Abstract
OBJECTIVES
Vancomycin weight-based dosing regimens often fail to achieve therapeutic trough serum concentration in children ≤12 years of age and rigorous studies evaluating efficacy and safety of body surface area (BSA)–based dosing regimens have not been performed. We compared vancomycin trough serum concentrations in pediatric patients receiving a weight- or BSA-based dosing regimen.
METHODS
This was a single-center, retrospective study evaluating pediatric patients, ages 1 to 12 years, who received vancomycin from September 2012 to October 2015. Patients received a minimum of 3 consecutive doses at the same scheduled interval within a dosing regimen prior to a measured vancomycin serum trough concentration. The primary outcome was percentage of initial vancomycin trough concentrations ≥10 mg/L. The secondary outcomes were percentage of supratherapeutic, therapeutic, and subtherapeutic vancomycin serum concentration for all patients, including a subset of overweight and obese patients, and number of nephrotoxic occurrences.
RESULTS
BSA-based dosing regimens resulted in 50% of the initial vancomycin trough concentrations ≥ 10 mg/L compared with 17% for the weight-based dosing regimens (p < 0.0001). No statistically significant differences were noted between the 2 dosing regimens for supratherapeutic, therapeutic, or subtherapeutic trough concentrations for all patients, and for the subset of overweight and obese patients. Nephrotoxic occurrences were noted in 7% of the weight-based dosing regimens compared with none in the BSA-based dosing regimens.
CONCLUSIONS
A BSA-based vancomycin dosing regimen resulted in significantly more initial vancomycin trough concentrations ≥10 mg/L and trended towards higher initial vancomycin trough concentrations without observable nephrotoxicity.
Keywords: antibiotic dosing, body surface area, pediatrics, therapeutic drug monitoring, vancomycin
Introduction
Vancomycin is a hydrophilic, glycopeptide antibiotic with slow bactericidal activity and variable tissue penetration. It is used most commonly in the treatment of Gram-positive infections with beta-lactam–resistant organisms.1 Efficacy has been linked to dosing that achieves an AUC/MIC (area under the curve to minimum inhibitory concentration) of ≥400. Vancomycin steady-state trough serum concentrations of 15 to 20 mg/L are used as a surrogate marker of AUC/MIC ≥400 in adult patients, and this is the recommended trough range for serious infections in pediatric patients per the Infectious Diseases Society of America guidelines. Dosing at 60 mg/kg/day divided every 6 hours is recommended to achieve trough serum concentrations of 15 to 20 mg/L for severe infections in pediatric patients; however, concentrations of 10 to 15 mg/L may be clinically appropriate for less severe infections.2 Currently, it is unclear if clinical benefit exists in achieving higher vancomycin troughs in the pediatric patient population.3,4
Despite optimal weight-based dosing regimens, subtherapeutic trough serum concentrations are often encountered in pediatric patients, particularly in those ≤12 years of age.5–11 Requirements of up to 90 mg/kg/day of vancomycin have been described given age-dependent pharmacokinetic variability in renal clearance.5,6 Pharmacokinetic modeling and simulation that examined the relationship of AUC/MIC in children receiving vancomycin 60 mg/kg/day who had a methicillin-resistant Staphylococcus aureus (MRSA) infection with a MIC of ≤1 mg/L found that a trough of 7 to 10 mg/L predicted achievement of AUC/MIC >400 in >90% of children.12 In overweight and obese patients, elevated initial vancomycin trough concentrations have occurred when total body weight–based dosing is used7; however, an optimal initial dosing regimen is unknown.
An alternative vancomycin dosing strategy using the linear relationship between body surface area (BSA) and drug clearance may achieve isometric vancomycin AUC for all body sizes and age groups of pediatric patients because height remains a consistent factor. Dosing based on BSA is used for many pediatric chemotherapy regimens. The daily vancomycin dose to achieve an adequate AUC is estimated to be 1500 to 2000 mg/m2/day.13 BSA dosing may be more effective and less likely to result in higher concentration exposures in obese patients because excess weight has less of an impact on the dose when compared with weight-based dosing regimens. However, BSA-based dosing has not been evaluated in pediatric patients.
Nephrotoxicity is a concern when evaluating new vancomycin dosing regimens. Vancomycin is renally cleared, and nephrotoxicity is a potential side effect. Nephrotoxicity in pediatric patients has been correlated with larger doses, duration of therapy beyond 4 days, and trough concentrations above >15 mg/L.14–16 While the exact mechanism is unclear, it is possibly due to oxidative stress in the proximal renal tubule.14
Because traditional vancomycin weight-based dosing regimens often fail to achieve therapeutic troughs in younger children, our study aimed to compare vancomycin trough concentrations in pediatric patients 1 to 12 years of age receiving a weight-based dosing regimen and a newly developed BSA-based dosing regimen. The purpose of this study was to compare vancomycin trough concentrations in pediatric patients who received vancomycin on a weight-based dosing regimen with those of patients who received a BSA-based dosing regimen.
Materials and Methods
This was a single-center, retrospective Institutional Review Board–approved study evaluating pediatric patients 1 to 12 years of age who received vancomycin from September 2012 to October 2015. Decision to initiate empiric vancomycin was based on provider discretion, with dosing and monitoring guided by an institutional protocol (Table 1). Prior to October 2014, a weight-based vancomycin dosing protocol was used and patients with normal renal function would have received 45 to 60 mg/kg/day (15 mg/kg/dose every 6 to 8 hours). A BSA-based dosing protocol, designed and implemented in October 2014, used 500 mg/m2/dose in patients 1 to 12 years of age, based on actual body weight and height using the Mosteller method.17 All dosing intervals were determined by age, disease severity, and renal function. Initial vancomycin trough concentration was targeted for 10 to 20 mg/L with later refinement of goal trough concentrations as defined by clinical disease severity established by Infectious Diseases Society of America guidelines for MRSA infection.2 Vancomycin troughs of 15 to 20 mg/L were considered therapeutic for the following types of infection: severe Gram-positive infections of skin and soft tissue (i.e., necrotizing fasciitis), bone (i.e., osteomyelitis), and infections of the pulmonary (i.e., community- and hospital-acquired pneumonia), cardiovascular (i.e., endocarditis, bacteremia), or central nervous (i.e., meningitis) system. Trough serum concentrations between 10 to 15 mg/L were considered therapeutic for any other types of infection.
Table 1.
Initial Dosing Guidelines for Vancomycin in Pediatric Patients
| BSA-Based Guideline Based on CrCL in Patients 1–12 Years of Age Given 500 mg/m2/dose | |||||
| CrCL (mL/min/1.73 m2) | 100 | 100–70 | 69–46 | 45–30* | 29–15* |
| Trough goal: 10–15 mg/L | q 6 hr | q 8 hr | q 12 hr | q 18 hr | q 24 hr |
| Trough goal: 15–20 mg/L | q 6 hr | q 8 hr | q 12 hr | q 18 hr | q 24 hr |
| Oncology patients | q 6 hr | q 8 hr | q 12 hr | q 18 hr | q 24 hr |
| Weight-Based Initial Dosing Guidelines in Infants and Pediatric Patients Given an Initial Dose of 15 mg/kg/dose | |||||
| CrCL (mL/min/1.73 m2) | >70 | 69–46 | 45–30 † | 29–15† | |
| Infants > 1 mo and children | q 8 hr | q 12 hr | q 18 hr | q 24 hr | |
| Non-obese pediatric cancer patients and CNS infections | q 6 hr | q 12 hr | q 18 hr | q 24 hr | |
CNS, central nervous system; CrCL, creatinine clearance; q, every.
* Renal dysfunction, end-stage renal disease, or on dialysis: subsequent dosages and frequency of administration are best determined by measurement of serum concentrations.
† Renal dysfunction, end-stage renal disease, or on dialysis: 10 to 20 mg/kg; subsequent dosages and frequency of administration are best determined by measurement of serum concentrations.
Study patients between the ages of 1 and 12 years were identified by a query of the institution's electronic database to identify patients receiving a dose of vancomycin. Patients were included if they received a minimum of 3 consecutive vancomycin doses at the same initial dose and interval, with a vancomycin trough serum concentration being drawn within 1 hour prior to the next dose. Patients were excluded if there was insufficient information to calculate creatinine clearance with the Schwartz equation,18 or if they received dialysis, extracorporeal membrane oxygenation, or developed >50% increase in serum creatinine from baseline on admission prior to initiation of vancomycin. Information collected included patient demographic information, baseline serum creatinine and creatinine clearance, vancomycin regimen (e.g., dose, interval, and start and stop dates), culture data, use of concomitant nephrotoxic medications, vancomycin trough concentrations, and the serum creatinine value at day 5.
The primary outcome was percentage of initial vancomycin trough serum concentrations ≥10 mg/L. The secondary outcomes were percentage of supratherapeutic, therapeutic, and subtherapeutic vancomycin serum concentration in comparison with goal trough concentrations for all patients, including a subset of overweight and obese patients, and number of nephrotoxic occurrences within 5 days of initiating vancomycin. Goal vancomycin trough concentrations were defined as 10 to 15 mg/L and 15 to 20 mg/L. Patients were considered overweight or obese as defined by Centers for Disease Control and Prevention percentiles of body mass index.19 Nephrotoxicity was defined by a serum creatinine increase of ≥0.5 mg/dL or ≥50% increase from baseline after 5 days of vancomycin therapy.9,20–22
All data collected were analyzed by using descriptive statistics to assess demographics, primary outcome, and secondary outcomes. The original study design was created to detect a 35% difference between weight-based dosing proportion, p1, of 0.25 and BSA-based dosing proportion, p2, of 0.60 (odds ratio of 4.5), with a sample size of 36 in each arm, using 80% power for the primary outcome, and a 2-group continuity corrected χ2 test with a 0.05 two-sided significance level. The study exceeded the original accrual goals. To test for differences in categorical data, Fisher exact tests were used; for continuous data, independent t tests were used.
Results
A total of 528 regimens were identified through the electronic database query, with 267 regimens meeting inclusion criteria. Of these 267 regimens, 77 were excluded, most commonly owing to insufficient information to calculate creatinine clearance. Of the included 190 regimens, 126 were weight-based regimens and 64 were BSA-based regimens, as displayed in Figure 1.
Figure 1.
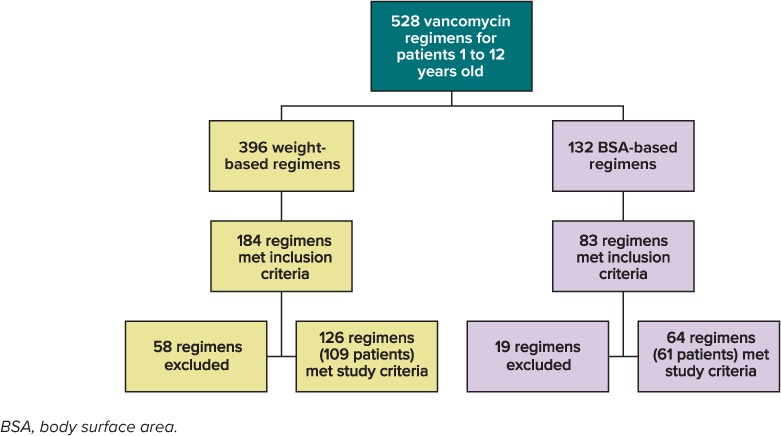
Flowchart of study population selection process.
Baseline characteristics for the 190 regimens are displayed in Table 2. Most patients were in the 1- to 4-year-old age group. Statistically significant differences existed with regard to dose and dosing interval. The mean weight-based dose was higher in patients receiving the BSA-based dosing regimen than in those receiving the weight-based regimen (19.6 mg/kg vs. 15.1 mg/kg, respectively) (p < 0.0001). Most patients had an initial creatinine clearance ≥ 70 mL/min/1.73 m2 with 97.7% of weight-based dosing patients and 100% of BSA-based dosing patients. BSA-based patients received an every-6-hour interval regimen 98% of the time, and weight-based patients received an every-6-hour interval regimen 42% of the time (p < 0.00001), which reflected the respective protocol's initial dosing interval recommendation.
Table 2.
Baseline Characteristics
| Characteristic | Weight-Based (n = 126) | BSA-Based (n = 64) | p value |
|---|---|---|---|
| Sex, male, n (%) | 70 (56) | 31 (49) | 0.35 |
| Age, yr, n (%) | |||
| 1–4 | 66 (52) | 31 (49) | 0.61 |
| 5–8 | 27 (17) | 15 (23) | 0.75 |
| 9–12 | 33 (21) | 18 (28) | 0.78 |
| Dose, mg/kg, mean ± SD | 15.1 ± 0.8 | 19.6 ± 3.0 | <0.0001 |
| Dosing interval, n (%) | |||
| 6 hr | 54 (42.9) | 63 (98.4) | <0.00001 |
| 8 hr | 69 (54.8) | 1 (1.6) | <0.00001 |
| 12 hr | 3 (2.4) | 0 (0) | 0.56 |
| Serum creatinine, mg/dL, mean ± SD | 0.37 ± 0.19 | 0.37 ± 0.15 | 0.91 |
| Creatinine clearance, mL/min/1.73 m2, mean ± SD | 180.7 ± 62.3 | 180.7 ± 58.7 | >0.99 |
| Number of concomitant nephrotoxic medications, mean ± SD | 0.75 ± 0.78 | 0.8 ± 0.73 | 0.71 |
| Overweight and obese patients, n (%) | 33 (26) | 12 (19) | 0.28 |
In evaluating the primary outcome, 50% of the BSA-based dosing regimens achieved vancomycin trough concentrations ≥10 mg/L compared with 17% of the weight-based dosing regimens (p < 0.0001) (Figure 2). Median trough serum concentrations of the interquartile ranges were 10 mg/L compared with 5.7 mg/L in the BSA- and weight-based dosing regimens, respectively (p < 0.0001) (Figure 3). The interquartile range of the weight-based regimens did not exceed 10 mg/L, whereas 50% to 75% of interquartile ranges of the BSA-based dosing regimens included troughs ≥10 mg/L.
Figure 2.
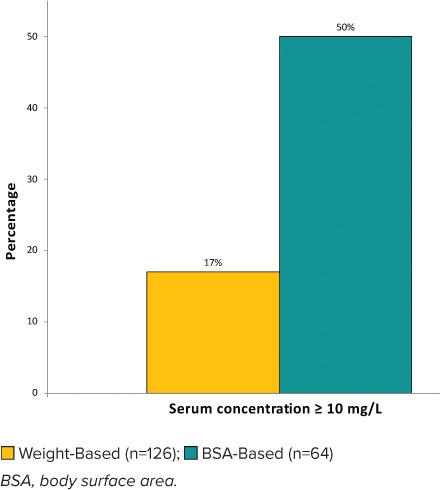
Percentage of regimens achieving vancomycin serum concentrations ≥10 mg/L.
Figure 3.
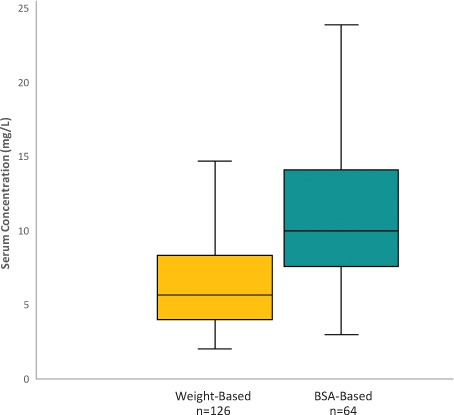
Initial vancomycin serum trough concentrations.
There were a decreased number of weight-based regimens evaluated in the secondary outcomes owing to lack of documented goal trough concentrations. Most patients, including the subset of overweight and obese patients, had subtherapeutic troughs despite differing dosing regimens (Table 3, Figures 4 and 5). Serum creatinine at day 5 was available in 45% of weight-based regimens and 34% of BSA-based regimens. Of these available regimens for evaluation, there were no nephrotoxic occurrences with BSA-based dosing regimens compared with 4 (7%) in the weight-based group (p = 0.57) (Table 3).
Table 3.
Vancomycin Serum Concentration Evaluation Based on Goal Level and Nephrotoxic Occurrences
| Secondary Outcomes | Weight-Based (n = 41) | BSA-Based (n = 64) | p value |
|---|---|---|---|
| Serum vancomycin trough concentrations for all patients | |||
| Supratherapeutic, n (%) | 1 (2) | 9 (14) | 0.084 |
| Therapeutic, n (%) | 8 (20) | 12 (19) | >0.99 |
| Subtherapeutic, n (%) | 32 (78) | 43 (67) | 0.27 |
| Serum vancomycin concentrations for overweight and obese patients | n = 15 | n = 12 | |
| Supratherapeutic, n (%) | 1 (7) | 1 (8) | 0.99 |
| Therapeutic, n (%) | 3 (20) | 4 (33) | 0.66 |
| Subtherapeutic, n (%) | 11 (73) | 7 (58) | 0.45 |
| All patients | n = 57 | n = 22 | |
| Nephrotoxic occurrences, n (%) | 4 (7) | 0 (0) | 0.57 |
Figure 4.
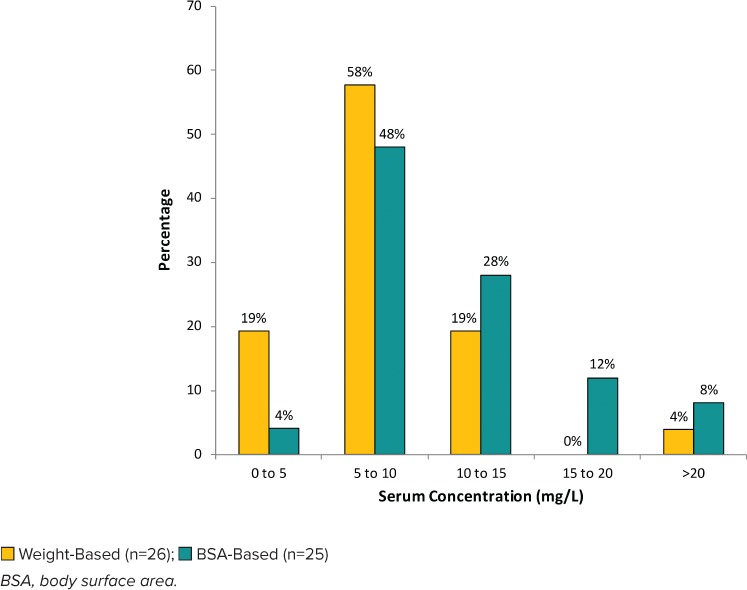
Distribution of initial vancomycin serum concentrations for regimens with goal trough of 10 to 15 mg/L.
Figure 5.
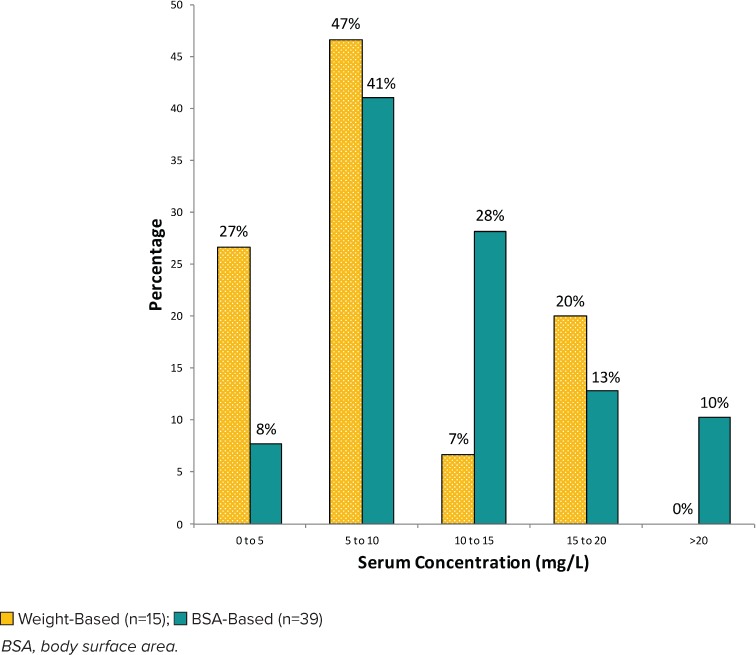
Distribution of initial vancomycin serum concentrations for regimens with goal trough of 15 to 20 mg/L.
The distributions of initial vancomycin serum trough concentrations achieved by each dosing regimen for each clinically defined goal trough concentration (10–15 mg/L or 15–20 mg/L) are shown in Figures 4 and 5, respectively. For regimens with a goal trough of 10 to 15 mg/L (Figure 4), 28% of BSA-based regimens were within goal, compared with 19% of weight-based regimens. For regimens with a goal trough of 15 to 20 mg/L (Figure 5), 13% of the BSA-based regimens were within goal, compared with 20% of the weight-based regimens.
Subtherapeutic vancomycin trough concentrations in weight-based regimens were 77% for goal trough of 10 to 15 mg/L and 81% for goal trough of 15 to 20 mg/L compared with BSA-based regimens, which were 52% and 77%, respectively. Supratherapeutic vancomycin troughs in weight-based regimens occurred in 4% of patients with a goal trough of 10 to 15 mg/L. Supratherapeutic vancomycin troughs in BSA-based regimens occurred in 20% of patients with a goal trough of 10 to 15 mg/L and 10% of patients with a goal trough of 15 to 20 mg/L.
Discussion
BSA-based vancomycin dosing resulted in significantly more initial vancomycin trough concentrations ≥10 mg/L for patients 1 to 12 years old compared with patients in the weight-based dosing regimen. Further analysis of the initial vancomycin trough concentration compared with the goal trough illustrated a trend for BSA-based regimens to result in higher initial vancomycin trough concentrations than for the weight-based regimens. A clinically meaningful finding was the decrease in BSA-based regimens within the 0 to 5 mg/L trough range, compared with weight-based regimens, and fewer subtherapeutic regimens, which has been common in this age group. Although not significant, there were a higher number of supratherapeutic troughs with BSA-based dosing for the goal trough of 15 to 20 mg/L. When evaluating the subset of overweight and obese patients, there was a higher percentage of therapeutic serum concentrations and lower percentage of subtherapeutic serum concentrations with BSA-based dosing than weight-based dosing. In addition, there were no nephrotoxic occurrences observed with BSA-based dosing.
Subtherapeutic troughs have been a common problem in younger pediatric patients and it is unclear what the optimal dose is to achieve goal trough concentrations, which are currently extrapolated from adult literature. Le et al8 discussed a pharmacokinetic age break for dosing at 12 years old, which in clinical practice has appeared true. This study illustrates patient data with protocol-driven dosing, including overweight and obese patients, based on the BSA and vancomycin clearance modeling described by Camaione et al.13 The BSA-based dosing demonstrated significantly more vancomycin trough concentrations ≥10 mg/L for all patients, including the subset of overweight and obese patients. One challenge with weight-based dosing protocols that use a standard recommended dose (i.e., 15 mg/kg/dose) is that the same initial dose is given to patients without regard to age or body habitus. BSA-based dosing provides an initial dosing that is potentially more appropriate for the patient with a higher mg/kg/dose given to younger patients and less risk of inappropriately increased initial doses for overweight and obese patients.
This study has limitations associated with its retrospective nature. With adoption of BSA-based dosing regimen, interval changes were also incorporated to reflect new standard-of-care practice with every-6-hour dosing becoming the standard for patients with normal renal function, compared with the previous every-8-hour interval that was more common for patients with normal renal function. Previously, in our institution, every-6-hour interval was recommended only for non-obese cancer patients and patients with central nervous system infections who had normal renal function. This resulted in most BSA-based patients receiving every-6-hour interval dosing, compared with the weight-based patients receiving every-6-hour or every-8-hour interval dosing. Inconsistent documentation of vancomycin trough concentration goals for weight-based regimens led to fewer regimens eligible to be evaluated for the secondary outcomes. Most patients had a normal renal function at baseline, which limits the significance of the low number of nephrotoxic occurrences that occurred. In addition, serum creatinine values at day 5 were not available for all patients. This could be attributed towards efforts to limit blood draws and monitor patients' renal function through urinary output and/or to patients not continuing vancomycin therapy through day 5, and serum creatinine monitoring not being necessary. This study is not designed to evaluate the clinical impact of BSA-based dosing in patients with poor renal function. Because vancomycin dosing was at provider discretion, it is worth considering that a provider bias could exist in the patient groups. Limitations related to interpatient variability include variety of concomitant conditions, care acuity, and renal function.
Future analysis is needed to determine an optimal vancomycin dosing protocol for patients 1 to 12 years old that incorporates age, dose, interval, and goal vancomycin trough serum concentration. Optimal dosing of vancomycin to adequately treat patients and avoid clinical complications continues to be evaluated. Overall, BSA-based vancomycin dosing regimen resulted in significantly more initial vancomycin trough concentrations ≥10 mg/L without observable nephrotoxicity. However, it remains unclear if obtaining higher troughs results in better clinical outcomes.
Acknowledgments
Pediatric Infectious Disease physicians, pharmacists, medical teams, and nurses at Wake Forest Baptist Health Brenner Children's Hospital. Lauren Wyatt, PharmD, and Coco Perry, PharmD, for reviewing. Preliminary results were presented at Midyear Clinical Meeting on December 8, 2015; PPAG Annual Meeting Resident Project Presentations in Minneapolis, MN, on April 30, 2015; and UHC Midyear Poster Presentation on December 6, 2014.
ABBREVIATIONS
- AUC
area under the curve
- BSA
body surface area
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Beth Sawrey and Mary Subramanian have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Dehority W. Use of vancomycin in pediatrics. Pediatr Infect Dis J. 2010;29(5):462–464. doi: 10.1097/INF.0b013e3181db7398. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):1–38. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.McNeil JC, Kok EY, Forbes AR et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infec Dis J. 2016;35(3):263–268. doi: 10.1097/INF.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil JC, Kaplan SL, Vallego JG. The influence of the route of antibiotic administration, methicillin susceptibility, vancomycin duration and serum trough concentration on outcomes of pediatric Staphylococcus aureus bacteremic osteoarticular infection. Pediatric Infec Dis J. 2017;36(6):572–577. doi: 10.1097/INF.0000000000001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madigan T, Sieve RM, Graner KK et al. The effect of age and weight on vancomycin serum trough concentrations in pediatric patients. Pharmacotherapy. 2013;33(12):1264–1272. doi: 10.1002/phar.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders SJ, Bijleveld YA, Sinkeler F et al. Clinical evaulation of vancomycin dosage in pediatric oncology patients. Pediatr Infect Dis J. 2014;33(7):731–733. doi: 10.1097/INF.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 7.Heble DE, McPherson C, Nelson MP et al. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacothearpy. 2013;33(12):1273–1277. doi: 10.1002/phar.1321. [DOI] [PubMed] [Google Scholar]
- 8.Le J, Bradley JS, Murray W et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(4):e155–e163. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiland LS, English TM, Eiland EH., III. Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients. Ann Pharmacother. 2011;45(5):582–589. doi: 10.1345/aph.1P588. [DOI] [PubMed] [Google Scholar]
- 10.Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Ped Infect Dis. 2013;2(3):259–262. doi: 10.1093/jpids/pis083. [DOI] [PubMed] [Google Scholar]
- 11.Miloslavsky M, Galler MF, Moawad I et al. The impact of pediatric-specific vancomycin dosing guidelines: a quality improvement initiative. Pediatrics. 2017;139(6) doi: 10.1542/peds.2016-2423. e20162423. doi:10.1542/peds.2016–2423. [DOI] [PubMed] [Google Scholar]
- 12.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J. 2013;32(10):1077–1079. doi: 10.1097/INF.0b013e318299f75c. [DOI] [PubMed] [Google Scholar]
- 13.Camaione L, Elliott K, Mitchell-Van Steele A et al. Vancomycin dosing in children and young adults: back to the drawing board. Pharmacotherapy. 2013;33(12):1278–1287. doi: 10.1002/phar.1345. [DOI] [PubMed] [Google Scholar]
- 14.Bonazza S, Bresee LC, Kraft T et al. Frequency of and risk factors for acute kidney injury associated with vancomycin use in the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2016;21(6):486–493. doi: 10.5863/1551-6776-21.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinclair EA, Yenokyan G, McMunn A et al. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014;48(12):1555–1562. doi: 10.1177/1060028014549185. [DOI] [PubMed] [Google Scholar]
- 16.Knoderer CA, Nichols KR, Lyon KC et al. Are elevated vancomycin serum trough concentrations achieved within the first 7 days of therapy associated with acute kidney injury in children? J Pediatric Infect Dis Soc. 2014;3(2):127–131. doi: 10.1093/jpids/pit076. [DOI] [PubMed] [Google Scholar]
- 17.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. doi:10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Obesity and overweight: defining childhood obesity. https://www.cdc.gov/obesity/childhood/defining.html Accessed April 2, 2019.
- 20.McKamy S, Hernandez E, Jahng M et al. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;168(3):422–426. doi: 10.1016/j.jpeds.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of vancomycin in adult patients: a consensus of the American Society of Health-Systems Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 22.McKamy S, Chen T, Lee M et al. Evaluation of a pediatric continuous-infusion vancomycin therapy guideline. Am J Health Syst Pharm. 2012;69(23):2066–2071. doi: 10.2146/ajhp120072. [DOI] [PubMed] [Google Scholar]


