Abstract
Acute stress is associated with a sensitized amygdala. Corticosteroids, released in response to stress, are suggested to restore homeostasis by normalizing/desensitizing brain processing in the aftermath of stress. Here, we investigated the effects of corticosteroids on amygdala processing using functional magnetic resonance imaging. Since corticosteroids exert rapid nongenomic and slow genomic effects, we administered hydrocortisone either 75 min (rapid effects) or 285 min (slow effects) before scanning in a randomized, double-blind, placebo-controlled design. Seventy-two healthy males were scanned while viewing faces morphing from a neutral facial expression into fearful or happy expressions. Imaging results revealed that hydrocortisone desensitizes amygdala responsivity rapidly, while it selectively normalizes responses to negative stimuli slowly. Psychophysiological interaction analyses suggested that this slow normalization is related to an altered coupling of the amygdala with the medial prefrontal cortex. These results reveal a temporarily fine-tuned mechanism that is critical for avoiding amygdala overshoot during stress and enabling adequate recovery thereafter.
Introduction
It is of vital importance to an organism to respond adequately to potential threats during the exposure to a stressful experience, but also to subsequently recover when the threat has subsided. The immediate central release of norepinephrine (NE) during the initial phase of the stress response is known to induce a surge of vigilance, which optimizes the detection and assessment of these threats by prioritizing sensory processing (de Kloet et al., 2005) and activating the key modulator of vigilance and emotional processing in the brain: the amygdala (Phelps and LeDoux, 2005; van Marle et al., 2009). Whereas this amygdala-mediated hypervigilant state of processing is highly beneficial during an initial fight-or-flight response, it may become maladaptive and culminate in mental diseases such as depression or post-traumatic stress disorder (PTSD) if this sensitization is not properly controlled (McEwen, 2004; de Kloet et al., 2005).
Corticosteroids, released at a slightly slower time scale in response to stress, have been suggested to be crucial factors in this regulation of the stress response (de Kloet et al., 2005). They restore homeostasis by diverting energy supply to challenged tissues and control the excitability of neuronal networks (de Kloet et al., 1999). Therefore, it can be hypothesized that they regulate amygdala activation and thus normalize vigilance in the aftermath of stress. Initial evidence for such a regulatory role of corticosteroids was derived from animal studies showing that corticosteroids induce anxiolytic effects in rodents (File et al., 1979; Andreatini and Leite, 1994). Corticosteroid administration resulted in more explorative and socially interactive behavior in rats, which was the exact opposite effect of acute stress. Recent studies have extended these findings to humans (Soravia et al., 2006; Het and Wolf, 2007; Putman et al., 2007). Remarkably, these anxiolytic effects occur relatively instantly. This goes against the general assumption that the normalizing effects occur gradually by a process involving gene transcription (de Kloet et al., 2005), but suggests that rapid nongenomic effects are involved as well.
To elucidate the role of corticosteroids in vigilance regulation, we targeted both the rapid nongenomic and the slow genomic effects of corticosteroids on amygdala function. To assess the dynamic corticosteroid effects over time, we used a randomized, double-blind, placebo-controlled design, in which healthy male participants received either 10 mg of hydrocortisone at 75 min (targeting the rapid effects) or 285 min (targeting the slow effects), or placebo before a task probing amygdala reactivity (van Marle et al., 2009) during functional magnetic resonance imaging (fMRI). Timing of hydrocortisone administration was based on animal work targeting the nongenomic and genomic effects of corticosteroids. The task consisted of passive viewing of photographed faces morphing from a neutral expression into a fearful or happy facial one (supplemental Fig. S1, available at www.jneurosci.org as supplemental material), allowing us to test whether the normalization is specific for certain emotional input. Additionally, we used functional connectivity analyses to test whether corticosteroids affect amygdala coupling to brain regions involved in its control.
Materials and Methods
Participants
Seventy-two young (age range, 18–29 years; median age, 21 years), right-handed, healthy male volunteers gave informed consent to participate in the study. To ensure stable effects of hydrocortisone over all participants, women were excluded from participation. Women are known to display different hypothalamic-pituitary-adrenal (HPA) axis activity than men, exhibiting smaller and more variable cortisol responses to stress (Kajantie and Phillips, 2006), depending on menstrual cycle phase and the use of hormonal contraceptives (Kirschbaum et al., 1999; Bouma et al., 2009). Furthermore, individuals who met any of the following criteria were excluded from participation: history of head injury; autonomic failure; history of or current psychiatric, neurological, or endocrine disorders; current periodontitis; acute inflammatory disease; acute peptic or duodenal ulcers; regular use of corticosteroids; treatment with psychotropic medications, narcotics, β-blockers, steroids, or any other medication that affects CNS or endocrine system; medical illness within the 3 weeks before testing; self-reported mental or substance use disorder; daily tobacco or alcohol use; regular night-shift work; or current stressful episode or major life event. Moreover, volunteers with high scores on depression (score >8 on the Beck Depression Inventory) (Beck et al., 2002) were excluded from participation. Four participants were excluded from analyses because they either displayed abnormal basal salivary cortisol levels (>3 SDs above mean; 1 participant) or showed no elevation in salivary cortisol level in response to hydrocortisone (CORT) intake, which means we ended up with 23 men in the placebo group, 23 in the slow CORT group, and 22 in the rapid CORT group. The study was approved by the local ethics committee [Commissie Mensgebonden Onderzoek (CMO) region Arnhem-Nijmegen, The Netherlands] and in accordance with the declaration of Helsinki.
Study design
Participants were scanned in a randomized, double-blind, placebo-controlled, parallel-group design. To target the time-differential effects of CORT, participants were divided over three groups, receiving 10 mg of CORT at either 75 min (rapid CORT effects) or 285 min (slow CORT effects), or placebo before viewing an emotional processing task in the MRI scanner. Physiological (cortisol level) and psychological (mood and attention) indices were measured to confirm cortisol level manipulation without additional side effects.
Procedure
Before arrival.
Before inclusion, all eligible participants received an extensive information brochure, listing all inclusion and exclusion criteria and explaining the setup of the experiment. If criteria were met (according to the participant's own insights), an appointment was made. To minimize differences in baseline cortisol levels, we instructed participants not to use any recreational drugs for 3 d, and to refrain from drinking alcohol, exercising, and smoking for 24 h before the appointment. Furthermore, participants were requested not to brush their teeth, floss, or eat and drink anything but water for 1 h before the session, enabling adequate saliva sampling for cortisol assessment. They were asked to take a light lunch and do so no later than 1 h before arrival; their lunch could not contain any citrus products, coffee, tea, milk, or sweets (Maheu et al., 2005). Throughout the entire study period, participants were given only water to drink, except for a scheduled lunch at 135 min after arrival. To reduce the impact of diurnal variation in cortisol levels, all testing was performed in the afternoon, between 12:00 P.M. (±30 min) and 6:00 P.M. (±30 min), when hormone levels are relatively stable.
Arrival.
Upon arrival, participants received an information brochure about the procedure, they gave written informed consent, and completed an intake questionnaire to ensure that inclusion and exclusion criteria were met. Thirty minutes after arrival, a first saliva sample was taken, followed by another one 15 min later, to measure a reliable baseline level. Participants were then asked to complete the NEO-Five Factor Inventory (FFI) Personality Inventory (Costa and McCrae, 1992), the Spielberger Trait Anxiety Inventory (trait anxiety) (van der Ploeg et al., 1980; van der Ploeg, 1981), and a first Profile of Mood States (POMS) questionnaire (Reddon et al., 1985; Wald and Mellenbergh, 1990; de Groot, 1992). Immediately after the second saliva sample (at t = 45 min) participants received the first capsule, containing either 10 mg of CORT or placebo. During the entire period (∼3.5 h) before scanning, the participants had to wait in a quiet room where they were free to conduct any activities except for anything potentially arousing (e.g., video games). At 255 min after arrival, participants were asked to complete a second POMS questionnaire and received the second capsule. Both drug capsules, containing either 10 mg of CORT or placebo (cellulose), were administered orally. This dose is known to elevate salivary cortisol levels to high-stress levels (Kirschbaum et al., 1996; Gröschl et al., 2002; Tops et al., 2003). Depending on the group to which participants were (randomly) assigned, they received the first capsule containing placebo, the second containing placebo (placebo condition); the first capsule placebo, the second CORT (rapid CORT condition); or the first capsule CORT, the second placebo (slow CORT condition).
Scanning.
At ∼4.5 h after arrival, participants were taken to the scanner room and the procedures were explained. Participants lay supine in the scanner and viewed the screen through a mirror positioned on the head coil. They were asked to lie as still as possible, keep their eyes open, and look directly and continuously at the center of the screen in front of them.
Dynamic facial expression task.
The dynamic facial expression task started 75 min after administration of the second capsule (at t = 330 min) (Fig. 1). In brief, participants were asked to passively view blocks of faces morphing dynamically into either a fearful or happy facial expression. The perceptual processing of emotional faces has been shown to robustly engage the amygdala (Vuilleumier and Pourtois, 2007) and even more so with a dynamic rather than static presentation (Sato et al., 2004). Stimuli consisted of short 133 ms animation clips for each of 10 different faces [taken from a standardized set (Ekman and Friesen, 1976) and equalized in luminance and contrast], showing a morphing sequence consisting of four frames (55, 70, 85, and 100% emotional expression). Within a block, each of these morphing sequences was immediately followed by the morphing sequence of a different face, resulting in the presentation of distinct faces every 0.5 s. An experimental session lasted 8 min and consisted of six blocks of each emotion (25 s, 50 morphing sequences each) and six blocks of fixation cross (25 s, baseline for analysis). Blocks were presented in a mirrored design avoiding covariation with linear drift, and adjacent blocks of the same emotion were avoided. Participants were asked to make a right index finger response on a button box whenever the fixation cross appeared, as a control for attention.
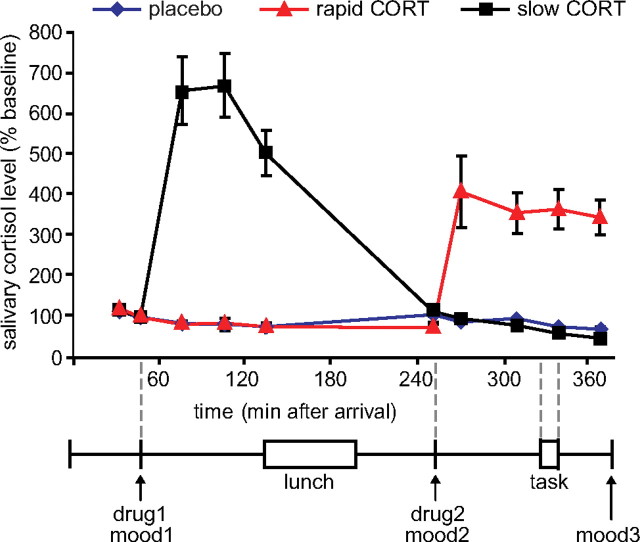
Figure 1.
Experimental design and salivary cortisol curves. Participants received two capsules (drug1 and drug2) containing either 10 mg of CORT or placebo at different time points before the emotional processing task. Hydrocortisone intake significantly elevated salivary cortisol levels in both hydrocortisone administration conditions to levels observed during moderate-to-severe stress. mood, POMS questionnaire (Reddon et al., 1985; Wald and Mellenbergh, 1990; de Groot, 1992). Error bars represent SEM.
Physiological and psychological measures
Saliva collection and analysis.
Cortisol levels were measured from saliva at 10 time points: baseline measurements at the beginning of the experiment (t = 30, 45 min), and eight samples (t = 75, 105, 135, 255, 275, 315, 345, and 375 min) to assess cortisol changes throughout the experiment. Saliva was collected using a commercially available collection device (Salivette, Sarstedt). For each sample, the participant first placed the cotton swab provided in each Salivette tube in his mouth and chewed gently on it for 1 min to produce saliva. The swab was then placed back in the Salivette tube, and the samples were stored in a freezer at −25°C until assayed. Laboratory analyses were performed at the Department of Biopsychology, Technische Universität Dresden (Dresden, Germany). After thawing, Salivettes were centrifuged at 3000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary free cortisol concentrations were subsequently measured using a commercially available chemiluminescence immunoassay with a high sensitivity of 0.16 ng/ml (IBL).
Mood state.
Mood state was assessed using the POMS questionnaire (Reddon et al., 1985; Wald and Mellenbergh, 1990; de Groot, 1992) at three time points: at the beginning of the experiment (t = 30 min); just before the intake of the second capsule (t = 255 min); and at the end of the experiment (t = 375 min).
Attention.
Average reaction times to appearance of the fixation cross were calculated to assess the participant's attentiveness.
Physiological and psychological statistical analysis
Behavioral and physiological data were analyzed in SPSS 15.0 (SPSS) using repeated measures ANOVAs with emotion type (fearful vs happy) as the within-subject factor and drug condition (placebo vs rapid CORT vs slow CORT) as the between-subject factor. The level of neuroticism (as assessed by the NEO-FFI Personality Inventory) (Costa and McCrae, 1992) was included as covariate. Due to the high levels of skewness and kurtosis of the POMS questionnaire (Reddon et al., 1985; Wald and Mellenbergh, 1990; de Groot, 1992), mood data were analyzed using nonparametric tests. Changes over time in mood state were assessed by Friedman tests, and Kruskal–Wallis tests were used to assess potential drug effects on mood. The α was set at 0.05 throughout.
MRI acquisition
Participants were scanned by a Siemens MAGNETOM Avanto 1.5 tesla MRI scanner equipped with an eight-channel head coil. A series of blood oxygenation level-dependent T2-weighted gradient echo planar imaging (EPI) images was acquired with the following parameters: repetition time (TR) = 2340 ms; echo time (TE) = 35 ms; flip angle (FA) = 90 °; 32 axial slices approximately aligned with anterior commissure-posterior commissure plane; slice matrix size = 64 × 64; slice thickness = 3.5 mm; slice gap = 0.35 mm; and field of view (FOV) = 212 × 212 mm2. Because of its relatively short TE, this sequence yields optimal contrast-to-noise ratio in the medial temporal lobes (Stöcker et al., 2006). High-resolution anatomical images were acquired for individuals by a T1-weighted three-dimensional magnetization-prepared rapid gradient echo sequence, which used the following parameters: TR = 2250 ms; TE = 2.95 ms; FA = 15 °; orientation, sagittal; FOV = 256 × 256 mm2; and voxel size = 1.0 mm isotropic.
fMRI data analysis
Data were analyzed using Statistical Parametric Mapping software (SPM5; UCL). The first five EPI volumes were discarded to allow for T1 equilibration. Before analysis, the images were motion corrected using rigid body transformations and least sum of squares minimization. Subsequently, they were temporally adjusted to account for differences in sampling times across different slices. All functional images were then coregistered with the high-resolution T1-weighted structural image using normalized mutual information maximization. The anatomical image was subsequently used to normalize all scans into Montreal Neurological Institute (MNI) 152 space. All functional images were resampled to a voxel size of 2 mm isotropic. Finally, all images were smoothed with an isotropic 8 mm full-width-at-half-maximum Gaussian kernel to accommodate residual functional/anatomical variance between subjects.
Data were analyzed using a general linear model, in which blocks were modeled based on emotion type. Regressors were temporally convolved with the canonical hemodynamic response function of SPM5. The six covariates corresponding to the movement parameters obtained from the realignment procedure were also included in the model. To reduce unspecific differences between scan sessions, and to correct for any unspecific, global effects of drug intake on hemodynamic response instead of neuronal activation (Desjardins et al., 2001; Peeters and Van der Linden, 2002), global normalization using proportional scaling was applied. Although this method might induce certain artifacts when local effects are strong enough to contribute substantially to global signal changes (Junghofer et al., 2005), all critical comparisons in this study (those between drug conditions) remain valid since this potential problem is similarly present in all drug conditions. The single subject parameter estimates from each session and condition obtained from the first-level analysis were included in subsequent random-effects analyses. For the second-level analysis, a factorial ANOVA was used, with emotion type (fearful vs happy) as the within-subject factor, drug condition (placebo vs rapid CORT vs slow CORT) as the between-subject factor, and level of neuroticism (as assessed by the NEO-FFI Personality Inventory) (Costa and McCrae, 1992), which is known to influence amygdala activity (Haas et al., 2007), as the covariate.
Given the abundance of glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) in both the amygdala (de Kloet, 1991) and medial prefrontal cortex (mPFC), and their involvement in emotional processing (Joëls et al., 2004; Ochsner and Gross, 2005), these regions were considered regions of interest. Data concerning these a priori regions of interest were corrected for reduced search volumes through anatomical masks as defined by the WFU PickAtlas Tool (version 2.4). A threshold of p < 0.05 whole-brain corrected was applied to all other regions. Visualizations of activations were created in SPM5 by superimposing statistical parametric maps thresholded at p < 0.001 uncorrected (unless specified otherwise) onto a canonical T1-weighted image in a standard MNI 152 space.
Functional connectivity analysis: psychophysiological interaction
Psychophysiological interaction (PPI) analyses were used to assess how activity in a brain region of interest covaried with a source region in response to the experimental condition (Friston et al., 1997). We examined functional connectivity from the drug × emotion type interaction cluster in the left amygdala as a source region to investigate whether this interaction was related to altered connectivity due to CORT administration. To test this, we extracted the deconvolved time series from this cluster (thresholded at p < 0.001 uncorrected). The PPI was calculated as the element-by-element product of this interaction cluster (the first eigenvariate from the time series of all voxels) and a vector coding for the effect of task (the contrast “faces > fixation”) was entered. This product was subsequently reconvolved with the hemodynamic response function, and the resulting interaction term was entered as a regressor in a first-level model together with the time series of the amygdalar interaction cluster and the vector coding for the task effect. The model was estimated and contrasts generated to test the effects of positive and negative PPIs. This analysis identified regions that display stronger functional connectivity with the amygdala during face processing. Next, the contrast images for the PPI effects were entered in a second-level analysis for which we used a factorial ANOVA with drug condition (placebo vs rapid CORT vs slow CORT) as the between-subject factor, and neuroticism as the covariate. Similar to the conventional fMRI analyses, regions of interest were corrected for reduced search regions through anatomical masks as defined by the WFU PickAtlas Tool (version 2.4). A threshold of p < 0.05 whole brain corrected was applied to all other regions.
Results
Endocrine and psychological measures
As expected, oral administration of 10 mg of hydrocortisone increased salivary cortisol levels to those observed during severe stress (Morgan et al., 2000) (Fig. 1) (for absolute values, see supplemental Table S1, available at www.jneurosci.org as supplemental material), which was evidenced by a significant time × group interaction (F(18,114) = 28.43; p < 0.001). Increased levels were observed from 30 min after administration onward in both hydrocortisone administration conditions, and the levels remained elevated for at least 90 min. This resulted in elevated cortisol levels during fMRI scanning in the rapid hydrocortisone condition, whereas the levels in the slow condition had already returned to baseline.
Postexperiment debriefing revealed that participants were not able to identify the substance received. Furthermore, drug administration did not affect mood as assessed three times during the experiment using the POMS questionnaire (supplemental Table S1, available at www.jneurosci.org as supplemental material) (Reddon et al., 1985; Wald and Mellenbergh, 1990; de Groot, 1992). Although significant reductions in levels of depression scores (Friedman's ANOVA; χ2(2) = 10.53; p = 0.005), anger scores (χ2(2) = 9.09; p = 0.011), vigor scores (χ2(2) = 78.79; p < 0.001), and tension scores (χ2(2) = 21.88; p < 0.001) were observed over the course of the experiment, and levels of fatigue (χ2(2) = 51.18; p < 0.001) increased, none of these factors was affected by drug administration. Groups did not differ on any aspect of mood state at baseline, nor at any other time point during the experiment (all p > 0.05). Changes in mood over time were also not affected by drug administration (all p > 0.05). The drug manipulation did not affect the participant's attentiveness since average reaction times to the appearance of the fixation cross were not different across groups (F(2) = 1.54, n.s.). Thus, differences in brain activity found after drug administration cannot readily be explained by changes in mood or attention.
Brain activation
We first identified brain regions activated by viewing emotional faces in general. As expected, the face-processing task activated the amygdala bilaterally. Furthermore, increased activity was observed in a widespread visual processing network, including the primary visual cortex, extrastriate cortex, and occipitotemporal regions like the fusiform gyrus, as well as the inferior frontal gyrus, and the angular and precentral gyrus (supplemental Table S2, available at www.jneurosci.org as supplemental material). Second, we looked into the effect of emotion type on brain activation. The left amygdala was the only brain region that displayed stronger responses toward fearful than happy faces, whereas the opposing contrast (happy > fearful) did not yield any significant differences in brain activity (supplemental Table S1, available at www.jneurosci.org as supplemental material).
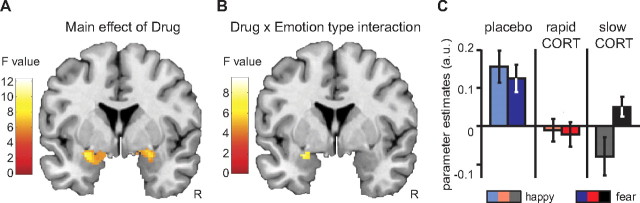
To examine how corticosteroids affect emotional processing over time, we first identified those brain regions whose activity was modulated by any of the drug conditions. The main effect of drug revealed that hydrocortisone affected amygdala responsivity bilaterally (x = −28, y = −4, z = −12; F(2,129) = 9.64; pcorrected = 0.009; and x = 26, y = −4, z = −12; F(2,129) = 7.43; pcorrected = 0.048). Further testing using directed t tests showed that both hydrocortisone administration conditions significantly reduced responses in the amygdala but did not significantly differ from each other. Thus, hydrocortisone administration in general reduced amygdala responsivity regardless of timing (Table 1, Fig. 2A).
Table 1.
Results for main effects of drug and drug × emotion interaction
| Region | MNI coordinates |
t value | ||
|---|---|---|---|---|
| x | y | z | ||
| Main effects of drug | ||||
| Slow CORT < placebo | ||||
| Amygdala, L | −22 | −8 | −12 | 3.85** |
| Amygdala, R | 26 | −4 | −12 | 3.82** |
| Rapid CORT < placebo | ||||
| Amygdala, L | −28 | −4 | −12 | 3.91** |
| Drug × emotion interaction | ||||
| Val(slow CORT) > Val(placebo) | ||||
| Amygdala, L | −26 | −4 | −12 | 3.69** |
| Amygdala, R | 24 | −2 | −12 | 3.24* |
| Val(slow CORT) > Val(rapid CORT) | ||||
| Amygdala, L | −26 | −4 | −12 | 3.31* |
Peak voxel and corresponding t values of significantly activated clusters. R, Right; L, left; Val, emotional valence contrast: fearful > happy.
*p < 0.05 small volume corrected for region of interest;
**p < 0.01 small volume corrected for region of interest.
Figure 2.
Hydrocortisone affected amygdala responsivity in a time- and emotion-specific manner. A, Main effect of hydrocortisone administration on activity in the amygdala (y = −4). Hydrocortisone administration reduced amygdala responsivity to faces in general, regardless of the timing of administration. B, Drug × emotion type interaction in the amygdala (y = −4). The effects of hydrocortisone administration depended on the emotion type. C, Extracted parameter estimates from the anatomically defined bilateral amygdala revealed that the drug × emotion type interaction was driven by a larger emotion effect (fearful > happy) in the slow hydrocortisone condition. Error bars represent SEM. For visualization purposes, both statistical parametric maps are thresholded at p < 0.005 uncorrected. For statistical tests, see Table 1.
Next, we assessed whether this corticosteroid modulation of brain activity was emotion specific, and tested for an interaction between drug condition and emotion type. This analysis revealed a significant interaction in the left amygdala (x = −26, y = −4, z = −12; F(2,129) = 8.17; pcorrected = 0.028) (Fig. 2B). Further testing showed that this interaction was caused by an emotion-specific response of the amygdala in the slow hydrocortisone condition only. Whereas corticosteroids rapidly reduced amygdala responsivity toward all emotional input, the slow corticosteroid effects enabled responses to emotionally negative information, while responses to positive stimuli remained reduced, resulting in emotion specificity of the amygdala response (Table 1, Fig. 2C).
Brain connectivity
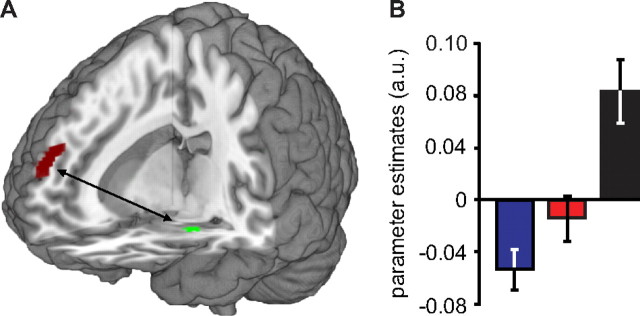
To investigate whether these corticosteroid effects on amygdala responsivity were related to altered amygdala coupling with brain regions involved in its regulation, we performed additional PPI analyses, seeding the drug by emotion type interaction in the amygdala. These analyses revealed that the slow effects of corticosteroids increased the coupling between the amygdala and the mPFC compared to the placebo condition (x = 10, y = 60, z = 24; t(64) = 4.38; pcorrected = 0.032) (Table 2, Fig. 3A). This effect was specific for the slow hydrocortisone condition, since it also differed from the rapid hydrocortisone condition (x = 12, y = 38, z = 48; t(64) = 4.26; pcorrected = 0.045) (Table 2, Fig. 3B), with the latter not being significantly different from placebo. Thus, the slow effects of corticosteroids induced both the emotion specificity in amygdala responses and altered its connectivity to the mPFC.
Table 2.
Results of PPI analysis seeding the amygdala
| Main effects of drug | MNI coordinates |
t value | ||
|---|---|---|---|---|
| x | y | z | ||
| Slow CORT > placebo | ||||
| Medial prefrontal cortex, R | 10 | 60 | 24 | 4.38* |
| Slow CORT > rapid CORT | ||||
| Medial prefrontal cortex, R | 12 | 38 | 48 | 4.26* |
Peak voxel and corresponding t values of significantly activated clusters in main effects of drug. R, Right.
*p < 0.05 small volume corrected for region of interest.
Figure 3.
The slow effects of corticosteroids strengthened connectivity between the amygdala and mPFC. A, PPI analyses showed that the slow effects of corticosteroids strengthened connectivity between the amygdala and a cluster within the mPFC. B, Analysis of the parameter estimates of the observed mPFC cluster showed that this altered connectivity was specific to the slow hydrocortisone condition, since it significantly differed from that observed in the rapid hydrocortisone condition (t(43) = 3.22; p = 0.002). Error bars represent SEM. For visualization purposes, the statistical parametric map is thresholded at p < 0.001 uncorrected. For other statistical tests, see Table 2.
To test whether these two effects were actually associated rather than independent, we extracted the parameter estimates of both the emotion effect in amygdala responsivity and the amount of amygdala–mPFC coupling, and tested whether these measures were correlated across participants. Even though the PPI analysis was corrected for amygdala activity fluctuations within each participant, this analysis showed that these measures were positively correlated across participants (r = 0.223). Although this correlation just failed to reach significance (p = 0.067), this suggests that stronger amygdala–mPFC coupling was related to stronger emotion-specific amygdala responses.
Discussion
In this study, we targeted the time-specific effects of corticosteroids on human amygdala functioning by administering hydrocortisone at two different time points before an emotional processing task during fMRI scanning. We found that corticosteroids downregulate amygdala responsivity to emotional stimuli in a time- and emotion-specific manner; whereas corticosteroids rapidly suppress amygdala responsivity toward all emotional stimuli, they only suppress responses to positive stimuli later on, while responses to negative emotional stimuli are normal again. This emotion-specific recovery of amygdala activity appears related to altered amygdala connectivity to the medial prefrontal cortex.
Previous work in animals has indicated that corticosteroids exert both rapid nongenomic and slow genomic effects that are functionally distinct (Joëls et al., 2006). At high concentrations, corticosteroids are shown to rapidly enhance hippocampal plasticity by binding to an MR thought to reside in the plasma membrane, leading to an increase in glutamate release (Karst et al., 2005). At the same time, a corticosteroid-induced genomic cascade is initiated by the binding of primarily intracellular GRs that upon binding translocate to the nucleus where they function as transcription factors to modulate the expression of >200 genes (Datson et al., 2001). These slow genomic effects of corticosteroids have been shown to inhibit hippocampal plasticity (Pavlides et al., 1995; Wiegert et al., 2005). Here, we dissociated these two effects experimentally by administering 10 mg of hydrocortisone at either 75 or 285 min before the emotional processing task. The timing of the rapid corticosteroid condition was based on (1) previous studies in rodents revealing a delay between elevations in corticosteroid level in plasma versus brain (Droste et al., 2008) and (2) the observation in humans that salivary cortisol levels peak at 1 h after intake (Abercrombie et al., 2003). Once in the brain, these nongenomic corticosteroid effects are rapid in onset and quickly reversible (Karst et al., 2005). The genomic effects of corticosteroids, on the other hand, generally do not start earlier than at least 3 h after exposure to high corticosteroid levels in vivo (Joëls et al., 2003; Morsink et al., 2006) and these effects last for hours (Joëls and de Kloet, 1992, 1994; Joëls et al., 2003). Thus, administration of hydrocortisone at 75 min before scanning probably caused sufficiently high levels of the hormone in the brain to evoke rapid nongenomic effects, although this delay was much too short to allow development of gene-mediated events. Conversely, when hydrocortisone was applied at 285 min before testing, hormone levels were so low during the behavioral task that nongenomic actions were not likely to happen, yet it allowed enough time for the gene-mediated actions to occur.
Here, we show that corticosteroids rapidly desensitize human amygdala responses to emotional stimuli. Corticosteroids may therefore be a crucial factor in terminating a critical feed-forward loop in the amygdala: acute stress sensitizes the amygdala, and the amygdala boosts vigilance/anxiety and drives in turn the stress response. This positive feed-forward loop constitutes a powerful mechanism leading to progressively augmented amygdala sensitization with repeated stress exposure. The fact that the HPA axis is dysregulated in stress-related mental disorders such as depression and PTSD, but also that corticosteroids seem to be effective in preventing (Schelling et al., 2006) and treating (Aerni et al., 2004; de Quervain, 2008) PTSD, may speak for their crucial role in interrupting this positive feed-forward loop.
The anxiolytic effects of corticosteroids observed in previous studies are in line with the proposed corticosteroid-induced desensitization and thus suppression of vigilance/anxiety. Behavioral studies in humans have shown that corticosteroids reduce the anxiety-driven selective attention to threat (Putman et al., 2007; van Peer et al., 2009), attenuate fear responses (Soravia et al., 2006), and protect mood during exposure to stressful situations (Het and Wolf, 2007). Here, we provide a mechanistic account for these observations by showing that the rapid, nongenomic corticosteroid effects unspecifically desensitize the amygdala. This claim is supported by a previous study showing a tonic suppression of the acoustic startle reflex in humans, thought to be modulated by the amygdala, which was independent of emotional modulation (Buchanan and Lovallo, 2001).
Two possible, but not mutually exclusive, molecular mechanisms could underlie this corticosteroid-associated reduction in amygdala activation. First of all, corticosteroids might modulate amygdala activity in a direct manner by binding to its MRs and GRs (Sapolsky et al., 1983; Reul and de Kloet, 1985). Corticosteroids have been shown to act in such direct manner in the hippocampus, where they rapidly increase neuronal excitability in a nongenomic fashion by binding to a low-affinity membrane MR (Karst et al., 2005; Olijslagers et al., 2008) and slowly impair hippocampal function by binding intracellular MRs and GRs. Corticosteroids could affect amygdala function in a similar manner, but supporting evidence for this idea is so far scarce (Karst et al., 2002; Duvarci and Pare, 2007; Pu et al., 2009). Alternatively, the corticosteroid effects might be mediated by a reduction in brain levels of corticotrophin-releasing hormone (CRH). CRH is a coordinator of the central stress response, and is known to induce anxious behavior by activating the human amygdala both directly (Liang and Lee, 1988) and indirectly by increasing locus ceruleus norepinephrine signaling (Valentino et al., 1983; Valentino and Foote, 1988). Since CRH levels are known to be inhibited by the negative feedback actions of corticosteroids on the hypothalamus (Keller-Wood and Dallman, 1984; Herman et al., 1996; Tasker, 2006; Aguilera et al., 2007), corticosteroid-induced reductions in circulating CRH levels could also explain our findings. Thus, corticosteroids rapidly inhibit amygdala activity either by direct modulation or by reducing circulating CRH levels, and thereby protect the amygdala during stress from potential overshoot by the sensitizing actions of NE and CRH.
One might also argue that an altered mood state during scanning could underlie the observed changes in amygdala response. We cannot exclude this possibility since we did not assess mood just before or during scanning. However, mood state was assessed three times during the study and appeared not to be affected by either a history of elevation in cortisol levels (mood measure 2 and 3 for the slow CORT group) (Fig. 1) or an acutely elevated level (mood measure 3 rapid CORT group) (Fig. 1). Therefore, we consider it unlikely that mood during scanning was different between groups.
The slow effects of corticosteroids, on the other hand, normalized responses to negative input, whereas responses to positive input remained suppressed. Moreover, the induction of this emotion specificity in the amygdala seemed to be related to increased coupling with the mPFC. The mPFC is known to play a significant role in emotion regulation (Ochsner and Gross, 2005), and suppresses the amygdala during the regulation of emotional responses to negative stimuli (Beauregard et al., 2001; Kompus et al., 2009). Further, the mPFC is known to play a critical role in the control over the HPA axis. The mPFC expresses high levels of glucocorticoid receptors (Diorio et al., 1993; Sánchez et al., 2000) and is a prominent target for the negative feedback control over the HPA axis (Sullivan and Gratton, 2002; Radley et al., 2009). Activation of the mPFC has been shown to reduce stress-induced salivary cortisol increases, but also amygdala activity and dispositional mood state (Kern et al., 2008). Here, we show that the connectivity between the amygdala and mPFC is strengthened by the slow, putatively genomic, actions of corticosteroids. Moreover, this strengthening seemed to enhance the preferential processing of negative over positive emotional stimuli. This suggests that the slow actions of corticosteroids ensure recovery of the rapid effects of corticosteroids on amygdala responses to negative input specifically by changing regulatory actions of the mPFC. This could entail a highly adaptive mechanism for survival, since it is most important to be capable to respond adequately to dangerous stimuli first.
Here we reveal an adaptive mechanism of time-dependent amygdala modulation by corticosteroids; rapid nongenomic effects of corticosteroids suppress overall amygdala activity in an unspecific manner, whereas slow genomic actions of corticosteroids upregulate (i.e., normalize) responses to negative input specifically by altered prefrontal control. In response to stress, corticosteroids thereby rapidly guard amygdala activation from potential overshoot by the sensitizing actions of NE and CRH, and normalize amygdala response later on, prioritizing negative emotional processing. Thus, corticosteroids control amygdala responsivity and vigilance/anxiety, and appear therefore as a crucial factor when the stress response has to be terminated adequately in the aftermath of traumatic experiences.
Footnotes
This work was supported by a grant (021.002.053) from The Netherlands Organization for Scientific Research.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Kiss A, Liu Y, Kamitakahara A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress. 2007;10:153–161. doi: 10.1080/10253890701391192. [DOI] [PubMed] [Google Scholar]
- Andreatini R, Leite JR. The effect of corticosterone in rats submitted to the elevated plus-maze and to to pentylenetetrazol-induced convulsions. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1333–1347. doi: 10.1016/0278-5846(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown GK. Handleiding. De Nederlandse versie van de Beck Depression Inventory. Ed 2. Lisse, The Netherlands: Swets Test Publishers; 2002. Beck Depression Inventory-II-NL. [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents' cortisol responses to awakening and social stress; Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Odessa, FL: Psychological Assessment Resources; 1992. Revised NEO Personality Inventory (NEO-PI-R) and the Five Factor Inventory (NEO-FFI): professional manual. [Google Scholar]
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- de Groot MH. Psychometrische aspecten van een stemmingsschaal (Verkorte POMS) Gedrag en Gezondheid. 1992;20:46–51. [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Paré D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen V. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Publishing; 1976. [Google Scholar]
- File SE, Vellucci SV, Wendlandt S. Corticosterone—an anxiogenic or an anxiolytic agent? J Pharm Pharmacol. 1979;31:300–305. doi: 10.1111/j.2042-7158.1979.tb13505.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gröschl M, Rauh M, Dörr HG. Cortisol and 17-hydroxyprogesterone kinetics in saliva after oral administration of hydrocortisone in children and young adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2002;87:1200–1204. doi: 10.1210/jcem.87.3.8297. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Het S, Wolf OT. Mood changes in response to psychosocial stress in healthy young women: effects of pretreatment with cortisol. Behav Neurosci. 2007;121:11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Joëls M, Velzing E, Nair S, Verkuyl JM, Karst H. Acute stress increases calcium current amplitude in rat hippocampus: temporal changes in physiology and gene expression. Eur J Neurosci. 2003;18:1315–1324. doi: 10.1046/j.1460-9568.2003.02845.x. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Schupp HT, Stark R, Vaitl D. Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. Neuroimage. 2005;25:520–526. doi: 10.1016/j.neuroimage.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, Joëls M. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett. 2009;467:76–80. doi: 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Liang KC, Lee EH. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology (Berl) 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. The perfect time to be stressed: a differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1281–1288. doi: 10.1016/j.pnpbp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Mason J, Southwick SM, Fox P, Hazlett G, Charney DS, Greenfield G. Hormone profiles in humans experiencing military survival training. Biol Psychiatry. 2000;47:891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joëls M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joëls M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Kimura A, Magariños AM, McEwen BS. Hippocampal homosynaptic long-term depression/depotentiation induced by adrenal steroids. Neuroscience. 1995;68:379–385. doi: 10.1016/0306-4522(95)94332-s. [DOI] [PubMed] [Google Scholar]
- Peeters RR, Van der Linden A. A data post-processing protocol for dynamic MRI data to discriminate brain activity from global physiological effects. Magn Reson Imaging. 2002;20:503–510. doi: 10.1016/s0730-725x(02)00513-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pu Z, Krugers HJ, Joëls M. Beta-adrenergic facilitation of synaptic plasticity in the rat basolateral amygdala in vitro is gradually reversed by corticosterone. Learn Mem. 2009;16:155–160. doi: 10.1101/lm.1272409. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans EJ, Koppeschaar H, van Schijndel A, van Honk J. A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology. 2007;32:793–802. doi: 10.1016/j.psyneuen.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddon JR, Marceau R, Holden RR. A confirmatory evaluation of the Profile of Mood States: convergent and discriminant item validity. J Psychopathol Behav Assess. 1985;7:243–259. [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res. 1983;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Brain Res Cogn Brain Res. 2004;20:81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DE Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöcker T, Kellermann T, Schneider F, Habel U, Amunts K, Pieperhoff P, Zilles K, Shah NJ. Dependence of amygdala activation on echo time: results from olfactory fMRI experiments. Neuroimage. 2006;30:151–159. doi: 10.1016/j.neuroimage.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring) 2006;14(Suppl 5):259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- Tops M, van der Pompe G, Baas D, Mulder LJ, Den Boer JA, Meijman TF, Korf J. Acute cortisol effects on immediate free recall and recognition of nouns depend on stimulus valence. Psychophysiology. 2003;40:167–173. doi: 10.1111/1469-8986.00018. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- van der Ploeg HM. Zelf-Beoordelings Vragenlijst. Handleiding: Addendum 1981. Lisse, The Netherlands: Swets and Zeitlinger; 1981. [Google Scholar]
- van der Ploeg HM, Defares PB, Spielberger CD. Lisse, The Netherlands: Swets and Zeitlinger; 1980. Handleiding bij de Zelf-Beoordelings Vragenlijst, ZBV: Een Nederlandse vertaling van de Spielberger State-Trait Anxiety Inventory. [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- van Peer JM, Spinhoven P, van Dijk JG, Roelofs K. Cortisol-induced enhancement of emotional face processing in social phobia depends on symptom severity and motivational context. Biol Psychol. 2009;81:123–130. doi: 10.1016/j.biopsycho.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wald FD, Mellenbergh GJ. De verkorte versie van de Nederlandse vertaling van de Profile of Mood States (POMS) Ned Tijdschr Psychol. 1990;45:86–90. [Google Scholar]
- Wiegert O, Pu Z, Shor S, Joëls M, Krugers H. Glucocorticoid receptor activation selectively hampers N-methyl-D-aspartate receptor dependent hippocampal synaptic plasticity in vitro. Neuroscience. 2005;135:403–411. doi: 10.1016/j.neuroscience.2005.05.039. [DOI] [PubMed] [Google Scholar]