Abstract
Background: β2-adrenoceptors (β2-ARs) are expressed on the surface of immune cells, including tumor-associated macrophages (TAMs). Previous studies have demonstrated that the expression of β2-ARs in hepatocellular carcinoma (HCC) is significantly increased in vitro. However, the role of β2-AR in M2-polarized macrophages remains unclear. G protein-coupled receptor kinase 2 (GRK2) can regulate G protein-coupled receptor (GPCR). Previous studies showed that down-regulation of GRK2 in HCC contributes the HCC progression, but it still remains unclear whether the regulation of β2-AR in M2-polarized macrophages by GRK2 can promote HCC.
Purpose: The present study was designed to investigate the role of activated β2-AR in M2-polarized macrophages in the HCC progression and GRK2 regulatory effect, as well as the underlying mechanisms involved.
Results: The results demonstrated that the M2-polarized macrophages were increased with HCC progression. In vitro, the activation of β2-AR by terbutaline in M2-polarized macrophages elevated the proliferative, migratory and invasive attributes of HCC cells. Furthermore, GRK2 down-regulation in β2-AR activated M2-polarized macrophages activated the downstream cyclic adenosine monophosphate (cAMP)/protein kinase A/cAMP-response element binding protein and cAMP/interleukin-6/signal transducer and the activator of transcription 3 signaling pathways, contributing to the secretion of tumor-associated cytokines, and thus resulting in the promotion of malignant biological behavior in HCC cells.
Conclusion: These findings suggest that the regulation of β2-AR occurs through the silencing of GRK2 in M2-polarized macrophages, which is conducive to HCC development, through its engagement in the activation of downstream signaling.
Keywords: hepatocellular carcinoma, tumor microenvironment, macrophage, beta2-adrenoceptor, G protein-coupled receptor kinase 2, therapeutic target
Introduction
Hepatocellular carcinoma (HCC) is a highly malignant type of cancer and the second leading cause of cancer-associated mortality.1 Although the progress on surgical procedures, including minimally invasive treatment and liver transplantation for HCC have been progressed, patients with HCC still have a poor prognosis with frequent recurrences and metastases. The molecular targeting agent, sorafenib, are used to improve the overall survival rates of patients at late stage HCC, but with limited effect. Therefore, novel therapeutic targets are required to improve the survival of patients with HCC.
Tumor associated macrophages (TAMs) are a type of immune cells present in the tumor microenvironment. They resemble M2 subtype, and produce large quantities of tumor-associated cytokines and chemokines, and differing from M1 cells.2,3 It has become increasingly evident that the presence of intratumor macrophages in a large number is associated with the high vessel density and the progression of various tumors, including urinary bladder and breast cancer.4–7 β2-adrenoceptors (β2-ARs) as a subtype of ARs, belong to the G protein-coupled receptor (GPCR) family with a common structural signature of seven membrane-spanning helices.8 β2-AR is overexpressed in HCC, the activation of β2-AR leads to the HCC growth progression as demonstrated in various HCC cell lines, like SMMC-7721, MHCC97L, HepG2 and MHCC97H.9–11 Furthermore, ARs are distributed on the surface of macrophages. A previous study demonstrated that β-adrenergic signaling modulates the growth and metastasis of tumors by producing changes to the tumor microenvironment, including macrophage recruitment.12 Grailer et al13 reported that catecholamine-induced activation of the β2-AR modified the entire macrophage phenotype, resulting in the type of M2-polarized macrophage in mice. However, the effect of activated β2-AR in M2-polarized macrophages on HCC cells remains unclear.
G protein-coupled receptor kinase 2 (GRK2) has a total-body expression and is primarily identified as the kinase that phosphorylates and desensitizes agonist-bound GPCRs, including β2-AR.14,15 Our previous study revealed that the inhibition of GRK2 significantly promotes HCC cell growth.16 Nevertheless, whether GRK2 regulates β2-AR in M2-polarized macrophages and contributes to the progression of HCC requires further investigation.
In the present study, terbutaline (Terb) was used as a selective agonist to active β2-ARs in M2-polarized macrophages. The results demonstrated that CD163 and CD206 (markers of M2-polarized macrophages) were increased with HCC progression. Furthermore, the down-regulation of GRK2 in Terb-stimulated M2-polarized macrophages delayed the β2-AR internalization, activated the downstream cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP-response element binding protein (CREB) and cAMP/interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) signaling pathways in macrophages, and promoted the secretion of pro-tumor cytokines. Finally, the proliferative, migratory and invasive abilities of HCC cells were elevated. These results suggested that GRK2 targeting at M2- polarized macrophages may be a potential therapeutic approach for the treatment of the patients with HCC through the blocking of tumor progression.
Materials and methods
Reagents
Phorbol-12-myristate-13-acetate (PMA) was bought from Sigma Aldrich (St. Louis, Missouri, USA), human IL-4 and human IL-13 were purchased from Peprotech (200-04 and 200-13, Rocky Hill, USA).The anti-CD163, anti-CD206, anti-β2-AR and anti-PKA antibodies were purchased from Santa Cruz Biotechnology(sc-33559, sc-58986, sc-271322 and sc-98951, Ca, USA). Phenylmethylsulfonyl fluoride (PMSF) was bought from beyotime biotechnology (Shanghai, China). Primary antibodies against STAT3, pSTAT3, pCREB were purchased from Cell Signaling Technology (4904s, 9131s and 9198s, Danvers, MA, USA). Anti-CREB antibody was purchased from Abcam (ab32515, Cambridge, MA, USA). AlexaFluor488 donkey anti-mouse antibody and AlexaFluor594 goat anti-rabbit antibody were purchased from ThermoFisher Scientific (A11037, A21202, Waltham, MA, USA). Fluorescein isothiocyanate dextran (FITC-dextran) was from Sigma Aldrich (FD40s, St. Louis, Missouri, USA). AlexFluor 647 was from Fcmacs (FMS-Msaf64701, Nanjing, Jiangsu, China).
Patients and samples
A total of 80 patients with HCC and 10 patients with hepatolithiasis (control group) who were diagnosed and underwent surgery at the First Affiliated Hospital of Anhui Medical University (Anhui, China) between Jan, 2012 and Dec, 2015, and had complete clinical data were included. Patients who received chemotherapy or radiation therapy prior to surgery were excluded from the present study. Fresh HCC specimens from patients with HCC and healthy liver tissue from those with hepatolithiasis were formalin-fixed, paraffin-embedded, and cut into 4 μm sections. Histopathological evaluation was performed independently by three pathologists. The present study was approved by the Research Ethics Committee of Anhui Medical University and written informed consents were provided by all patients. The study was performed in accordance with the Declaration of Helsinki.
Immunofluorescence double staining
In order to determine the inflitration of M2-polarized macrophages from HCC tissues, immunofluorescence double staining was performed. The paraffin-embedded tissue was sliced into 4 μm sections, heated at 70°C, dewaxed with xylene and rehydrated with graded alcohol washes. Subsequently, a pressure cooker was used to perform antigen retrieval, followed by blocking of endogenous peroxidase activity with 3% hydrogen peroxide for 15 min. Thereafter, slides were incubated with CD163 and CD206 (markers of M2-polarized macrophages) (dilution 1:50) primary antibodies overnight at 4°C. The next day, slides were incubated with goat-anti-rabbit IgG (dilution 1:100) and goat-anti-mouse IgG (dilution 1:100) secondary antibodies at room temperature for 1 h in the dark. Following washing twice with PBS, 4ʹ,6ʹ-diamidino-2-phenylindole (DAPI; Zhongshan Biotechnology Inc., Beijing, China, ZLI-9557) was used to stain the nuclei at room temperature for 10 min, and stored at 4°C. Laser scanning confocal microscope (SP8; Leica, Germany) was used to observe and image fluorescent sections. The same exposure time and microscope settings were used for all captured images.
M2-polarized macrophage induction and identification
Human monocytic cell line THP-1 was purchased from cell bank of Chinese Academy of Sciences (Shanghai, China), and was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS(Wisent Biomart,Quebec, Canada) at 37°C in a 5% CO2 nhumidified atmosphere. It was a widely used method that use monocyte, such as THP-1 to differentiate into macrophage.17 THP-1 was treated with 320 nmol/L PMA for 24 h. Once differentiated, PMA was removed. Then the cells were treated with IL-4 (20 ng/ml) simultaneously treated with IL-13 (20 ng/ml), as inducers of the M2 phenotype, for another 24 h.18 The M2 macrophages were used freshly.
Identification of M2-polarized macrophages was performed by immunofluorescence. THP-1 cells suspension (5×104 cells/well) were inoculated on coverslips that were placed previously into a 12-well plate. Then the cell induction was performed according to the aforementioned procedures. The induced cells were fixed with 4% formaldehyde in PBS for 20 min, followed by three times washing with PBS and then permeabilized with 0.1% Triton-X 100. After blocked with 1% fetal bovine serum (FBS) in PBS for 1 h, the cells were washed three times with PBS and incubated with the primary antibody CD206 (dilution 1:50) and CD163 (dilution 1:50) at 37 °C overnight at 4 °C. The 12-well plate was incubated with secondary antibodies: goat-anti-rabbit IgG (dilution 1:100) and goat-anti-mouse IgG (dilution 1:100) the next day. DAPI was used to stain the nuclei. Laser Scanning Confocal Microscope (SP8, Leica, Germany) was used to observe and photograph fluorescent sections.
Phagocytosis assays
After M2-polarized macrophage cells treated with Terb at different concentration (10−4, 10−5, 10−6 mol/L) for 10 min, phagocytosis was assayed by flow cytometry. The removed (5×104) cells were suspended in 200 µl PBS containing 0.5% bovine serum albumin, and incubated with FITC-dextran (1 mg/ml) at 37 °C or 4 °C for 90 min, followed by three times washing with PBS. Then, the cells were analyzed on CytoFLEX flow cytometer (Beckman Coulter, Inc., Brea, CA, USA). Data were analyzed using Cytexpert software. The phagocytosis of M2-polarized macrophages was analyzed by calculating the mean FITC value.
GRK2 small interfering RNA(siRNA) transfection
According to the GRK2 gene sequences, siRNA duplexe targeting the GKR2 gene was synthesized (GenePharma, Shanghai, China):GRK2-homo-1675 sense, 5ʹ-GCAUCAUGCAUGGCUACAUTT-3ʹ,GRK2-homo-1675 antisense, 5ʹ-AUGUAGCCAUGCAUGAUGCTT-3ʹ. 2×105 THP-1 cells were plated into 24-well plate and induced into M2-polarized macrophages. The transfection was conducted according to the guidance of Lipofectamine RNAiMAX protocol (Invitrogen, Life Technologies).Western blot was used to confirm the transfection.
Preparation of conditioned media (CM) of M2-polarized macrophages
Three types of CM were collected for future use: M2-polarized macrophages without any treatment; M2-polarized macrophages treated with Terb (10−5 mol/L); and M2-polarized macrophages transfected with GRK2 siRNA, followed by Terb stimulation (10−5 mol/L) for 12 h. Following the aforementioned treatments, the medium in each group was replaced with serum-free fresh mediumand incubated for 2 days prior to the collection of CM. Cell debris from the supernatants was removed by centrifugation prior to use in experiments.
Proliferation assay
The human liver cancer cell lines HepG2 and SMMC-7721 were purchased from the Type Culture Collection of the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2.19 An MTT assay was performed to investigate the viability of liver cancer cells co-cultured with CM from M2-polarized macrophages transfected with GRK2 siRNA. The 100 μl liver cancer cells were plated in 96-well culture plate at a density of 5×104 cells/ml. The cells were cultured in medium containing 0.5% FBS overnight. Cells were then incubated with the CM from the M2-polarized macrophages for 48 h and subsequently with MTT solution (5.0 mg/ml, 20.0 μl/well) at 37°C in 5% CO2 for 4 h. Following the treatments, dimethyl sulfoxide (DMSO) (150 μl/well) was used to dissolve the MTT-formazan product. Following agitation for 10 min on a horizontal oscillator, the plates were analyzed on an Infinite M1000 PRO microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The proliferation of HCC cells was analyzed by calculating the OD value at 490 nm.
Migration assay
A wound-healing assay was performed to examine the effects of the CM from M2-polarized macrophages cultured under different conditions on the migration rate of liver cancer cells. HepG2 and SMMC-7721 cells were plated in 6-well plates and incubated until 80–90% confluence was achieved. A “scratch” was created by wounding the cell monolayer with a 200 μl-pipette in a straight line. Subsequently, the cells were washed three times with PBS to remove cell debris and cultured in fresh medium supplemented with 0.5% FBS, followed by incubation with the CM from each group. An inverted microscope (IX71; Olympus Corporation, Tokyo, Japan) was used to capture images at 0 and 48 h after incubation. Three fields from each well were documented and each experiment was repeated three times. Cell migration was determined by measuring the movement of cells into the scraped area, and quantitative analysis was evaluated using ImageJ software with the mean percentage wound closure area relative to the area of the initial wound.
Invasion assay
Transwell cell culture chambers (24 wells, 8-μm pore size; Corning Incorporated, Corning, NY, USA) were used for invasion assay. The Transwell membranes were first coated with 100 μl Matrigel matrix. The liver cancer HepG2 or SMMC-7721 cell lines suspended in 100 μl serum-free medium were plated in the upper chambers (5×103 cells), and 500 µl CM from each group supplemented with 10% FBS was added to lower chambers. After 24 h, a cotton swab was used to remove the non-invasive cells on the top surface of the chambers, while the cells that invaded the lower surface of the membranes were fixed withmethanol and stained with 0.1% crystal violet for 15 min. The invasive cells on the lower side of the membrane were quantified by counting the number of cells in four random fields with a light microscope (BX5; Olympus Corporation).
Cytokine assays
The expression levels of cytokines in CM from M2-polarized macrophages were evaluated by Human Cytokine Array (QAH-CYT-1-1, Raybiotech, Norcross, GA, USA). The microarray was performed according to the manufacturer’s instructions. The fluorescent images were scanned by an Axon GenePix laser scanner (GenePix 4000B; Molecular Devices, Orleans, USA), and the data were analyzed with GenePix Pro 7.0 software. The cytokines were quantified according to the standard curve calibrated from the same array.
Western blot analysis
To confirm the successful transfection of GRK2, and measure the expression of β2-AR on the cell membrane and its downstream signaling pathways, Western blot analysis was performed. Total protein was extracted using RIPA protein extraction reagent supplemented with phenylmethylsulfonyl fluoride (PMSF) (1:100) following by centrifuged at 12,000×g for 10 min at 4 °C. The supernatants of cultures were collected and maintained at −80 °C until use. Protein concentrations were determined using a BCA Protein Assay kit. A total of 20 μg protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Thereafter, the proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Shanghai, China), blocked with 5% non-fat milk in Tris-buffered saline (0.1 mol/L, pH 7.4) and then incubated at 4°C overnight with anti-GRK2 (dilution 1:500), anti-β2-AR (dilution 1:200), anti-PKA (dilution 1:500), anti-STAT3, anti-pSTAT3 (dilution 1:1,000), anti-CREB (dilution 1:500), anti-pCREB (dilution 1:1,000) and anti-β-actin (dilution 1:1,000) primary antibodies. Subsequently, the protein was further incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (dilution 1:10,000) for 2 h at room temperature. Following each step, blots were washed three times with TBST. An Electro-Chemi-Luminescence (ECL) detection kit (Pierce; Thermo Fisher Scientific, Inc.) was used to visualize the bands. Autoradiographs were scanned using ImageQuant LAS 4,000 mini (GE Healthcare Life Sciences, Pittsburgh, USA) and the images were analyzed by Image J software. All the experiments performed in the current study were repeated three times.
Measurement of β2-AR on the membrane of M2-polarized macrophages
Western blot assay was performed to detect the β2-AR on the membrane of M2-polarized macrophages, which stimulated with Terb for 5, 10, 15 or 20 min following transfection with GRK2 siRNA. In order to extract membrane proteins, the total protein of the M2-polarized macrophages was centrifuged at 12,000×g for 10 min at 4°C. Some of supernatants were centrifuged at 100,000×g for 1 h. Then, the precipitation was collected and maintained at −80°C until use. The following steps were performed according to the procedures of Western blot.
Flow cytometry analysis
Following transfection with GRK2 siRNA, the M2-polarized macrophages were stimulated with Terb (10−5 mol/L) for 10 or 20 min, and flow cytometry was used to assess the difference in β2-AR expression on the cell membrane. The treated cells were suspended in 100 µl PBS containing 0.5% bovine serum albumin, and incubated with anti-β2-AR mAb at room temperature for 1 h. Subsequently, the cells were washed with PBS once. The cells at the bottom of the Eppendorf tubes were collected following centrifugal separation and stained with AlexFluor 647 (dilution 1:100) for 1 h, then analyzed with a CytoFLEX flow cytometer (Beckman Coulter, Inc.) using Cytexpert software package. The expression of β2-AR of membrane on M2-polarized macrophage cells was analyzed by calculating the mean fluorescence intensity of β2-AR.
Measurement of β2-AR downstream signaling
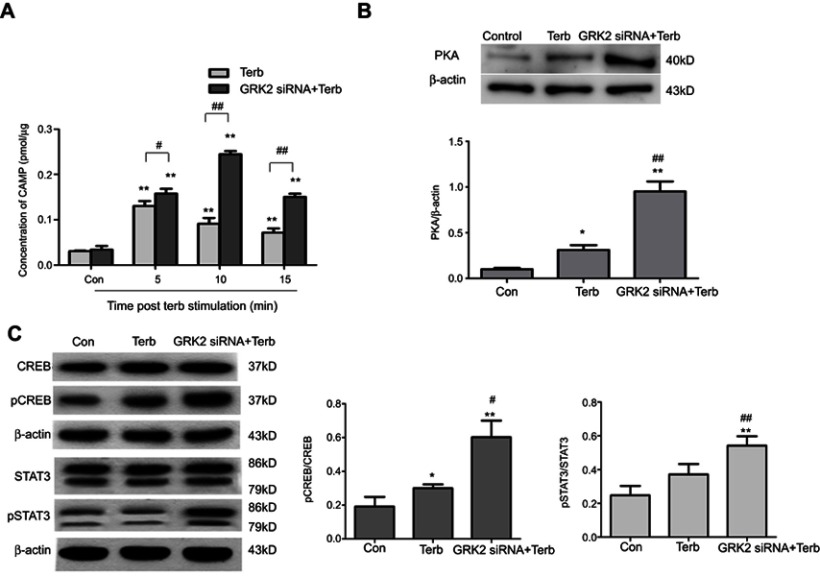
An ELISA kit (k317, BioVision, Inc.) was used to determine the cAMP level in M2-polarized macrophages. M2-polarized macropohages stimulated with Terb for 5, 10 or 15 min in 24-well plate were the first group. The macrophages transfected with GRK2 siRNA and then stimulated with Terb for 5, 10 or 15 min were the second group. The following procedures were performed according to the manufacturer’s protocol. The plates were analyzed using an Infinite M1000 PRO microplate reader at 450 nm. The standard curve was acquired according to the standards, and the level of cAMP in the samples was calculated. Western blot analysis was performed as aforementioned to determine the PKA, CREB, p-CREB, STAT3 and p-STAT3 levels of M2-polarized macrophages stimulated with Terb following the transfection with GRK2 siRNA.
Statistical analysis
Data are expressed as the mean ± standard deviation and statistical analysis was performed using one-way analysis of variance (S-N-K test or Dunnett’s test) and independent sample t-tests. The statistical significance between control and experimental groups was analyzed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Infiltration of M2-polarized macrophages were elevated in patients with HCC
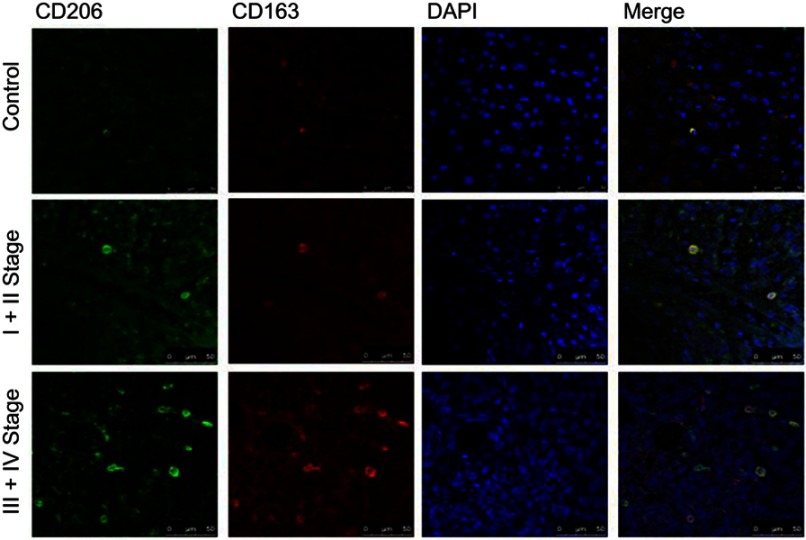
Immunofluorescence staining was performed on the paraffin-embedded sections from such patients with HCC to investigate the expressions of CD163 and CD206 (markers of M2-polarized macrophages) in different stage of HCC. As shown in Figure 1, few cells had the positive co-expression of CD163 and CD206 in the control group. While in HCC group, cells in III+IV stage group had an increase of co-expression of CD163 and CD206 than those of I+II stage group.
Figure 1.
Expression of CD163 and CD206 were increased in HCC group compared with control group. Few cells had the co-expression of CD163 and CD206 in control group, while many more cells had the co-expression of CD163 and CD206 in Ⅰ+Ⅱ stage group of HCC. The number of co-expression cells was elevated in Ⅲ+Ⅳ stage group compared with Ⅰ+Ⅱ stage group.
Induction and identification of M2-polarized macrophages
THP-1 cells are round and regular-shaped cells. After 48 h of induction, the cells became adherent and differentiated into a heterogeneous cell population comprising round and spindle-shaped ones (Figure 2A). CD163 and CD206 are considered as typical M2-like markers.20–22 Immunofluorescence double staining confirmed that the induction was successful following the treatments with PMA, IL-4 and IL-13. The results demonstrated that CD163 and CD206 were expressed in >90% cells (Figure 2B).
Figure 2.
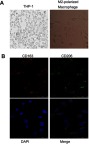
Induction and identification of M2-polarized macrophages. (A) THP-1 cells were cultured under a normal condition and with PMA (320 nmol/L) for 24 h, and then PMA was removed following the differentiation. Then, the cells were cultured with IL-4 (20 ng/ml) and IL-13 (20 ng/ml) for another 24 h (magnification fold: 400×). (B) Immunofluorescence double staining showed that CD163 and CD206 was co-expressed in >90% of cells indicating that a successful induction.
M2-polarized macrophages exhibit a higher phagocytic capacity compared with THP-1 cells and treatment with Terb reduces phagocytosis
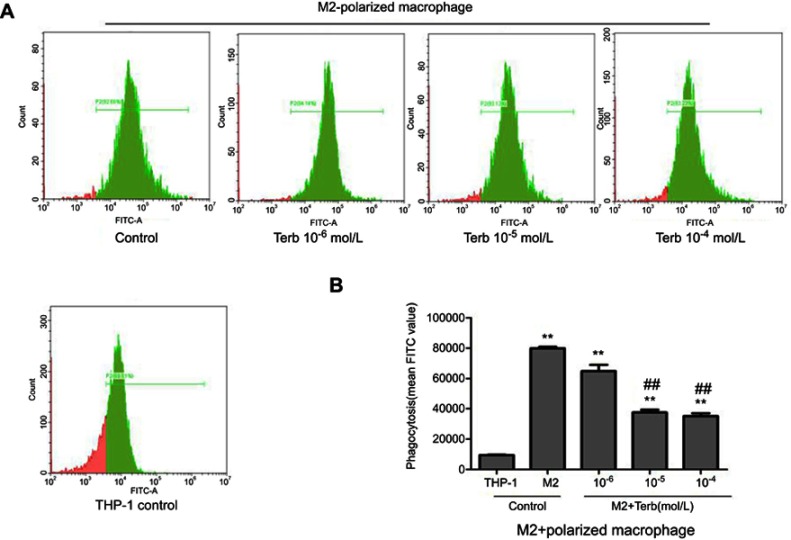
The capacity of taking up FITC-dextran was significantly higher in M2-polarized macrophages compared with that in THP-1 cells. The effects of Terb on phagocytic capacity were further evaluated. As demonstrated in Figure 3, there was a significant reduction in phagocytosis following stimulation with Terb at a dose of 10−5 mol/L compared with 10−6 mol/L. Nevertheless, when the Terb concentration increased to 10−4 mol/L, no significant reduction was observed compared with the 10−5 mol/L group. M2-polarized macrophages phagocytose cancer cells and the decline in phagocytic capacity may lead to the growth of tumor cells. Due to the significant reduction in phagocytosis, the concentration of Terb at the dose of 10−5 mol/L was selected for the subsequent studies.
Figure 3.
Phagocytic ability of M2-polarized macrophages was stronger compared with that in THP-1 cells, and the phagocytic ability of M2-polarized macrophages declined after the treatment of Terb. (A) The phagocytic ability of M2-polarized macrophages was elevated compared with that of THP-1 cells. Besides, following the treatment of M2-polarized macrophages with Terb, the phagocytic ability was decreased compared with the untreated control group. (B) Histogram represents the mean FITC value in each group as determined by flow cytometry. **P<0.01 compared with the THP-1 group; ##P<0.01 compared with the M2 group.
Activation of β2-AR in M2-polarized macrophages elevates the proliferation, migration and invasion of HCC cells, and the regulatory effect of GRK2
MTT, wound-healing and transwell assays were performed to detect the proliferation, migration and invasion of HepG2 and SMMC-7721 cell lines. In our preliminary study, the proliferation, migration and invasion of liver cancer cell lines cultured in blank medium group, in the CM of M2-polarized macrophages group and in the CM of Terb-stimulated M2-polarized macrophages group were all observed. As shown in Figures S1–S3, the liver cancer cell lines cultured with CM of M2-polarized macrophages group had a higher absorbance at 490 nm, an increasing wound closure and an elevated number of migrated HCC cells compared with the blank group. Those attributes of liver cancer cells cultured with CM of Terb-stimulated M2-polarized macrophages group were further elevated compared with CM of M2-polarized macrophages group. These preliminary study results showed that the proliferative, migratory and invasive attributes of the liver cancer cell lines cultured with CM of M2-polarized macrophages were elevated and M2-polarized macrophages which stimulated with Terb promoted the elevation. According to the preliminary study results, in the present study, we mainly focus on the regulatory effect of GRK2 on the beta2-AR activation in M2-polarized macrophages. GRK2 siRNAs were used to knock down GRK2 expression in human M2-polarized macrophages. The efficiency of GRK2 siRNA was determined using Western blotting. Following transfection of GRK2 siRNA in M2-polarized macrophages for 48 h, the expression of GRK2 decreased compared with the negative control group (Figure 4A). As shown in Figure 4B–D, the liver cancer cells incubated with CM from M2-polarized macrophages in the GRK2 siRNA+Terb-CM group had a higher absorbance at 490 nm, an increasing wound closure and an elevated number of migrated HCC cells compared with the Terb-CM group. These results revealed that inhibition of GRK2 by siRNA in M2-polarized macrophages significantly elevated the growth and metastasis of co-cultured liver cancer cells compared with non-siRNA-treated macrophages.
Figure 4.
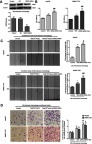
Down-regulation of GRK2 in M2-polarized macrophages elevated the proliferation, migration and invasion of liver cancer HepG2 and SMMC-7721cell lines. (A) GRK2 siRNA was used to knockdown the expression of GRK2 in M2-polarized macrophages. Western blotting was performed to detect the relative protein expression levels of GRK2 in the control, negative control and GRK2 siRNA groups, as presented in the histogram. (B) An MTT assay was performed to investigate the proliferation rate of liver cancer cells co-cultured with three types of CM from M2-polarized macrophages. The proliferation of liver cancer cells was elevated following activation of β2-AR in M2-polarized macrophages, and down-regulation of GRK2 in M2-polarized macrophages further promoted such elevation. Histogram represents the OD (490 nm) of liver cancer HepG2 and SMMC-7721 cell lines. (C) A wound-healing assay was performed to investigate the migration of liver cancer cells co-cultured with three types of CM from M2-polarized macrophages. The migratory ability of the cells was elevated following activation of β2-AR in M2-polarized macrophages, and down-regulation of GRK2 in M2-polarized macrophages further promoted this elevation. The histogram represents the fold change in scratch closure of HepG2 and SMMC 7721 cells. (D) A transwell assay was performed to investigate the invasion of liver cancer cells co-cultured with three types of CM from M2-polarized macrophages. The invasive ability of liver cancer cells was elevated following the activation of β2-AR in M2-polarized macrophages, and GRK2 down-regulation in M2-polarized macrophages further promoted the elevation. The histogram represents the rate of HepG2 and SMMC7721 cell invasion. *P<0.05 and **P<0.01 compared with the control group; #P<0.05 and ##P<0.01 compared with the Terb-CM group.
Activation of β2-ARs increases the IL-6, vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP-9) levels secreted by M2-polarized macrophages and the regulatory effect of GRK2
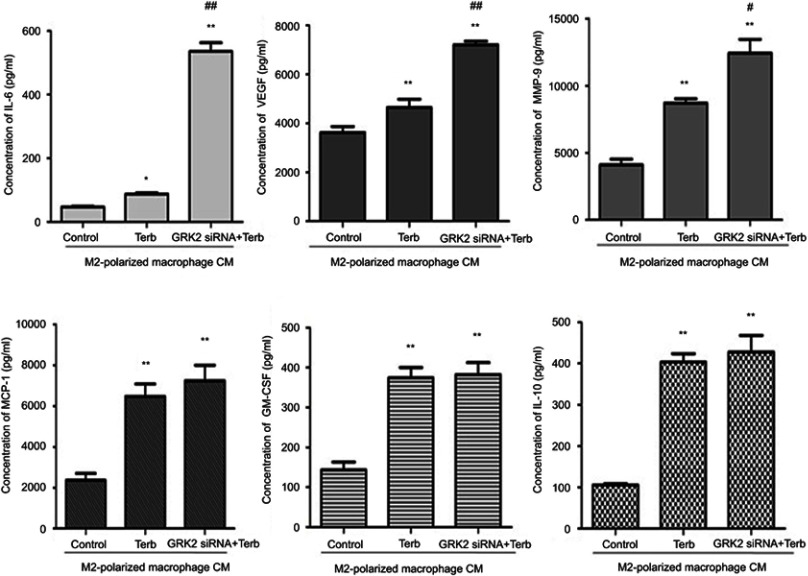
To determine the cytokines that M2-polarized macrophages secreted, the cytokines assays were performed. The expression levels of IL-6,VEGF, MMP-9, granulocyte-macrophage colony stimulating factor (GM-CSF), IL-10 and monocyte chemotactic protein-1 (MCP-1) were significantly elevated in the supernatant of Terb-stimulated M2-polarized macrophages compared with the control group (Figure 5). As it was observed that down-regulation of GRK2 in M2-polarized macrophages elevated the proliferation, migration and invasion of liver cancer cells, it was hypothesized that the enhancement of functions of liver cancer cells may be associated with the elevation of tumor-associated cytokines in M2-polarized macrophages transfected with GRK2 siRNA. As shown in Figure 5, the expression levels of IL-6, VEGF and MMP-9 were significantly enhanced in the GRK2 siRNA+Terb-CM group compared with the Terb-alone group. These results indicated that the GRK2 down-regulation increased the secretion of IL-6, VEGF and MMP-9 in β2-AR-activated M2-polarized macrophages compared with the control group, and the expression levels of GM-CSF, MCP-1 and IL-10 may not associated with GRK2 regulation.
Figure 5.
Down-regulation of GRK2 in M2-polarized macrophages elevated the expression levels of IL-6, MMP-9 and VEGF in the supernatant. A cytokine assay was performed to evaluate the cytokine levels in the supernatant of M2-polarized macrophages pretreated with Terb alone or combined with GRK2 siRNA. MCP-1. The expression levels of IL-10, GM-CSF, IL-6, MMP-9 and VEGF were elevated when M2-polarized macrophages were stimulated with selective β2-AR agonist Terb. Whereas only the expression levels of IL-6, MMP-9 and VEGF were elevated significantly in M2-polarized macrophages following transfection with GRK2 siRNA. No significant elevation was observed in other cytokines. *P<0.05 and **P<0.01 compared with the control group; #P<0.05 and ##P<0.01 compared with the Terb-CM group.
Down-regulation of GRK2 delays the internalization of β2-AR in M2-polarized macrophages
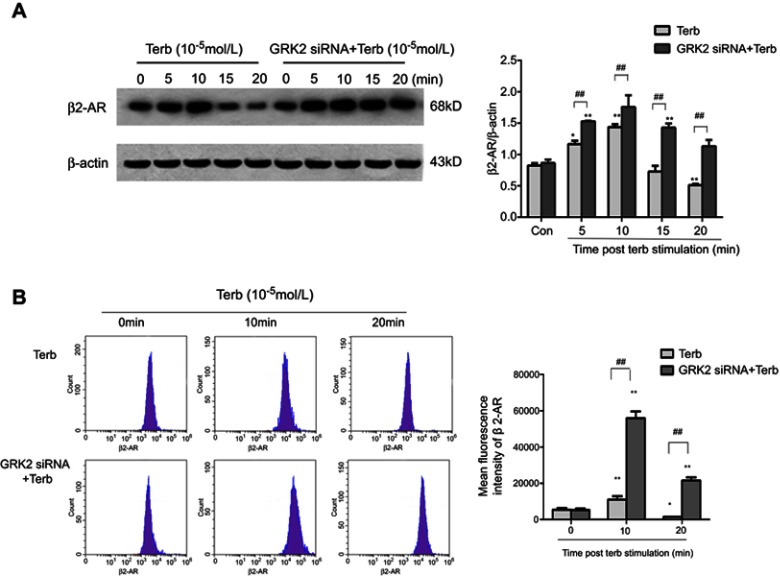
Western blot assay was performed to detect the internalization of β2-AR in M2-polarized macrophages which stimulated with Terb for 5, 10, 15, 20 mins following the transfection with GRK2 siRNA. At 0 min, only a few β2-ARs were on the membrane of macrophages, and following Terb stimulation for 5 min, a rapid elevation of β2-AR was observed on the cell membrane, reaching a peak at 10 mins in the Terb and GRK2 siRNA+Terb groups. Besides, in the GRK2 siRNA+Terb group, there was an increase in the expression of β2-ARs on macrophage membranes compared with the Terb-alone group. Thereafter, β2-AR began to translocate into the cytosol rapidly, and at 20 min, the majority of the β2-ARs were located in the cytosol of the Terb-alone group. While in the GRK2 siRNA+Terb group, the expression of β2-ARs gradually decreased from 10 mins to 20 mins at a slower rate compared with the Terb-alone group, and following 20-min of Terb stimulation, a significant fraction of β2-ARs were still displayed on the membrane surface (Figure 6A). The results regarding the expression of β2-ARs on the membrane surface performed by flow cytometry analysis complied with those of the Western blot analysis (Figure 6B).The results demonstrated that GRK2 down-regulation increased β2-ARs expression on the macrophages membrane surface, and delayed the desensitization and internalization of β2-ARs, resulting in a prolonged effect.
Figure 6.
Down-regulation of GRK2 elevated the expression of β2-ARs on the M2-macrophage membrane and delayed the internalization of β2-AR. (A) A Western blot assay was performed to detect the expression of β2-AR on the membrane of M2-polarized macrophages stimulated with Terb for 5, 10, 15 or 20 min following transfection with GRK2 siRNA. The expression of β2-AR on the membrane of M2-polarized macrophages peaked at 10 min in the Terb-alone and GRK2 siRNA+Terb groups. While the expression of β2-AR in the GRK2 siRNA+Terb group was higher compared with that in the Terb-alone group at 10 min. The expression of β2-AR in the Terb-alone group declined rapidly from 10 min to 20 min following Terb stimulation, while in the GRK2 siRNA+Terb group, the expression of β2-AR declined gradually and at 20 min there was still a significant fraction of β2-ARs on the membrane. Histogram represents the relative level of β2-AR in Terb group and GRK2 siRNA+Terb group. (B) Flow cytometry was performed to detect β2-AR expression on the membrane of M2-polarized macrophages stimulated with Terb for 10, or 20 min after transfection with GRK2 siRNA. Though the expression of β2-AR peaked at 10 min in the Terb-alone and GRK2 siRNA+Terb groups, there was a significant elevation in β2-AR formation of the GRK2 siRNA+Terb group compared with Terb-alone group. Furthermore, the decline in β2-AR expression on the cell membrane was slower in the GRK2 siRNA+Terb group compared with Terb-alone group. The histogram represents the relative expression of β2-AR on cell membrane in the Terb-alone and GRK2 siRNA+Terb groups. *P<0.05 and **P<0.01 compared with the 0 min group; ##P<0.01 compared with each other group at the same time point.
Down-regulation of GRK2 in M2-polarized macrophages promotes the activation of β2-AR downstream signaling
It was observed that GRK2 down-regulation in M2-polarized macrophages promoted the HCC progression. The underlying mechanisms were further investigated. Engagement of β2-ARs activates a cascade of signaling intermediates, including cAMP, an important second messenger and PKA.23 Terb is a selective agonist of β2-AR that activates adenylyl cyclase to form cAMP. Following the stimulation of Terb for 5, 10 or 15 min, a rapid elevation in cAMP was observed. The concentration of cAMP reached a peak at 5 min and then gradually declined from 5 min to 15 min. To further investigate the effect of GRK2 on cAMP levels, M2-polarized macrophages were transfected with GRK2 siRNA simultaneously stimulated with Terb. The concentration of cAMP peaked after 10 min with Terb stimulation of GRK2 siRNA+Terb group, which was increased more than 1-fold compared with the peak of the Terb group, as shown in Figure 7A. In addition, at 15 min, the concentration of cAMP in the GRK2 siRNA+Terb group was higher compared with that in the Terb-alone group. Therefore, it was evident that inhibition of GRK2 amplified the formation of cAMP in M2-polarized macrophages compared with the Terb-alone group.
Figure 7.
Down-regulation of GRK2 in M2-polarized macrophages activated the β2-AR downstream signaling pathway. (A) An ELISA was performed to determine the cAMP level in M2-polarized macrophages stimulated with Terb for 5, 10 or 15 min after the transfection with GRK2 siRNA. In the Terb-alone group, the formation of cAMP peaked at 5 min, and declined from 5 min to 15 min. While in GRK2 siRNA+Terb group, there was a significant elevation in cAMP formation and the peak time was delayed to 10 min. Furthermore, at 15 min, the level of cAMP in the GRK2 siRNA+Terb group was higher compared with the Terb-alone group. *P<0.05 and **P<0.01 compared with the control group; #P<0.05 and ##P<0.01 compared with each other group at the same time point. (B) Western blot analysis was performed to determine the level of PKA in M2-polarized macrophages stimulated with Terb for 10 min after transfection with GRK2 siRNA. The expression of PKA was increased in the GRK2 siRNA+Terb group compared with Terb-alone group. (C)Western blot analysis was performed to determine the expression levels of CREB, p-CREB, STAT3, p-STAT3 levels in M2-polarized macrophages stimulated with Terb for 10 min after the transfection with GRK2 siRNA. No significant elevation was observed in the expression of CREB and STAT3 in the GRK2 siRNA+Terb group compared with the Terb-alone group, while p-CREB and p-STAT3 levels were significantly increased. *P<0.05 and **P<0.01 compared with the control group; #P<0.05 and ##P<0.01 compared with the Terb group.
PKA and CREB signaling are downstream of cAMP. Thereafter, the expression levels of PKA, CREB and pCREB in the three groups were measured. As shown in Figure 7B, the expression level of PKA was higher in the GRK2 siRNA+Terb group compared with the Terb-alone group. Furthermore, no significant elevation of CREB was observed in the GRK2 siRNA+Terb group compared with the control and Terb-alone groups. Nevertheless, it was demonstrated that p-CREB was remarkablely increased in the GRK2 siRNA+Terb group, which indicated that the activated PKA resulted in the increased CREB phosphorylation levels (Figure 7C).
β2-AR stimulation also elevates IL-6 production through the classical β2-AR/cAMP pathway.24 In the present study, the expression level of IL-6 was elevated significantly in the supernatant from M2-polarized macrophages in the GRK2 siRNA+Terb group compared with Terb alone group. IL-6 which binds to its receptor and GP130 can activate STAT3 and downstream signaling pathways.22,25 As shown in Figure 7C, no significant elevation in STAT3 expression level was observed in the GRK2 siRNA+Terb group, whereas p-STAT3 expression levels in cells pretreated with GRK2 siRNA+Terb increased significantly. These results indicated that compared with β2-AR-activated M2-polarized macrophages, the activation of the β2-AR/cAMP/PKA/CREB and the β2-AR/cAMP/IL-6/STAT3 signaling pathways were significantly activataed following the knockdown of GRK2.
Discussion
HCC is one of the most common malignancies of digestive system with poor prognostic outcome. The tumor microenvironment has been proved to play an important role in modulating the metastasis and recurrence of cancer. Macrophages are an important type of immune cells infiltrated in the tumor microenvironment. In this study, the number of M2-polarized macrophages in the late-stage of HCC was elevated compared with that of early stage of HCC. The results indicated that the infiltration of M2-polarized macrophages in tumor microenvironment may be related to the malignancy of HCC.
Macrophages functioning as executive cells of the innate immune system, phagocytose bacteria, ingest and degrade debris of debris, tumor cells and foreign materials meanwhile secrete antimicrobial and pro-inflammatory mediators as the effecter cells of the innate immune system.26 In the current study, compared with THP-1 cells, the phagocytic ability of M2-polarized macrophages was stronger. However, following stimulation with Terb at a dose of 10−5 mol/L, the phagocytosis rate decreased significantly. These results indicated that the activation of β2-AR in M2-polarized macrophages suppressed phagocytosis, which may lead to the HCC progression.
Our previous study reported that the decreased GRK2 expression could promote proliferative, migratory and invasive abilities of liver cancer cells.16 Besides, Chen et al27 reported that β2-AR was positively associated with the aforementioned abilities in HCC. In our preliminary study, the results showed that CM of M2-polarized macrophages could elevate the proliferative, migratory and invasive attributes of co-cultured liver cancer cells. And those attributes of liver cancer cells were further elevated when co-cultured with CM of Terb-stimulated M2-polarized macrophages. To further investigate the role of GRK2 in β2-AR signaling. GRK2 siRNA was transfected into human M2-polarized macrophages. The results demonstrated that following GRK2 knockdown in β2-AR-activated macrophages, the co-cultured HepG2 and SMMC-7721 cell lines exhibited a prominent elevation in the proliferative, migratory and invasive abilities.
Previous studies have reported that TAMs could promote the migration and invasion of cancer cells by secreting various cytokines, which serve as tumor promoters through their support in neovascularization, immunosuppression and invasion in cancer.2,28–31 The present study revealed that the expression levels of IL-6, VEGF and MMP-9 in the CM of macrophages treated with GRK2 siRNA and Terb were increased significantly. A previous study also reported that the expression levels of IL-6 in patients with HCC were elevated, which could be used to distinguish HCC from benign liver lesions.2 In addition, increased VEGF expression secreted by macrophages accelerates the malignant progression of tumors, and possesses a critical role in HCC angiogenesis.3,32 Furthermore, macrophages exhibit enhanced production of MMP-9 in tumor environments, which remodels the extracellular matrix (ECM), These facilitate cancer cells to invade through the ECM and consequently promotes the metastasis of cancer cells.33 The results of the current study demonstrated that down-regulation of GRK2 in M2-polarized macrophages, which promoted the progression of HCC, was due to the involvement of cytokines secreted by M2 macrophages, including IL-6, VEGF and MMP-9.
The effect of GRK2 on the internalization of β2-AR was determined. Phosphorylation of β2-AR by GRK is a major step in its desensitization and the subsequent step is receptor internalization.34,35 Previous studies have reported that the kinase activity of GRK2 is primarily responsible for the desensitization of β2-AR during the first 30 min of stimulation.36,37 Malach et al35,38 revealed that silencing of GRK2 activity abolished β2-AR phosphorylation, as well as the sensitivity to β2-AR activity. The results of the present study demonstrated that following pretreatment of macrophages with Terb for 10 min, β2-ARs in the cytomembrane reached a peak and transferred to the cytoplasm rapidly. While following the down-regulation of GRK2 in M2-polarized macrophages, the formation of β2-ARs also reached a peak on membranes with higher expression compared with the Terb-alone group since 10 min following Terb pretreatment and the internalization of β2-ARs slowed down. These results indicated that down-regulation of GRK2 amplified β2-AR activity and delayed its internalization in M2-polarized macrophages.
The underlying mechanisms were investigated next. Previous studies have reported that decreased expression and activity of GRK2 significantly enhances cAMP formation. Activated β2-AR elevates the activity of adenylcyclase on macrophage membranes to form cAMP via Gs, which suppresses the innate and adaptive immune responses.24,38,39 Concomitantly, the current investigation suggested that following GRK2 silencing, the formation of cAMP in M2-polarized macrophages was elevated significantly compared with the Terb-alone group. As a result, cAMP thereafter activated downstream components, the PKA and CREB. In this study, the expression level of PKA was also elevated in the GRK2 siRNA+Terb group compared with Terb-alone group. CREB is a nuclear transcription factor and has been demonstrated to be up-regulated in various tumor types, including non-small cell lung carcinoma, colorectal cancer and glioma.40–42 CREB has been reported to be a mediator between several signaling pathways and regulate expression of downstream genes, including MMP-9, Bcl-2 and the cell cycle-associated genes cyclinA1, cyclinB1 and cyclinD1.43 In the present research, the expression level of p-CREB increased remarkablely in M2-polarzied macrophages transfected with GRK2 siRNA simultaneously stimulated with Terb. These observations suggested that down-regulation of GRK2 in β2-AR-activated M2-polarized macrophages amplified the activity of the β2-AR/cAMP/PKA/CREB signaling pathway, leading to the elevation of MMP-9 levels.
cAMP activates downstream signaling pathways and participates in the secretion of inflammatory cytokines.44,45 Madden et al46 reported that β2-AR stimulation regulated IL-6 production through the classical β2-AR/cAMP signaling pathway. Consistent with previous study findings, our data indicated that IL-6 levels in the CM of β2-AR activated macrophages pretreated with GRK2 siRNA were increased significantly. IL-6 is known to active STAT3 through GP130/janus kinase and binds to a recognition sequence to promote the downstream genes, including VEGF, Cyclin D1 and B-cell lymphoma-extra large (Bcl-XL).22,47,48 In the present research, phosphorylation of STAT3 and the expression of VEGF were elevated in the GRK2 siRNA+Terb group compared with the Terb-alone group. The results indicated that the STAT3 signaling pathway was activated in M2-polarized macrophages transfected with GRK2 siRNA, followed by the enhancement of VEGF expression. MCP-1, GM-CSF and IL-10 were all related to the progression of malignant tumor.49–52 The secretion of MCP-1, GM-CSF and IL-10 of M2-polarized macrophages were all elevated when macrophages were treated with Terb, indicating that the agitation of β2-AR in M2-polarized macrophages could promote the secretion of these cytokines by M2-polarized macrophages. However, they showed no obvious changes after GRK2 silencing in β2-AR agitated M2-polarized macrophages. The secretion of cytokines in immune cells and tumor cells are complicated and were involved with multiple signaling pathways and mechanisms. There may still be other proteins except for GRK2 which play critical roles in regulating β2-AR to secrete these cytokines.
Taken together, our study demonstrated that the infiltration of M2-polarized macrophages was increased in the late-stage HCC samples compared with the early stage group and control group. This study also provided insight into the mechanism of β2-AR regulation by silencing GRK2 in M2-polarized macrophages, which contributes to the progression of HCC. More HCC tissue samples will be collected in the future and M2-polarized macrophages which isolated from human fresh liver cancer tissues by fluorescence-activated cell sorting will be used to further confirm the results. The effect of conditioned media collected from GRK2 over-expressed M2-polarized macrophages on co-cultured liver cancer cells will be further investigated in vitro in our next work. The targeting on GRK2 in M2-polarized macrophages to block tumor progression may be a potential therapeutic approach for HCC patients.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (No. 81330081, 81673444, 81770605), the Science Foundation of Anhui Medical University (No.2015xkj015), a program for Young Excellent Talents in Universities of Anhui Province (No. gxyqZD2018024).
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Anhui Medical University and written informed consents were provided by all patients. The present study was performed in accordance with the Declaration of Helsinki.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 2.Coskun U, Bukan N, Sancak B, et al. Serum hepatocyte growth factor and interleukin-6 levels can distinguish patients with primary or metastatic liver tumors from those with benign liver lesions. Neoplasma. 2004;51:209–213. [PubMed] [Google Scholar]
- 3.Yang JC, Teng CF, Wu HC, et al. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology. 2009;49:1962–1971. doi: 10.1002/hep.22889 [DOI] [PubMed] [Google Scholar]
- 4.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7 [DOI] [PubMed] [Google Scholar]
- 6.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2. doi: 10.1038/npjbcancer.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aljabery F, Olsson H, Gimm O, Jahnson S, Shabo I. M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer. Urol Oncol. 2018;36:159.e19–159.e26. doi: 10.1016/j.urolonc.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 8.Cannavo A, Liccardo D, Koch WJ. Targeting cardiac beta-adrenergic signaling via GRK2 inhibition for heart failure therapy. Front Physiol. 2013;4:264. doi: 10.3389/fphys.2013.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan A, Li Z, Li X, et al. The mitogenic effectors of isoproterenol in human hepatocellular carcinoma cells. Oncol Rep. 2010;23:151–157. [PubMed] [Google Scholar]
- 10.Kassahun WT, Guenl B, Ungemach FR, Jonas S, Abraham G. Expression and functional coupling of liver beta2 - adrenoceptors in the human hepatocellular carcinoma. Pharmacology. 2012;89:313–320. doi: 10.1159/000337381 [DOI] [PubMed] [Google Scholar]
- 11.Li J, Yang XM, Wang YH, et al. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014;60:1225–1234. doi: 10.1016/j.jhep.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 12.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun. 2014;6:607–618. doi: 10.1159/000358524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653 [DOI] [PubMed] [Google Scholar]
- 15.Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu ZW, Yan SX, Wu HX, et al. The influence of TNF-alpha and Ang II on the proliferation, migration and invasion of HepG2 cells by regulating the expression of GRK2. Cancer Chemother Pharmacol. 2017;79:747–758. doi: 10.1007/s00280-017-3267-z [DOI] [PubMed] [Google Scholar]
- 17.Tjiu JW, Chen JS, Shun CT, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310 [DOI] [PubMed] [Google Scholar]
- 18.Rahal OM, Wolfe AR, Mandal PK, et al. Blocking Interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100:1034–1043. doi: 10.1016/j.ijrobp.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 19.Liu SQ, Zhang JL, Li ZW, Hu ZH, Liu Z, Li Y. Propofol inhibits proliferation, migration, invasion and promotes apoptosis through down-regulating miR-374a in hepatocarcinoma cell lines. Cell Physiol Biochem. 2018;49:2099–2110. doi: 10.1159/000493814 [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Zhang J, Sui L, et al. Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis. Sci Rep. 2017;7:14982. doi: 10.1038/s41598-017-14808-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebelo SP, Pinto C, Martins TR, et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials. 2018;163:185–197. doi: 10.1016/j.biomaterials.2018.02.030 [DOI] [PubMed] [Google Scholar]
- 22.Liao Q, Zeng Z, Guo X, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33:2098–2109. doi: 10.1038/onc.2013.161 [DOI] [PubMed] [Google Scholar]
- 23.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation–overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 25.Zou M, Zhang X, Xu C. IL6-induced metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of 3,3ʹ-diindolylmethane in ovarian cancer cells. Cell Oncol (Dordr). 2016;39:47–57. doi: 10.1007/s13402-015-0251-7 [DOI] [PubMed] [Google Scholar]
- 26.Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Xing W, Hong J, et al. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2012;19:3556–3565. doi: 10.1245/s10434-012-2396-1 [DOI] [PubMed] [Google Scholar]
- 28.Rolny C, Capparuccia L, Casazza A, et al. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med. 2008;205:1155–1171. doi: 10.1084/jem.20072509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. doi: 10.1084/jem.20150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123–135. doi: 10.1038/nrc3449 [DOI] [PubMed] [Google Scholar]
- 32.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912 [DOI] [PubMed] [Google Scholar]
- 33.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–1807. doi: 10.1074/jbc.M705747200 [DOI] [PubMed] [Google Scholar]
- 35.Malach E, Shaul ME, Peri I, et al. Membrane-permeable tastants amplify beta2-adrenergic receptor signaling and delay receptor desensitization via intracellular inhibition of GRK2’s kinase activity. Biochim Biophys Acta. 2015;1850:1375–1388. doi: 10.1016/j.bbagen.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 36.Nobles KN, Xiao K, Ahn S, et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731 [DOI] [PubMed] [Google Scholar]
- 38.Lombardi MS, Kavelaars A, Schedlowski M, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Faseb J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715 [DOI] [PubMed] [Google Scholar]
- 39.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao M, Zhu Y, Cong X, Li Q. Knockdown of CREB1 inhibits tumor growth of human gastric cancer in vitro and in vivo. Oncol Rep. 2017;37:3361–3368. doi: 10.3892/or.2017.5636 [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Chen T, Wang J, et al. Methamphetamine modulates the production of interleukin-6 and tumor necrosis factor-alpha via the cAMP/PKA/CREB signaling pathway in lipopolysaccharide-activated microglia. Int Immunopharmacol. 2018;56:168–178. doi: 10.1016/j.intimp.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 42.Park JK, Park SH, So K, Bae IH, Yoo YD, Um HD. ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via Akt and CREB. Int J Oncol. 2010;36:181–192. [PubMed] [Google Scholar]
- 43.Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15:2583–2587. doi: 10.1158/1078-0432.CCR-08-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mack EM, Smith JE, Kurz SG, Wood JR. cAMP-dependent regulation of ovulatory response genes is amplified by IGF1 due to synergistic effects on Akt phosphorylation and NF-kappaB transcription factors. Reproduction. 2012;144:595–602. doi: 10.1530/REP-12-0225 [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–56. doi: 10.1152/ajpcell.00508.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madden KS, Szpunar MJ, Brown EB. beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747–758. doi: 10.1007/s10549-011-1348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu XL, Duan W, Su CY, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;66:1597–1608. doi: 10.1007/s00262-017-2052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130 [DOI] [PubMed] [Google Scholar]
- 49.Ma Y, Han CC, Huang Q, Sun WY, Wei W. GRK2 overexpression inhibits IGF1-induced proliferation and migration of human hepatocellular carcinoma cells by downregulating EGR1. Oncol Rep. 2016;35:3068–3074. doi: 10.3892/or.2016.4641 [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–528. doi: 10.1093/jnci/djq044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pena CG, Nakada Y, Saatcioglu HD, et al. LKB1 loss promotes endometrial cancer progression via CCL2-dependent macrophage recruitment. J Clin Invest. 2015;125:4063–4076. doi: 10.1172/JCI82152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51:170–182. doi: 10.1007/s12026-011-8262-6 [DOI] [PubMed] [Google Scholar]