Abstract
While it is well established that Semaphorin family proteins function as axon guidance ligands in invertebrates and vertebrates, several recent studies indicate that the Drosophila Semaphorin-1a (Sema1a), a transmembrane Semaphorin, can also function as a receptor during neural development. The regulator of Sema1a reverse signaling, however, remains unknown. In this study, we show that like Sema1a, the well known Semaphorin receptor Plexin A (PlexA), is required for the proper guidance of photoreceptor (R cell) axons in the Drosophila visual system. Loss of PlexA, like loss of semala, disrupted the association of R-cell growth cones in the optic lobe. Conversely, overexpression of PlexA, like overexpression of sema1a, induced the hyperfasciculation of R-cell axons. Unlike Sema1a, however, the cytoplasmic domain of PlexA is dispensable. Epistasis analysis suggests that PlexA functions upstream of semala. And PlexA and sema1a interact genetically with Rho1. We propose that PlexA regulates Semala reverse signaling in the Drosophila visual system.
Introduction
Neuronal growth cones respond to cues present in the surrounding environment in guiding axons toward their target region (Tessier-Lavigne and Goodman, 1996). The Semaphorin family proteins are well known repulsive guidance cues (Tamagnone and Comoglio, 2000; Pasterkamp and Kolodkin, 2003), while some Semaphorins can also induce attractive responses (Wong et al., 1999; Polleux et al., 2000; Dalpé et al., 2005). The action of Semaphorins is mediated by two families of growth-cone receptors, plexins and neuropilins (Fujisawa and Kitsukawa, 1998). Several cell surface receptor proteins such as Off-track (Otk) (Winberg et al., 2001), L1 (Castellani et al., 2000), Gyc76C (Ayoob et al., 2004), heparan sulfate proteoglycans and chondroitin sulfate proteoglycans (Kantor et al., 2004), have been shown to function as part of the receptor complex for Semaphorins in axon guidance.
Recent studies on the Drosophila transmembrane Sema1a demonstrate that Semaphorin can also function as a receptor (for review, see Zhou et al., 2008). Sema1a was originally identified as a repulsive ligand in mediating motor axon guidance in the fly embryo (Yu et al., 1998). Sema1a binds to its receptor Plexin A (PlexA) (Winberg et al., 1998), which forms a complex with the receptor tyrosine kinase Otk (Winberg et al., 2001) and regulates the flavoprotein monooxygenase MICAL (Terman et al., 2002) and the A kinase anchoring protein Nervy (Terman and Kolodkin, 2004) during motor axon guidance. Our recent work shows that Sema1a functions as a receptor to regulate photoreceptor (R cell) axon guidance (Cafferty et al., 2006). Similarly, Luo and colleagues show that Sema1a also functions as a receptor to mediate the targeting of dendrites from projection neurons in the olfactory system (Komiyama et al., 2007). It has also been shown that Sema1a is involved in synaptic formation by mediating bidirectional signaling in the adult giant fiber system (Godenschwege et al., 2002). The identity of the upstream protein that activates Sema1a reverse signaling in axon guidance, however, remains unclear.
Here we show that PlexA interacts with Sema1a to regulate R-cell axon guidance. The Drosophila compound eye consists of ∼800 repeating units called ommatidia, each contains eight different R cells (i.e., R1–R8) (Tomlinson and Ready, 1987). During the third-instar larval stage R cells extend their axons into the optic lobe (Meinertzhagen and Hanson, 1993; Clandinin and Zipursky, 2002; Tayler and Garrity, 2003). After reaching the lamina, R1–R6 growth cones terminate at appropriate topographic locations, where they form close contacts with neighboring growth cones. Whereas R7 and R8 axons pass through the lamina and elaborate a precise topographic map in the medulla.
In this study, we show that PlexA displayed phenotypes similar to that of semala. Epistasis analysis suggests that PlexA functions upstream of sema1a to mediate the interaction between neighboring R-cell axons for the establishment of appropriate topographic projections in the optic lobe. The cytoplasmic domain of PlexA is dispensable. PlexA and Sema1a interact genetically with the cytoskeletal regulator Rho1. These results support a role for PlexA as a regulator of Semala reverse signaling to regulate R-cell axon-axon interaction.
Materials and Methods
Genetics.
UAS-Rho1.N19 flies were obtained from the Bloomington Drosophila Stock Center. To examine PlexA loss-of-function phenotype, genetic crosses were performed to generate PlexADf(4)C3/GAL4; TM6, Tb/UAS-GFP. Nonfluorescent larvae (genotype: PlexADf(4)C3/PlexADf(4)C3) from the resulting line was selected for dissection. To knock down the expression level of PlexA, UAS-PlexA-RNAi (pWIZ) or UAS-PlexA-RNA (pMF3) (provided by L. Luo, Stanford University, Stanford, CA) flies were crossed with GMR-GAL4 flies. To overexpress full-length PlexA or cytoplasmic-domain truncated PlexA (i.e., plexAΔcyt), UAS-PlexA or UAS-PlexAΔcyt flies were crossed with GMR-GAL4 flies. To examine the potential genetic interaction between sema1a and PlexA, genetic crosses were performed to generate sema1aP1/UAS-PlexA-RNAi; longGMR-GAL4/+. To remove semala in flies overexpressing PlexA, genetic crosses were performed to generate sema1aP1, GMR-GAL4/sema1aDf(2)N22–5,UAS-PlexA. To express UAS-PlexAΔcyt in R cells in which endogenous PlexA is knocked down, genetic crosses were perform to generate GMR-GAL4/+; UAS-PlexAΔcyt/UAS-PlexA-RNAi (pWIZ). To investigate potential genetic interactions between Sema1a and a set of intracellular signaling proteins, GMR-GAL4, UAS-sema1a/Bc flies were crossed with flies carrying the mutations, thus reducing the dosage of these genes by 50% in flies overexpressing sema1a. To express the dominant-negative form of Rho1 (i.e., Rho1.N19) in R cells, genetic crosses were performed to generate GMR-GAL4/UAS-Rho1.N19.
Histology.
Dissection, fixation and staining of the eye–brain complexes from third-instar larvae were performed similarly as described previously (Ruan et al., 1999). MAb 24B10 and anti-GFP antibodies were used at 1:100 and 1:1000 dilutions, respectively. The secondary antibodies (Jackson Immunochemicals) were used at 1:200 dilution. Anti-PlexA antibody was used at 1:1000 dilution. Epifluorescent images were captured using a high-resolution fluorescence imaging system (Canberra Packard) and analyzed by two-dimensional deconvolution using MetaMorph imaging software (Universal Imaging).
Molecular biology.
To generate GST-PlexA fusion protein for raising anti-PlexA antibody, a 350 bp sequence encoding for amino acid sequence Thr701 to Leu818 in the PlexA extracellular region was amplified by PCR using two primers 5′cagcgaattcacagctgaaaactgccgg3′ and 5′atgactcgagaagtggcttcgaccc3′. This fragment was subcloned into the EcoRI and XhoI sites of pGEX-4T-1 vector.
To generate UAS-PlexA-RNAi transgenic construct, a ∼600 bp fragment encoding for a portion of PlexA cytoplasmic domain was amplified by PCR using two primers 5′gccatctagaggagttcgaatagctgg3′ and 5′ccgatctagagcttgcatacatctctcc3′. This fragment was subcloned into the pWIZ vector to generate a construct containing an inversely repeated sequence.
To generate the UAS-PlexAΔcyt construct, two primers 5′ggtgacaccatttactgcgatagc3′ and 5′ccttgctagcgtagctccagaatgtccatctg3′ were used to amplify the fragment encoding for the amino acid sequence G785 to R1326 followed by a stop codon TAG by PCR. The PCR fragment was digested by XbaI and NheI, and subsequently used to replace the XbaI-XbaI fragment in the full-length UAS-PlexA construct. The resulting plasmid lacks the sequence encoding for almost entire cytoplasmic domain (i.e., amino acid 1327–1945).
Results
PlexA is required in R cells for the proper association of R-cell growth cones at the intermediate target region
That Sema1a functions as a receptor in R-cell axon guidance raises the interesting possibility that PlexA, the well known Semaphorin receptor, activates Sema1a reverse signaling in R-cell axons. To determine the potential role of PlexA in the developing Drosophila visual system, we performed loss-of-function analysis. While most of mutants homozygous for a deficiency chromosome (i.e., PlexADf(4)C3) lacking the PlexA gene died at late embryonic stage, occasionally some mutants could reach the third-instar larval stage, which allowed us to examine the effect of PlexA deletion on R-cell axonal projection pattern.
In wild type (Fig. 1A), the differentiating R-cells project axons through the optic stalk into the developing optic lobe. After migrating over the superficial lamina, R1–R6 axons terminate in between two layers of lamina glial cells, and their growth cones associate closely with each other to form a continuous and dense terminal layer at the intermediate target region. R7 and R8 axons within the same bundle extend through the lamina into the deeper medulla layer.
Figure 1.
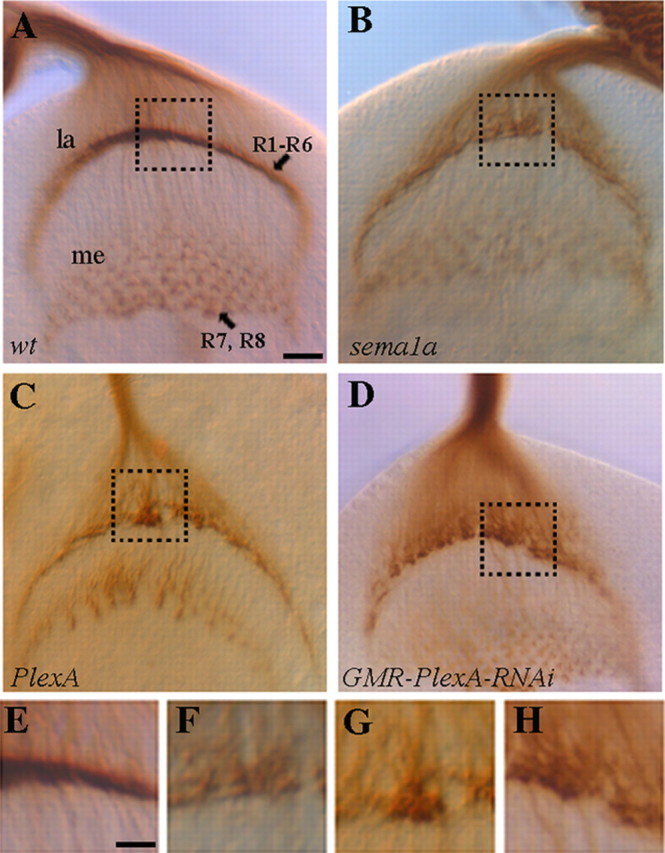
PlexA is required for the proper formation of R1–R6 termination layer in the developing optic lobe. Third-instar larval eye–brain complexes were stained with MAb 24B10 to visualize R-cell axonal projection pattern. A, Wild type. R1–R6 axons stop at the intermediate target region in the lamina (la), where their growth cones expand and associate closely with each other to form a smooth and dense terminal layer. Whereas R7 and R8 axons project through the lamina into the medulla (me). B, sema1aP1 homozygote. R1–R6 terminal layer was disrupted. R1–R6 growth cones associated loosely with neighboring growth cones and failed to pack into a dense layer. C, D, A similar phenotype was observed in PlexA deficiency mutants (C) and eye-specific PlexA knockdown mutants (D). E–H are enlarged views of the boxed regions in A–D, respectively. Scale bar: A–D, 10 μm; E–H, 5 μm.
In our previous study (Cafferty et al., 2006), we showed that Sema1a functions as a receptor to mediate attractive interactions between R1–R6 growth cones at the lamina intermediate target region. Mutations in the sema1a gene disrupted the association of R1–R6 growth cones at the intermediate target region, leading to the appearance of a discontinuous termination layer in the lamina, where R1–R6 growth cones scattered around the lamina termination region (Fig. 1B,F). Interestingly, we found that homozygous PlexA mutants displayed a similar phenotype as R1–R6 growth cones failed to form a continuous and dense termination layer in all mutants examined (100%, n = 9, Fig. 1C,G).
Our previous study showed that Sema1a functions in R cells to mediate the association between R1–R6 growth cones (Cafferty et al., 2006). If PlexA functions in the same pathway, one would predict that PlexA is also required in R cells. Since PlexA is located on the fourth chromosome, it is not feasible to perform FRT-mediated genetic mosaic analysis to determine whether PlexA is also required in R cells. To circumvent this problem, we performed gene knockdown analysis. We examined R-cell axonal projection pattern in fly larvae in which the expression of PlexA was specifically knocked down in R cells by expressing a UAS-PlexA-RNAi transgene (i.e., pWIZ-UAS-PlexA-RNAi) under control of the eye-specific driver GMR-GAL4. Compared with that of PlexA mutants (Fig. 1C,G), eye-specific PlexA knockdown mutants displayed a similar defect at the lamina termination layer (∼20%, n = 41; Fig. 1D,H). Similar results were obtained when PlexA was knocked down by using another PlexA-RNAi transgenic construct (pMF3) that targets a different sequence in the PlexA gene (∼19%, n = 21), which was generated by Luo and colleagues (Komiyama et al., 2007).
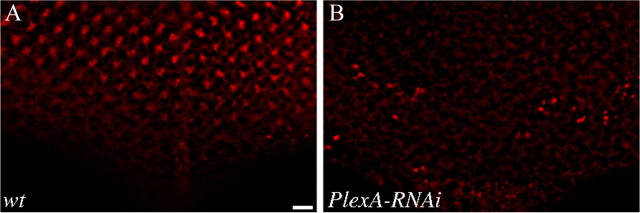
To confirm that eye-specific expression of UAS-PlexA-RNAi effectively knocked down the level of PlexA, we performed immunohistochemical analysis. Third-instar larval eye discs were stained with a rabbit anti-PlexA antibody. At third-instar larval stage, precursor cells in the eye-imaginal disc begin to differentiate into R cells. In wild type (Fig. 2A), like Sema1a (Cafferty et al., 2006), PlexA is detected in R cells in the posterior region of the eye disc (Fig. 2A). We found that the level of PlexA staining was significantly reduced when UAS-PlexA-RNAi was expressed in the developing eye disc (Fig. 2B).
Figure 2.
Eye-specific expression of the UAS-PlexA-RNAi transgene effectively decreased the level of PlexA in R cells. Third-instar eye discs were stained with anti-PlexA antibody. A, Wild-type. Strong PlexA staining was detected in developing R-cell clusters in the posterior region of the eye disc. B, PlexA staining was significantly reduced when the level of PlexA was knocked down by expressing a UAS-PlexA-RNAi transgene under control of the eye-specific GMR-GAL4 driver. Scale bar, 5 μm.
Thus, like sema1a, PlexA is also required in R cells for the proper interaction of R1–R6 growth cones in the intermediate target region.
PlexA interacts genetically with sema1a
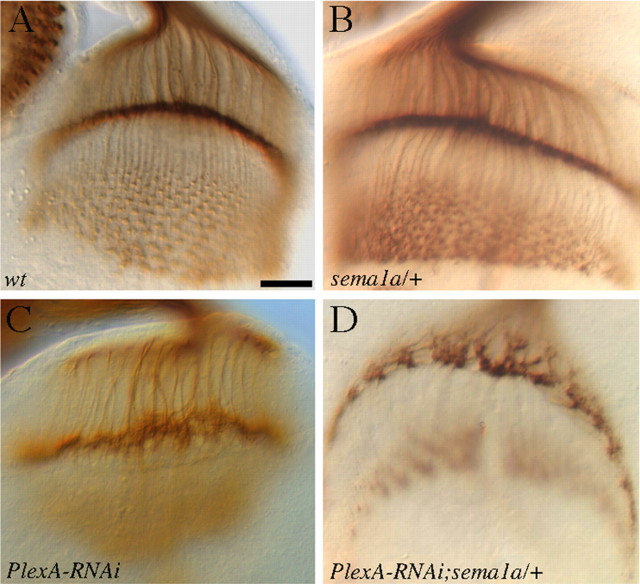
To determine whether PlexA and sema1a function in the same pathway, we examined the potential genetic interaction between PlexA and sema1a. The dosage of sema1a was reduced by 50% in PlexA knockdown larvae in which the reduction in PlexA level caused a low-penetrant phenotype. If PlexA and sema1a function in the same pathway, the prediction is that reducing the dosage of sema1a would further weaken the pathway leading to the enhancement of the phenotype. Indeed, we found that reducing the dosage of sema1a by 50% significantly enhanced the PlexA knockdown phenotype (Fig. 3D). The penetrance of the phenotype was increased from ∼20% (n = 41) to ∼60% (n = 25), and the severity of the phenotype was also increased (Fig. 3D). This result is consistent with that PlexA and sema1a function in the same pathway.
Figure 3.
PlexA interacts genetically with sema1a. R-cell axonal projection pattern in third-instar larval eye–brain complexes was visualized with MAb 24B10 staining. A, Wild type. B, Normal R-cell projection pattern was observed in sema1a heterozygotes. C, Larvae in which the level of PlexA was specifically knocked down in the eye displayed a mild phenotype. D, Reducing the dosage of sema1a by 50% in PlexA knockdown larvae increased both penetrance and severity of the phenotype. Scale bar, 20 μm.
Overexpression of PlexA induced the hyperfasciculation of R-cell axons
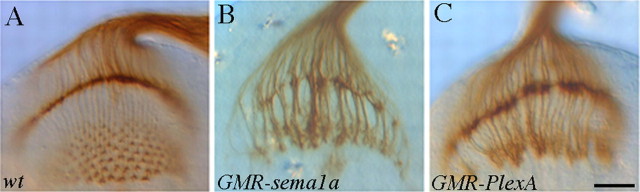
Our previous study showed that Sema1a functions as a receptor to mediate an attractive interaction between R-cell axons (Cafferty et al., 2006), and overexpression of Sema1a could induce the hyperfasciculation of R-cell axons (Fig. 4B) (Cafferty et al., 2006). If PlexA functions in the same pathway, one would predict that overexpression of PlexA should induce a similar phenotype. To address this, we examined R-cell projection pattern in third-instar larvae in which PlexA was overexpressed in R-cell axons under control of the eye-specific driver GMR-GAL4. Like overexpression of semala (Fig. 4B), we found that overexpression of PlexA also induced R-cell axonal hyperfasciculation in all hemispheres examined (n = 20, Fig. 4C). Compared with wild type (Fig. 4A), overexpression of sema1a (Fig. 4B) or PlexA (Fig. 4C) caused the formation of thicker axonal bundles, which led to the decrease in the number of separate axonal bundles between lamina and medulla. Unlike that of wild type in which R-cell axons form a precise array of separate and expanded “Y”-shape terminal structure in the medulla (Fig. 4A), most of R-cell axons in larvae ovexpressing sema1a (Fig. 4B) or PlexA (Fig. 4C) fused with each other to form large clumps in the medulla.
Figure 4.
Overexpression of PlexA induced the hyperfasciculation of R-cell axons. A, Wild type. B, Overexpression of Sema1a in R-cell axons induced the formation of thicker R-cell axonal bundles between lamina and medulla. In medulla, R-cell axons formed large clumps. C, Overexpression of PlexA caused a similar hyperfasciculation phenotype. Scale bar, 20 μm.
The cytoplasmic domain of PlexA is dispensable for inducing R-cell axonal hyperfasciculation
Our previous study showed that the cytoplasmic domain of Sema1a is essential for its function in R-cell axon guidance (Cafferty et al., 2006). Similarly, Luo and colleagues showed that the function of Sema1a in the olfactory system also requires its cytoplasmic domain (Komiyama et al., 2007). To further determine the action of PlexA in R-cell axon guidance, we examined whether the cytoplasmic domain of PlexA is necessary for its action in R-cell axon guidance.
We generated a UAS-PlexAΔcyt transgene in which the cytoplasmic domain of PlexA was deleted. This transgene was overexpressed in R-cell axons under control of the eye-specific GMR-GAL4 driver. Interestingly, we found that like full-length PlexA (Fig. 5B), PlexAΔcyt was also able to induce the hyperfasciculation of R-cell axons (100%, n = 21 hemispheres, Fig. 5C). We then tested whether expression of PlexAΔcyt is able to rescue the phenotype induced by PlexA RNAi treatment. PlexAΔcyt was expressed in R cells in which endogenous PlexA was knocked down by the PlexA RNAi transgene targeting the sequence encoding a portion of the cytoplasmic domain. We found that expression of PlexAΔcyt largely restored the R1–R6 termination pattern in the lamina (Fig. 5F, n = 39). These results are in marked contrast to the essential requirement of the Sema1a cytoplasmic domain (Cafferty et al., 2006), and argue against that PlexA functions as a receptor in R-cell axon guidance.
Figure 5.
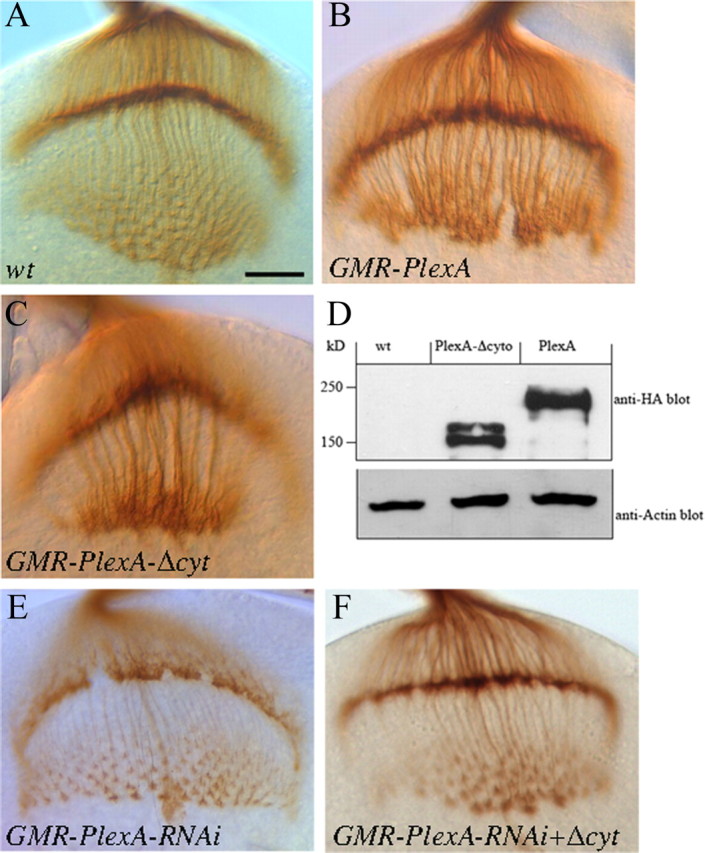
The cytoplasmic domain of PlexA is dispensable. A, Wild type. B, Overexpression of PlexA under control of GMR-GAL4 caused the formation of thicker axonal bundles. C, Overexpression of PlexAΔcyt driven by GMR-GAL4 caused an identical hyperfasciculation phenotype. D, Western blot analysis using anti-HA antibody showed the expression of HA-tagged full-length and cytoplasmic-domain-truncated PlexA in flies under control of GMR-GAL4. The size of transgenic proteins is consistent with the predicted size. E, An eye-specific PlexA knockdown mutant. F, Expression of PlexAΔcyt largely restored the R1–R6 growth-cone organization pattern in the lamina in PlexA knockdown mutants. Scale bar, 20 μm.
Loss of sema1a suppressed the PlexA overexpression phenotype
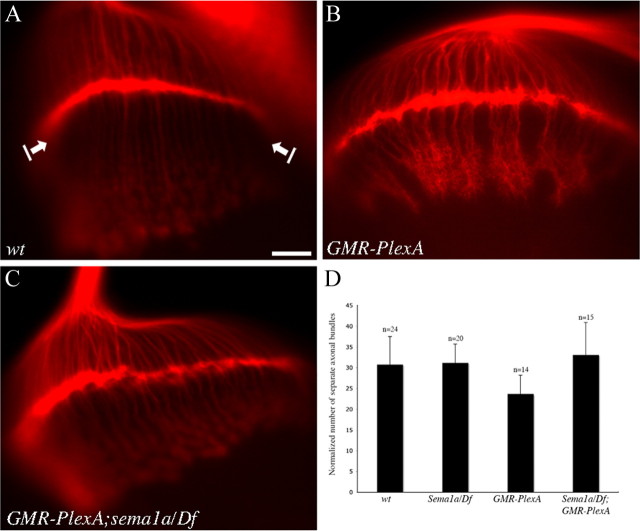
To further determine the functional relationship between PlexA and sema1a in R-cell axon guidance, we performed epistasis analysis. Since overexpression of PlexA induced R-cell axonal hyperfasciculation, we tested whether loss of sema1a could modify this phenotype. Interestingly, we found that loss of sema1a largely suppressed the PlexA-induced R-cell axonal hyperfasciculation phenotype (Fig. 6C,D). This result suggests that PlexA functions upstream of Sema1a in the pathway, and consistent with that PlexA regulates Sema1a reverse signaling in R-cell axon guidance.
Figure 6.
PlexA functions upstream of sema1a. A, Wild type. B, Overexpression of PlexA under control of GMR-GAL4-induced R-cell axonal hyperfasciculation. C, The PlexA-induced hyperfasciculation phenotype was suppressed when sema1a was disrupted. D, The number of separate axonal bundles that are located between lamina and medulla was counted. The data were normalized with the row number of R-cell clusters in the eye disc. Compared with wild type, overexpression of PlexA induced the formation of thicker bundles and thus significantly decreased the number of separate R-cell axonal bundles (p = 0.0012). Compared with that of PlexA overexpression in wild-type background, the number of separate axonal bundles in PlexA-overexpression mutants in which the sema1a gene was disrupted, was increased significantly (p = 0.0005). Error bars denote SE. Scale bar, 20 μm.
Sema1a interacts genetically with Rho1
To gain further insights into the mechanism of Sema1a reverse signaling, we examined the potential genetic interaction between Sema1a and a number of intracellular signaling proteins (supplemental Table 1, available at www.jneurosci.org as supplemental material). Among them, we found that reducing the dosage of small GTPase Rho1 by 50% significantly enhanced the Sema1a-overexpression-induced hyperfasciculation phenotype (Fig. 7C; supplemental Table 1, available at www.jneurosci.org as supplemental material). Whereas reducing the dosage of other members of Rho family small GTPases such as Cdc42 and Rac did not show any obvious effect (supplemental Table 1, available at www.jneurosci.org as supplemental material). A previous study showed that a putative Enabled (Ena)-binding motif in the cytoplasmic domain of Sema1a is essential for its function in synaptic formation in the adult giant fiber system (Godenschwege et al., 2002). However, we did not detect any obvious interaction between Sema1a and Ena or between Sema1a and Abl (Fig. 7B; supplemental Table 1, available at www.jneurosci.org as supplemental material).
Figure 7.
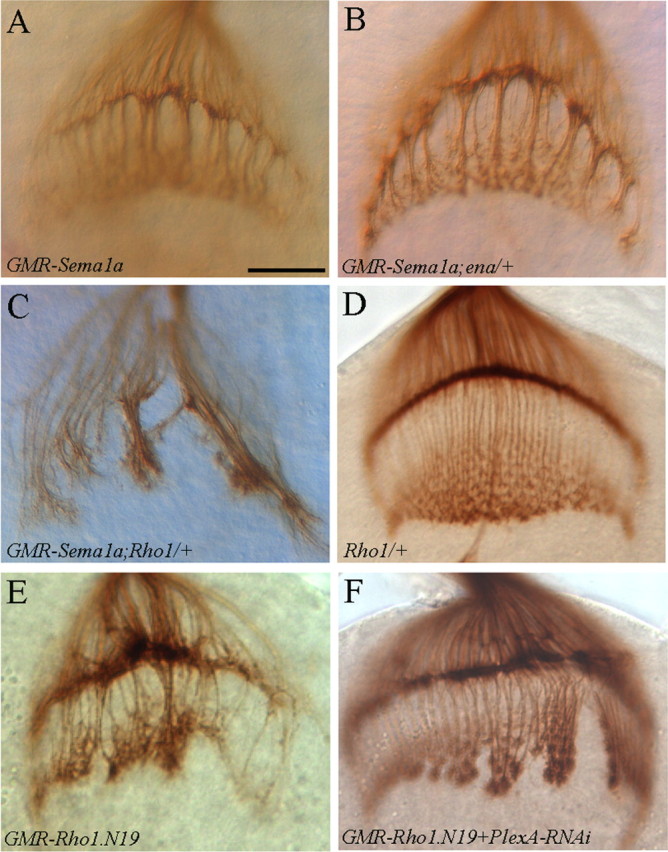
Sema1a and PlexA interact genetically with Rho1. A, Overexpression of Sema1a induced the formation of thicker axonal bundles. B, Reducing the dosage of ena by half did not modify the Sema1a-induced hyperfasciculation phenotype. C, Reducing the dosage of Rho1 by half significantly enhanced the Sema1a-overexpression phenotype. D, Reducing the dosage of Rho1 by half in wild-type background did not affect R-cell projection pattern. E, Expressing the dominant-negative form Rho1.N19 in R cells also induced an axonal hyperfasciculation phenotype. F, Knocking down PlexA suppressed the Rho1.N19-induced phenotype. Scale bar, 20 μm.
To further investigate the role of Rho1, we examined whether interfering with the function of Rho1 affects R-cell axonal projections. A dominant-negative form of Rho1 (i.e., Rho1.N19) was expressed in R cells under control of the GMR-GAL4 driver. Interestingly, we found that eye-specific expression of Rho1.N19 induced an axonal-hyperfasiculation phenotype similar to overexpression of Sema1a (∼55%, n = 20, Fig. 7E). We then examined whether PlexA, like sema1a, interacts genetically with Rho1. PlexA was knocked down by RNAi in flies expressing Rho1.N19. We found that the severity of the Rho1.N19-induced hyperfasciculation phenotype was significantly suppressed by PlexA-RNAi treatment (n = 23, Fig. 7F). These results support that PlexA-Sema1a reverse signaling regulates the function of Rho1 in R-cell axon guidance.
Discussion
Recent studies indicate that Sema1a, the Drosophila transmembrane Semaphorin, can function as a receptor or a component of a receptor complex to mediate axon/dendrite guidance and synaptic formation (Godenschwege et al., 2002; Cafferty et al., 2006; Komiyama et al., 2007). However, it is unclear what molecules activate Sema1a to trigger downstream signaling events. In this study, we provide genetic evidence to support that PlexA functions as a regulator of Sema1a reverse signaling in the Drosophila visual system. First, PlexA and sema1a mutants displayed similar loss-of-function and gain-of-function phenotypes. Second, sema1a displayed dosage-sensitive genetic interaction with PlexA, consistent with that they function in the same pathway. Third, expression of PlexAΔcyt mutant lacking the cytoplasmic domain was still able to induce R-cell axon hyperfasciculation, and largely rescued the PlexA-RNAi phenotype. And fourth, epistasis analysis suggests that PlexA functions upstream of Sema1a.
Our results suggest that in addition to functioning in the classic Sema1a-to-PlexA signaling pathway (i.e., Forward signaling) in motor axon guidance (Winberg et al., 1998; Yu et al., 1998), PlexA and its cognate ligand Semala are also capable of mediating PlexA-to-Sema1a reverse signaling to regulate R-cell axon guidance in the fly visual system. Such ligand/receptor bidirectional signaling is not unprecedented. For instance, it has been shown that Eph receptor tyrosine kinases and their ligands ephrins exhibit both forward and reverse signaling in neural development (Murai and Pasquale, 2003; Davy and Soriano, 2005). Recent studies also indicate a role for ErbB receptors and their ligands Neuregulins bidirectional signaling in neural development (Chen et al., 2008). Such bidirectional signaling mediated by a ligand and receptor complex greatly increases the plasticity of intercellular communications during neural development.
We propose that PlexA interacts with Sema1a to regulate the communication between neighboring R1–R6 growth cones, which is necessary for the establishment of an appropriate retinotopic termination pattern at the intermediate target region in the developing lamina. Previous studies show that the Sema1a-to-PlexA forward signaling pathway mediates axon-axon repulsion in motor axon guidance (Winberg et al., 1998; Yu et al., 1998) and the targeting of olfactory receptor neurons (Sweeney et al., 2007). However, our results are not consistent with a model in which the interaction between PlexA and Sema1a induce axon-axon repulsion. First, loss of PlexA, like loss of Sema1a, appeared to disrupt R-cell axon association leading to the formation of discontinuous termination layer. And second, like overexpression of Sema1a, overexpression of PlexA induced a R-cell axonal hyperfasciculation phenotype. Since both PlexA and Sema1a are expressed and genetically required in R cells, we favor a model in which PlexA interacts with Sema1a to mediate attractive axon-axon interaction for the proper organization of R-cell growth cones at the intermediate target region.
What is the nature of Sema1a-dependent downstream signaling in R-cell axons? Previous study identified a putative Enabled (Ena)-binding motif in the cytoplasmic domain of Sema1a that is essential for its function in synaptic formation in the adult giant fiber system (Godenschwege et al., 2002). In vertebrates, it has been shown that some transmembrane Semaphorins use their cytoplasmic domain to recruit intracellular signaling proteins such as EVL (Ena/Vasp-like protein) (Klostermann et al., 2000), PSD-95 (Inagaki et al., 2001; Ohoka et al., 2001; Schultze et al., 2001), c-Src (Eckhardt et al., 1997), Abl kinase and Enabled (Toyofuku et al., 2004). Among them, Abl kinase and Enabled are key components of the Semaphorin6D reverse signaling pathway for regulating the migration of myocardial cells in the chick embryo (Toyofuku et al., 2004). However, we could not detect any genetic interaction between Sema1a and Enabled in R-cell axon guidance (supplemental Table 1, available at www.jneurosci.org as supplemental material). Instead, we found that Sema1a and PlexA interact genetically with small GTPase Rho1 (Fig. 7; supplemental Table 1, available at www.jneurosci.org as supplemental material), raising the interesting possibility that Sema1a reverse signaling involves negative regulation of Rho1. One attractive model is that Sema1a, activated by PlexA, downregulates the function of Rho1. A decrease in Rho1 function may prevent Rho1 from inhibiting the function of certain cell adhesion molecules, thus promoting the attractive interaction between R1–R6 growth cones at the intermediate target region. Future studies will be necessary to test this model and elucidate the exact downstream signaling events activated by the PlexA and Sema1a interaction in the fly visual system.
Footnotes
This work was supported by an operating grant (MOP-14688) awarded to Yong Rao from Canadian Institutes of Health Research. Y.R. is a Le Fonds de la Recherche en Santé de Québec senior research scholar. Y.L. is a recipient of Le Fonds de la Recherche en Santé de Québec studentship. We thank people in the laboratories of Yong Rao and Don van Meyel for comments and suggestions; the Bloomington Stock Center for fly stocks; the Developmental Studies Hybridoma Bank at University of Iowa for MAb 24B10; Liqun Luo for anti-PlexA antibody and pMF3-UAS-PlexA-RNAi transgenic line; Corey Goodman for UAS-sema1a; and Alex Kolodkin for sema1aP1 and UAS-PlexA.
References
- Ayoob JC, Yu HH, Terman JR, Kolodkin AL. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24:6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty P, Yu L, Long H, Rao Y. Semaphorin-1a functions as a guidance receptor in the Drosophila visual system. J Neurosci. 2006;26:3999–4003. doi: 10.1523/JNEUROSCI.3845-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu AK, Ip NY. Bidirectional signaling of ErbB and Eph receptors at synapses. Neuron Glia Biol. 2008;4:211–221. doi: 10.1017/S1740925X09990287. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Dalpé G, Brown L, Culotti JG. Vulva morphogenesis involves attraction of plexin 1-expressing primordial vulva cells to semaphorin 1a sequentially expressed at the vulva midline. Development. 2005;132:1387–1400. doi: 10.1242/dev.01694. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- Eckhardt F, Behar O, Calautti E, Yonezawa K, Nishimoto I, Fishman MC. A novel transmembrane semaphorin can bind c-src. Mol Cell Neurosci. 1997;9:409–419. doi: 10.1006/mcne.1997.0644. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Kitsukawa T. Receptors for collapsin/semaphorins. Curr Opin Neurobiol. 1998;8:587–592. doi: 10.1016/s0959-4388(98)80085-8. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Ohoka Y, Sugimoto H, Fujioka S, Amazaki M, Kurinami H, Miyazaki N, Tohyama M, Furuyama T. Sema4c, a transmembrane semaphorin, interacts with a post-synaptic density protein, PSD-95. J Biol Chem. 2001;276:9174–9181. doi: 10.1074/jbc.M009051200. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem. 2000;275:39647–39653. doi: 10.1074/jbc.M006316200. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor; 1993. pp. 1363–1491. [Google Scholar]
- Murai KK, Pasquale EB. ‘Eph’ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Ohoka Y, Hirotani M, Sugimoto H, Fujioka S, Furuyama T, Inagaki S. Semaphorin 4C, a transmembrane semaphorin, [corrected] associates with a neurite-outgrowth-related protein, SFAP75. Biochem Biophys Res Commun. 2001;280:237–243. doi: 10.1006/bbrc.2000.4080. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Ruan W, Pang P, Rao Y. The SH2/SH3 adaptor protein dock interacts with the Ste20-like kinase misshapen in controlling growth cone motility. Neuron. 1999;24:595–605. doi: 10.1016/s0896-6273(00)81115-0. [DOI] [PubMed] [Google Scholar]
- Schultze W, Eulenburg V, Lessmann V, Herrmann L, Dittmar T, Gundelfinger ED, Heumann R, Erdmann KS. Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95. J Neurochem. 2001;78:482–489. doi: 10.1046/j.1471-4159.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Sweeney LB, Couto A, Chou YH, Berdnik D, Dickson BJ, Luo L, Komiyama T. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron. 2007;53:185–200. doi: 10.1016/j.neuron.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- Tayler TD, Garrity PA. Axon targeting in the Drosophila visual system. Curr Opin Neurobiol. 2003;13:90–95. doi: 10.1016/s0959-4388(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, Goodman CS. The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron. 2001;32:53–62. doi: 10.1016/s0896-6273(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Wong JT, Wong ST, O'Connor TP. Ectopic semaphorin-1a functions as an attractive guidance cue for developing peripheral neurons. Nat Neurosci. 1999;2:798–803. doi: 10.1038/12168. [DOI] [PubMed] [Google Scholar]
- Yu HH, Araj HH, Ralls SA, Kolodkin AL. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]