Abstract
Many aspects of human behavior are driven by rewards, yet different people are differentially sensitive to rewards and punishment. In this study, we show that white matter microstructure in the uncinate/inferior fronto-occipital fasciculus, defined by fractional anisotropy values derived from diffusion tensor magnetic resonance images, correlates with both short-term (indexed by the fMRI blood oxygenation level-dependent response to reward in the nucleus accumbens) and long-term (indexed by the trait measure sensitivity to punishment) reactivity to rewards. Moreover, trait measures of reward processing were also correlated with reward-related functional activation in the nucleus accumbens. The white matter tract revealed by the correlational analysis connects the anterior temporal lobe with the medial and lateral orbitofrontal cortex and also supplies the ventral striatum. The pattern of strong correlations suggests an intimate relationship between white matter structure and reward-related behavior that may also play a role in a number of pathological conditions, such as addiction and pathological gambling.
Introduction
Individual differences in self-regulation—such as reward seeking, fear avoidance, and inhibitory control—have been associated with the probability of engaging in addictive behaviors such as pathological gambling or substance abuse. Understanding the neurobiological processes and dynamics that are responsible for such individual differences (normal and pathological) will help us to grasp the causes of such behavior and to develop intervention strategies.
A key brain structure in the regulation of reward-appetitive and consummatory behaviors is the ventral striatum [including the nucleus accumbens (NAcc)]. Its activity, as assessed by the hemodynamic response in functional imaging, is modulated by the following: (1) the presence of positive and negative reward outcomes (e.g., monetary gains and losses) (Camara et al., 2008), (2) the size of the potential loss (decreasing its amplitude) (Tom et al., 2007), (3) learning, decision-making, and motivation manipulations (Camara et al., 2009), and (4) individual differences in the preferences of delayed versus immediate rewards (Hariri et al., 2006). The NAcc has also been implicated in addictive and impulsive decision making (Reuter et al., 2005). Recent investigations have shown that individual response differences in this region might be directly related to reward-related behavioral patterns (Cohen et al., 2009).
Here, we consider the question of whether individual differences in reward-related behavior and in the reward-related reactivity of the NAcc might be brought about by this structure's anatomical connectivity. Just as the size and capacity of roadways can restrict the flow of traffic between different cities, the properties of white matter fiber connections between brain structures should constrain the flow of information between these regions and the influence that one region can exert over others. Thus, brain networks, which comprise the different regions connected through white matter pathways, might constrain the nature of information processing locally and across brain regions (Behrens and Johansen-Berg, 2005). Specifically, we asked to which degree local white matter microstructure differences surrounding a discrete functional region, i.e., the NAcc, drive the reward-related hemodynamic response in this region. Moreover, we also assessed whether white matter properties explain interindividual variance with regard to psychometrically determined long-term personality traits related to reward processing.
Several indices [e.g., fractional anisotropy (FA)] derived from diffusion tensor magnetic resonance imaging (DTI) have been proposed to characterize the microstructure of the studied medium with differences in diffusional properties (Beaulieu, 2002). In particular, FA has been shown to mirror axonal microstructure in vivo (e.g., axon size, extent of myelination) (Basser and Jones, 2002). Thus, it has been used to describe the quality of axonal connectivity (Waxman and Bennett, 1972), which in turn might constrain the activity within the connected brain regions.
We predicted a tridirectional correlation, expecting FA to be related to both the functional response of the NAcc and reward-related personality traits. Also, we expected a relationship between personality traits and the reward-response of the NAcc.
Materials and Methods
All procedures were cleared by the ethical review board of the University of Barcelona.
Participants.
Thirty-five healthy students (24 women; mean age, 21.8 ± 2.2 years) from the University of Barcelona (Barcelona, Spain) gave informed consent to participate.
Psychometric measurements.
Participants completed the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (Torrubia et al., 2001), which assesses temperamental dimensions derived from the personality model of impulsiveness and anxiety by Gray (1982). Sensitivity to punishment (SP) identifies persons selectively responsive to anxiety and fear stimuli, whereas sensitivity to reward (SR) identifies subjects selectively responsive to stimuli suggesting emotional well-being, reward, and consummatory behavior [internal consistency of the scales is 0.83 (SP) and 0.78 (SR)].
Gambling task.
Each trial of the gambling task began with a warning signal (an asterisk appearing on the screen for 500 ms), followed by the presentation of two numbers (5 and 25) displayed in white against a black background in the two possible combinations, [5 25] or [25 5]. Participants had to select one of the two numbers by pressing a spatially corresponding button with the left or right index finger. One second after the choice, one of the numbers turned green and the other turned red. If the number selected by the participant changed to red, the participant incurred a loss of the corresponding amount of money in Euro cents. In contrast, if the number turned into green, this indicated a gain.
In addition to the standard trials described above (80% of all trials), two additional conditions were created to assess brain responses to unexpected rewards and losses. In 10% of the trials (boost trials) the number 125 appeared in either red or green, signaling an unexpectedly large loss or gain. This change in magnitude occurred equally often for 5 and 25 trial bets to avoid positive or negative biases in choosing the number 25. To control for the fact that boost trials were both large and unexpected, in an additional 10% of the trials (similar unexpected), the chosen number turned to either 7 (instead of 5) or 27 (instead of 25). Although these trials were unexpected, the magnitude of the gain or loss was virtually unchanged. Additionally, each run included 12 randomized fixation trials that lasted 20 s.
Participants were provided with an initial 10 € sum and were encouraged to win as much as possible. The experiment comprised four blocks of 140 trials each. At the end of each block, participants were informed about the accumulated amount of money and were paid the final amount at the end of the experiment.
fMRI scanning methods.
fMRI data were collected using a 3T whole-body MRI scanner (Siemens Magnetom Trio). Visual images were back-projected onto a screen using an LED projector and participants viewed the images through a mirror on the head coil. Magnet-compatible response buttons were used. Conventional high-resolution structural images [magnetization-prepared rapid-acquisition gradient echo sequence, 192 slice sagittal, repetition time (TR) = 2500 ms, echo time (TE) = 4.77 ms, inversion time = 1100 ms, flip angle = 7°, 1 mm thickness (isotropic voxels)] were followed by functional images sensitive to blood oxygenation level-dependent contrast (BOLD; echo planar T2*-weighted gradient echo sequence, TR = 2000 ms, TE = 30 ms, flip angle = 80°). Each functional run consisted of 336 sequential whole-brain volumes comprising 32 axial slices aligned to the plane intersecting the anterior and posterior commissures, 3.5 mm in-plane resolution, 4 mm thickness, no gap, positioned to cover all but the most superior region of the brain and the cerebellum.
Functional images were analyzed using standard procedures implemented in the Statistical Parameter Mapping software (SPM2, http://www.fil.ion.ucl.ac.uk/spm). The preprocessing included slice timing, realignment, normalization, and smoothing, as described previously (Camara et al., 2008).
The statistical evaluation was based on a least-square estimation using the general linear model by modeling the different conditions with a regressor waveform convolved with a canonical hemodynamic response function (Friston et al., 1998). Thus, an event-related design matrix was created including the conditions of interest: gain 5, gain 25, gain 7/27, gain 125, loss 5, loss 25, loss 7/27, loss 125, and fixation. The data were high-pass filtered (to a maximum of 1/90 Hz), and serial autocorrelations were estimated using an autoregressive model. Resulting estimates were used for nonsphericity correction during the model estimation. Confounding effects in global mean were removed by proportional scaling, and signal-correlated motion effects were minimized by including the estimated movement parameters. The individual contrast images were entered into a second-level analysis using a one-sample t test using a random-effects analysis within the general linear model.
DTI data were collected using a diffusion tensor spin echo echoplanar imaging sequence. Diffusion weighting was conducted using the standard twice refocused spin echo method. Images were measured using 2-mm-thick slices, no gap, TR = 4200 ms, TE = 74 ms, 128 × 128 acquisition matrix, interpolated by zero padding to 256 × 256, field of view = 28 cm, 64 axial slices. To obtain diffusion tensors, diffusion was measured along 12 noncollinear directions, chosen according to the DTI acquisition scheme proposed by Papadakis et al. (1999), and the values specified by Skare et al. (2000) using a single b-value of 1000 s/mm2. Three runs were acquired per slice and diffusion gradient direction.
The FA was calculated from the directionally dependent signal decay due to diffusion (Pierpaoli and Basser, 1996; Beaulieu, 2002). FA approximates the degree to which water diffuses preferentially in one direction.
DTI data were processed using SPM2, as reported previously (Càmara et al., 2007; Fuentemilla et al., 2009). To ensure that the observed effects were restricted to white matter regions, a mask image was computed (constrained to those voxels with FA values >0.15 for all individuals) and results were masked to such a threshold value. This cutoff allowed us to reliably isolate white matter from other brain tissues (Jones et al., 1999).
Main analyses.
The current analysis was constrained to the boost trials in which the unexpectedness and thereby the corresponding reward outcome activity was maximal [gain (125) vs loss (125)]. The contrast was thresholded at p < 0.05, corrected for multiple comparisons (Worsley et al., 1995; Ashburner and Friston, 1999). The maxima of suprathreshold regions were localized by rendering them onto the mean volunteers' normalized T1 structural images on the MNI reference brain. Maxima and all coordinates are reported in MNI coordinates, as used by SPM and labeled in line with the Talairach atlas.
Voxelwise t tests were performed to detect those voxels in which the slope of FA data against BOLD-related (or psychometric) measures was significantly different from zero. Previously normalized FA images were individually correlated on gains, losses, and the difference of the β values derived from the loss-versus-gain contrast by applying a simple correlation SPM2 model. The gain-versus-loss contrast was inverted to loss-versus-gain based on previous results (Tom et al., 2007; Sokol-Hessner et al., 2009) for the sake of interpretation. Only the correlation peak significant at the cluster level (uncorrected p < 0.001) is reported and discussed in the text. However, for illustration purposes, significance levels from the correlation analysis are displayed at two different uncorrected thresholds: p < 0.001 and p < 0.01. The use of this gradual threshold allows the visualization of the underlying white matter path. The maxima of suprathreshold regions were labeled by using the white matter fiber tracts from the DTI brain atlas by Wakana et al. (2004) and Catani and Thiebaut de Schotten (2008). Maxima and all coordinates are reported in MNI coordinates.
Additionally, a region of interest analysis was performed to confirm voxel-based findings. Therefore, from the main significant cluster, FA value was averaged across the whole region of interest and correlated with the difference of the β values derived from the loss and gain unexpected boost conditions. Significance was determined by using a standard two-tailed t test analysis and Pearson's correlation was used to determine the correlation coefficients.
Additional correlations were performed between FA values and psychometric measures and between BOLD response and psychometric measures.
Results
fMRI results
The gain-versus-loss contrast showed revealed the left ventral striatum/NAcc (peak coordinates: −8, 4, −8 mm; T = 6.48, p < 0.05 family-wise error-corrected at whole-brain level; 20 voxels spatial extent) (Fig. 1A). In contrast, we did not find any area which was more activated for the loss-versus-gain trials, which suggests that the same brain network is involved in processing positive and negative outcomes, although with a differential amount of activation (supplemental Fig. S1 and supplemental Table S1, available at www.jneurosci.org as supplemental material) (Dreher, 2007; Tom et al., 2007; Camara et al., 2008).
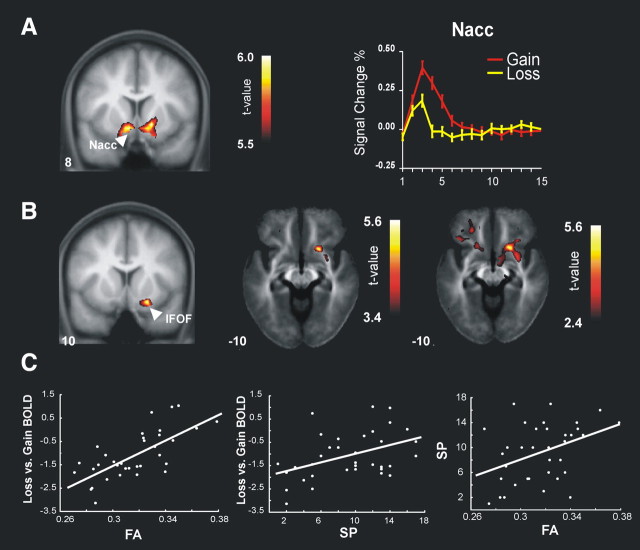
Figure 1.
A, Gain-versus-loss contrast superimposed on the group-averaged T1 MRI image in standard stereotactic space. Right, BOLD time course at the activation peak in the NAcc. B, Correlation between the difference of the β values (loss minus gain) and FA at the whole brain (p < 0.001, peak 22, 11, −12). t scores superimposed both on a coronal group-averaged T1 MRI image (left) and on axial views of the group-averaged FA image (right) using two different thresholds (p < 0.001 and p < 0.01) for the sake of visualization of the underlying white matter tracks in the region of interest. FA values at the right uncinate/IFOF predict the hemodynamic response at the ventral striatum region. C, Scatter plots showing the correlations between the difference in β value (loss vs gain) and FA value in the IFOF (R2 = 0.703, p < 0.00001) (left), the difference in β value (loss vs gain) and sensitivity to punishment (SP; R2 = 0.43, p < 0.001) (middle), and the IFOF FA value and SP (R2 = 0.43, p < 0.001) (right). All FA and β values are computed at the peak coordinates (22, 11, −12) of the uncinate/IFOF.
DTI-fMRI correlations
A positive correlation was found between the differential BOLD response (loss vs gain) and the corresponding FA values at the whole-brain level (p < 0.00001; peak coordinates: 22, 11, −12; R2 = 0.703, T = 5.68, p < 0.05 corrected at cluster level; 60 voxels spatial extent) (Fig. 1B). Specifically, FA values in the right uncinate/inferior fronto-occipital fasciculus (IFOF) predicted the hemodynamic response in the ventral striatum region (Fig. 1C, left). This result was observed bilaterally. When the BOLD response to gains and the BOLD response to losses (rather than their difference) were correlated with FA at the whole-brain level (p < 0.001, uncorrected), no significant regions emerged.
Correlations between fMRI, DTI, and reward-seeking tendencies
Those participants scoring higher on the SP dimension tended to show larger FA values (Fig. 1C, right). In addition, greater SP was associated to a larger differential activation between gain and loss trials in the gambling task. A similar significant correlation was observed only for the BOLD response in loss trials and SP.
Discussion
In the present study, we evaluated the hypothesis that reward-related activity in the NAcc and habitual tendencies in reward-related behavior are constrained by the structural properties of brain white matter, in particular in the vicinity of the ventral striatum, specifically the NAcc. The first major finding of this study is that reward-related activity in the NAcc was correlated with the microstructural properties of the uncinate/IFOF. The uncinate fasciculus is a ventral associative white matter pathway that connects the anterior temporal lobe with the medial and lateral orbitofrontal cortex (Ungerleider et al., 1989; Catani and Thiebaut de Schotten, 2008) and thus might mediate information flow between these structures. Also, interconnected frontotemporal regions supplied by the uncinate fasciculus have been implicated in emotion processing and memory (Gaffan and Wilson, 2008; Ross, 2008) and the encoding of prediction errors for monetary rewards (Ramnani et al., 2004). Importantly, both structural and functional MRI measures were also linked to stable behavioral tendencies related to SP.
Although it seems that the peak of the correlation is seen in the uncinate/IFOF fibers, it is important to note that several additional pathways, such as the anterior thalamic projections between ventral anterior thalamic nuclei and the orbitofrontal cortex as well as frontostriatal connections to the nucleus accumbens, also pass near this region (Wakana et al., 2004). Previous DTI tracking studies centered on the ventral striatum have shown fibers connecting the medial orbitofrontal cortex, the ventromedial frontal pole, the uncus, and the temporal pole (Lehéricy et al., 2004). Specific connections also exist between the subgenual part of the anterior cingulate cortex, the orbitofrontal cortex, and the NAcc and from there to the medial temporal lobe, either via a direct path running laterally through the amygdala or via a more posterior path along the fornix to the hippocampus (Johansen-Berg et al., 2008). These corticolimbic pathways identified in humans by using DTI measurements are in agreement with the information provided from tracing studies in monkeys. In these studies, it has been shown that the ventral striatum receives afferents projections from the medial and orbitomedial prefrontal cortex (Haber et al., 1995; Groenewegen et al., 1999) and limbic structures (Alheid and Heimer, 1988), including the amygdala (Fudge et al., 2002), the entorhinal cortex and hippocampus (Carmichael and Price, 1996), and the anterior cingulate cortex (Friedman et al., 2002). The ventral striatum is also target of dense dopaminergic innervations from the ventral mesencephalon.
The strong correlation observed between FA, BOLD response, and SP suggests that differences in reward processing are related to the quality of the anatomical connections of the reward-related regions mentioned above. Regarding the correlation between BOLD and FA, it has been proposed that the BOLD response reflects local field potential activity, which is thought to represent the averaged synaptic input to the dendritic tree rather than its spiking output (Logothetis, 2002). Thus, the correlation between BOLD and FA can be attributed to the constraints imposed by the anatomical characteristics of the connecting white matter tracts on gray matter functioning. FA values are thought to reflect the axonal microstructure (e.g., axon size, caliber, fiber density, and the extent of myelinization, and indirectly the quality of axonal transmission) (Waxman and Bennett, 1972), which is susceptible to experience-dependent changes (Demerens et al., 1996; Fields, 2005). Results similar to the present findings have been previously obtained in other domains, where DTI-FA measures predicted brain activations related to visual processing (Toosy et al., 2004) or personality measures (Cohen et al., 2009). Crucially, FA changes have been reported as a function of long-term musical training or a short training of a specific skill (Bengtsson et al., 2005; Scholz et al., 2009; Ullen, 2009).
We speculate that the correlation between reward-related BOLD response in the ventral striatum and FA measures in the surrounding white matter reflect neural plasticity of the white matter tracts induced by individual variations in experience. Larger FA values in the present sample might reflect increased efficiency in transmitting information within the cortical–limbic network involved in reward processing, which should be reflected in a specific change at the level of the averaged synaptic input to a given area. Importantly, the BOLD response in the ventral striatum is supposed to be influenced by the firing of the dopaminergic midbrain neurons (Schott et al., 2008). Previous pharmacological MRI studies also suggest a positive relationship between striatal dopamine levels and hemodynamic reward responses (Knutson and Gibbs, 2007). Indeed, the decrease of the BOLD response in the ventral striatum as the size of a potential loss increased is consistent with primate electrophysiological recordings showing decreased midbrain dopamine neural firing for negative events (Mirenowicz and Schultz, 1996). Thus, the reduction of the dopaminergic input to the NAcc could lead to a reduced BOLD signal. Notice that midbrain dopaminergic responses are also regulated by an indirect excitatory influence of the ventral striatum to ventral pallidum neurons, which in turn tonically inhibit the substantia nigra/ventral tegmental area (Grace et al., 2007). In addition, the amygdala and the hippocampus show reward-related activations and because of their connections with the NAcc (see above), they could also modulate the striatal BOLD response (Camara et al., 2009; Lisman and Grace, 2005). The prefrontal cortex, in particular the orbitofrontal, ventromedial and dorsolateral prefrontal, and insular cortexes could also modulate the striatal BOLD response via frontostriatal connections.
To reiterate, larger FA values in our study were associated with a decreased difference between gains and losses (Table 1; Fig. 1C). A decreased BOLD response to monetary losses might reflect individual differences in loss aversion, most probably related to differences in dopamine function (Tom et al., 2007). In the present study, FA showed a positive correlation to the BOLD response in loss trials (Table 2) and a (nonsignificant) negative correlation to the BOLD response in gain trials. Thus, the correlation between the (loss-minus-gain)-BOLD difference and FA is partly due to the increased BOLD response observed in loss trials for those participants showing larger FA values. In such participants, the larger BOLD response for loss trials resulted in a loss-minus-gain difference close to zero, which implies that larger FA values in the uncinate fasciculus (UF)/IFOF or nearby white matter modulate the transmission of the information of negative events to the nucleus accumbens.
Table 1.
Correlations between the psychometric measures and the BOLD response
| Gain | Loss | Loss − gain | |
|---|---|---|---|
| Sensitivity to punishment | −0.09 | 0.32 | 0.43 |
| n.s. | p < 0.065 | p < 0.01 | |
| Sensitivity to reward | 0.37 | 0.28 | −0.162 |
| p < 0.03 | n.s. | n.s. |
n.s., Not significant.
Table 2.
Correlations between FA, psychometric measures and the BOLD response
| Sensitivity to punishment | Sensitivity to reward | Gain | Loss | Loss − gain | |
|---|---|---|---|---|---|
| FA | 0.43 | 0.162 | −0.258 | 0.39 | 0.703 |
| p < 0.01 | n.s | n.s | p < 0.02 | p < 0.0001 |
n.s., Not significant.
In relation to the coupling between FA and SP, our findings are nicely complemented by a recent study (Craig et al., 2009) reporting a significant reduction of FA values in the right uncinate fasciculus in psychopathic subjects when compared with a control group. This was interpreted in terms of a possible reduction of connectivity in the amygdala–orbitofrontal limbic network through the UF. As disinhibitory behavior in psychopaths is characterized by a decreased SP (Fowles, 1980), our results indeed agree with the previous ones, as we encountered a positive correlation between SP and FA in similar UF/IFOF regions (Fig. 1C). Thus, the observed tridirectional pattern of correlations between white matter microstructure, reward-related BOLD response of the NAcc, and trait SP opens up new possibilities for the investigation of behavioral disorders, such as drug abuse, obesity, and pathological gambling, which have been associated to diminished activation in reward-related regions (Volkow et al., 2008).
Footnotes
E.C. is supported by a postdoctoral grant from the Catalan Government (Beatriu de Pinos program). A.R.F. is supported by a grant from the Spanish (PSI2008–03901) and the Catalan Governments (SGR 2009 SGR 93). T.F.M. is supported by grants from the Deutsche Forschungsgemeinschaft (SFB779 TP5) and the Bundesministerium für Bildung und Forschung.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis: a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H. Relating connectional architecture to grey matter function using diffusion imaging. Philos Trans R Soc Lond B Biol Sci. 2005;360:903–911. doi: 10.1098/rstb.2005.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Càmara E, Bodammer N, Rodríguez-Fornells A, Tempelmann C. Age-related water diffusion changes in human brain: a voxel-based approach. Neuroimage. 2007;34:1588–1599. doi: 10.1016/j.neuroimage.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Münte TF. Functional connectivity of reward processing in the brain. Front Hum Neurosci. 2008;2:19. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Ye Z, Münte TF. Reward networks in the brain as captured by connectivity measures. Front Neurosci. 2009;3:350–362. doi: 10.3389/neuro.01.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, McGuire PK, Fahy T, Murphy DG. Altered connections on the road to psychopathy. Mol Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC. Sensitivity of the brain to loss aversion during risky gambles. Trends Cogn Sci. 2007;11:270–272. doi: 10.1016/j.tics.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: implications of Gray's two factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Càmara E, Münte TF, Krämer UM, Cunillera T, Marco-Pallarés J, Tempelmann C, Rodriguez-Fornells A. Individual differences in true and false memory retrieval are related to white matter brain microstructure. J Neurosci. 2009;29:8698–8703. doi: 10.1523/JNEUROSCI.5270-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Wilson CR. Medial temporal and prefrontal function: recent behavioural disconnection studies in the macaque monkey. Cortex. 2008;44:928–935. doi: 10.1016/j.cortex.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray J. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal systems. Oxford: Oxford UP; 1982. [Google Scholar]
- Groenewegen HJ, Mulder AB, Beijer AVJ, Wright CI, Lopes da Silva FH, Pennartz CMA. Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology. 1999;27:149–164. [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;7:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SC, Markus HS. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke. 1999;30:393–397. doi: 10.1161/01.str.30.2.393. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Huang CL, Hall LD, Carpenter TA. A comparative study of acquisition schemes for diffusion tensor imaging using MRI. J Magn Reson. 1999;137:67–82. doi: 10.1006/jmre.1998.1673. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23:777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Ross ED. Sensory-specific amnesia and hypoemotionality in humans and monkeys: gateway for developing a hodology of memory. Cortex. 2008;44:1010–1022. doi: 10.1016/j.cortex.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson. 2000;147:340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hsu M, Curley NG, Delgado MR, Camerer CF, Phelps EA. Thinking like a trader selectively reduces individuals' loss aversion. Proc Natl Acad Sci U S A. 2009;106:5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Ciccarelli O, Parker GJ, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Characterizing function-structure relationships in the human visual system with functional MRI and diffusion tensor imaging. Neuroimage. 2004;21:1452–1463. doi: 10.1016/j.neuroimage.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Pers Individ Dif. 2001;31:837–862. [Google Scholar]
- Ullén F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biology. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Bennett MV. Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol. 1972;238:217–219. doi: 10.1038/newbio238217a0. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Vandal AC, Friston KJ. Tests for distributed, nonfocal brain activations. Neuroimage. 1995;2:183–194. doi: 10.1006/nimg.1995.1024. [DOI] [PubMed] [Google Scholar]