ABSTRACT
It is becoming increasingly evident that most cell types are capable of forming and releasing multiple distinct classes of membrane-enclosed packages, referred to as extracellular vesicles (EVs), as a form of intercellular communication. Microvesicles (MVs) represent one of the major classes of EVs and are formed by the outward budding of the plasma membrane. The second major class of EVs, exosomes, are produced as components of multivesicular bodies (MVBs) and are released from cells when MVBs fuse with the cell surface. Both MVs and exosomes have been shown to contain proteins, RNA transcripts, microRNAs and even DNA that can be transferred to other cells and thereby trigger a broad range of cellular activities and biological responses. However, EV biogenesis is also frequently de-regulated in different pathologies, especially cancer, where MVs and exosomes have been suggested to promote tumor cell growth, therapy resistance, invasion and even metastasis. In this Review, we highlight some of the recent advances in this rapidly emerging and exciting field of cell biology, focusing on the underlying mechanisms that drive MV and exosome formation and release, with a particular emphasis on how EVs potentially impact different aspects of cancer progression and stem cell biology.
KEY WORDS: Exosomes, Extracellular vesicles, Microvesicles
Summary: This Review highlights some of the recent findings regarding the roles of extracellular vesicles in physiological and pathological processes.
Introduction
Non-classical secretory vesicles, referred to as extracellular vesicles (EVs), have been steadily garnering attention from the cell biology community, as well as from the biotechnology and pharmaceutical industries. This is due to the promise they hold for new clinical strategies and because of their potential applications as diagnostic markers and therapeutic vehicles (Desrochers et al., 2016a; Agrahari et al., 2019; Kamerkar et al., 2017). The rapid growth of this field is made all the more remarkable by the fact that, not long ago, these vesicles were thought simply to represent a mechanism by which cells rid themselves of unwanted contents, or in other cases, were vesicular artifacts produced by apoptotic cells (Cocucci et al., 2009). However, with each passing month, new publications are appearing that implicate EVs in a spectrum of cellular activities, biological processes and diseases. However, some healthy skepticism lingers, particularly within the cell biology community, because of the difficulty in achieving a robust biochemical characterization of EVs, especially with regard to the specific nature of their cargo and how it contributes to their functions. Many of these types of questions and concerns are to be expected in any young and rapidly evolving field, and addressing them will undoubtedly help to further define their unique roles. In this Review, we will consider two areas, namely cancer progression and stem cell biology, where exciting findings are emerging that speak to EV biogenesis and their biological functions.
There have been some excellent reviews describing the general features and functions of EVs, and we refer the reader to the following recent examples (Mathieu et al., 2019; van Niel et al., 2018; Maas et al., 2017), while acknowledging that many others exist in the literature.
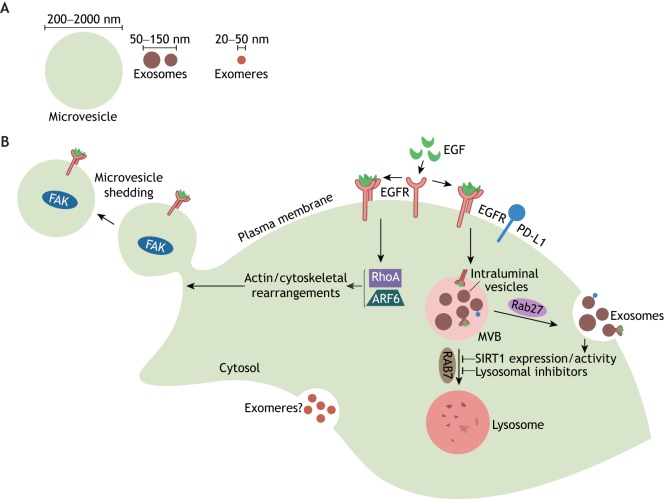
Most investigators in the field divide EVs into two broad sub-families, based on their size and the mechanisms responsible for their generation. One major sub-family is comprised of relatively ‘large’ vesicles, typically ranging in size from 200 nm to 1–2 µm in diameter (Fig. 1A). These EVs are generated at the plasma membrane, from which they bud off, and are most commonly referred to as microvesicles (MVs) (Fig. 1B), although the earlier literature gave these vesicles other names, including shedding vesicles, ectosomes and, when shown to contain transforming and/or oncogenic cargo, oncosomes (Desrochers et al., 2016a). The other major sub-family of EVs comprises vesicles that range from 30 to 150 nm in diameter (Fig. 1A). These smaller vesicles were first observed by Stahl and colleagues, who found that they formed as intraluminal vesicles within endosomal multivesicular bodies (MVBs), and were released from cells upon the fusion of MVBs with the plasma membrane (Harding et al., 1983); they are now referred to as exosomes (Fig. 1B).
Fig. 1.
Multiple distinct classes of EVs and non-vesicular nanoparticles are generated by cells, including microvesicles, exosomes and exomeres. (A) The relative sizes of each class of EV, as well as the major type of non-vesicular nanoparticle (i.e. exomers). (B) Schematic illustration depicting how different EVs are generated. MVs are formed as EGFRs, which signal through RhoA and Arf6, induce actin/cytoskeletal rearrangements that promote the outward budding and shedding (i.e. release) of microvesicles from the plasma membrane. Exosomes are formed from MVBs containing intraluminal vesicles that are trafficked to the cell surface in a Rab27-dependent manner. The MVBs then fuse with the plasma membrane and release their contents (i.e. exosomes) into the extracellular space. Inhibiting lysosomal function, for instance by treating cells with lysosomal inhibitors (i.e. chloroquine or Bafilomycin A) or by reducing SIRT1 expression and/or activity, causes more MVBs to fuse with the plasma membrane. The mechanisms underlying exomere biogenesis are unknown. Some important protein cargo found in microvesicles (i.e. EGFR and FAK) and exosomes (EGFR and PD-L1) are indicated.
Unfortunately, a good deal of confusion, and in some cases, mis-information exists regarding which class of EVs is responsible for specific biological functions. In particular, the term exosome is often used in reference to all EV-mediated processes, and many assume that only exosomes have significant biological functions and importance. Some of this has been due to the less than rigorous approaches used to isolate EVs, or because of a lack of precision in assigning functions to a particular class of these vesicles. Such practices have helped to cast doubt upon the credibility of the entire field and the important roles that EVs play in biology and disease. A further complication is that there are good reasons to suspect that there will be multiple sub-classes of EVs, as well as non-vesicular nanoparticles that often co-purify with exosomes. In fact, recent studies, in which great care was taken in the isolation of EVs, demonstrated that at least three distinct-sized EVs could be resolved from various cells (Kowal et al., 2016). Moreover, by using asymmetric flow field-flow fractionation to separate EVs of different sizes from cancer cells, it was recently reported that nanoparticles, of 20–50 nm in diameter, were resolved, which were given the name exomeres (Zhang et al., 2018) (Fig. 1A,B).
In light of these issues, it is incumbent upon the EV field that efforts be devoted to the type of biochemical classification and functional definition that was used earlier to characterize the fields of endocytosis and inter-organelle coatomer trafficking processes. Toward that end, the International Society of Extracellular Vesicles (ISEV) has established the Minimal Information for Studies of Extracellular Vesicles (MISEV 2018), a comprehensive guide for how to best isolate and classify the different types of EVs, as well as non-vesicular nanoparticles (i.e. exomeres) (Théry et al., 2018). Moreover, there are recent studies that are shedding new light on the important roles EVs have in different aspects of tumorigenesis, as well as identifying the regulatory signals responsible for their generation that appear to be broadly relevant to a number of cellular contexts (Mathieu et al., 2019; van Niel et al., 2018; Maas et al., 2017). There are also exciting findings implicating EVs in developmental processes, as well as in fundamental stem cell biology and in various neurodegenerative disorders (Rajendran et al., 2006; Desrochers et al., 2016b; Kim et al., 2019; Valadi et al., 2007; Ratajczak et al., 2006; Qiu et al., 2018; Yu et al., 2015). It seems likely that there will be common threads in EV biology that are responsible for their actions in these various biological settings, and that the commonalities in their biogenesis and regulation will help to contribute to a better definition of what types of EVs are responsible for specific functional outcomes.
In this Review, we will focus on two important examples of EV function in biology and disease, one involving the regulatory mechanisms responsible for the biogenesis of EVs from various types of cancer cells, and the other describing how EVs may play important roles in stem cell biology. We will also emphasize the future implications this could hold for regenerative medicine.
The roles of EVs in cancer progression
EVs and the primary tumor
EVs have been extensively studied in the context of cancer, where they have been implicated in the promotion of virtually every aspect of tumorigenesis. MVs and exosomes generated by highly aggressive cancer cells can be transferred to low grade and/or less malignant cancer cells and stimulate their growth, survival and migration (Zomer et al., 2015; Al-Nedawi et al., 2008; Sung et al., 2015). These EV-mediated effects might be particularly relevant within the setting of primary tumors, which consists of heterogeneous populations of cancer cells with varying levels of tumor-promoting capabilities (reviewed in Rackov et al., 2018; Zomer et al., 2015; Al-Nedawi et al., 2008; Sung et al., 2015). Thus, EVs provide a potential mechanism for aggressive cancer cells to communicate with lower grade/less malignant cells within a tumor, thereby conferring them with more aggressive behavior and promoting tumor expansion (Fig. 2A).
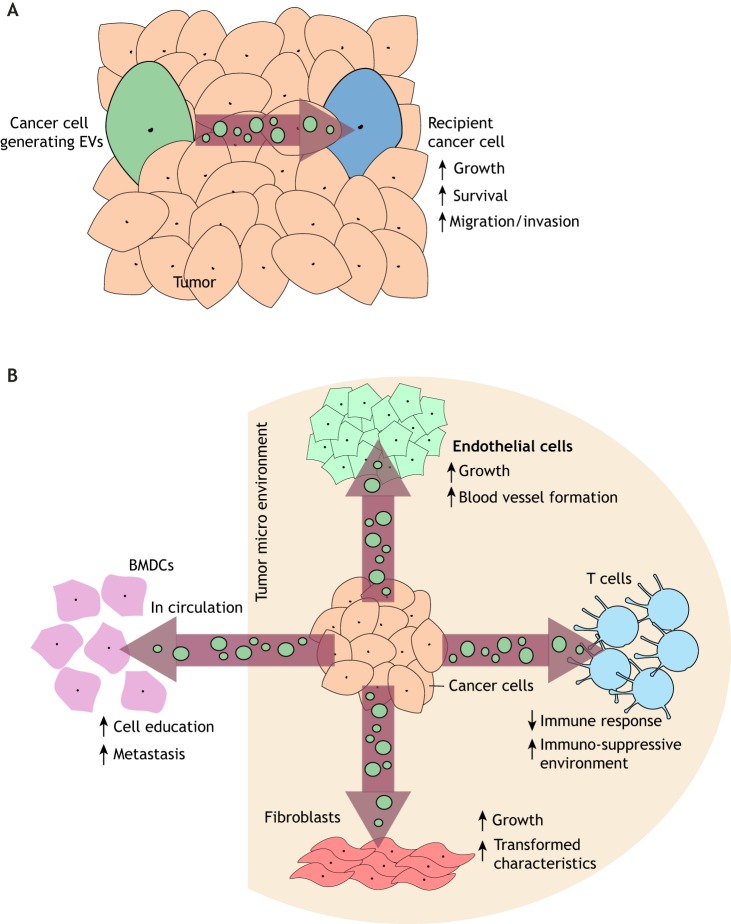
Fig. 2.
Schematic illustrations depicting how EVs mediate several different aspects of cancer progression. (A) EVs generated by a cancer cell can be transferred to other cancer cells within a tumor, and stimulate their growth, survival and ability to migrate. (B) EVs generated by cancer cells within a tumor influence the function of non-cancer cell types that comprise the tumor microenvironment (shaded area), including fibroblasts, endothelial cells, and immune cells (i.e. T-cells). Cancer cell-derived EVs can also enter the circulation to help promote metastasis. For example, these EVs can accumulate in the bone marrow and ‘educate’ bone marrow-derived cells (BMDCs) to migrate to and prepare secondary sites for the arrival of circulating cancer cells.
EVs generated by cancer cells have also been suggested to help shape the tumor microenvironment (Rackov et al., 2018) (Fig. 2B). Several years ago, we found that treatment of fibroblasts and mammary epithelial cells, two cell types frequently found in breast cancer microenvironments, with MVs derived from the triple-negative MDA-MB-231 breast cancer cell line, induced their ability to grow under anchorage-independent conditions (Antonyak et al., 2011). Additional work demonstrated that EVs derived from other cancer cell lines can recruit fibroblasts to tumor sites, differentiate mesenchymal stem cells into myo-fibroblasts and activate endothelial cells to form blood vessels (Feng et al., 2017; Dörsam et al., 2018; Song et al., 2017; Skog et al., 2008). The collective results of these effects are the production of growth factors and extracellular matrix proteins, combined with an increased blood supply, which are all critical for sustaining the growth and survival of tumor cells.
Another example of how EVs produced by cancer cells can markedly impact the function of cells within the local environment of a tumor came from recent studies examining the role of EVs in immunosuppression (Chen et al., 2018; Ricklefs et al., 2018; Poggio et al., 2019). It has been long appreciated that cancer cells evade the host immune system by upregulating the expression of programmed death ligand-1 (PD-L1; also known as CD274) (Chen and Han, 2015). PD-L1 then binds to its corresponding receptor, the receptor programmed death-1 (PD-1; also known as PDCD1), which is expressed on surveilling T-cells, and suppresses their function. Recently, it was discovered that exosomes generated by various types of highly aggressive cancer cells, which are enriched in PD-L1, can suppress the immune response and provide a protective barrier surrounding the tumor, engaging and inactivating T-cells before they are able to reach the cancer cells (Chen et al., 2018; Ricklefs et al., 2018; Poggio et al., 2019).
EVs and metastatic spread
The roles of EVs in promoting metastasis are complicated and only just beginning to be appreciated. For example, there are reasons to suspect that EVs produced by non-cancer cell types that populate the area immediately surrounding solid tumors can contribute to metastatic spread. Specifically, it has been shown that cancer-associated fibroblasts (CAFs) release exosomes that can be transferred to nearby breast cancer cells, enhancing their invasive activity and ability to metastasize (Luga et al., 2012). There is also a good deal known regarding how cancer cell-derived EVs affect this process. Exosomes generated by metastatic cancer cells are present in the circulation of patients, where they are thought to help prepare secondary sites of tumor formation for the arrival and proliferation of circulating cancer cells. For example, exosomes from metastatic melanoma cells were found to contain high levels of the Met tyrosine kinase (Met), and when injected into mice, a portion of these exosomes were taken-up by bone marrow stem cells, resulting in their expression of Met (Peinado et al., 2012). This induced a migratory phenotype in the bone marrow stem cells and caused them to re-locate to the lungs, the future site of metastasis for melanomas (Fig. 2B). Within the lung, the bone marrow stem cells differentiated into several different cell types, and formed new blood vessels, as a way to increase the probability that circulating melanoma cells will successfully colonize the lung (Peinado et al., 2012).
Another mechanism involving cancer cell-derived exosomes and their actions in the metastatic process is through their ability to accumulate at sites of secondary colonization (Hoshino et al., 2015). This is mediated by the expression of various members of the integrin family along the surfaces of exosomes. Because different integrins are known to interact with distinct extracellular matrix proteins, the integrins expressed on the surfaces of cancer-cell-derived exosomes were shown to preferentially bind to cells within a tissue that secreted a particular type of extracellular matrix protein, for example fibronectin in the lung (Hoshino et al., 2015). The exosomes were then taken-up by resident cells, causing them to produce a unique set of growth factors and cytokines that further altered the tissue, as well as recruiting additional cell types and generating new blood vessels, to make the site more receptive to circulating tumor cells (Hoshino et al., 2015). Although much more work is needed to fully understand the extent to which EVs contribute to the metastatic process, the findings from a recent study highlight just how important of a role EVs might play. Specifically, it was shown that treating mouse models of breast cancer with chemotherapy gives rise to the production of exosomes with a unique cargo, including annexin VI (Keklikoglou et al., 2019). These exosomes were then shown to accumulate in the lungs of the animal and were taken-up by endothelial cells and monocytes, activating annexin VI-dependent signaling events that promoted cell growth and blood vessel formation. This resulted in the formation of a pre-metastatic niche that increased the rates of lung metastasis (Keklikoglou et al., 2019).
The mechanisms regulating EV biogenesis
A role for cancer cell metabolism
The production of both exosomes and MVs is increased in diseased, or damaged, cells. This is exemplified in cancer, where aggressive and high-grade cancer cells have been shown to generate greater numbers of EVs compared to their normal cellular counterparts (Martins et al., 2013; Al-Nedawi et al., 2008; Antonyak et al., 2011). Similarly, treating cancer cells with chemotherapeutic agents, or radiation, significantly enhances the number of EVs produced and shed by the cells (Kreger et al., 2016a; Lv et al., 2012; Pavlyukovms et al., 2018; Yu et al., 2006). A possible explanation for these observations comes from the findings that an important contributor to the ability of cancer cells to generate increased numbers of EVs is the metabolic re-programming that these cells undergo (Song et al., 2017; Santana et al., 2014; Dorai et al., 2018). The vast majority of cancer cells exhibit marked changes in their metabolic activities, including significantly increased glycolytic fluxes (Lukey et al., 2018). This is accompanied by an uncoupling of their glycolytic pathway from the tricarboxylic acid (TCA) cycle, changes are referred to as the ‘Warburg effect’ (Vander Heiden et al., 2009). In order to satisfy the metabolic requirements imposed by the Warburg effect, cancer cells often compensate by developing alternative mechanisms for inputting into the TCA cycle, with one of the most common being their ability to significantly increase the metabolism of the amino acid glutamine (Lukey et al., 2018). This occurs through a significantly accelerated first step in glutamine metabolism, the conversion of glutamine to glutamate, which is mediated by the mitochondrial enzyme glutaminase. Interestingly, we have found that specific allosteric inhibitors of this metabolic enzyme can markedly attenuate the production of both exosomes and MVs (Song et al., 2017; Santana et al., 2014). What remains to be determined is how the metabolic changes characteristic of cancer cells are translated into an increased production of both classes of EVs, although a plausible possibility is that they give rise to the generation of metabolites and building blocks (e.g. fatty acids) that contribute to the production of membranes coating these vesicles (Lukey et al., 2018).
Signals that stimulate MV biogenesis in cancer cells
Given the importance of MVs in cancer progression, a good deal of work has been dedicated towards understanding how they are formed and shed from transformed cells. Signaling proteins that influence actin cytoskeletal dynamics have been identified as critical mediators of MV biogenesis. Indeed, several groups have shown that epidermal growth factor (EGF) stimulation of prostate, cervical and breast cancer cells can dramatically increase the number of MVs detected along the surfaces of these cells or released into the medium (Antonyak et al., 2011; Schlienger et al., 2014; Di Vizio et al., 2009). Moreover, expression of an activated form of the EGF receptor (EGFR) in glioma cells, similarly affected MV production (Al-Nedawi et al., 2008; Skog et al., 2008). It was then discovered that EGFRs mediated this effect by activating the small GTPases RhoA and Arf6 (Muralidharan-Chari et al., 2009; Li et al., 2012). Each of these proteins influence signaling events that induce actin rearrangements, such that they help coordinate the outward budding of MVs from the surface of a cell and their subsequent fission and release (Fig. 1B). MVs generated from transformed cells have also been shown to contain specific types of protein cargo. For example, MVs derived from fibroblasts that express an oncogenic form of the diffuse B-cell lymphoma protein (i.e. onco-Dbl; Dbl is also known as MCF2) were found to be highly enriched in the non-receptor tyrosine kinase focal adhesion kinase (FAK, also known as PTK2), whereas MVs from non-transformed cells had undetectable amounts of FAK, despite both cell types expressing FAK to a similar extent (Kreger et al., 2016b). However, as yet little is known regarding the detailed mechanisms that underlie the accumulation of specific proteins, as well as nucleic acids, in these vesicles and thus how cargo sorting is achieved (Fernández-Messina et al., 2010; Shurtleff et al., 2016).
Regulation of exosome biogenesis
Like MVs, exosomes have been implicated in different stages of cancer progression, and in particular, in metastasis (Mathieu et al., 2019). MVBs containing intraluminal vesicles within the endocytic pathway are either directed to the lysosome for degradation, or they fuse with the plasma membrane and release their contents (i.e. exosomes) into the extracellular space (Fig. 1B). Several proteins involved in MVB maturation and trafficking, including the endosomal sorting complexes required for transport (ESCRT) machinery, and several members of the Rab family of small GTPases, have also been shown to be required for exosome biogenesis (Mathieu et al., 2019; van Niel et al., 2018; Maas et al., 2017). It is also worth noting that ESCRT-independent mechanisms of exosome formation and release have also been identified (Kosaka et al., 2010; Trajkovic et al., 2008; Guo et al., 2015). Foremost among these involves the ability of neutral sphingomyelinases (nSMases) to generate the bioactive lipid ceramide (Shamseddine et al., 2015). Increases in ceramide levels can promote different aspects of MVB maturation. Thus, knocking down the expression of specific nSMase family members in cells, or blocking their ability to generate ceramide using the inhibitor GW4869, has been shown to reduce the release of exosomes (Kosaka et al., 2010; Guo et al., 2015). However, the mechanisms that determine which MVBs will be directed to the cell surface, versus the lysosome, are not well understood. One potentially intriguing idea is that changes in lysosomal function may help determine the fate of MVBs. This was initially suggested from studies showing that treating cells with lysosomal inhibitors increased the number of exosomes detected in their media (Miao et al., 2015; Vingtdeux et al., 2007; Alvarez-Erviti et al., 2011).
Recently, we discovered an unexpected connection between the NAD+-dependent lysine deacetylase sirtuin 1 (SIRT1) and exosome biogenesis (Latifkar et al., 2019). SIRT1 is best known for its role in extending lifespan (Schmeisser et al., 2013), but its expression level is also frequently downregulated in highly aggressive breast tumors (Latifkar et al., 2019; Simic et al., 2013; Wang et al., 2008). How reductions in SIRT1 expression enhances transformed phenotypes has been an open question, and we now know that siRNA-mediated knockdown of SIRT1 expression in breast cancer cells impairs lysosomal activity by inhibiting the function of the vacuolar-type H+ ATPase (V-ATPase), the proton pump responsible for maintaining proper lysosomal pH (Colacurcio and Nixon, 2016). This resulted in MVBs that would normally be targeted for degradation in lysosomes to instead fuse with the plasma membrane and release exosomes (Fig. 1). Furthermore, the exosomes derived from SIRT1-depleted cancer cells were enriched in several types of protein and RNA cargo, compared to exosomes from control cells, and capable of strongly promoting cell survival, cell migration and invasion (Latifkar et al., 2019). Collectively, these findings highlight how reductions in SIRT1 expression can disrupt lysosomal function and alter the secretome of breast cancer cells, such that it promotes aggressive phenotypes. Since SIRT1 expression levels also decrease during cell senescence and aging, it is intriguing to consider that these cells may use a similar mechanism to produce exosomes that can be transferred to other cells to negatively impact aging and lifespan. Moreover, certain neurodegenerative diseases such as Alzheimer's disease (AD) are characterized by the loss of SIRT1 expression (Kim et al., 2007; Min et al., 2010) and lysosomal impairment (Fraldi et al., 2016; Nixon, 2017), and neurons have also been shown to form exosomes that have been implicated in the spread of the disease (Howitt and Hill, 2016).
Emerging roles for EVs in stem cell biology and their potential uses in regenerative medicine
EVs in regenerative medicine
Recent evidence has suggested that EVs are not only produced by diseased or damaged cells, but that EVs can be generated by most cell types and play important roles in a number of biological processes, ranging from development to tissue homeostasis. There may be no better example of this than the emerging roles of stem cell-derived EVs in promoting cell survival and helping to maintain pluripotency (Desrochers et al., 2016a; Kim et al., 2019; Valadi et al., 2007; Ratajczak et al., 2006; Qiu et al., 2018; Yu et al., 2015) (Fig. 3). Thus, one area for new potential applications of EVs is the field of regenerative medicine, where the goal has been to restore cells or tissues that are diseased, or have been damaged, to their original functions (Mao and Mooney, 2015). This is most often accomplished by either stimulating the body's own repair mechanisms to correct the deficiencies exhibited by dysfunctional cells and/or tissues, or by replacing these tissues with cells grown in the laboratory. For this purpose, the transplantation of stem cells, or specialized and/or differentiated cell types that are derived from stem cells, into damaged tissues is a commonly used approach (Giri et al., 2019). Mesenchymal stem cells (MSCs) are considered one of the best cell types to use for tissue regenerative therapies, because they can be readily derived and propagated, as well as differentiated into a variety of cell types (Kassem et al., 2004). Moreover, when these multi-potent stem cells were used to treat animal models of stroke, myocardial ischemia and diabetes, they showed strong regenerative capabilities (Murphy et al., 2013). As an example, MSC transplantation into rodent models of heart diseases, such as dilated cardiomyopathy and myocardial infarction, was found to improve cardiac function (Miyahara et al., 2006; Nagaya et al., 2005), possibly by generating new heart tissue and promoting angiogenesis. It was initially thought that these effects were due to the migration of the engrafted MSCs to the damaged heart tissue, and their subsequent differentiation into cardiomyocytes and vascular cells (Miyahara et al., 2006; Nagaya et al., 2005). However, it was subsequently reported that the engrafted MSCs did not efficiently differentiate, suggesting that their therapeutic benefits were not due to their ability to change into cell lineages that could directly replace the damaged tissue (reviewed in Phinney and Prockop, 2007). An important breakthrough came when it was shown that the conditioned medium from MSCs was sufficient to recapitulate the effects of the engrafted cells (Timmers et al., 2011). Indeed, upon injecting MSC-conditioned medium into mice with myocardial infarction, there was an improvement in cardiac function and a reduction in the infarct size, as an outcome of stimulating angiogenesis and increasing myocardial perfusion (Timmers et al., 2011). These findings suggested that factors secreted by MSCs, rather than the cells themselves, were able to provide significant therapeutic benefits.
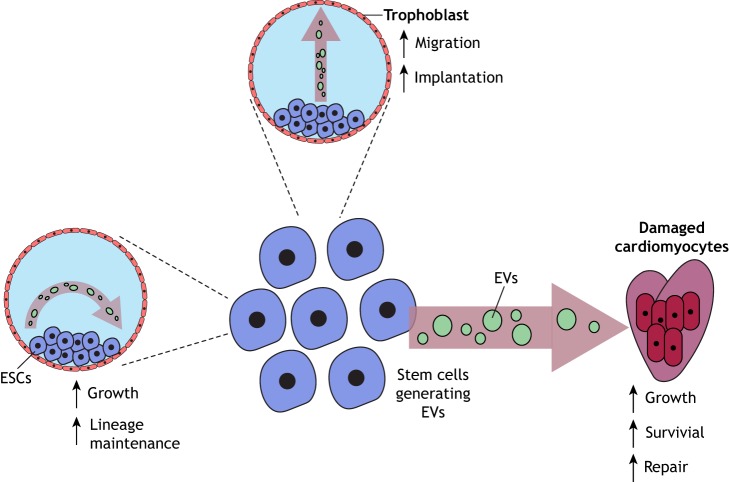
Fig. 3.
Illustration showing the different ways EVs from stem cells can impact recipient cells. The EVs generated by MSCs can help regenerate damaged tissue (a damaged heart is depicted) by promoting the survival, growth and repair of heart tissue. ESCs also generate large amounts of microvesicles and exosomes. These EVs have been shown to help the trophoblasts in a blastocyst to migrate and invade into the uterus, an important step in early pregnancy referred to as implantation. EVs shared between stem cells may also serve as a mechanism to help maintain stem cell identity.
Additional studies showed that EVs in the MSC-conditioned medium were responsible for mediating these effects. Using EVs isolated from MSCs to treat a mouse with myocardial infarction resulted in a reduction in infarction size and improved cardiac function by decreasing the levels of oxidative stress and inflammation at the site of damaged tissue (Arslan et al., 2013; Teng et al., 2015). These beneficial effects were linked to the ability of EVs to increase the activation of AKT family proteins and glycogen synthase kinase 3β (GSK-3β), thereby suppressing the activation of apoptosis-inducing signaling proteins in mouse cardiomyocytes when administered intravenously after myocardial ischemia-reperfusion injury. These findings highlight how MSC-derived EVs can help restore the function of damaged tissues by altering the local environment in a manner that promotes the survival of damaged cells until they have time to repair themselves (Fig. 3).
How these vesicles mediate their effects is still not well understood. Recent studies have suggested that microRNAs (miRNAs) contained in EVs from MSCs might help promote cell and tissue repair and/or regeneration (Qiu et al., 2018). miR-22, which is best known for promoting cell survival by downregulating the expression of methyl CpG binding protein 2 (MECP2), was identified in an RNA-seq screen performed on MSC-derived EVs (Yu et al., 2015). In the context of a mouse model of myocardial infarction, it was shown that the EV-mediated transfer of miR-22 to recipient cardiomyocytes prevented fibrosis and inhibited cell death, leading to a better recovery in heart function. miR-19a was identified as another miRNA in EVs from MSCs that might help to restore cardiac function, as it was demonstrated to inhibit the expression of phosphatase and tensin homolog (PTEN), which dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to phosphatidylinositol 4,5-bisphosphate (PIP2) and inhibits AKT signaling (Yu et al., 2015). Therefore, by decreasing the levels of PTEN in primary rat neonatal cardiomyocytes, AKT signaling was enhanced with a concomitant increase in cell survival. As EVs from cancer cells also have been shown to induce the activation of AKT signaling and subsequently to increase cell survival (Al-Nedawi et al., 2008; Antonyak et al., 2011), it will be interesting to see if EVs from MSCs share similar cargo with EVs from cancer cells.
EVs and early development
We and other groups have shown that embryonic stem cells (ESCs) generate EVs, which have some interesting biological consequences (Desrochers et al., 2016b; Nawaz et al., 2016; Khan et al., 2015). ESCs, which are derived from the inner cell mass of the blastocyst, are characterized by self-renewal and an ability to differentiate into all three germ layers of the developing embryo (Desrochers et al., 2016a). Interestingly, it has been suggested that ESCs, as well as a few other cell types expressed in maternal and embryonic tissues during pregnancy, use EVs as a mechanism to communicate with their surroundings (Simon et al., 2018). Several recent studies have begun to reveal the important roles played by these vesicles in development. Here, we will highlight a few studies whose findings provide new insights into how this form of intercellular communication is used in pregnancy.
Implantation is an early stage of pregnancy, where the embryo attaches and invades into the uterine wall (Desrochers et al., 2016a). Successful implantation requires a high degree of coordination between the embryo and the endometrium of the uterus of the mother, and a variety of soluble factors secreted by the endometrium help mediate this process (Paiva et al., 2009). However, it has also been shown that the endometrial epithelium releases EVs into the uterine fluid, where they might influence implantation by transferring certain cargo to either the blastocyst or to adjacent endometrial epithelial cells (Ng et al., 2013). One study performed in sheep showed that endogenous β-retroviruses are transferred from the endometrium to the embryo via EVs (Burns et al., 2014). The EV-mediated transfer of retroviruses to the embryo regulates trophoblast development, which is necessary for endometrial receptivity and for ensuring a successful pregnancy.
Although implantation is considered as a process primarily regulated by signals originating from the mother (Paiva et al., 2009), it has been recently suggested that the embryo may also contribute to this process by generating MVs (Desrochers et al., 2016b). Here, the authors discovered that the ESCs within the inner cell mass of the blastocyst release MVs that are transferred to the surrounding layer of trophoblasts, stimulating their ability to migrate and invade into the uterine lining (Fig. 3). This process was mediated by two extracellular matrix proteins, laminin α5 and fibronectin, which are present along the surfaces of the ESC-derived MVs and bind to the laminin receptor and α5β1 integrin on trophoblasts, resulting in the activation of FAK and c-Jun N-terminal kinase (JNK) family proteins. Blocking the binding of these MVs to the trophoblasts, or inhibiting MV-stimulated JNK activation, prevented trophoblast migration in vitro. Moreover, injecting blastocysts with ESC-derived MVs caused enhanced rates of implantation (Desrochers et al., 2016b).
Effects of ESC-derived EVs in cellular reprogramming
EVs generated by stem cells have been suggested to help influence cellular reprogramming. Surprisingly, studies have shown that EVs produced by ESCs, and other types of progenitor cells, contain proteins or RNA transcripts that encode proteins, known to have essential roles in maintaining ‘stemness’, including Oct3/4 (also known as POU5F1), Sox2, Nanog and Rex-1 (also known as ZFP42) (Desrochers et al., 2016a; Nawaz et al., 2016; Khan et al., 2015; Valadi et al., 2007; Ratajczak et al., 2006). It has been generally assumed that EVs can transfer these transcripts to recipient cells, where they are translated into functional proteins (Desrochers et al., 2016a; Nawaz et al., 2016; Valadi et al., 2007; Ratajczak et al., 2006). We feel that this raises the interesting possibility that EVs might provide a potential mechanism by which ESCs undergo a type of intercellular communication within the inner cell mass of the blastocyst to help maintain a pluripotent niche, until such time that they receive signals to differentiate (Fig. 3).
Because EVs cause recipient cells to acquire many of the phenotypes of the cells that generated the vesicles, the field is becoming increasingly attracted to the idea that EVs generated by ESCs can potentially cause differentiated cells to behave more like a stem cell, or even possibly convert them into stem cells.
In a landmark study that culminated in the Nobel Prize in 2012, it was shown that introducing four stemness-related genes into fibroblasts reprogrammed them into pluripotent stem cells, a process called induced pluripotency (Takahashi and Yamanaka, 2006). This technology provides a source of genetically identical cells that can be potentially differentiated into any cell type. Therefore, induced pluripotency provides a one-of-a-kind approach to generate disease models using patient-specific-derived stem cells, which are especially helpful for the development of new drugs, as well as individualized regenerative therapy approaches. However, this approach requires a significant amount of time to derive stem cells from somatic cells, and the efficiency of this process is extremely low (Takahashi and Yamanaka, 2006; Rony et al., 2015). Moreover, the introduction of stemness genes into somatic cells involves viral infection, making it highly unlikely that the cells could ever be used for therapeutic applications (Rony et al., 2015). Thus, there is a need for better and more efficient approaches to induce pluripotency.
EVs from ESCs could potentially be used for this purpose. The basis for this idea comes from the findings that ESC-derived EVs contain several proteins and RNA transcripts linked to the induction of pluripotency (Nawaz et al., 2016; Valadi et al., 2007; Ratajczak et al., 2006). There is some recent evidence showing that ESC EVs are able to transfer the transcripts encoding Oct3/4 and Sox2, as well as the 290 cluster of miRNAs, to retinal progenitor Müller cells (Katsman et al., 2012). This caused the Müller cells to upregulate the expression of genes involved in promoting de-differentiation, including cyclin D2 and bone morphogenic protein 7, while downregulating the expression of miRNAs involved in differentiation and cell cycle arrest, such as the miR-let-7 cluster (Katsman et al., 2012). Similarly, EVs generated by ESCs were found to enhance the survival and growth of murine hematopoietic progenitor cells by transferring mRNAs and proteins related to maintaining pluripotency to these cells (Ratajczak et al., 2006). Taken together, these findings suggest that ESC-derived EVs can cause differentiated cells to start to behave like stem/progenitor cells, and potentially can even induce pluripotency.
Concluding remarks
As is often the case in a rapidly moving research field, the questions tend to outpace the answers. This is certainly true for the field of EVs. We have learned a great deal over the past five years regarding the roles these vesicles have in different aspects of biology and disease. Many exciting discoveries now add credence to a cell biological phenomenon that was once considered an artifact. The challenge that lies ahead will be to provide the necessary biochemical characterizations to make it possible to rigorously define the functional roles played by MVs and exosomes in different physiological and disease settings. Among the more interesting questions will be to determine the specific proteins present within the membrane coatings of these vesicles, versus those present within their vesicular lumen, and identifying which RNA transcripts, microRNAs and even DNA might be colocalized together with their protein cargo. In this context, the question arises as to what types of signaling events within cells are required to achieve the specific accumulation of these protein and nucleic acid cargo. Still other questions that will need to be answered include addressing the roles that the different classes of EVs play in development, neuronal function, the immune system and in life span. How similar or distinct are the roles of EVs in these ‘normal’ biological processes, versus their roles in pathological conditions, such as cancer and neurodegeneration? Finally, once we begin to obtain answers to these questions, we can turn our attention to taking advantage of this information to develop therapeutic strategies that can be directed toward altering EV production or the cargo they contain, in order to achieve clinical benefits. All of this seems to ensure that the EV field will be an exciting area of research for years to come.
Acknowledgements
We would like to thank Cindy Westmiller for helping prepare this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work is supported by funding from the National Institutes of Health (R35 GM122575, R01 CA201402 and U54 CA210184) to R.C. and F99 CA234921 to A.L. Deposited in PMC for release after 12 months.
References
- Agrahari V., Agrahari V., Burnouf P.-A., Chew C. H. and Burnnouf T. (2019). Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. 10.1016/j.tibtech.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A. and Rak J. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619-624. 10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L., Seow Y., Schapira A. H., Gardiner C., Sargent I. L., Wood M. J. A. and Cooper J. M. (2011). Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 42, 360-367. 10.1016/j.nbd.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak M. A., Li B., Boroughs L. K., Johnson J. L., Druso J. E., Bryant K. L., Holowka D. A. and Cerione R. A. (2011). Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA 108, 4852-4857. 10.1073/pnas.1017667108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F., Lai R. C., Smeets M. B., Akeroyd L., Choo A., Aguor E. N. E., Timmers L., van Rijen H. V., Doevendans P. A., Pasterkamp G. et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301-312. 10.1016/j.scr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Burns G., Brooks K., Wildung M., Navakanitworakul R., Christenson L. K. and Spencer T. E. (2014). Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 9, e90913 10.1371/journal.pone.0090913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. and Han X. (2015). Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 125, 3384-3391. 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Huang A. C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H. et al. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382-386. 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E., Racchetti G. and Meldolesi J. (2009). Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43-51. 10.1016/j.tcb.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Colacurcio D. J. and Nixon R. A. (2016). Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 32, 75-88. 10.1016/j.arr.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers L. M., Antonyak M. A. and Cerione R. A. (2016a). Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev. Cell 37, 301-309. 10.1016/j.devcel.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers L. M., Bordeleau F., Reinhart-King C. A., Cerione R. A. and Antonyak M. A. (2016b). Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 7, 11958 10.1038/ncomms11958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D., Kim J., Hager M. H., Morello M., Yang W., Lafargue C. J., True L. D., Rubin M. A., Adam R. M., Beroukhim R. et al. (2009). Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601-5609. 10.1158/0008-5472.CAN-08-3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai T., Shah A., Summers F., Mathew R., Huang J., Hsieh T.-C. and Wu J. M. (2018). NRH:quinone oxidoreductase 2 (NQO2) and glutaminase (GLS) both play a role in large extracellular vesicles (LEV) formation in preclinical LNCaP-C4-2B prostate cancer model of progressive metastasis. Prostate 78, 1181-1195. 10.1002/pros.23693 [DOI] [PubMed] [Google Scholar]
- Dörsam B., Bösl T., Reiners K. S., Barnert S., Schubert R., Shatnyeva O., Zigrino P., Engert A., Hansen H. P. and von Strandmann E. P. (2018). Hodgkin lymphoma-derived extracellular vesicles change the secretome of fibroblasts toward a CAF phenotype. Front. Immunol. 9, 1358 10.3389/fimmu.2018.01358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Zhang C., Lum D., Druso J. E., Blank B., Wilson K. F., Welm A., Antonyak M. A. and Cerione R. A. (2017). A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 8, 14450 10.1038/ncomms14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Messina L., Ashiru O., Boutet P., Agüera-González S., Skepper J. N., Reyburn H. T. and Valés-Gómez M. (2010). Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J. Biol. Chem. 285, 8543-8551. 10.1074/jbc.M109.045906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A., Klein A. D., Medina D. L. and Settembre C. (2016). Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. 39, 277-295. 10.1146/annurev-neuro-070815-014031 [DOI] [PubMed] [Google Scholar]
- Giri T. K., Alexander A., Agrawal M., Saraf S., Saraf S. and Ajazuddin (2019). Current status of stem cell therapies in tissue repair and regeneration. Curr. Stem Cell. Res. Ther. 14, 117-126. 10.2174/1574888X13666180502103831 [DOI] [PubMed] [Google Scholar]
- Guo B. B., Bellingham S. A. and Hill A. F. (2015). The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J. Biol. Chem. 290, 3455-3467. 10.1074/jbc.M114.605253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J. and Stahl P. (1983). Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329-339. 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S. et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329-335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt J. and Hill A. F. (2016). Exosomes in the pathology of neurodegenerative diseases. J. Biol. Chem. 291, 26589-26597. 10.1074/jbc.R116.757955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S., LeBleu V. S., Sugimoto H., Yang S., Ruivo C. F., Melo S. A., Lee J. J. and Kalluri R. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498-503. 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M., Kristiansen M. and Abdallah B. M. (2004). Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin. Pharmacol. Toxicol. 95, 209-214. 10.1111/j.1742-7843.2004.pto950502.x [DOI] [PubMed] [Google Scholar]
- Katsman D., Stackpole E. J., Domin D. R. and Farber D. B. (2012). Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS ONE 7, e50417 10.1371/journal.pone.0050417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikoglou I., Cianciaruso C., Güç E., Squadrito M. L., Spring L. M., Tazzyman S., Lambein L., Poissonnier A., Ferraro G. B., Baer C. et al. (2019). Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 21, 190-202. 10.1038/s41556-018-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Nickoloff E., Abramova T., Johnson J., Verma S. K., Krishnamurthy P., Mackie A. R., Vaughan E., Garikipati V. N. S., Benedict C. et al. (2015). Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 117, 52-64. 10.1161/CIRCRESAHA.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Nguyen M. D., Dobbin M. M., Fischer A., Sananbenesi F., Rodgers J. T., Delalle I., Baur J. A., Sui G., Armour S. M. et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169-3179. 10.1038/sj.emboj.7601758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Steinberg D. R., Burdick J. A. and Mauck R. L. (2019). Extracellular vesicles mediate improved functional outcomes in engineered cartilage produced from MSC/chondrocyte cocultures. Proc. Natl. Acad. Sci. USA 116, 1569-1578. 10.1073/pnas.1815447116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y. and Ochiya T. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442-17452. 10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J., Arras G., Colombo M., Jouve M., Morath J. P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M. and Théry C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 113, E968-E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger B. T., Johansen E. R., Cerione R. A. and Antonyak M. A. (2016a). The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers (Basel) 8, E111 10.3390/cancers8120111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger B. T., Dougherty A. L., Greene K. S., Cerione R. A. and Antonyak M. A. (2016b). Microvesicle cargo and function changes upon induction of cellular transformation. J. Biol. Chem. 291, 19774-19785. 10.1074/jbc.M116.725705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifkar A., Ling L., Hingorani A., Johansen E., Clement A., Zhang X., Hartman J., Fischbach C., Lin H., Cerione R. A. et al. (2019). Loss of Sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev. Cell 49, 393-408.e7. 10.1016/j.devcel.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Antonyak M. A., Zhang J. and Cerione R. A. (2012). RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740-4749. 10.1038/onc.2011.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V., Zhang L., Viloria-Petit A. M., Ogunjimi A. A., Inanlou M. R., Chiu E., Buchanan M., Hosein A. N., Basik M. and Wrana J. L. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151, 1542-1556. 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Lukey M. J., Katt W. P. and Cerione R. A. (2018). Targeting therapy resistance: when glutamine catabolism becomes essential. Cancer Cell 33, 795-797. 10.1016/j.ccell.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L.-H., Wan Y.-L., Lin Y., Zhang W., Yang M., Li G.-L., Lin H.-M., Shang C.-Z., Chen Y.-J. and Min J. (2012). Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro . J. Biol. Chem. 287, 15874-15885. 10.1074/jbc.M112.340588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S. L. N., Breakefield X. O. and Weaver A. M. (2017). Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172-188. 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao A. S. and Mooney D. J. (2015). Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. USA 112, 14452-14459. 10.1073/pnas.1508520112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V. R., Dias M. S. and Hainaut P. (2013). Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 25, 66-75. 10.1097/CCO.0b013e32835b7c81 [DOI] [PubMed] [Google Scholar]
- Mathieu M., Martin-Jaular L., Lavieu G. and Théry C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9-17. 10.1038/s41556-018-0250-9 [DOI] [PubMed] [Google Scholar]
- Miao Y., Li G., Zhang X., Xu H. and Abraham S. N. (2015). A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell 161, 1306-1319. 10.1016/j.cell.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S.-W., Cho S.-H., Zhou Y., Schroeder S., Haroutunian V., Seeley W. W., Huang E. J., Shen Y., Masliah E., Mukherjee C. et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953-966. 10.1016/j.neuron.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara Y., Nagaya N., Kataoka M., Yanagawa B., Tanaka K., Hao H., Ishino K., Ishida H., Shimizu T., Kangawa K. et al. (2006). Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 12, 459-465. 10.1038/nm1391 [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G. and D'Souza-Schorey C. (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875-1885. 10.1016/j.cub.2009.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. B., Moncivais K. and Caplan A. I. (2013). Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 45, e54 10.1038/emm.2013.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N., Kangawa K., Itoh T., Iwase T., Murakami S., Miyahara Y., Fujii T., Uematsu M., Ohgushi H., Yamagishi M. et al. (2005). Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 112, 1128-1135. 10.1161/CIRCULATIONAHA.104.500447 [DOI] [PubMed] [Google Scholar]
- Nawaz M., Fatima F., Vallabhaneni K. C., Penfornis P., Valadi H., Ekström K., Kholia S., Whitt J. D., Fernandes J. D., Pochampally R. et al. (2016). Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016, 1073140 10.1155/2016/1073140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y. H., Rome S., Jalabert A., Forterre A., Singh H., Hincks C. L. and Salamonsen L. A. (2013). Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 8, e58502 10.1371/journal.pone.0058502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon R. A. (2017). Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729-2743. 10.1096/fj.201700359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva P., Menkhorst E., Salamonsen L. and Dimitriadis E. (2009). Leukemia inhibitory factor and interleukin-11: critical regulators in the establishment of pregnancy. Cytokine Growth Factor. Rev. 20, 319-328. 10.1016/j.cytogfr.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Pavlyukov M. S., Yu H., Bastola S., Minata M., Shender V. O., Lee Y., Zhang S., Wang J., Komarova S., Wang J. et al. (2018). Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell 34, 119-135. 10.1016/j.ccell.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C. et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883-891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney D. G. and Prockop D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 25, 2896-2902. 10.1634/stemcells.2007-0637 [DOI] [PubMed] [Google Scholar]
- Poggio M., Hu T., Pai C.-C., Chu B., Belair C. D., Chang A., Montabana E., Lang U. E., Fu Q., Fong L. et al. (2019). Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 17, 414-427.e13. 10.1016/j.cell.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G., Zheng G., Ge M., Wang J., Huang R., Shu Q. and Xu J. (2018). Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 9, 320 10.1186/s13287-018-1069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackov G., Garcia-Romero N., Esteban-Rubio S., Carrión-Navarro J., Belda-Iniesta C. and Ayuso-Sacido A. (2018). Vesicle-mediated control of cell function: the role of extracellular matrix and microenvironment. Front. Physiol. 9, 651 10.3389/fphys.2018.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P. and Simons K. (2006). Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 103, 11172-11177. 10.1073/pnas.0603838103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P. and Ratajczak M. Z. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847-856. 10.1038/sj.leu.2404132 [DOI] [PubMed] [Google Scholar]
- Ricklefs F. L., Alayo Q., Krenzlin H., Mahmoud A. B., Speranza M. C., Nakashima H., Hayes J. L., Lee K., Balaj L., Passaro C. et al. (2018). Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 10.1126/sciadv.aar2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rony I. K., Baten A., Bloomfield J. A., Islam M. E., Billah M. M. and Islam K. D. (2015). Inducing pluripotency in vitro: recent advances and highlights in induced pluripotent stem cells generation and pluripotency reprogramming. Cell Prolif. 48, 140-156. 10.1111/cpr.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana S. M., Antonyak M. A., Cerione R. A. and Kirby B. J. (2014). Cancerous epithelial cell lines shed extracellular vesicles with a bimodal size distribution that is sensitive to glutamine inhibition. Phys. Biol. 11, 065001 10.1088/1478-3975/11/6/065001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger S., Campbell S. and Claing A. (2014). ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol. Biol. Cell 25, 17-29. 10.1091/mbc.e13-06-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser K., Mansfeld J., Kuhlow D., Weimer S., Priebe S., Heiland I., Birringer M., Groth M., Segref A., Kanfi Y. et al. (2013). Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 9, 693-700. 10.1038/nchembio.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddine A. A., Airola M. V. and Hannun Y. A. (2015). Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv. Biol. Regul. 57, 24-41. 10.1016/j.jbior.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff M. J., Temoche-Diaz M. M., Karfilis K. V., Ri S. and Schekman R. (2016). Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 5, e19276 10.7554/eLife.19276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic P., Williams E. O., Bell E. L., Gong J. J., Bonkowski M. and Guarente L. (2013). SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep. 3, 1175-1186. 10.1016/j.celrep.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C., Greening D. W., Bolumar D., Balaguer N., Salamonsen L. A. and Vilella F. (2018). Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 39, 292-332. 10.1210/er.2017-00229 [DOI] [PubMed] [Google Scholar]
- Skog J., Würdinger T., van Rijn S., Meijer D. H., Gainche L., Curry W. T. Jr, Carter B. S., Krichevsky A. M. and Breakefield X. O. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470-1476. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. H., Warncke C., Choi S. J., Choi S., Chiou A. E., Ling L., Liu H.-Y., Daniel S., Antonyak M. A., Cerione R. A. et al. (2017). Breast cancer-derived extracellular vesicles stimulate myofibroblast differentiation and pro-angiogenic behavior of adipose stem cells. Matrix Biol. 60-61, 190-205. 10.1016/j.matbio.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B. H., Ketova T., Hoshino D., Zijlstra A. and Weaver A. M. (2015). Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 6, 7164 10.1038/ncomms8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Teng X., Chen L., Chen W., Yang J., Yang Z. and Shen Z. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. 37, 2415-2424. 10.1159/000438594 [DOI] [PubMed] [Google Scholar]
- Théry C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G. K. et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers L., Lim S. K., Hoefer I. E., Arslan F., Lai R. C., van Oorschot A. A. M., Goumans M. J., Strijder C., Sze S. K., Choo A. et al. (2011). Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 6, 206-214. 10.1016/j.scr.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B. and Simons M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244-1247. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J. and Lötvall J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654-659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- Van Niel G., D'Angelo G. and Raposo G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213-228. 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Cantley L. C. and Thompson C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029-1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V., Hamdane M., Loyens A., Gelé P., Drobeck H., Bégard S., Galas M.-C., Delacourte A., Beauvillain J.-C., Buée L. et al. (2007). Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 282, 18197-18205. 10.1074/jbc.M609475200 [DOI] [PubMed] [Google Scholar]
- Wang R.-H., Sengupta K., Li C., Kim H.-S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B. et al. (2008). Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312-323. 10.1016/j.ccr.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Harris S. L. and Levine A. J. (2006). The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 66, 4795-4801. 10.1158/0008-5472.CAN-05-4579 [DOI] [PubMed] [Google Scholar]
- Yu B., Kim H. W., Gong M., Wang J., Millard R. W., Wang Y., Ashraf M. and Xu M. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 182, 349-360. 10.1016/j.ijcard.2014.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Freitas D., Kim H. S., Fabijanic K., Li Z., Chen H., Mark M. T., Molina H., Martin A. B., Bojmar L. et al. (2018). Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332-343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A., Maynard C., Verweij F. J., Kamermans A., Schäfer R., Beerling E., Schiffelers R. M., de Wit E., Berenguer J., Ellenbroek S. I. J. et al. (2015). In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046-1057. 10.1016/j.cell.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]