the insula. However, the individually identified target areas showed equal activation during the neurofeedback run (as already shown by the ROI analysis; Fig. 2E). The whole-brain contrast map for localizer versus neurofeedback (p < 0.001, corrected) confirmed this pattern of overall higher activation for the localizer runs.
Figure 4.
A, Whole-brain analysis revealed the modulation of cortico-subcortical circuits through neurofeedback. Whole-brain analysis for the neurofeedback runs shows activation in the SMA, PCG, GP (y = ±3/z = 7), and a contiguous cluster covering STN and cerebellum (CB). For detailed coordinates, see Table 3. B, Plot showing β values of brain areas (whole-brain analysis for EG) for the neurofeedback (NF) and localizer (LOC) runs. All areas were found to be more active during the localizer runs compared with the neurofeedback runs. C, Contrast map of the two groups during the NF runs. A and C show coronal and axial views in radiological convention. For detailed coordinates, see Table 4 (y = 0; z = −4).
Table 3.
Areas of the brain activated and deactivated during neurofeedback
| Peak x/y/z | t value | p value | Cluster size (mm3) | |
|---|---|---|---|---|
| Brain areas activated | ||||
| Supplementary motor area/medial frontal gyrus | −1/−5/55 | 18.971279 | 0.000000 | 10,711 |
| Left precentral gyrus | −36/−15/48 | 12.780833 | 0.000000 | 24,791 |
| Right precentral gyrus | 29/−17/51 | 12.950804 | 0.000000 | 11,800 |
| Left STN region | −13/−14/−3 | 11.916059 | 0.000000 | 1680 |
| Right STN region | 17/−14/−2 | 9.442472 | 0.000000 | 1006 |
| Left globus pallidus | −19/0/9 | 9.907552 | 0.000000 | 734 |
| Right globus pallidus | 17/−5/6 | 10.168879 | 0.000000 | 668 |
| Left insula | −31/19/9 | 11.160399 | 0.000000 | 10,811 |
| Right insula | 32/19/3 | 12.689801 | 0.000000 | 5824 |
| Brain areas deactivated | ||||
| Posterior cingulate | 2/−50/27 | −14.176578 | 0.000000 | 1666 |
| Anterior medial frontal cortex | −4/46/34 | −13.245537 | 0.000000 | 10,233 |
This table summarizes the activated and deactivated areas for the contrast between the upregulation predictor and baseline in the EG.
The CG showed bilateral activation in the SMA, PCG, and the insula and GP but no activation in the STN during the upregulation blocks. We also found areas of deactivation in the cingulate gyrus, middle temporal gyrus, and parietal areas in the CG. To extract whole-brain effects that were specific for the EG, we also contrasted the upregulation maps from the EG and CG and found higher activation in the EG compared with the CG in SMA, PCG, STN, and cerebellum (Fig. 4B, Table 4).
Table 4.
Group activation differences
| Brain areas | Peak x/y/z | t value | p value | Cluster size (mm3) |
|---|---|---|---|---|
| Supplementary motor area | −1/−5/54 | 8.791275 | 0.000000 | 676 |
| Right precentral gyrus | 41/−11/48 | 10.25540 | 0.000000 | 691 |
| Right STN | 5/−20/−6 | 10.92679 | 0.000000 | 318 |
| Insula | 32/16/3 | 8.540370 | 0.000000 | 22 |
| Left STN | −7/−20/−3 | 8.799783 | 0.000000 | 92 |
| Left precentral gyrus | −49/−2/30 | 13.15048 | 0.000000 | 3639 |
This table summarizes the areas showing significantly different activation between the upregulation blocks of the EG and the imagery blocks of the CG.
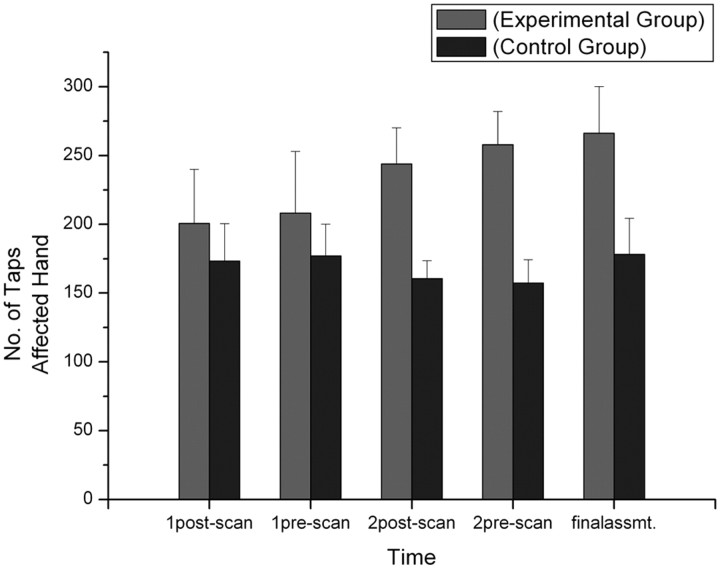
There was no baseline difference between the scores on the UPDRS, a standard global measure of PD state of the EG and CG (t(8) = −0.263; p = 0.799), but only the EG showed clinical and functional improvements. On the UPDRS, the EG had a 37% improvement of motor functions from session 1 (mean score, 14.2) to session 3 (mean score, 9) (p = 0.042, Wilcoxon's signed-rank test). No such effect was seen in the CG (mean scores: session 1, 15; session 3, 13.4; p = 0.336). Lower scores denote better motor function. Patients in the EG group improved across the five symptom clusters defined by Hagell and Brundin (2009) (axial/postural/gait, rest tremor, postural tremor, rigidity, limb bradykinesia), but no patient showed improvement on all five. The cluster with the most consistent improvement (four of five patients) was limb bradykinesia. A comparison of the number of finger taps attained in session 1 (before scan, mean number of taps, 210.6) with that in session 3 (mean number of taps, 266.2) (Fig. 5) for the affected hand showed that participants in the experimental group improved significantly over time (p = 0.043), whereas the control group did not (session 1, before scan, mean number of taps, 177; session 3, mean number of taps, 178.2; p = 0.686).
Figure 5.
The functional improvement was apparent from the increase in finger-tapping frequency. Mean number of finger taps is shown for all sessions, with error bars showing the SD. Patients in the experimental group were able to increase the number of finger taps from session 1 to session 3 (the final assessment) (p < 0.05).
Discussion
Neurofeedback is an attractive tool for any disorder in which imbalance between neural circuits is suspected. In the present proof-of-principle study, patients who received neurofeedback successfully upregulated a higher cortical motor area and concomitantly influenced activity in basal ganglia circuits implicated in PD. This training resulted in significant and clinically relevant improvement of motor functions. Neurofeedback in PD might work in analogy to physical stimulation techniques, such as transcranial magnetic or direct current stimulation, but with the advantage of being less invasive. Assuming that patients can learn to transfer the strategies used during neurofeedback into real-life settings, it might also become possible to sustain the clinical benefits without regular stimulation sessions. We chose fMRI- rather than EEG-based neurofeedback because fMRI has the potential advantage of allowing participants specifically to target subcomponents of the motor network. Furthermore, with whole-brain coverage, it is possible to detect the effects of successful self-regulation of the SMA in other parts of the brain, including subcortical networks, and thus to identify potential compensatory circuits in PD.
Our patients achieved fast and consistent upregulation of a higher motor area, the SMA. Successful upregulation of the SMA during neurofeedback constitutes a notable difference to mere mental imagery paradigms, in which activation in higher motor areas was consistently lower than during motor execution (Pascual-Leone et al., 1995). Yet SMA activity during motor imagery is detectable at single subject level, even in neurological patients (Monti et al., 2010). It may therefore seem puzzling that our CG produced no significant SMA activation increase over baseline in the imagery blocks. However, the scatter plots show that the deficit in the CG was mainly produced by higher variability across runs and patients than in the EG. Neurofeedback may thus aid more consistent activation of higher motor areas during imagery, especially in PD. Previous studies of motor imagery in PD have reported both altered (Thobois et al., 2000) and normal (Cunnington et al., 2001) recruitment of the SMA. These studies used predefined or cued imagery, whereas the motor imagery used in the present study was self-initiated, which was likely more difficult for patients. Indeed, a recent study shows that PD patients benefit from external cues in obtaining more vivid motor imagery (Heremans et al., 2011). They may therefore have acquired additional guidance or support from neurofeedback to obtain reliable SMA activation.
The results presented here show that PD patients can increase activity in the SMA by motor imagery with the help of real-time neurofeedback training. The neurofeedback session and the practice of the successful upregulation strategy at home resulted in improvements in motor function. Effects were seen over a 2 month interval and were at least as strong as those obtained with noninvasive stimulation [37% improvement in UPDRS-III compared with 20% in the study by Hamada et al. (2008)], although our study was too small to allow for formal quantitative comparisons of treatment effects.
Modulation of cortical areas with transcranial magnetic (Lefaucheur, 2006) or direct current stimulation (Fregni et al., 2006), which has been proposed as an alternative, or adjunct, to invasive stimulation, and direct epidural stimulation have recently been piloted as well (Lefaucheur, 2009). Here, too, one of the key target areas has been the SMA (Fregni et al., 2006; Lefaucheur, 2006, 2009; Hamada et al., 2008, 2009). The clinical effects of stimulation of primary and higher motor areas, especially on motor functions, have been promising, although short-lived and strongly dependent on stimulation parameters. The ability and willingness of patients to practice the neurofeedback strategy at home may be important for long-lasting symptom improvement, which has so far been one of the weaknesses of the external stimulation methods. In any clinical application, neurofeedback would not be intended as an alternative to medication but as an add-on therapy, possibly allowing saving on the dose of dopaminergic medication. The practicability of the current procedure is supported by the patients' positive subjective responses and high compliance. However, further studies, following the framework for the evaluation of complex interventions (Craig et al., 2008) are needed to address its efficacy and cost effectiveness.
What then are the potential mechanisms for motor improvement afforded by neurofeedback? In PD, compensatory mechanisms in the cortico-basal-ganglia-thalamocortical network may sustain neurological functions in the face of the progressive loss of dopaminergic input (Park and Reuter-Lorenz, 2009), slowing down the progression of the clinical symptoms of the disorder. This compensation may be enabled by neural degeneracy, whereby normally redundant neural pathways become functionally relevant when the original pathways cease to function (Edelman and Gally, 2001). Through the upregulation of the SMA and the associated network of thalamic/subthalamic areas and GPi, patients may have increased the compensatory activity of such a pathway. The SMA controls the programming of complex movements (Roland et al., 1980; Stephan et al., 1995), and its training may thus be particularly beneficial for patients with difficulties initiating movements (hypokinesia) or motor slowing (bradykinesia), as observed in the present study. The SMA sends direct signals to the subthalamic nucleus through a hyperdirect pathway (Nambu et al., 1996). It has also been shown that deep brain stimulation of the subthalamic nucleus increases GPi activity and improves symptoms (Hashimoto et al., 2003; Montgomery and Gale, 2008; Vitek, 2008). Therefore, increased activation of the SMA would lead to an increase in direct input to the subthalamic nucleus, in turn leading to increased GPi activity, causing a change in the pattern of neural activity within the basal ganglia circuit or increasing the regularity of GPi neurons potentially resulting in an improvement of symptoms. This model is in keeping with recent results from a motor learning study in healthy individuals, where the functional coupling between SMA and basal ganglia increased with practice (Ma et al., 2010). However, with the present design, we cannot single out any particular component of the activated network as being responsible for the potential clinical benefits.
We are confident that the clinical effects were not just produced by the attention the patients received from researchers or by the time on task because numerous trials have shown that motor symptoms do not improve in sham conditions of equally time-consuming trials (Boggio et al., 2006; Fregni et al., 2006; Lefaucheur, 2009), which was also not the case in our CG, who received no feedback with motor imagery and showed no clinical improvement. However, it is impossible to disentangle the effects of the direct neurofeedback sessions from those of the mental training. We would assume that the mental training at home, which involved the same strategy that was effective for the upregulation of SMA during scanning, delivered regular “boosts” to the SMA-subcortical network. Thus, the brain-focused approach to mental training, which was only possible because of the initial neurofeedback session, sets the present approach apart from traditional mental imagery training, which has consistently shown weaker effects than those observed in the present study (Herbert et al., 1998; Lotze and Halsband, 2006). However, larger trials with longer observation periods, will be needed for a definitive assessment of this issue. Participants reported no side effects, and the safety and tolerability of fMRI neurofeedback has recently been confirmed in a large study (Hawkinson et al., 2011).
A limitation of the study is the small sample size and reliance on null effects in imaging and clinical outcomes for the CG. Absence of a record of the imagery practiced at home, lack of a blinded UPDRS rating, and the inability to assess motor functions during the off medication state are further limitations that will have to be addressed in the next stage of the evaluation cycle (Craig et al., 2008). A comparison of the effects of neurofeedback on patients with early and late onset of the disease and patients on different medication regimens might also be of interest.
In conclusion, this new intervention program holds promise for clinical practice and should now undergo further rigorous clinical testing. This neurofeedback protocol aims to maximize use of surviving neurons in the brains of PD patients and improve compensatory mechanisms without any known side effects, and, most importantly, directly involves the patients, giving them a sense of control over their treatment and eventual improvement of symptoms. Although it may not stop the progression of the disease, it has the potential to alter the course of motor symptoms and possibly reduce drug requirements in early disease. This may have the effect of delaying more severe motor complications and improve the quality of life of the patients affected by PD.
Footnotes
This research was supported by the Wales Institute of Cognitive Neuroscience and the North Wales Grants Committee. M.H. is supported by the Wellcome Trust. L.S. is supported by a Strategic Fund of Cardiff University. We thank Lorraine Woods for help designing the figures.
The authors declare no competing financial interests.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J. Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback. 2006;31:3–20. doi: 10.1007/s10484-006-9001-y. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Egan GF, O'Sullivan JD, Hughes AJ, Bradshaw JL, Colebatch JG. Motor imagery in Parkinson's disease: a PET study. Mov Disord. 2001;16:849–857. doi: 10.1002/mds.1181. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage. 2004;21:436–443. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Mov Disord. 2006;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Hagell P, Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry. 2009;80:489–492. doi: 10.1136/jnnp.2008.159772. [DOI] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R. Real-time fMRI feedback training may improve chronic tinnitus. Eur Radiol. 2010;20:696–703. doi: 10.1007/s00330-009-1595-z. [DOI] [PubMed] [Google Scholar]
- Hamada M, Ugawa Y, Tsuji S Effectiveness of rTMS on Parkinson's Disease Study Group, Japan. High-frequency rTMS over the supplementary motor area for treatment of Parkinson's disease. Mov Disord. 2008;23:1524–1531. doi: 10.1002/mds.22168. [DOI] [PubMed] [Google Scholar]
- Hamada M, Ugawa Y, Tsuji S Effectiveness of rTMS on Parkinson's Disease Study Group, Japan. High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson's disease: subanalysis of double-blind sham-controlled study. J Neurol Sci. 2009;287:143–146. doi: 10.1016/j.jns.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, Ross AJ, Parthasarathy S, Scott DJ, Laramee EA, Posecion LJ, Rekshan WR, Sheau KE, Njaka ND, Bayley PJ, deCharms RC. Quantification of adverse events associated with functional MRI scanning and with real-time fMRI-based training. Int J Behav Med. 2011 doi: 10.1007/s12529-011-9165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert RD, Dean C, Gandevia SC. Effects of real and imagined training on voluntary muscle activation during maximal isometric contractions. Acta Physiol Scand. 1998;163:361–368. doi: 10.1046/j.1365-201X.1998.t01-1-00358.x. [DOI] [PubMed] [Google Scholar]
- Heremans E, Nieuwboer A, Feys P, Vercruysse S, Vandenberghe W, Sharma, Helsen WF. External cueing improves motor imagery quality in patients with Parkinson disease. Neurorehabil Neural Repair. 2011 doi: 10.1177/1545968311411055. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): insights into the treatment of Parkinson's disease by cortical stimulation. Neurophysiol Clin. 2006;36:125–133. doi: 10.1016/j.neucli.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Treatment of Parkinson's disease by cortical stimulation. Expert Rev Neurother. 2009;9:1755–1771. doi: 10.1586/ern.09.132. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Boroojerdi B, MacMahon D, Patton J, Jankovic J. Overnight switch from oral dopaminergic agonists to transdermal rotigotine patch in subjects with Parkinson disease. Clin Neuropharmacol. 2007;30:256–265. doi: 10.1097/wnf.0b013e318154c7c4. [DOI] [PubMed] [Google Scholar]
- Lotze M, Halsband U. Motor imagery. J Physiol Paris. 2006;99:386–395. doi: 10.1016/j.jphysparis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci Biobehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, Owen AM, Laureys S. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 2009;60:306–326. doi: 10.1016/j.brainresrev.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J, Rodríguez-Oroz M, Benitez-Temino B, Blesa F, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. 2008;23(Suppl 3):S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy—Psychophysics software in Python. J Neurosci Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. Generating stimuli for neuroscience using PsychoPy. Front Neuroinform. 2008;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekadar S, Caria A, Veit R, Kircher TT, Birbaumer N, Sitaram R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21427. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, Le Bars PD, Broussolle E. Motor imagery in normal subjects and in asymmetrical Parkinson's disease: a PET study. Neurology. 2000;55:996–1002. doi: 10.1212/wnl.55.7.996. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Deep brain stimulation: how does it work? Cleve Clin J Med. 2008;75(Suppl 2):S59–S65. doi: 10.3949/ccjm.75.suppl_2.s59. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003;19:577–586. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]