ABSTRACT
Centrins are EF-hand containing proteins ubiquitously found in eukaryotes and are key components of centrioles/basal bodies as well as certain contractile fibers. We previously identified three centrins in the human parasite Toxoplasma gondii, all of which localized to the centrioles. However, one of them, T. gondii (Tg) Centrin2 (CEN2), is also targeted to structures at the apical and basal ends of the parasite, as well as to annuli at the base of the apical cap of the membrane cortex. The role(s) that CEN2 play in these locations were unknown. Here, we report the functional characterization of CEN2 using a conditional knockdown method that combines transcriptional and protein stability control. The knockdown resulted in an ordered loss of CEN2 from its four compartments, due to differences in incorporation kinetics and structural inheritance over successive generations. This was correlated with a major invasion deficiency at early stages of CEN2 knockdown, and replication defects at later stages. These results indicate that CEN2 is incorporated into multiple cytoskeletal structures to serve distinct functions that are required for parasite survival.
KEY WORDS: Toxoplasma, Centrin, Invasion, Parasite, Replication
Highlighted Article: Centrin2 from the human parasite Toxoplasma gondii is incorporated into multiple cytoskeletal structures to serve distinct functions in invasion and replication that are required for its survival.
INTRODUCTION
Centrin-like proteins were first discovered in the ciliated protozoan Zoothamnium geniculatum as a component of the spasmoneme, an organelle that contracts in response to Ca2+ binding (Amos et al., 1975). Subsequently, centrins were found to be components of the centrioles/basal bodies and to play an important role in regulating centrosomal duplication, and flagellar biogenesis and function in the cells of many animals and protozoa (Salisbury, 1995, 2007; Sanders and Salisbury, 1994; Wright et al., 1985).
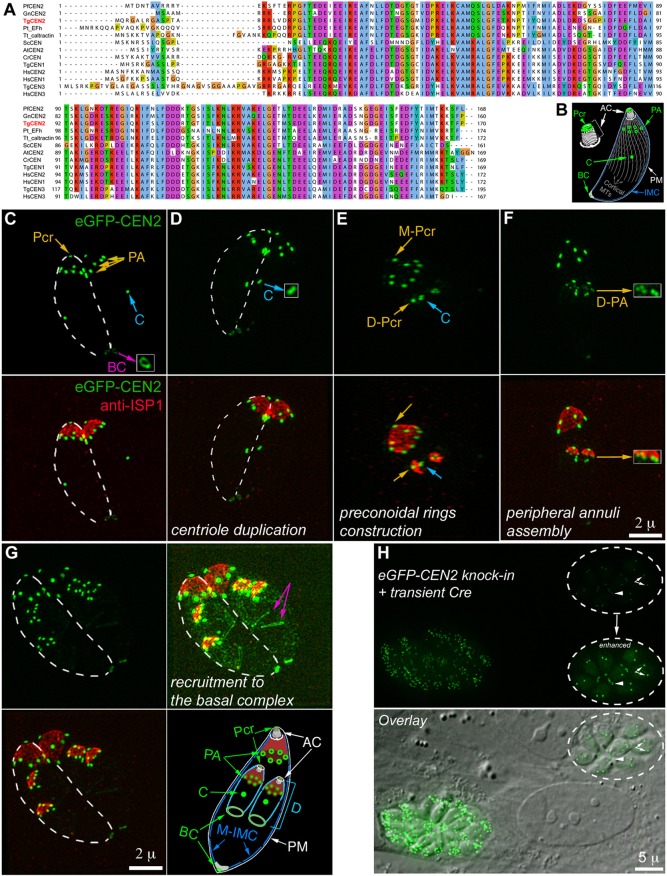
We previously identified three centrins in Toxoplasma gondii, a human parasite that causes devastating toxoplasmic encephalitis in immunocompromised individuals and unprotected fetuses. As with canonical centrins in other systems, T. gondii (Tg) Centrin 1 and 3 (CEN1 and CEN3) are predominantly localized to the centrioles. Although TgCentrin2 (CEN2) shares a high degree of sequence similarity with CEN1 and CEN3 (Fig. 1A), its localization is remarkably different (Hu et al., 2006). In addition to the centrioles, ectopically expressed CEN2 tagged with eGFP also localized to three other structures: a ring-shaped complex at the apex of the parasite (the preconoidal rings), a capping structure at the basal end (the basal complex), as well as approximately five or six peripheral annuli located about one-quarter of a parasite length below the apex (Hu et al., 2006) (Fig. 1B). The location of the CEN2 annuli coincides with the boundary between the apical cap and the rest of the parasite membrane cortex, as marked by the inner membrane complex (IMC) sub-compartment proteins (ISPs) (Beck et al., 2010).
Fig. 1.
Phylogenetic analysis of selected centrin homologs and the localization of CEN2 in T. gondii. (A) Alignment of T. gondii CEN1, CEN2 and CEN3 and selected centrin homologs in other organisms using the multiple alignment program MUSCLE. PfCEN2 XP_001348617.1 (GenBank), GnCEN2 XP_011129982.1, TgCEN2 TGGT1_250340 (ToxoDB), Pt_Efh XP_001441649.1, Tt_caltractin XP_001023350.1b, ScCEN NP_014900.3, AtCEN2 NP_190605.1, CrCEN XP_001699499.1, TgCEN1 TGGT1_247230, HsCEN2 NP_004335.1, HsCEN1 NP_004057.1, TgCEN3 TGGT1_260670, HsCEN3 NP_004356.2, Pf, Plasmodium falciparum; Gn, Gregarina niphandrodes; Tg, Toxoplasma gondii; Pt, Paramecium tetraurelia; Tt, Tetrahymena thermophila; Sc, Saccharomyces cerevisiae; At, Arabidopsis thaliana; Cr, Chlamydomonas reinhardtii; Hs, Homo sapiens. (B) Diagram of an interphase parasite in which CEN2-containing structures are highlighted in green. Pcr, preconoidal rings; PA, peripheral annuli; C, centrioles; BC, basal complex; AC, apical complex; PM, plasma membrane; IMC, inner membrane complex; Cortical MTs, cortical microtubules. (C–G) Projections of 3D-SIM images of eGFP-CEN2 knock-in parasites at different stages of the cell cycle labeled with a mouse anti-ISP1 antibody. Insets (2×) in C, D and F are contrast enhanced and include regions indicated by the arrows. Dashed lines in C, D and G indicate the approximate outline of one of the two parasites in the same vacuole. The diagram in G highlights the localization of CEN2 (green) and ISP1 (red) with respect to the IMC and the plasma membrane. ‘M-’, mother structures; ‘D-’, daughter structures; D, daughter. Other abbreviations are the same as in B. Green, eGFP–CEN2; red, anti-ISP1. Scale bars: 2 µm. (H) Fluorescence (top) and fluorescence/DIC overlay (bottom) images of eGFP-CEN2 knock-in parasites at ∼48 h after transfection with a plasmid expressing Cre recombinase. There are two vacuoles in the field. Dashed circles indicate the vacuole in which eGFP–CEN2 expression has decreased significantly. Inset (1×) is contrast enhanced to visualize residual eGFP–CEN2 signal in the centrioles (arrows) and basal complex (arrowheads) of the parasites in this vacuole. Scale bar: 5 µm.
We were interested in understanding what function(s) CEN2 plays in the four distinct structures that it targets. After many failed attempts with established gene manipulation approaches to delete the CEN2 gene or downregulate its expression, we knocked down CEN2 expression in T. gondii by using a dual-regulation approach that combines anhydrotetracycline (ATc)-mediated transcription suppression and ligand-regulatable FKBP protein destabilization domain (ddFKBP)-mediated protein degradation. We discovered that CEN2 was depleted from its four locations with different kinetics. The loss of CEN2 from the two more apically located structures, the preconoidal rings and peripheral annuli, occurred earlier, followed by significant CEN2 depletion from the centrioles and basal complex. This was correlated with a major inhibition of parasite invasion evident at early stages of CEN2 knockdown followed by replication defects that developed at later stages. This suggests that CEN2 is critical for multiple aspects of the parasite lytic cycle and that its associated structures comprise potential targets for anti-parasitic measures.
RESULTS
The localization of CEN2 as determined in eGFP–CEN2 knock-in parasites
In the previous studies, the localization of CEN2 was characterized by ectopic expression. To confirm its localization pattern, we replaced the endogenous CEN2 gene with eGFP–CEN2 through homologous recombination. The resulting eGFP–CEN2 knock-in parasite line shows that CEN2 is localized to the preconoidal rings, peripheral annuli, centrioles and basal complex (Fig. 1B–G), the same pattern as ectopically expressed eGFP–CEN2 (Hu, 2008; Hu et al., 2006). Fig. 1C–G includes images of parasites (cultured in human foreskin fibroblasts) at different stages of cell division - a ‘born-within’ type of replication during which the cortical cytoskeleton of the daughter parasite is built inside the mother (Hu et al., 2002; Nishi et al., 2008; Sheffield and Melton, 1968). Of the CEN2-containing structures, duplication of the centrioles occurs first (Fig. 1D, inset), followed by construction of the preconoidal rings (Fig. 1E), and then the peripheral annuli, which appear after the ISP1 cap is assembled (Fig. 1F,G). Recruitment of CEN2 to the daughter basal complex becomes evident only at a late stage of daughter assembly (Fig. 1G).
Unsuccessful attempts to generate CEN2 knockout and knockdown mutants using several established methods
In the knock-in line, the eGFP-CEN2 coding sequence and the HXGPRT drug selection cassette are flanked with LoxP sites, which allows for the excision of this locus upon transfection with Cre recombinase-expressing plasmids. Indeed, significant loss of eGFP–CEN2 fluorescence was observed in many parasites at ∼48 h after Cre transfection (Fig. 1H). However, numerous attempts to isolate CEN2-knockout clones failed. We also failed to recover CEN2-knockout clones using a CRISPR-Cas9 based gene deletion strategy (data not shown), suggesting that the complete loss of CEN2 results in lethality. This result is consistent with the fitness score for CEN2 (−4.4) obtained by Sidik et al. (2016).
The functions of many presumed essential genes have been characterized by using the anhydrotetracycline (ATc)-based knockdown method (Meissner et al., 2001, 2002; Mital et al., 2005). Therefore, we attempted to regulate CEN2 expression by generating a line in which the expression of mAppleFP-CEN2 was driven by the TetO7Sag4 promoter and could be downregulated with ATc, which binds to the tetracycline-inducible transactivator (TATi) to inhibit transcription initiation by the promoter. We found that while the ATc treatment significantly downregulated mAppleFP-CEN2 expression in the initial passages, the ATc control of CEN2 expression waned considerably over time (data not shown), indicating that ATc-based transcriptional control alone is not sufficient to maintain robust regulation of CEN2 expression.
Combining transcriptional and translational controls allow for more robust and stable knockdown of CEN2
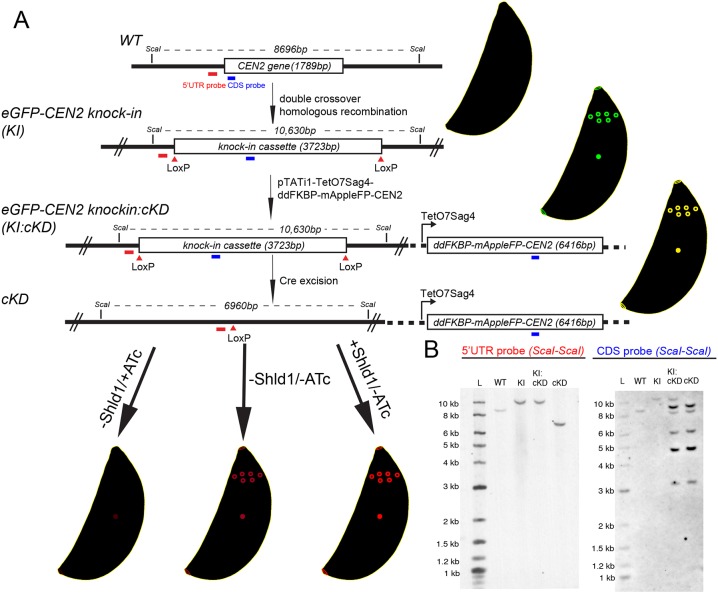
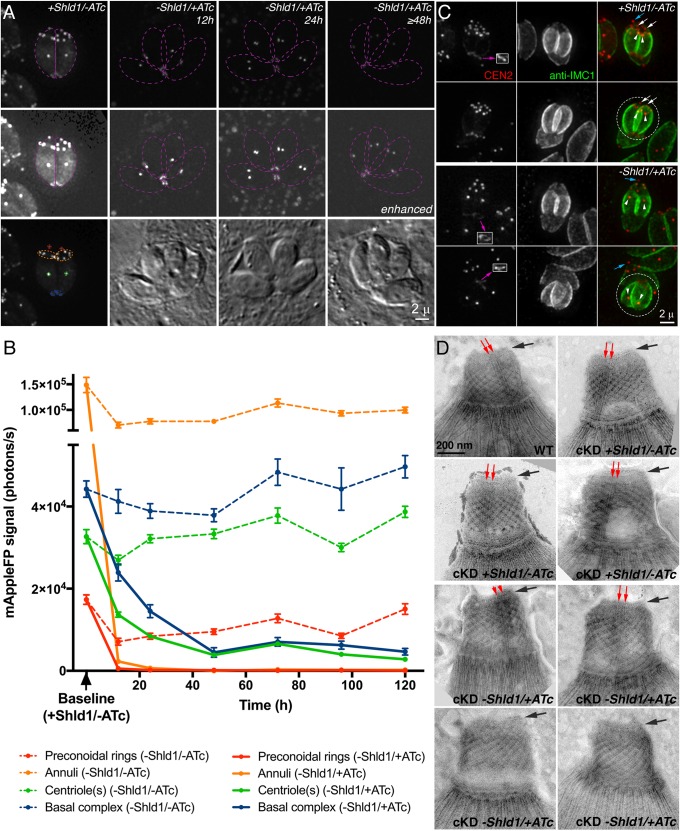
To achieve tighter regulation of CEN2 expression, we combined the ATc transcriptional regulation system with translational control. Specifically, the eGFP-CEN2 knock-in parasites were stably transfected with a plasmid that contains a ddFKBP-mAppleFP-CEN2 expression cassette controlled by the TetO7Sag4 promoter, as well as an expression cassette for TATi (eGFP-CEN2 knock-in: ddFKBP-mAppleFP-CEN2 cKD parasites, referred to as ‘KI:cKD’ for simplicity). Upon excision of eGFP-CEN2 from the endogenous locus through transient Cre recombinase expression, clones (ddFKBP-mAppleFP-CEN2 cKD parasites, referred to as ‘cKD’) were isolated and maintained in the presence of Shield-1 (Shld1), which binds to and stabilizes the ddFKBP domain, thus blocking degradation of ddFKBP–mAppleFP–CEN2 (Fig. 2). The cellular level of ddFKBP–mAppleFP–CEN2 from the TetO7Sag4 promoter therefore can be controlled not only with ATc (regulating transcription), but also with Shld1, the absence of which leads to proteasome-mediated degradation of the fusion protein (Banaszynski et al., 2006; Herm-Götz et al., 2007). Fig. 3A shows that ddFKBP–mAppleFP–CEN2 correctly localized to all of the CEN2-containing structures, and downregulation of ddFKBP–mAppleFP–CEN2 expression was achieved by withdrawing Shld1 from the culture and adding ATc (−Shld1/+ATc) to a final concentration of 270 nM. To measure the kinetics of CEN2 loss, images of parasitophorous vacuoles that contained two to eight parasites were captured after 12 to 120 h of Shld1 withdrawal and ATc treatment, and mAppleFP fluorescence signals in the preconoidal rings, peripheral annuli, centriole(s) and basal complex were quantified. While Shld1 withdrawal alone (−Shld1/−ATc) resulted in a modest reduction of the mAppleFP–CEN2 signal in the preconoidal rings and annuli, CEN2 localization in these structures became undetectable 12 h after Shld1 withdrawal and ATc addition (−Shld1/+ATc) (Fig. 3B). The depletion kinetics for CEN2 in the centrioles and the basal complex were very different. Withdrawal of Shld1 alone (−Shld1/−ATc) did not result in a significant decrease of the mAppleFP signal in these structures. After 12 h of −Shld1/+ATc treatment, the level of mAppleFP was ∼42±2% (centrioles) and 54±5% (basal complex) of the fluorescence in the corresponding structures in cKD parasites without downregulation (mean±s.e.m.) [Fig. 3B, Baseline (+Shld1/−ATc)], and ∼12±1% (centrioles) and ∼10±3% (basal complex) by 48 h. This low level of mAppleFP–CEN2 signal remained detectable even after 120 h of ATc treatment (Fig. 3B). As it is unlikely that a protein can persist for ∼15–20 generations of the parasite [the parasite doubling time has been estimated to be ∼6–8 h (Black and Boothroyd, 2000)], this lingering signal might reflect a residual level of continuing CEN2 synthesis. We observed a similar ordered and differential loss of eGFP–CEN2 from the four compartments in our attempts to generate CEN2-knockout mutants when using the Cre-LoxP-based method (Fig. 1H).
Fig. 2.
Generation of the CEN2 knock-in and cKD parasite lines. (A) Schematic for generating eGFP-CEN2 knock-in (KI), eGFP-CEN2 knock-in:cKD (KI:cKD) and cKD parasites, and Southern blotting strategy. Positions of the ScaI restriction sites, CDS probe (blue) and the probe annealing upstream of the CEN2 coding sequence (5′UTR probe, red) used in the Southern blotting analysis and the corresponding expected DNA fragment sizes are indicated as shown. (B) Southern blotting analysis of the CEN2 locus in RHΔhx parasites (WT), KI, KI:cKD, and cKD parasites. For the CDS probe, the expected parasite genomic DNA fragment sizes after ScaI digestion are 8696 base pairs for the WT (i.e. wild-type CEN2 locus), 10,630 base pairs for the eGFP-CEN2 cassette in the KI and KI:cKD parasites, and variable for the multiple, random integrations of the cKD plasmid (pTATi1-TetO7Sag4-ddFKBP-mAppleFP-CEN2) since the fragment sizes would depend on where the plasmid integrated relative to ScaI sites in the parasite genome. The expected DNA fragment sizes for the upstream probe are the same as for the CDS probe for the WT, KI and KI:cKD parasites, and 6960 base pairs for the cKD parasites after Cre excision of the eGFP-CEN2 cassette. L, ladder.
Fig. 3.
Quantification of CEN2 downregulation in cKD parasites, and ultrastructural analysis of the cytoskeletal apical complex in CEN2-depleted parasites. (A) Representative fluorescence and DIC images of cKD parasites in two to four parasite vacuoles cultured in the presence of Shld1 (+Shld1/−ATc), or 12, 24 and 48 h after withdrawal of Shld1 and addition of ATc (−Shld1/+ATc). The image of the vacuole cultured in −Shld1/+ATc medium for 48 h is representative of vacuoles imaged at subsequent time points (i.e. 72, 96 and 120 h in −Shld1/+ATc medium). Individual parasites are outlined in dashed purple lines. The image of the cKD +Shld1/−ATc parasites is additionally marked with dashed lines to mark the four compartments that CEN2 targets: the preconoidal rings (red), peripheral annuli (orange), centrioles (green) and basal complex (blue). Images in each row were acquired and processed under the same conditions. Images in the middle row were highly contrast enhanced to display low mAppleFP–CEN2 signal in the parasites. Scale bar: 2 µm. (B) Intensity measurements of mAppleFP–CEN2 in the preconoidal rings (red), peripheral annuli (orange), centrioles (green) and basal complex (blue) in cKD parasites 12–120 h after −Shld1/−ATc (dashed lines) or −Shld1/+ATc treatment (solid lines). Baseline, average mAppleFP intensity of individual CEN2-containing structures in cKD parasites cultured in +Shld1/−ATc. Note that the two segments of the y-axis are scaled differently to facilitate better visualization of the lower photon counts in the later time points. Results are mean±s.e.m. (C) Fluorescence images of cKD parasites cultured in the presence of Shld1 (+Shld1/−ATc) or treated for 14 h with ATc and no Shld1 (−Shld1/+ATc), in single parasite vacuoles before or undergoing the first round of cytokinesis post-invasion. Dashed circles indicate daughters emerging from the mother parasite. The blue and white arrows indicate the preconoidal rings in the maternal cytoskeleton and newly assembled daughters, respectively, and the white arrowheads indicate daughter centrioles. The daughter basal complexes indicated by the purple arrows are included in the insets (1×, contrast enhanced). Scale bar: 2 µm. (D) Negative staining of whole mount detergent-extracted parasites to visualize the apical cytoskeletal structure of wild-type parasites (WT), cKD parasites cultured in the presence of Shld1 (cKD, +Shld1/−ATc) or treated for 144 h with ATc and no Shld1 (cKD, −Shld1/+ATc). The structure of the preconoidal rings (black arrows) appears to be normal after CEN2 depletion, but the intra-conoid microtubules (red arrows) are not detectable in some CEN2 knockdown parasites (cKD, −Shld1/+ATc, bottom row). Scale bar: 200 nm.
The role of structural inheritance in the differential depletion of CEN2 from the four compartments
As shown in Fig. 1D–G, the daughter cortical cytoskeleton is built anew inside the mother during the replication of Toxoplasma and other apicomplexan parasites, but some cytoskeletal structures, such as the centrioles, are replicated and inherited from the mother (Hu, 2008; Hu et al., 2002). The kinetics of depletion for a protein component from a structure are therefore affected by the synthesis of new protein, the dissociation and degradation rates of pre-existing protein, ‘dilution’ of a finite amount of protein over successive rounds of cellular replication, as well as whether the structure itself is synthesized de novo or inherited from the mother. To determine the effect of structural inheritance on CEN2 depletion from the four compartments during knockdown, cKD parasites maintained in Shld1 were allowed to invade, Shld1 was then further maintained or withdrawn, and 270 nM ATc was added to downregulate ddFKBP–mAppleFP–CEN2 for 14 h. Vacuoles containing a single parasite before or undergoing cytokinesis (i.e. before completion of the first round of replication post-invasion) were examined. In contrast to cKD parasites maintained in Shld1 where the mAppleFP signal was prominent in all CEN2-containing structures (Fig. 3C, +Shld1/−ATc), the ATc-treated parasites assembled daughters with CEN2 that was invariably undetectable in the preconoidal rings and peripheral annuli but prominent in the duplicated centrioles and the basal complex (Fig. 3C, −Shld1/+ATc). In some instances, the daughters emerged in an orientation that permitted observation of mAppleFP signal still present in the preconoidal rings and peripheral annuli of the disintegrating mother cytoskeleton (Fig. 3C, −Shld1/+ATc, bottom row), strongly suggesting that the maternal CEN2 in these structures was not reused to build the corresponding structures in the daughters.
Although the localization of the CEN2 annuli coincides with the posterior edge of the ISP1 ‘cap’ (Fig. 1), depletion of CEN2 from the peripheral annuli did not affect the localization of ISP1 even after 120 h of ATc treatment (Fig. S1A, −Shld1/+ATc). Taken together with a previous finding showing that the loss of ISP1 has no impact on CEN2 localization (Beck et al., 2010), these data suggest that the assembly and maintenance of the CEN2- and ISP1-containing compartments are independent processes. We then examined the ATc-treated cKD parasites by electron microscopy to investigate whether the depletion of CEN2 from the preconoidal rings and peripheral annuli affected the ultrastructure of the cytoskeletal apical complex. The CEN2-knockdown parasites (cKD, −Shld1/+ATc) were capable of extruding the conoid (Fig. 3D). The structure of the preconoidal rings in the extruded conoid of these parasites also appeared to be normal. However, in slightly under one-third of the parasites imaged (four out of 14), the intra-conoid microtubules were not detectable (Fig. 3D, bottom row). This is worth noting because the intra-conoid microtubules were always clearly visible in both wild-type parasites and cKD (+Shld1/−ATc) parasites prepared under the same conditions.
CEN2 is critical for the parasite lytic cycle
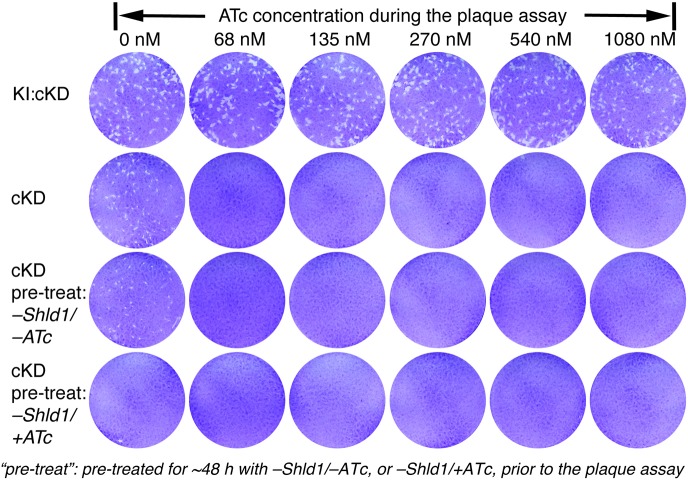
Toxoplasma is an obligate intracellular parasite. Its dissemination in the host relies on its ability to progress through multiple rounds of the lytic cycle, which consists of host cell invasion, parasite replication and egress. To determine the impact of the loss of CEN2 on the Toxoplasma lytic cycle, we examined the efficiency of the parasite in forming plaques when CEN2 is knocked down in cKD parasites using a range of ATc concentrations, with different pre-treatment conditions for 2 days prior to the plaque assay (+Shld1/−ATc, −Shld1/−ATc, or −Shld1/+ATc) (Fig. 4). For all pre-treatment conditions, the cKD parasites did not form plaques when subsequently cultured in the absence of Shld1 with an ATc concentration of 68 nM or higher, while the parental strain (KI:cKD) had comparable plaque-forming efficiencies under all ATc concentrations tested (0 to 1080 nM). No plaques were observed when the cKD parasites were pre-treated with 270 nM ATc for 2 days, even when there was no ATc added to the culture medium used in the plaque assay. This suggests that CEN2 plays an important role in the ability of the parasite to progress through multiple rounds of the lytic cycle.
Fig. 4.
The effect of CEN2 downregulation on the parasite lytic cycle. Plaques formed in the presence of 0, 68, 135, 270, 540 and 1080 nM ATc and without Shld1 by the KI:cKD and cKD parasites. The KI:cKD parasites were maintained in −Shld1/−ATc medium and the cKD parasites were maintained in +Shld1/−ATc medium prior to the plaque assay. Pre-treat, the cKD parasites were pre-treated for ∼48 h with no drug (−Shld1/−ATc), or 270 nM ATc (−Shld1/+ATc). Each well of HFF monolayers was infected with 100 parasites, grown for 6 days at 37°C, and then fixed and stained with Crystal Violet. Host cells that remained intact absorbed the Crystal Violet staining, whereas regions of host cells lysed by the parasites (‘plaques’) are clear.
Parasites deficient in CEN2 are significantly impaired in invasion but not egress
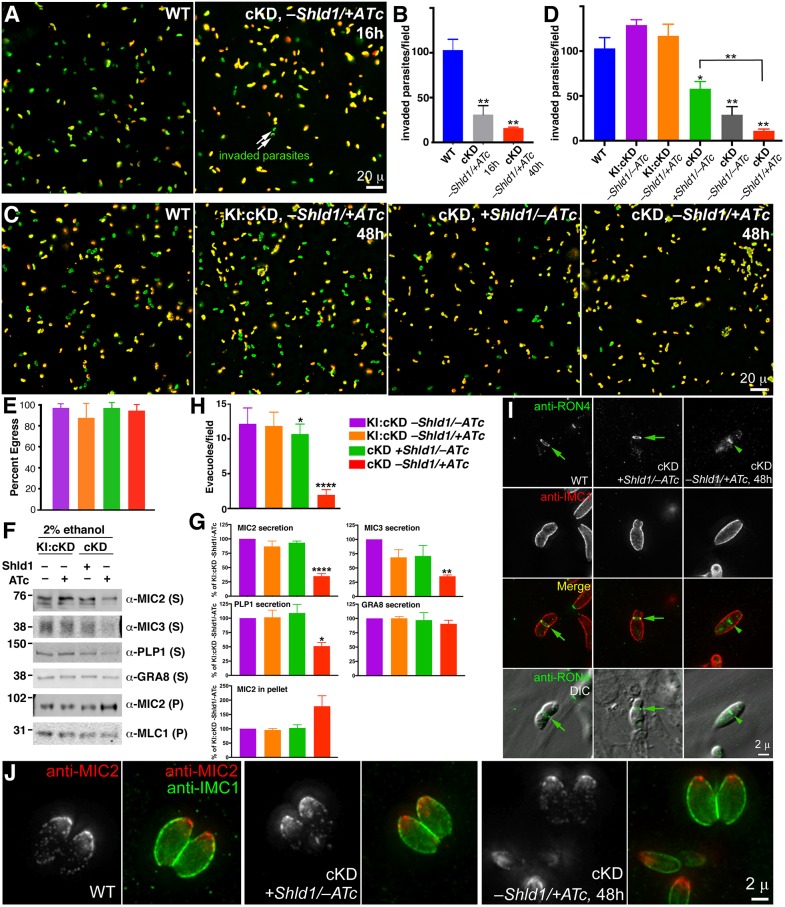
The lytic cycle consists of invasion, replication and egress. To determine how CEN2 might contribute to the steps of the lytic cycle, we first examined the ability of CEN2-knockdown parasites to invade host cells, using an established two-color immunofluorescence-based invasion assay. In this assay, the extracellular (non-invaded) and invaded parasites are differentially labeled due to a difference in antibody accessibility (Carey et al., 2004; Mital and Ward, 2008). Extracellular parasites have the outer surface of their plasma membrane fully exposed, and therefore can be directly labeled by an antibody against a surface antigen (SAG1). On the other hand, parasites that have invaded a host cell can only be labeled after permeabilization of the host cell. We discovered that after ∼16 h of −Shld1/+ATc treatment (a time point when CEN2 was nearly completely depleted from the preconoidal rings and peripheral annuli, and partially reduced in the centrioles and basal complex), the invasion efficiency of the cKD parasites was ∼30% relative to the wild type (P=0.009, Fig. 5A,B, Table 1). Upon ∼48 h of −Shld1/+ATc treatment, invasion by the cKD parasites decreased to ∼10% that of the wild-type parasite (P=0.0014) and ∼19% that of the cKD +Shld1/−ATc control (P=0.005) (Fig. 5C,D, Table 2).
Fig. 5.
The effect of CEN2 downregulation on parasite invasion, microneme secretion, egress, evacuole formation and moving junction formation. (A) Representative wide-field epifluorescence images of invasion by RHΔhx parasites (WT), and cKD parasites treated with ATc (−Shld1/+ATc) for 16 h. Parasites that are intracellular (i.e. have successfully invaded) are labeled green, and parasites that did not invade are labeled both green and red (i.e. appear yellow). Scale bar: 20 µm. (B) Quantification of the mean±s.e.m. number of invaded parasites per field for RHΔhx parasites (WT), and cKD parasites treated with ATc (−Shld1/+ATc) for 16 h or 40 h after Shld1 withdrawal. **P<0.01. (C) Representative wide-field epifluorescence images of invasion by RHΔhx parasites (WT), KI:cKD treated with ATc for 48 h, and cKD parasites cultured with Shld1 (+Shld1/−ATc), or treated with ATc (−Shld1/+ATc) for 48 h. Scale bar; 20 µm. (D) Quantification of the mean±s.e.m. number of invaded parasites per field for RHΔhx parasites (WT), KI:cKD cultured in −Shld1/−ATc, treated with ATc (−Shld1/+ATc) for 48 h, and cKD parasites cultured with Shld1 (+Shld1/−ATc), no drug (−Shld1/−ATc), or treated with ATc (−Shld1/+ATc) for 48 h. *P<0.05; **P<0.01 relative to WT or as indicated. See also Tables 1, 2 for quantification of invasion efficiency relative to WT parasites and the matrix of pairwise P values. (E) Percentage of vacuoles (mean±2× s.e.m.) from which parasites activated motility to egress within ∼6 min of treatment with 5 µM A23187. For each sample, a total of 12 randomly selected fields in two different dishes were analyzed. The color coding for each condition is as indicated, and the same as in G and H. (F) The unsecreted (pellet, P) and secreted (supernatant, S) fractions of eGFP-CEN2 knock-in:cKD (KI:cKD, −Shld1/−ATc or −Shld1/+ATc) and cKD (+Shld1/−ATc or −Shld1/+ATc) parasites upon ethanol stimulation, as probed by antibodies against MIC2, MIC3, PLP1 and GRA8, with MLC1 in the pellets as a loading control. The numbers on the left indicate molecular masses in kDa. (G) Levels of MIC2, MIC3, PLP1 and GRA8 in the secreted fractions, and MIC2 in the pellet fractions relative to those from KI:cKD, −Shld1/−ATc. The levels shown for MIC2 in the pellet were normalized to the levels of MLC1 in the pellet for each sample. Results are mean±s.e.m. from at least three independent biological replicates. *P<0.05; **P≤0.01; ****P<0.0001 relative to KI:cKD, −Shld1/−ATc. (H) Mean±s.e.m. number of evacuoles observed per field, formed by parasites upon treatment with cytochalasin D. The color coding for each condition is the same as in E. *P<0.05; ****P<0.0001 relative to KI:cKD, −Shld1/−ATc. Note that for KI:cKD −Shld1/±ATc, and cKD +Shld1/−ATc, the mean±s.e.m. were calculated from 10 randomly selected fields. For cKD −Shld1/+ATc, the mean±s.e.m. were calculated from 10 fields that had at least one evacuole each. The mean thus overestimates the efficiency of evacuole formation under this condition as most fields did not contain any evacuoles. (I) Wide-field deconvolution images of RHΔhx parasites (WT) and cKD parasites cultured with Shld1 (+Shld1/−ATc), or treated with ATc for 48 h after Shld1 withdrawal (−Shld1/+ATc), in which RON4 (green), and IMC1 (red, a marker for the cortex of mature and daughter parasites) were labeled by immunofluorescence after extracellular parasites were incubated with host cells for a short period of time (i.e. ‘pulse invasion’). Moving junctions (arrows) were readily observed for the WT and the cKD +Shld1/−ATc parasites, but never for CEN2-depleted parasites (cKD, −Shld1/+ATc, 48 h). The RON4 signal (arrowhead) close to the middle of the CEN2 depleted parasite might associate with the Golgi or other organelles in the secretory pathway. Scale bar: 2 µm. (J) Representative wide-field deconvolution images of RHΔhx parasites (WT) and cKD parasites cultured with Shld1 (+Shld1/−ATc), or treated with ATc for 48 h after Shld1 withdrawal (−Shld1/+ATc), in which IMC1 (green) and MIC2 (red) were labeled by immunofluorescence. Scale bar: 2 µm.
Table 1.
Quantification of invasion for the wild-type and cKD (−Shld1/+ATc) parasites
Table 2.
Quantification of invasion for the wild-type, KI:cKD and cKD parasites
Comparison among the wild-type, parental (KI:cKD) and cKD parasites under various Shld1 and ATc treatment conditions further supports that parasite invasion efficiency is linked to the level of CEN2 expression (Fig. 5C,D, Table 2). The parental line (KI:cKD, −Shld1/−ATc), which expresses fluorescent protein-tagged CEN2 from both the endogenous locus and the ectopic TetO7Sag4 promoters, invades at ∼125% of the level of the wild-type parasite (P=0.1208). Derived from the KI:cKD line by deletion of the eGFP-CEN2 gene from the endogenous locus, the cKD line invades at a significantly lower efficiency than KI:cKD. Consistent with the differences in the mAppleFP–CEN2 levels (Fig. 3B), the cKD parasites invade with different efficiencies under the +Shld1/−ATc (∼56% of the wild-type, P=0.033), −Shld1/−ATc (∼28%, P=0.0071) and −Shld1/+ATc (∼10%, P=0.0014) conditions. The lower level of invasion for the cKD +Shld1/−ATc parasites compared to the wild type is most likely because expression of ddFKBP–mAppleFP–CEN2 in the cKD parasites is driven by the TetO7Sag4 promoter, which is known to be weak. Similar differences among the wild-type, parental (KI:cKD) and cKD parasites in TATi-based systems have been observed previously (Huynh and Carruthers, 2006).
In contrast to the significant defect in invasion, CEN2 depletion did not result in a delay in Ca2+-induced egress. Upon stimulation with the Ca2+ ionophore A23187, CEN2-knockdown parasites (cKD, −Shld1/+ATc, ∼48 h) in more than 90% of vacuoles activated motility to egress within 6 min, similar to what was observed for the parental line (KI:cKD) (Fig. 5E).
Parasites deficient in CEN2 are significantly impaired in micronemal secretion and form significantly fewer evacuoles and moving junctions
Parasite invasion requires the secretion of adhesins from the micronemes, which allows the parasite to attach to and move into the host cell (Carruthers et al., 1999; Carruthers and Sibley, 1997; Huynh and Carruthers, 2006; Huynh et al., 2003). The adhesins, such as the micronemal protein MIC2, are secreted onto the parasite surface and then cleaved, releasing the ectodomain into the supernatant (Huynh and Carruthers, 2006; Huynh et al., 2003). A deficiency in secretion of these adhesins results in severely impaired host cell invasion. Upon examining the amount of released ectodomain in the supernatant by western blotting, we discovered that after ∼40 h of CEN2 knockdown, ethanol-stimulated MIC2 secretion was partially inhibited. This was reflected in the reduced amount of MIC2 in the secreted fraction and a corresponding increase in the pellet fraction (Fig. 5F,G). The secretion of two other micronemal proteins, PLP1 and MIC3, was also significantly reduced, indicating that the impact of CEN2 depletion on micronemal secretion is global and not limited to a specific micronemal protein. In contrast, the secretion of the dense granule protein GRA8 was not affected by CEN2 depletion (Fig. 5F,G).
During parasite invasion, micronemal secretion is followed by protein discharge from the rhoptries, another set of apically located organelles. Rhoptry discharge is typically visualized and assessed by performing the evacuole assay (Hakansson et al., 2001), in which the parasites are prevented from completing host cell invasion by treatment with cytochalasin D. The CEN2-knockdown parasites (cKD, −Shld1/+ATc, ∼48 h) generated significantly fewer evacuoles compared with the parental line (Fig. 5H), consistent with the invasion defect. Secretion from the micronemes and rhoptries also both contribute to the formation of the moving junction, a structure critical for parasite invasion (Alexander et al., 2005; Beck et al., 2014; Lamarque et al., 2011; Mital et al., 2005; Tonkin et al., 2011). This ring-shaped constriction forms during parasite invasion to line the opening of the nascent parasitophorous vacuole (Fig. 5I). As the parasite moves forward during invasion, it squeezes through this constriction, and eventually the moving junction caps the basal end of the parasite when invasion is complete. While the moving junction (labeled by anti-RON4 antibody) was readily observable for wild-type or cKD +Shld1/−ATc parasites in pulse invasion assays (Fig. 5I), we were never able to see a single case of moving junction formation when CEN2 was knocked down (cKD, −Shld1/+ATc, 48 h).
Despite the defect in secretion, CEN2 depletion does not appear to perturb the localization of the secretory organelles in intracellular parasites. Similar to what is seen for the wild-type parasite, the micronemal vesicles (marked by anti-MIC2 antibody) were concentrated in the apical portion of the parasite when CEN2 was depleted (Fig. 5J), indicating that the CEN2-containing structures play a role in the mechanics of micronemal secretion rather than the biogenesis or distribution of micronemal organelles. CEN2 depletion also did not affect the distribution of the rhoptries [labeled by an antibody that recognizes ROP2, ROP3 and ROP4 (anti-ROP2,3,4) and one that recognizes RON2 (anti-RON2-4)] and dense granules (labeled by anti-GRA8 antibody) (Fig. S1B–G).
CEN2 knockdown results in abnormal replication patterns
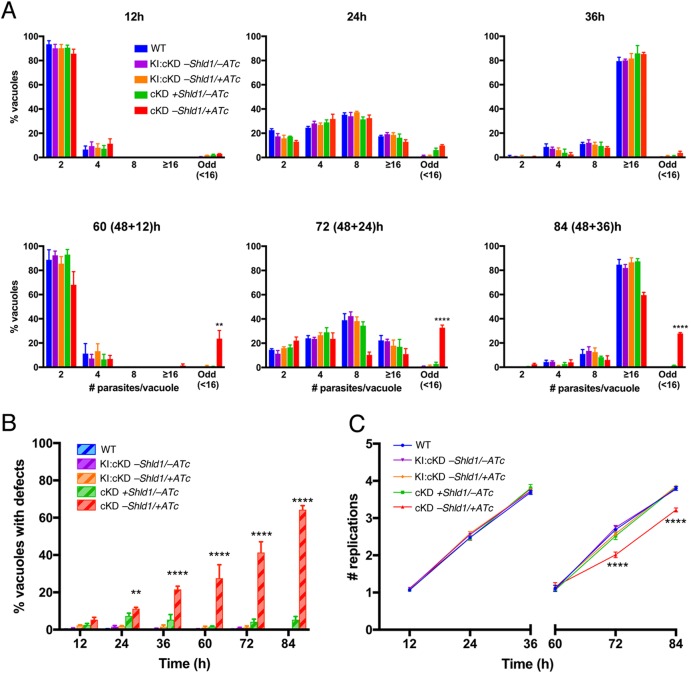
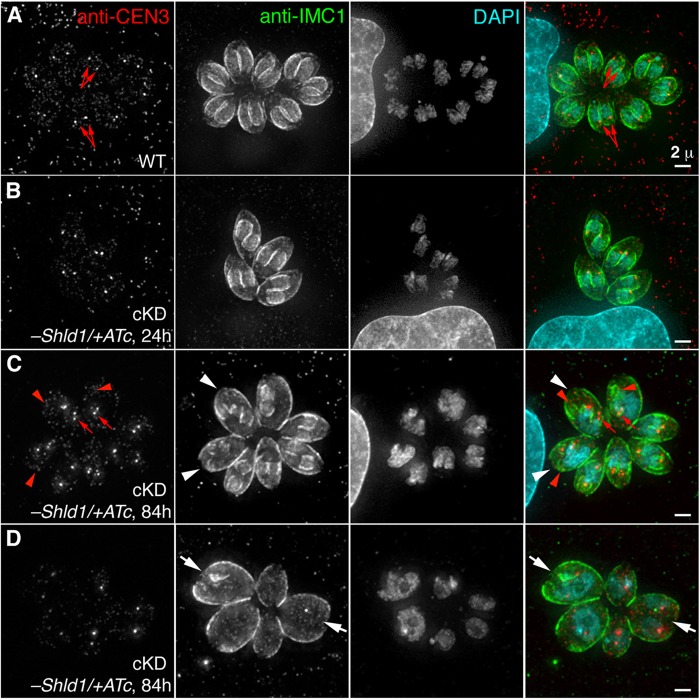
The centrosome (including the centrioles and the spindle pole) is a critical organelle for cell division, and the basal complex has been shown to be involved in parasite cytokinesis (Heaslip et al., 2010). We therefore examined the impact of CEN2 depletion on parasite replication (Fig. 6). As the level of CEN2 decreased due to ATc treatment and Shld1 withdrawal, an increasing proportion of vacuoles contained parasites that deviated from the canonical one-to-two division process (Fig. 6A). After ∼24 h of ATc treatment (Figs 6B, 7A,B), only ∼11% of the vacuoles had parasites displaying abnormal division or growth. At this point, CEN2 had been depleted from the preconoidal rings and peripheral annuli to an undetectable level (Fig. 3). We thus propose that the CEN2 pools in the preconoidal rings and peripheral annuli do not play a major role in parasite replication. By ∼84 h of ATc treatment, ∼64% of the vacuoles contained parasites that showed signs of abnormal replication (Fig. 6B). In addition to multiple (more than two) daughters assembling in mother parasites (Fig. 7C), the parasites were often swollen in size and some had undergone nuclear division without cytokinesis, that is had multiple nuclei but no daughters (Fig. 7D). Although the kinetics of CEN2 depletion from the centrioles and the basal complex are similar, these replication defects are most likely due to CEN2 depletion from the centrioles, because the presence and appearance of the basal complex marker IAP1 (Frenal et al., 2014) were not significantly affected (Fig. S2A,B). In addition, while centriole duplication occurred in CEN2-depleted parasites, segregation of the centrioles appeared to be perturbed (Fig. 7C), as indicated by the unequal distribution of foci that contained high anti-CEN3 antibody signals among the daughter parasites. In some of the daughter parasites, no centriole signal was observed (red arrowheads). In the vacuoles containing parasites with replication defects, there was considerable variation in the size and cell cycle stage of the parasites, indicating that CEN2 depletion does not affect a specific checkpoint and that the cumulative effect of knockdown is stochastic in nature. Interestingly, the partitioning and inheritance of membrane-bound organelles, such as the apicoplast (Fig. S2C,D), were not severely perturbed. Although the appearance of the apicoplast was more heterogeneous in misshapen CEN2-knockdown parasites (Fig. S2D) when compared to parasites expressing stabilized CEN2 (Fig. S2C), almost all of these parasites inherited an apicoplast.
Fig. 6.
The effect of CEN2 downregulation on parasite replication. (A) Comparison of the intracellular growth of cKD parasites (mean±s.e.m. percentage of vacuoles containing indicated number of parasites) treated with ATc (cKD, −Shld1/+ATc) with that of RHΔhx (WT), KI:cKD (−Shld1/−ATc or −Shld1/+ATc) and cKD parasites cultured with Shld1 (cKD +Shld1/−ATc) parasites at 12, 24, 36, 60 (48+12), 72 (48+24) and 84 (48+36) h after drug removal/addition. To facilitate parasite counting after extended (>48 h) drug treatment times, dishes for the last three time points were inoculated with parasites that had been cultured for 48 h in T12.5 cm2 flasks under the indicated conditions, and allowed to invade a fresh HFF monolayer and proliferate for 12, 24 and 36 h under the corresponding conditions. Odd, vacuoles in which the number of parasites was less than 16, and not an integral power of 2; ≥16, vacuoles that contained 16 or more parasites. (B) Percentage (mean±s.e.m.) of vacuoles containing parasites with replication defects. Phenotypes include an odd number of parasites, enlarged parasites, parasites with multiple nuclei or none at all, and parasites with a single daughter or more than two daughters. (C) Mean±s.e.m. replication (doubling) rate. Note that particularly at the 36 h and 84 (48+36) h time points, there were many very large vacuoles containing from 16 to upwards of ∼128 parasites. However, since the precise number of parasites within each of these large vacuoles could not be calculated, they were grouped into the ‘16 or more parasites’ bin and assigned four doublings (four rounds of replication, equivalent to 16 parasites). Therefore, the replication rates for these time points are an underestimate of the actual replication rate. **P<0.01; ****P<0.0001 (two-way ANOVA and Bonferroni's multiple comparisons test), when cKD parasites were compared with RHΔhx (WT) or KI:cKD parasites as measured in three independent biological replicates.
Fig. 7.
CEN2 knockdown results in abnormal replication patterns. (A) Representative images of untreated RHΔhx parasites (WT) at the 24 h time point for the replication assays in Fig. 6. CEN3 is a marker for the centrioles (red arrows); IMC1 is a marker for the cortex of mature and daughter parasites; DAPI, fluorescent nucleic acid stain. Scale bar: 2 µm. (B) Images of a vacuole of cKD parasites treated with ATc (−Shld1/+ATc) for 24 h undergoing normal replication. Scale bar: 2 µm. (C,D) Images of one vacuole of cKD parasites treated with ATc (−Shld1/+ATc) for 84 h with more than two daughters forming inside the mother (C, white arrowheads) and another vacuole with parasites that are swollen with large or multiple nuclei (D, white arrows). Notice the uneven distribution of the centrioles (red arrows). Red arrowheads indicate some daughter parasites that receive no centrioles. Scale bars: 2 µm.
DISCUSSION
In this work, we explore the function of TgCentrin2 (CEN2), a protein that localizes to four different cytoskeletal structures in T. gondii. By using a dual system that regulates transcription through ATc/TATi and protein stability through Shld1/ddFKBP, we determined the time course of CEN2 depletion by quantitative fluorescence imaging and used it as a guideline to dissect the functional consequences of CEN2 depletion from the individual structures. We found that CEN2 is depleted to an undetectable level from the preconoidal rings and peripheral annuli much earlier than from the centrioles and basal complex. The ordered depletion of CEN2 from the two sets of structures is correlated with the development of defects in two distinct steps of the parasite lytic cycle: invasion and replication. This indicates that CEN2 is recruited to multiple cytoskeletal structures where it functions in distinct aspects of parasite physiology.
The dual-regulation system enables tight control of genes critical for the parasite lytic cycle
Numerous attempts to generate a knockout mutant of CEN2 using Cre-LoxP- and CRISPR-Cas9-based methods failed. Furthermore, a CEN2 knockdown line in which CEN2 downregulation solely relied on transcriptional control with ATc quickly became unresponsive to ATc treatment, probably as a result of selecting for mutants that had lost ATc sensitivity within the population. While essentiality cannot be positively proven (i.e. essential genes cannot be deleted, but unsuccessful attempts to generate knockout mutants do not prove essentiality), these results suggest that the parasite is highly sensitive to altered CEN2 expression and that CEN2 is likely to be essential. By implementing a dual-regulation system that simultaneously utilizes ATc/TATi to control transcription and Shld1/ddFKBP to control protein stability, the expression of CEN2 can be consistently downregulated. Although growth adaptation can still be detected over long-term passage (>3 months), the phenotype is sufficiently stable for analysis as long as care is taken to use low passage number cultures. Given that a single conditional regulation technique (e.g. ATc-based transcriptional regulation or ddFKBP-based protein degradation) is always ‘leaky’ to some extent, a knockdown strategy that employs orthogonal methods can be useful for characterizing the functions of genes that are otherwise difficult to manipulate.
The kinetics of protein knockdown are affected by structural inheritance through cellular replication
In addition to the half-life of transcripts and their protein products, the kinetics of protein knockdown are affected by structural inheritance through cellular replication. Given that Toxoplasma and other apicomplexans divide by building daughters inside the mother, if everything else is equal, components of the cortical cytoskeleton or an organelle regenerated de novo with every replication should be depleted much faster than those of structures inherited from the mother parasite. For proteins such as CEN2 that are components of multiple structures, this is an important consideration when delineating their functions. For instance, our data indicate that structural inheritance plays an important role in the differential depletion of CEN2 from the four structures: the CEN2 populations in the preconoidal rings and peripheral annuli are depleted much earlier, because these structures are made de novo during parasite replication. In contrast, the CEN2 population in the centrioles persists over many generations after downregulation, because the daughters inherit the centrioles from the mother. However, factors other than structural inheritance must also contribute to the differential depletion of CEN2, as the CEN2 population in the basal complex does not fit this pattern. A new basal complex is built for each daughter with every generation (Hu, 2008; Hu et al., 2006), but the depletion of CEN2 from the basal complex follows a trend similar to that of the centrioles rather than the preconoidal rings or peripheral annuli. One possible explanation is that as complete CEN2 removal is lethal, only parasites that still express a small amount of CEN2 protein can survive under −Shld1/+ATc conditions. The residual CEN2 protein in these parasites is then preferentially incorporated into the basal complex and centrioles instead of the preconoidal rings and peripheral annuli, perhaps due to differences in binding affinity.
The role of CEN2 in regulating parasite invasion
The downregulation of CEN2 results in pronounced defects in both parasite invasion and replication. The invasion defect emerged at an early stage of CEN2 knockdown (∼16 h of ATc treatment and Shld1 withdrawal) when CEN2 had disappeared from the preconoidal rings and peripheral annuli, and been partially depleted from the centrioles and the basal complex. The timing of depletion suggests that the preconoidal rings and peripheral annuli likely play an important role in parasite invasion. However, the other pools of CEN2 in the centrioles, basal complex and cytoplasm might also be involved in invasion. In support of this hypothesis, we found that the invasion efficiency of cKD parasites decreased from 30% to 10% upon further CEN2 depletion (∼16 h versus 48 h of ATc treatment and Shld1 withdrawal) after it had already disappeared from the preconoidal rings and peripheral annuli but was still in the process of being depleted from the centrioles and basal complex. However, it is also possible that further depletion of a very small amount of CEN2 remaining at the apical structures (not detectable by mAppleFP fluorescence) was responsible for the further decrease in invasion efficiency from 16 h to 48 h of CEN2 downregulation.
Part of the invasion defect upon CEN2 knockdown can be explained by the impaired secretion of MIC2, a major adhesin that mediates parasite attachment to the host cell. As CEN2 is an EF-hand containing protein, it will be of interest to determine whether CEN2 is part of the Ca2+ signaling pathway that controls parasite secretion and invasion. One potential link is Toxoplasma phosphoinositide phospholipase C, which is also concentrated in the apical end of the parasite (Fang et al., 2006; Hortua Triana et al., 2018) and has been hypothesized to impact micronemal secretion through regulating Ca2+ homeostasis and the production of phosphatidic acid (Bullen et al., 2016). It is also tempting to speculate that some of the CEN2-containing structures (e.g. the preconoidal rings and peripheral annuli) might be Ca2+-sensitive contractile structures that gate the release of micronemal contents, but we have not been able to detect specific enrichment of MIC2 at these locations before or after CEN2 depletion. In a subset (∼29%) of the CEN2-knockdown parasites, the intra-conoid microtubules were not detectable. The intra-conoid microtubules have been proposed to serve as tracks for micronemal secretion. It is thus conceivable that structural perturbation of the intra-conoid microtubules interferes with micronemal secretion. However, the lack of intra-conoid microtubule detection in this subset of parasites could also be the result rather than the cause for perturbed micronemal secretion, since the lack of vesicle or protein association might result in decreased stain deposition along the intra-conoid microtubules in electron microscopy.
It is important to note that secretion of the three micronemal proteins tested (MIC2, MIC3 and PLP1) is not completely blocked by the depletion of CEN2, and the invasion defect of the CEN2 knockdown is more severe than that of the knockout mutants for individual micronemal proteins (Cérède et al., 2005; Gras et al., 2017; Kafsack et al., 2009). Furthermore, the cKD parasites cultured in +Shld1/−ATc had lower invasion efficiency compared to the parental line (KI:cKD), but secreted MIC2 at a similar level. Therefore, CEN2 downregulation must affect other processes that also contribute to invasion. One such process might be moving junction formation, which requires protein secretion from both the micronemes and the rhoptries (Alexander et al., 2005; Beck et al., 2014; Lamarque et al., 2011; Mital et al., 2005; Tonkin et al., 2011). Indeed, we found that CEN2 knockdown parasites generated significantly fewer evacuoles and moving junctions compared with the wild-type and cKD +Shld1/−ATc parasites. Furthermore, similar to the CEN2-knockdown parasite, mutants of moving junction components also have low invasion efficiency, but no obvious egress defect (Beck et al., 2014; Mital et al., 2005). It is therefore possible that CEN2 controls parasite invasion partially by regulating moving junction formation. While this is an appealing hypothesis, we should point out that rhoptry secretion occurs after the parasites make contact and form an intimate attachment with the host cell. Therefore, the apparent deficiency in rhoptry discharge and moving junction formation might instead be a consequence of upstream invasion processes disrupted by CEN2 knockdown rather than the direct cause of the invasion defect.
The role of CEN2 in regulating parasite replication
CEN2 is a component of the centrioles, and the depletion of CEN2 from the centrioles correlates with an increase in abnormal parasite replication. The centrosome (including the centrioles and the spindle pole) has been hypothesized to orchestrate daughter construction and organelle biogenesis in Toxoplasma (Dhara et al., 2017; Francia et al., 2012; Hu et al., 2002; Nishi et al., 2008; Suvorova et al., 2015). For instance, knockdown of the centrosome-associated striated fiber assemblins (SFAs) impairs the initiation of construction of the daughter cytoskeleton (Francia et al., 2012). When a temperature-sensitive mutant of MAPKL was cultured at the non-permissive temperature, the parasite generated an abnormally high number of daughters without completion of cytokinesis (Suvorova et al., 2015). Significant depletion of CEN2 from the centrioles results in a heterogeneous population of multi-nucleated (without daughters), multi-daughter (more than two), as well as normal looking parasites. This suggests that CEN2 does not control a specific ‘checkpoint’ or coupling point of the regulatory circuit of the cell cycle. However, it is worth noting that in the parasites that survive prolonged (∼120 h) ATc treatment, the CEN2 signal in the centrioles remains detectable, likely because complete CEN2 removal is lethal. Therefore, the consequence of total loss of CEN2 for parasite replication remains unknown. It would also be of interest to determine the functions of other centrin homologs (CEN1 and CEN3) in the centrioles, and whether they have roles in replication that overlap with CEN2.
The CEN2-containing structures are critical for parasite invasion, replication and survival. Future identification and characterization of structure-specific proteins will facilitate more precise function designation, and aid in the discovery of promising ‘druggable’ targets that lead to multiple points of vulnerability in the parasite.
MATERIALS AND METHODS
T. gondii, host cell cultures and parasite transfection
T. gondii tachyzoites were maintained by serial passage in confluent human foreskin fibroblast (HFFs, ATCC# SCRC-1041) monolayers in Dulbecco's modified Eagle's medium (DMEM, Life Technologies-Gibco, Cat# 10569-010), supplemented with 1% (v/v) heat-inactivated Cosmic calf serum (Hyclone, Cat# SH30087) as previously described (Leung et al., 2017; Liu et al., 2016; Roos et al., 1994). African green monkey renal epithelial cells (BS-C-1, ATCC# CCL-26) used for the invasion assays and rat aortic smooth muscle cells (A7r5, ATCC# CRL-1444) used for live imaging and immunofluorescence assays were cultured in the same manner as HFFs. The ddFKBP-mAppleFP-CEN2 cKD (‘cKD’) parasites were cultured in the presence of 125 nM Shield-1 (a kind gift from Dr Tom Wandless, Stanford University, Stanford, CA) (Banaszynski et al., 2006) to stabilize the ddFKBP–mAppleFP–CEN2 fusion. Downregulation of ddFKBP–mAppleFP–CEN2 was achieved by removal of Shld1 and incubation in medium supplemented with 270 nM anhydrotetracycline (ATc) (Clontech) unless noted otherwise. T. gondii transfections were carried out as previously described (Liu et al., 2013).
Alignment analysis of selected centrin homologs
Protein sequences for selected centrin homologs were aligned using the MUSCLE program accessed through JalView (v2.8.1, http://www.jalview.org) with default parameters and displayed using the Clustal X color scheme.
Cloning of plasmids
Primers used in cloning DNA fragments and for sequencing are listed in Table S1. Genomic DNA (gDNA) fragments were amplified using gDNA template prepared from RH, RHΔhx or RHΔku80Δhx (‘RHΔku80’) parasites (a kind gift from Dr Vern Carruthers, University of Michigan, Ann Arbor, MI) (Fox et al., 2009; Huynh and Carruthers, 2009) using the Wizard Genomic DNA Purification Kit (Cat# A1120, Promega, Madison, WI) according to the manufacturer's instructions. Similarly, coding sequences (CDS) were amplified from T. gondii cDNA. All DNA fragments generated by PCR were confirmed by sequencing.
pTKO2_II-eGFP-CEN2 – for generation of eGFP-CEN2 knock-in parasites
This plasmid was constructed in the pTKO2_II plasmid backbone (Heaslip et al., 2010) designed for replacement of genes in T. gondii by homologous recombination. Three fragments were generated and ligated into the pTKO2_II vector backbone using the corresponding sites. The 3′UTR of CEN2 (TgGT1_250340) was amplified using S1 and AS1 as primers and RH gDNA as the PCR template, and ligated via the NheI and ApaI sites. The 5′UTR was amplified using S2 and AS2 as primers and RH gDNA as the PCR template, and ligated via the NotI and EcoRI sites. The eGFP-CEN2 CDS with a Kozak sequence and flanking PmeI and RsrII sites was synthesized (GenScript Inc, NJ) and ligated via the PmeI and RsrII sites.
pmin-mCherryFP-CEN2 – intermediate plasmid for cloning pTATi1-TetO7Sag4-ddFKBP-mAppleFP-CEN2
This plasmid was constructed by Biomeans Inc (Sugar Land, TX). Briefly, the CEN2 CDS was amplified using primers S3 and AS3 and cloned into pCR-Blunt-II-TOPO (Invitrogen), prior to ligation with the plasmid backbone of pmin-mCherryFP-TgICMAP1 via the BamHI and AflII sites. [pmin-mCherryFP-TgICMAP1 has the same vector backbone as pmin-eGFP-TgICMAP1 (Heaslip et al., 2009) but with mCherryFP in place of eGFP.]
pTATi1-TetO7Sag4-ddFKBP-mAppleFP-CEN2 – for generation of eGFP-TgCEN2 knock-in:cKD and cKD parasites
To generate pTATi1-TetO7Sag4-ddFKBP-mAppleFP-CEN2, two synthesized DNA or plasmid digestion fragments were generated and ligated with the pTATi1-TetO7Sag4 vector backbone using the corresponding sites. The ddFKBP–mAppleFP fragment features a MfeI restriction site in between the ddFKBP and mAppleFP coding sequences, and was synthesized (GenScript Inc, NJ) and ligated via flanking NheI-BamHI sites into the NheI-BglII sites on the vector backbone. The CEN2 fragment was derived from the pmin-mCherryFP-CEN2 plasmid and ligated via the flanking BamHI-AflII sites into the BglII-AflII sites on the vector backbone. The pTATi1-TetO7Sag4 vector backbone was constructed from a four-component HiFi assembly (Cat# E5520, New England Biolabs): (1) the vector backbone, which contains the bacterial origin of replication, a β-lactamase gene for ampicillin resistance, the Cre recombinase CDS and the DHFR 3′UTR, was generated from an ApaI and BmtI double restriction digest of the plasmid ptub1200bp-mAppleFP-Cre (see below); (2) the TATi1 CDS expression cassette was amplified by PCR using primers S4 and AS4 with the plasmid ptub8-TATi1-HX (a kind gift from Dr Dominique Soldati-Favre, University of Geneva, Geneva, Switzerland) (Meissner et al., 2002) as the template; (3) the sagCATsag chloramphenicol transferase expression cassette was released from the plasmid ptub1200bp-mAppleFP-Cre by digestion with PpuMI and HindIII; and (4) the ATc-responsive TetO7Sag4 mini-promoter was amplified by PCR using primers S5 and AS5 with the plasmid TetO7Sag4_mycGFP (a kind gift from Dr Dominique Soldati-Favre, University of Geneva, Geneva, Switzerland) (Meissner et al., 2002) as the template. The ptub1200bp-mAppleFP-Cre plasmid intermediate was constructed by ligating, via the PpuMI and NheI sites, a truncated tubulin promoter amplified by PCR using primers S6 and AS6 with the plasmid ptub-mAppleFP-TLAP2 (Liu et al., 2016) as the template, and ligating via the BglII and AflII sites the plasmid backbone ptub-mAppleFP-TLAP2 with the Cre recombinase CDS, which was amplified by PCR using primers S7 and AS7 with the plasmid ptub-Cre-EGFP (Heaslip et al., 2010) as the template.
pTKO2_II_mCherryFP-Cre-EGFP – for excision of the LoxP-flanked eGFP-TgCEN2 knock-in expression cassette
This plasmid was constructed by ligating the Cre-EGFP expression cassette from pmin-Cre-EGFP (Heaslip et al., 2010) with the plasmid backbone of pTKO2_II_mCherryFP (Liu et al., 2013) via the NotI and ApaI sites.
Generation of knock-in, conditional knockdown and transgenic parasites
eGFP–CEN2 knock-in parasites
Approximately 107 RHΔhx parasites were electroporated with 50 μg of pTKO2_II-eGFP-CEN2 linearized with NotI and selected with 25 μg/ml mycophenolic acid and 50 μg/ml xanthine for four passages, and enriched by fluorescence-activated cell sorting (FACS) for parasites with an intermediate level of eGFP signal to reduce the number of parasites in the population that had not undergone double homologous recombination. Clones were screened by fluorescence light microscopy, and confirmed by diagnostic gDNA PCRs to have the CEN2 endogenous locus replaced by a LoxP-flanked eGFP–CEN2 expression cassette. One clone was further verified by Southern blotting; this clone was used for the subsequent generation of eGFP-CEN2 knock-in:cKD and cKD parasites.
eGFP-CEN2 knock-in:cKD parasites
The eGFP-CEN2 knock-in parasites were transfected with 30 μg of pTATi1-TetO7Sag4-ddFKBP–mAppleFP–CEN2 that subsequently integrated multiple times, randomly into the parasite genome after selection with 20 µM chloramphenicol.
ddFKBP-mAppleFP-CEN2 cKD parasites
KI:cKD parasites (∼107) were electroporated with 20 μg of pTKO2-mCherryFP-Cre-eGFP to excise the knock-in expression cassette between the two LoxP sites, selected with 80 μg/ml of 6-thioxanthine for two passages, and screened for the loss of eGFP–CEN2 fluorescence. Clones were confirmed by diagnostic gDNA PCRs. One clone (clone 10) was further verified by Southern blotting and used in all of the experiments reported here. In the resultant parasite line (cKD), the expression level of ddFKBP–mAppleFP–CEN2 is controlled by ATc and Shld1. The parasite line was maintained in the presence of 125 nM Shld1 unless indicated otherwise.
Generation of the rat anti-TgCEN3 and anti-TgIAP1 antibodies
Purified recombinant TgCEN3 (TogoA.00877.a.A1.PS00788) and TgIAP1 (TogoA.17172.a.A1.PW29285) proteins (kind gifts from the Seattle Structural Genomics Center for Infectious Disease, Seattle, WA) were used to inject rats for antibody production (Cocalico Biologicals, Inc) and sera of the immunized animals were harvested for performing the immunofluorescence labeling of TgCEN3 and TgIAP1.
FACS and parasite cloning
FACS was performed using an AriaII flow cytometer (BD Biosciences, San Jose, CA) driven by FACSDiva software at the Indiana University Bloomington Flow Cytometry Core Facility (Bloomington, IN). To subclone parasites by limiting dilution, 3–25 parasites (depending on the survival rate of specific parasite lines after sorting) with the desired fluorescence profile were sorted per well into a 96-well plate containing confluent HFF monolayers. Wells were screened 7-9 days after sorting for single plaques.
Southern blotting
Southern blotting was performed as previously described (Liu et al., 2016, 2013) with probes synthesized using components based on the NEBlot Phototope Kit (New England BioLabs, Cat# N7550) and detected using components based on the Phototope-Star detection kit (New England BioLabs, Cat# N7020). All T. gondii gDNA was prepared from freshly egressed parasites and extracted using the Wizard Genomic DNA Purification kit (Cat# A1120, Promega, Madison, WI).
To probe and detect changes at the CEN2 genomic locus in the RHΔhx (WT), eGFP-CEN2 knock-in (KI), KI:cKD and cKD parasites, 5 µg of gDNA was digested with ScaI. A CDS probe (282 bp) specific for exon 1 of CEN2 was amplified from T. gondii RHΔhx gDNA using primers S8 and AS8, and used as a template in probe synthesis. A probe specific for the region upstream of the CEN2 genomic locus (168 bp) was amplified from plasmid pTKO2_II-eGFP-CEN2 using primers S9 and AS9 and used as a template in probe synthesis.
Plaque assay
Plaque assays were performed as previously described with some modifications (Liu et al., 2016). A total of 100, 200 or 500 freshly egressed, pre-treated parasites (see below) was added to each well of a 12-well plate containing a confluent HFF monolayer, and incubated undisturbed for 6–7 days. The pre-treatment medium and corresponding incubation medium were either the regular parasite growth medium, or the medium supplemented with the concentrations of either ATc or Shld1 as indicated in the text and figure legends. Infected monolayers were then fixed with 3.7% (v/v) formaldehyde at 25°C for 10 min, washed with Dulbecco's phosphate-buffered saline (DPBS), stained with 2% (w/v) Crystal Violet and 20% (v/v) methanol in DPBS for 15 min, gently rinsed with distilled water and air dried.
Wide-field deconvolution microscopy
Image stacks were acquired at 37°C using a DeltaVision imaging station (GE Healthcare/Applied Precision) fitted onto an Olympus IX-70 inverted microscope base. A 60× silicone oil immersion lens (Olympus 60X U-Apo N, NA 1.3) used with or without an auxiliary magnification of 1.5× or 100× oil immersion lens (Olympus 100× UPLS Apo, NA1.40) with immersion oil at a refractive index of 1.524 was used for imaging. 3D image stacks were collected with a z-spacing of 0.3 µm unless otherwise noted. Images were deconvolved using the point spread functions and software supplied by the manufacturer. All samples for wide-field deconvolution and for 3D-SIM (below) were prepared in 35-mm dishes (#1.5) with a 20- or 14-mm microwell (P35G-1.5-20-C or P35G-1.5-14-C; MatTek) in Phenol Red-free CO2-independent medium for live imaging and in DPBS with 10 mM sodium azide for fixed samples. Contrast levels were adjusted to optimize the display.
3D structured-illumination microscopy
3D structured-illumination microscopy (3D-SIM) image stacks were acquired using an OMX imaging station (GE Healthcare/Applied Precision, Seattle, WA). A 100× oil immersion lens (NA 1.40) with immersion oil at a refractive index of 1.516 or 1.518 was used; stacks were collected with a z-spacing of 0.125 µm. Images were deconvolved using the point spread functions and software supplied by the manufacturer.
Electron microscopy
Negative staining of whole mount, detergent-extracted parasites was performed as previously described (Leung et al., 2017). To determine the effect of CEN2 depletion on the parasite cytoskeleton, cKD parasites were treated with −Shld1/+ATc for 144 h prior to processing for electron microscopy analysis.
Quantification of mAppleFP–CEN2 fluorescence in cKD parasites
Parasite culture and imaging conditions
T. gondii cultures were grown in culture medium +Shld1/−ATc (125 nM Shld1), −Shld1/−ATc or −Shld1/+ATc (270 nM ATc) for 12, 24, 48, 72, 96 or 120 h prior to live imaging. The average intensity of individual CEN2-containing structures in cKD parasites cultured in +Shld1/−ATc was used to calculate the baseline fluorescence. Multiple flasks of parasites were coordinated with staggered timing such that a freshly lysed flask was used to inoculate 35-mm dishes (#1.5) with a 20-mm microwell (P35G-1.5-20-C; MatTek) ∼16 h prior to imaging. This yielded smaller vacuoles containing two, four or eight parasites to facilitate imaging each of the four CEN2-containing compartments within the parasite for the quantification of mAppleFP–CEN2 signal. The medium in each dish was replaced with pre-warmed, Phenol Red-free CO2-independent medium (custom order, SKU#RR060058; Gibco/Life Technologies) prior to imaging. Dishes were imaged in a humidified environmental chamber maintained at 37°C.
Twenty full fields of view (1024×1024 pixels) were collected with 2×2 binning for quantitative analysis. For each field of view, a reference differential interference contrast (DIC) image was acquired followed by a 3D stack of three z-slices, spaced 1.0 µm apart, of fluorescence images in the mAppleFP and eGFP channels. Irradiation of each field of view was minimized to prevent photobleaching and kept consistent to reduce variability in the quantification.
Image analysis
Image analysis strategies and procedures used here were carried out as previously described (Murray, 2017). The Semper software package [source code kindly provided by Dr Owen Saxton (Murray-Edwards College, University of Cambridge, UK; Saxton et al., 1979)] was used for image analysis.
Grouping of integrated fluorescence and statistical analysis
A minimum of 20 vacuoles were quantified per parasite line and drug treatment. Vacuoles containing a smaller number of intracellular parasites (two, four or eight parasites) were used for quantification, and the mAppleFP–CEN2 signal was classified into four groups corresponding to each of the compartments it localizes to in the parasite (preconoidal rings, peripheral annuli, centrioles and basal complex). All photons/s measurements shown are per individual structure, per parasite except for the peripheral annuli, which were quantified collectively (i.e. all annuli in one parasite were grouped as a single value). Since the basal complexes of parasites tend to cluster within a vacuole, these were quantified collectively for each vacuole and subsequently calculated per parasite, based on the number of parasites observed in the corresponding reference DIC image. Data were analyzed using two-way ANOVA and Dunnett's multiple comparisons tests with GraphPad Prism v7 (La Jolla, CA).
Immunofluorescence assay for intracellular parasites
T. gondii-infected HFF monolayers growing in a 3.5 cm glass-bottom dish were fixed with 3.7% (v/v) formaldehyde in DPBS for 10 min, permeabilized with 0.5% (v/v) Triton X-100 in DPBS for 15 min, and blocked in 1% (w/v) BSA in DPBS for 30-60 min, followed by antibody labeling (see below). Dishes were incubated with primary antibodies for 30–60 min followed by incubation with secondary antibodies for 30–60 min unless otherwise noted. Primary antibodies and dilutions used were as follows: rabbit anti-ACP, 1:500 (a kind gift from Dr David Roos, University of Pennsylvania, Philadelphia, PA and Dr Geoff McFadden, University of Melbourne, Melbourne, Australia; Waller et al., 1998); rabbit anti-IMC1, 1:1000 (a kind gift from Dr Con Beckers, University of North Carolina, Chapel Hill, NC; Mann and Beckers, 2001); mouse anti-ISP1, 1:1000 (a kind gift from Dr Peter Bradley, University of California, Los Angeles, CA; Beck et al., 2010); mouse anti-MIC2 6D10, 1:1000 (a kind gift from Dr Vern Carruthers, University of Michigan, Ann Arbor, MI; Carruthers et al., 2000); mouse monoclonal anti-GRA8 and mouse monoclonal anti-IMC1 45.36 antibodies, 1:1000 (kind gifts from Dr Gary Ward, University of Vermont, Burlington, VT; Carey et al., 2000; Ward and Carey, 1999); mouse anti-ROP2,3,4, 1:1000 (a kind gift from Dr Jean-François Dubremetz, Université de Montpellier, Montpellier, France; Leriche and Dubremetz, 1991); rabbit anti-RON2-4 and rabbit anti-RON4, 1:500 (kind gifts from Dr Maryse Lebrun, Université de Montpellier, Montpellier, France; Lamarque et al., 2011; Lebrun et al., 2005); rat anti-CEN3, 1:1000 (this study); and rat anti-IAP1, 1:1000 (this study). Secondary antibodies, all used at 1:1000 dilution were: goat anti-rabbit IgG conjugated to Alexa Fluor 488, 1:1000 (Cat# A11034, Molecular Probes), goat anti-rabbit IgG conjugated to Alexa Fluor 568 (Cat# A11036, Molecular Probes), goat anti-rat IgG conjugated to Alexa Fluor 488 (Cat# A11006, Molecular Probes), goat anti-rat IgG conjugated to Alexa Fluor 568 (Cat# A11077, Molecular Probes), goat anti-mouse IgG conjugated to Cy3 (Cat#115-166-003, Jackson ImmunoResearch), goat anti-mouse IgG conjugated to Alexa Fluor 488 (Cat# A11029, Molecular Probes); and goat anti-mouse IgG conjugated to Alexa Fluor 568 (Cat# A11031, Molecular Probes). To label the nucleus, 4′,6-diamidino-2-phenylindole (DAPI) was used at a final concentration of 1 µg/ml. All immunofluorescence labeling steps were performed at room temperature.
Invasion assays
Invasion assays were performed as previously described (Leung et al., 2017) with the following modifications. Since we observed during routine culturing that there was a clear invasion defect for a subset of the parasite lines, preparation of the cultures was adjusted in terms of the amount of inoculum, and timed such that all parasite preparations would be at the same stage at the time of harvest. For ATc treatment (−Shld1/+ATc), at ∼48, 40 or 16 h prior to harvest, the medium for the indicated subset of parasites was changed to that containing 270 nM ATc and no Shld1.
For the first round of immunolabeling, the primary antibody was mouse anti-SAG1 (Argene, Cat# 11-132; 1:1000 dilution for 30 min), followed by goat anti-mouse conjugated to Alexa Fluor 568 (1:1000 dilution for 30 min). For the second round of immunolabeling, the primary antibody was rabbit anti-SAG1 (a kind gift from Dr Lloyd Kasper, Dartmouth College, Lebanon, NH; 1:1000 dilution for 30 min), followed by goat anti-rabbit IgG conjugated to Alexa Fluor 488 (1:1000 dilution for 30 min). The dishes were imaged at low magnification (Olympus 20× UPlanApo, NA 0.70) for a total of 15 full-fields of view per sample for each of three independent experiments. Fields were randomly selected using the Alexa Fluor 488 channel. The mean number of parasites counted per sample, per replicate, was ∼3926.
Semi-automated quantification of invaded parasites was performed using FIJI [ImageJ v. 2.0.0-rc-65/1.51s; (Schindelin et al., 2012; Schneider et al., 2012)] as previously described (Leung et al., 2017). Pairwise comparisons were made with unpaired, two-tailed Student's t-tests using GraphPad Prism, v7 (GraphPad, La Jolla, CA).
Microneme secretion – excretory/secretory antigen assay
Microneme secretion assays were performed as previously described (Leung et al., 2017) with some modifications. Freshly egressed parasites were harvested and resuspended in DMEM supplemented with 1% (v/v) Cosmic calf serum (Hyclone, Cat# SH30087.3). Excreted/secreted antigen preparation was performed by incubating 108 parasites (adjusting the final volume to 500 µl) at 37°C for 7–15 min in 2% (v/v) ethanol to assess induced secretion. Tubes were placed on ice for 5 min immediately afterwards, and centrifuged at 1000 g for 6 min at 4°C. 450 µl of the supernatant was removed and centrifuged again, and 400 µl of the second supernatant was concentrated ∼10-fold using an Amicon Ultra 3000 MWCO centrifugal filter device (Millipore) before adding an equal volume of 4× NuPAGE sample buffer. The pellet was washed with DPBS and resuspended in 50 µl RIPA buffer [150 mM NaCl, 1% (v/v) NP-40, 0.5% (v/v) sodium deoxycholate, 0.1% (w/v) SDS, 50 mM Tris-HCl pH 7.4], and then treated with benzonase nuclease (Santa Cruz Biotechnology) for 15 min at 37°C before the addition of an equal volume of 4× NuPAGE sample buffer. Samples were incubated at 75°C for 10 min and resolved using NuPAGE 4-12% Bis-Tris gels, and blotted by wet transfer to nitrocellulose membrane. Membranes were processed for western blotting by probing with mouse mAb anti-MIC2 6D10, 1:5000; mouse mAb anti-MIC3 T4 2F3, 1:200 (a kind gift from Dr Maryse Lebrun, Université de Montpellier, Montpellier, France; Cérède et al., 2005), rabbit anti-PLP1, 1:1000 (a kind gift from Dr Vern Carruthers, University of Michigan, Ann Arbor, MI; Kafsack et al., 2009); mouse anti-GRA8 (1:5000), and rabbit anti-MLC1 (1:2000; a kind gift from Dr Con Beckers, University of North Carolina, Chapel Hill) (Gaskins et al., 2004) in Tris-buffered saline with 0.1% Tween 20 (TBS-T) with 5% (w/v) BSA or blocking solution [1% (w/v) casein, Hammarsten, 0.1 M maleate pH 7.5, 0.1 M NaCl], washed with TBS-T, followed by goat anti-mouse IRDye 680RD, goat anti-mouse IRDye 800CW, or goat anti-rabbit IRDye 800CW infrared dye-conjugated antibodies (1:10,000; LI-COR) in TBS-T with 5% (w/v) nonfat dried milk or blocking solution. Blots were scanned in the 700- and 800-nm channels using a LI-COR Odyssey Classic imaging system (LI-COR Biosciences, Lincoln, NE), and band intensities were plotted and then quantified with local background subtraction using the Gel Analyzer tool in FIJI (ImageJ v. 2.0.0-rc-65/1.51s).
Egress assay
Ca2+-induced egress assays were performed as previously described, using the Ca2+ ionophore A23187 (Cat#C7522, Sigma-Aldrich) for induction (Heaslip et al., 2011). KI:cKD parasites were maintained in −Shld1/−ATc medium and cKD parasites were cultured in +Shld1/−ATc medium. For ATc treatment (−Shld1/+ATc), KI:cKD and cKD parasites were treated with 270 nM ATc and no Shld1 for ∼46-52 h, prior to the egress assay.
Evacuole assay
Evacuole assays were performed as previously described (Hakansson et al., 2001) except the parasites were harvested and resuspended in ENDO buffer (44.7 mM K2SO4, 10 mM MgSO4, 106 mM sucrose, 5 mM glucose, 20 mM Tris-H2SO4, 3.5 mg/ml BSA, adjusted to pH 8.2; Endo and Yagita, 1990) prior to induction of evacuole formation with 1 µM cytochalasin D in DMEM supplemented with 1% (v/v) Cosmic calf serum. KI:cKD parasites were cultured in −Shld1/−ATc medium and cKD parasites were cultured in +Shld1/−ATc medium. For ATc treatment (−Shld1/+ATc), KI:cKD and cKD parasites were treated with 270 nM ATc and no Shld1 for ∼48 h prior to the evacuole assay. Anti-ROP2,3,4 antibody was used for visualizing rhoptry discharge. Note that for KI:cKD −Shld1/±ATc, and cKD +Shld1/−ATc, the mean±s.e.m. values were calculated from 10 randomly selected fields. For cKD −Shld1/+ATc, the mean±s.e.m. values were calculated from 10 fields that had at least one evacuole each. The mean thus overestimates the efficiency of evacuole formation under this condition as most fields did not contain any evacuoles.
Pulse invasion assay for analyzing moving junction formation
Pulse invasion assays were performed as previously described (Parussini et al., 2012) with some modifications. For ATc treatment (−Shld1/+ATc), cKD parasites were treated with 270 nM ATc and no Shld1 for 48 h prior to the pulse invasion assay. Parasites were harvested from large vacuoles and resuspended in ENDO (invasion non-permissive) buffer pre-warmed to 37°C, added to a ∼90% confluent HFF monolayer in a 3.5 cm glass-bottom dish, and incubated at 37°C for 30 min. The ENDO buffer was gently exchanged with invasion permissive medium [DMEM+1% (v/v) heat-inactivated Cosmic calf serum] pre-warmed to 37°C. RHΔhx (wild-type) parasites and cKD parasites maintained in +Shld1/−ATc medium were allowed to invade at 37°C for 1 min 30 s; cKD parasites treated with ATc (−Shld1/+ATc) were allowed to invade for 1 min 30 s, 2 min 30 s, 4 min, 8 min and 18 min. The dishes were washed once with DPBS prior to immunofluorescence labeling as described above.
Intracellular replication assay
Intracellular replication assays were performed as previously described (Heaslip et al., 2010), except parasites were grown for 12, 24, 36, 60, 72 or 84 h in medium with no drug supplementation (−Shld1/−ATc), with 125 nM Shld1 (+Shld1/−ATc) or with 270 nM ATc (−Shld1/+ATc). To ensure that the number of parasites in a vacuole was countable, for the last three time points (i.e. 60, 72 and 84 h), parasites were first cultured for 48 h in T12.5 cm2 flasks in the indicated conditions (−Shld1/−ATc, +Shld1/−ATc or −Shld1/+ATc), released by mechanical disruption of the host cell monolayer, then used to inoculate dishes containing the same medium conditions as the originating flasks, for an additional 12, 24 or 36 h. At each time point, dishes were processed for immunofluorescence as described above, using antibodies for markers of the mother and daughter cortex (IMC1), centrioles (CEN3), basal complex (IAP1) and a fluorescent nucleic acid stain (DAPI) to count the number of parasites per vacuole and to assess the percentage of replication defects. The number of parasites per vacuole was determined as 2, 4, 8, ≥16 or ‘odd’ (i.e. where the number of parasites was less than 16, and not an integral power of 2). For Fig. 6B, vacuoles with any of the following phenotypes were classified as having replication defects: an odd number of parasites, enlarged parasites, parasites with multiple nuclei or none at all, and parasites with a single daughter or more than two daughters. Note that for Fig. 6C, particularly at the 36 h and 84 h time points, there were many very large vacuoles containing from 16 to upwards of ∼128 parasites. However, since the precise number of parasites within each of these vacuoles could not be calculated, they were grouped into the ‘16 or more parasites’ bin and assigned four doublings (four rounds of replication, equivalent to 16 parasites). Therefore, the replication rates for these time points are an underestimate of the actual replication rate.
For every time point, a minimum of 200 vacuoles was assessed for each parasite line and condition in each of three independent biological replicates, except for the cKD parasites treated with ATc, in which 100 vacuoles were quantified per time point because its invasion deficiency resulted in many fewer vacuoles forming. Data were analyzed using two-way ANOVA and Bonferroni's multiple comparisons tests with GraphPad Prism v7 (La Jolla, CA). Where statistically significant, multiplicity adjusted P values for comparisons are indicated with asterisks.
Supplementary Material
Acknowledgements
We thank Dr Owen Saxton for providing image processing softwares, and Drs Con Beckers, Peter Bradley, Vern Carruthers, Jean-François Dubremetz, Lloyd Kasper, Maryse Lebrun, David Roos, Geoff McFadden, Gary Ward, Dominique Soldati-Favre, Tom Wandless, and the Seattle Structural Genomics Center for Infectious Disease for reagents. We thank Christiane Hassel of the Indiana University Bloomington (IUB) Flow Cytometry Core Facility for assistance with flow cytometry, and Dr. James Powers of the IUB Light Microscopy Imaging Facility for assistance and support with light microscopy (NIH S10-RR028697). We would also like to thank Dr. Amanda Rollins and Qing Zhang for technical support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.M.L., J.L., K.H.; Methodology: J.M.L., J.L., K.H.; Investigation: J.M.L., J.L., L.A.W., K.H.; Data curation: J.M.L., J.L., K.H.; Writing - original draft: J.M.L., J.L., K.H.; Writing - review & editing: J.M.L., J.L., L.A.W., K.H.; Supervision: K.H.; Funding acquisition: J.M.L., K.H.
Funding
This study was supported by American Heart Association Postdoctoral Fellowships (16POST31330004 and 18POST34090005) awarded to J.M.L., and funding from the March of Dimes Foundation (6-FY18-674), the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01-AI132463) awarded to K.H., and facility funding from the Indiana Clinical and Translational Sciences Institute to K.H., funded in part by Grant UL1 TR001108 from a National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.228791.supplemental
References
- Alexander D. L., Mital J., Ward G. E., Bradley P. and Boothroyd J. C. (2005). Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1, e17 10.1371/journal.ppat.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos W. B., Routledge L. M. and Yew F. F. (1975). Calcium-binding proteins in a vorticellid contractile organelle. J. Cell Sci. 19, 203-213. [DOI] [PubMed] [Google Scholar]
- Banaszynski L. A., Chen L.-C., Maynard-Smith L. A., Ooi A. G. L. and Wandless T. J. (2006). A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995-1004. 10.1016/j.cell.2006.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. R., Rodriguez-Fernandez I. A., Cruz de Leon J., Huynh M. H., Carruthers V. B., Morrissette N. S. and Bradley P. J. (2010). A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 6, e1001094 10.1371/journal.ppat.1001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. R., Chen A. L., Kim E. W. and Bradley P. J. (2014). RON5 is critical for organization and function of the Toxoplasma moving junction complex. PLoS Pathog. 10, e1004025 10.1371/journal.ppat.1004025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. W. and Boothroyd J. C. (2000). Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607-623. 10.1128/MMBR.64.3.607-623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen H. E., Jia Y., Yamaryo-Botté Y., Bisio H., Zhang O., Jemelin N. K., Marq J.-B., Carruthers V., Botté C. Y. and Soldati-Favre D. (2016). Phosphatidic acid-mediated signaling regulates microneme secretion in Toxoplasma. Cell Host Microbe 19, 349-360. 10.1016/j.chom.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Carey K. L., Donahue C. G. and Ward G. E. (2000). Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 105, 25-37. 10.1016/S0166-6851(99)00160-7 [DOI] [PubMed] [Google Scholar]
- Carey K. L., Westwood N. J., Mitchison T. J. and Ward G. E. (2004). A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 101, 7433-7438. 10.1073/pnas.0307769101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers V. B. and Sibley L. D. (1997). Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73, 114-123. [PubMed] [Google Scholar]
- Carruthers V. B., Giddings O. K. and Sibley L. D. (1999). Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1, 225-235. 10.1046/j.1462-5822.1999.00023.x [DOI] [PubMed] [Google Scholar]
- Carruthers V. B., Sherman G. D. and Sibley L. D. (2000). The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275, 14346-14353. 10.1074/jbc.275.19.14346 [DOI] [PubMed] [Google Scholar]
- Cérède O., Dubremetz J. F., Soête M., Deslée D., Vial H., Bout D. and Lebrun M. (2005). Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J. Exp. Med. 201, 453-463. 10.1084/jem.20041672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhara A., de Paula Baptista R., Kissinger J. C., Snow E. C. and Sinai A. P. (2017). Ablation of an ovarian tumor family deubiquitinase exposes the underlying regulation governing the plasticity of cell cycle progression in Toxoplasma gondii. mBio 8, e01846-17 10.1128/mBio.01846-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T. and Yagita K. (1990). Effect of extracellular ions on motility and cell entry in Toxoplasma gondii. J. Protozool. 37, 133-138. 10.1111/j.1550-7408.1990.tb05883.x [DOI] [PubMed] [Google Scholar]
- Fang J., Marchesini N. and Moreno S. N. J. (2006). A Toxoplasma gondii phosphoinositide phospholipase C (TgPI-PLC) with high affinity for phosphatidylinositol. Biochem. J. 394, 417-425. 10.1042/BJ20051393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. A., Ristuccia J. G., Gigley J. P. and Bzik D. J. (2009). Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot. Cell 8, 520-529. 10.1128/EC.00357-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia M. E., Jordan C. N., Patel J. D., Sheiner L., Demerly J. L., Fellows J. D., de Leon J. C., Morrissette N. S., Dubremetz J.-F. and Striepen B. (2012). Cell division in Apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella. PLoS Biol. 10, e1001444 10.1371/journal.pbio.1001444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenal K., Marq J. B., Jacot D., Polonais V. and Soldati-Favre D. (2014). Plasticity between MyoC- and MyoA-glideosomes: an example of functional compensation in Toxoplasma gondii invasion. PLoS Pathog. 10, e1004504 10.1371/journal.ppat.1004504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins E., Gilk S., DeVore N., Mann T., Ward G. and Beckers C. (2004). Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 165, 383-393. 10.1083/jcb.200311137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S., Jackson A., Woods S., Pall G., Whitelaw J., Leung J. M., Ward G. E., Roberts C. W. and Meissner M. (2017). Parasites lacking the micronemal protein MIC2 are deficient in surface attachment and host cell egress, but remain virulent in vivo. Wellcome Open Res. 2, 32 10.12688/wellcomeopenres.11594.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson S., Charron A. J. and Sibley L. D. (2001). Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20, 3132-3144. 10.1093/emboj/20.12.3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip A. T., Ems-McClung S. C. and Hu K. (2009). TgICMAP1 is a novel microtubule binding protein in Toxoplasma gondii. PLoS ONE 4, e7406 10.1371/journal.pone.0007406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip A. T., Dzierszinski F., Stein B. and Hu K. (2010). TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii. PLoS Pathog. 6, e1000754 10.1371/journal.ppat.1000754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip A. T., Nishi M., Stein B. and Hu K. (2011). The motility of a human parasite, Toxoplasma gondii, is regulated by a novel lysine methyltransferase. PLoS Pathog. 7, e1002201 10.1371/journal.ppat.1002201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herm-Götz A., Agop-Nersesian C., Münter S., Grimley J. S., Wandless T. J., Frischknecht F. and Meissner M. (2007). Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat. Methods 4, 1003-1005. 10.1038/nmeth1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortua Triana M. A., Marquez-Nogueras K. M., Chang L., Stasic A. J., Li C., Spiegel K. A., Sharma A., Li Z. H. and Moreno S. N. J. (2018). Tagging of weakly expressed Toxoplasma gondii calcium-related genes with high-affinity tags. J. Eukaryot. Microbiol. 65, 709-721. 10.1111/jeu.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K. (2008). Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog. 4, 108-121. 10.1371/journal.ppat.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Mann T., Striepen B., Beckers C. J. M., Roos D. S. and Murray J. M. (2002). Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol. Biol. Cell 13, 593-606. 10.1091/mbc.01-06-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Johnson J., Florens L., Fraunholz M., Suravajjala S., Dilullo C., Yates J., Roos D. S. and Murray J. M. (2006). Cytoskeletal components of an invasion machine-the apical complex of Toxoplasma gondii. PLoS Pathog. 2, e13 10.1371/journal.ppat.0020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M.-H. and Carruthers V. B. (2006). Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2, e84 10.1371/journal.ppat.0020084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M.-H. and Carruthers V. B. (2009). Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell 8, 530-539. 10.1128/EC.00358-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M.-H., Rabenau K. E., Harper J. M., Beatty W. L., Sibley L. D. and Carruthers V. B. (2003). Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22, 2082-2090. 10.1093/emboj/cdg217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack B. F. C., Pena J. D. O., Coppens I., Ravindran S., Boothroyd J. C. and Carruthers V. B. (2009). Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323, 530-533. 10.1126/science.1165740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque M., Besteiro S., Papoin J., Roques M., Vulliez-Le Normand B., Morlon-Guyot J., Dubremetz J.-F., Fauquenoy S., Tomavo S., Faber B. W. et al. (2011). The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 7, e1001276 10.1371/journal.ppat.1001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Michelin A., El Hajj H., Poncet J., Bradley P. J., Vial H. and Dubremetz J. F. (2005). The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell. Microbiol. 7, 1823-1833. 10.1111/j.1462-5822.2005.00646.x [DOI] [PubMed] [Google Scholar]
- Leriche M. A. and Dubremetz J. F. (1991). Characterization of the protein contents of rhoptries and dense granules of Toxoplasma gondii tachyzoites by subcellular fractionation and monoclonal antibodies. Mol. Biochem. Parasitol. 45, 249-260. 10.1016/0166-6851(91)90092-K [DOI] [PubMed] [Google Scholar]
- Leung J. M., He Y., Zhang F., Hwang Y. C., Nagayasu E., Liu J., Murray J. M. and Hu K. (2017). Stability and function of a putative microtubule organizing center in the human parasite Toxoplasma gondii. Mol. Biol. Cell 28, 1361-1378. 10.1091/mbc.e17-01-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wetzel L., Zhang Y., Nagayasu E., Ems-McClung S., Florens L. and Hu K. (2013). Novel thioredoxin-like proteins are components of a protein complex coating the cortical microtubules of Toxoplasma gondii. Eukaryot. Cell 12, 1588-1599. 10.1128/EC.00082-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., He Y., Benmerzouga I., Sullivan W. J. Jr, Morrissette N. S., Murray J. M. and Hu K. (2016). An ensemble of specifically targeted proteins stabilizes cortical microtubules in the human parasite Toxoplasma gondii. Mol. Biol. Cell 27, 549-571. 10.1091/mbc.e15-11-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T. and Beckers C. (2001). Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol. Biochem. Parasitol. 115, 257-268. 10.1016/S0166-6851(01)00289-4 [DOI] [PubMed] [Google Scholar]
- Meissner M., Brecht S., Bujard H. and Soldati D. (2001). Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res. 29, E115 10.1093/nar/29.22.e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M., Schluter D. and Soldati D. (2002). Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837-840. 10.1126/science.1074553 [DOI] [PubMed] [Google Scholar]
- Mital J. and Ward G. E. (2008). Current and emerging approaches to studying invasion in apicomplexan parasites. Subcell. Biochem. 47, 1-32. 10.1007/978-0-387-78267-6_1 [DOI] [PubMed] [Google Scholar]
- Mital J., Meissner M., Soldati D. and Ward G. E. (2005). Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16, 4341-4349. 10.1091/mbc.e05-04-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M. (2017). An icosahedral virus as a fluorescent calibration standard: a method for counting protein molecules in cells by fluorescence microscopy. J. Microsc. 267, 193-213. 10.1111/jmi.12559 [DOI] [PubMed] [Google Scholar]
- Nishi M., Hu K., Murray J. M. and Roos D. S. (2008). Organellar dynamics during the cell cycle of Toxoplasma gondii. J. Cell Sci. 121, 1559-1568. 10.1242/jcs.021089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parussini F., Tang Q., Moin S. M., Mital J., Urban S. and Ward G. E. (2012). Intramembrane proteolysis of Toxoplasma apical membrane antigen 1 facilitates host-cell invasion but is dispensable for replication. Proc. Natl. Acad. Sci. USA 109, 7463-7468. 10.1073/pnas.1114661109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D. S., Donald R. G. K., Morrissette N. S. and Moulton A. L. (1994). Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45, 27-78. 10.1016/S0091-679X(08)61845-2 [DOI] [PubMed] [Google Scholar]
- Salisbury J. L. (1995). Centrin, centrosomes, and mitotic spindle poles. Curr. Opin. Cell Biol. 7, 39-45. 10.1016/0955-0674(95)80043-3 [DOI] [PubMed] [Google Scholar]
- Salisbury J. L. (2007). A mechanistic view on the evolutionary origin for centrin-based control of centriole duplication. J. Cell. Physiol. 213, 420-428. 10.1002/jcp.21226 [DOI] [PubMed] [Google Scholar]
- Sanders M. A. and Salisbury J. L. (1994). Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 124, 795-805. 10.1083/jcb.124.5.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W., Pitt T. and Horner M. (1979). Digital image processing: the Semper system. Ultramicroscopy 4, 343-353. 10.1016/S0304-3991(79)80044-3 [DOI] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield H. G. and Melton M. L. (1968). The fine structure and reproduction of Toxoplasma gondii. J. Parasitol. 54, 209-226. 10.2307/3276925 [DOI] [PubMed] [Google Scholar]
- Sidik S. M., Huet D., Ganesan S. M., Huynh M.-H., Wang T., Nasamu A. S., Thiru P., Saeij J. P. J., Carruthers V. B., Niles J. C. et al. (2016). A Genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell 166, 1423-1435.e12. 10.1016/j.cell.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova E. S., Francia M., Striepen B. and White M. W. (2015). A novel bipartite centrosome coordinates the apicomplexan cell cycle. PLoS Biol. 13, e1002093 10.1371/journal.pbio.1002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin M. L., Roques M., Lamarque M. H., Pugniere M., Douguet D., Crawford J., Lebrun M. and Boulanger M. J. (2011). Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333, 463-467. 10.1126/science.1204988 [DOI] [PubMed] [Google Scholar]
- Waller R. F., Keeling P. J., Donald R. G. K., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S. and McFadden G. I. (1998). Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95, 12352-12357. 10.1073/pnas.95.21.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. E. and Carey K. L. (1999). 96-Well plates providing high optical resolution for high-throughput, immunofluorescence-based screening of monoclonal antibodies against Toxoplasma gondii. J. Immunol. Methods 230, 11-18. 10.1016/S0022-1759(99)00109-X [DOI] [PubMed] [Google Scholar]
- Wright R. L., Salisbury J. and Jarvik J. W. (1985). A nucleus-basal body connector in Chlamydomonas reinhardtii that may function in basal body localization or segregation. J. Cell Biol. 101, 1903-1912. 10.1083/jcb.101.5.1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.