ABSTRACT
Type I and type II classical cadherins constitute a family of cell adhesion molecules expressed in complex combinatorial profiles in the nervous system, suggesting that a cadherin code implements specific adhesive recognition events that control the development of neural circuits. In the spinal cord, classical cadherins define at a molecular level the positional organization of motor neuron subtypes into discrete nuclear structures termed motor pools. However, the roles and contributions of different members of the family in defining motor neuron spatial organization are not yet clear. By combining mouse genetics with quantitative positional analysis, we found that motor neuron organization into pools depends on type II cadherins. Type II cadherin function, however, does not strictly reflect the predictions arising from binding specificities at a molecular level, but instead relies on N-cadherin, a type I cadherin whose elimination is required to reveal type II contributions.
KEY WORDS: Classical cadherins, Motor neuron, Adhesion, Migration, Neuronal development
Summary: In mouse, type II cadherins control the organization of motor neurons into pools by functional interaction with N-cadherin rather than via the implementation of an adhesive code.
INTRODUCTION
The precision with which neurons are connected during development underpins the function of the nervous system. Several processes, such as neurogenesis, migration, dendritic elaboration, axon guidance and synapse formation, have to be coordinated to ensure the wiring of neural circuits. The mechanisms controlling these events often rely on expression of molecules promoting specific recognition, and the cadherin superfamily of cell adhesion molecules has been implicated in most of these processes (Takeichi, 2007; Jontes, 2018). In particular, type II classical cadherins are expressed in the central nervous system in complex patterns often delineating discrete structures and circuits, suggesting important roles for neuronal organization and connectivity (Hirano and Takeichi, 2012).
The characteristic of type II cadherins expression to define anatomical features of the nervous system is evident in the spinal cord, where motor neurons are organized into nuclear structures, termed motor pools, which are at the basis of muscolotopic organization of motor maps and the wiring of spinal circuits (Romanes, 1964; Sürmeli et al., 2011; Hinckley et al., 2015). Combinatorial expression of type II cadherins defines pools at a molecular level and manipulation of classical cadherin signaling perturbs segregation and clustering of motor neurons (Fig. S1A; Price et al., 2002; Demireva et al., 2011; Bello et al., 2012; Astick et al., 2014; Montague et al., 2017). The precise organization of motor pools in the lateral motor column (LMC) of the spinal cord is achieved by a two-step process; first, inside-out migration separates early- and late-born neurons in a two-layered structure on the mediolateral axis, the medial and lateral divisions (Palmesino et al., 2010; Bello et al., 2012). A second, independent process, organizes pools within divisions on the dorsoventral axis (Dewitz et al., 2018). Genetic analysis indicates that both processes are dependent on β- and γ-catenin adhesive function, but elimination of N-cadherin, a type I cadherin expressed by all motor neurons, selectively impairs the first phase without affecting relative organization of pools within divisions on the dorsoventral axis (Dewitz et al., 2018). The effectors controlling the second phase are unknown, and type II cadherins are obvious candidates because of their expression profiles and dependency on catenin activity (Nelson, 2008). However, elimination of individual type II cadherins does not perturb any aspect of motor neuron positioning (Demireva et al., 2011).
What controls the second phase of motor pool organization? What are the roles of type II cadherins? In order to address these questions, we combined mouse genetics and quantitative positional analysis to investigate classical cadherin contributions. We found that type II cadherins control dorsoventral organization of pools. Surprisingly, our data indicate that their function does not rely on the establishment of an adhesive code, but instead is only revealed in the absence of N-cadherin, thus suggesting that cross-talk between type I and type II cadherins is a crucial element orchestrating motor neuron positioning.
RESULTS AND DISCUSSION
Inactivation of a specificity group does not affect divisional organization
Recent studies indicate that functional redundancy in subsets of type II cadherins is at the basis of adhesive recognition properties in the nervous system (Duan et al., 2014, 2018; Basu et al., 2017). At a molecular level, type II cadherins can be divided into three specificity groups according to their adhesive preferences (Brasch et al., 2018). Cadherin (cad)-8 and cad-11; cad-6, cad-9 and cad-10; cad-7, cad-12, cad-18, cad-20 and cad-22 represent three groups displaying a clear preference for heterophilic interactions according to their binding affinities. Functional compensation between members of a group could explain the lack of positioning phenotypes in single type II cadherin mutants. To test this hypothesis we eliminated all members of a specificity group by crossing cad-8 and cad-11 single mutant mice (8/11−/−) (Horikawa et al., 1999; Suzuki et al., 2007).
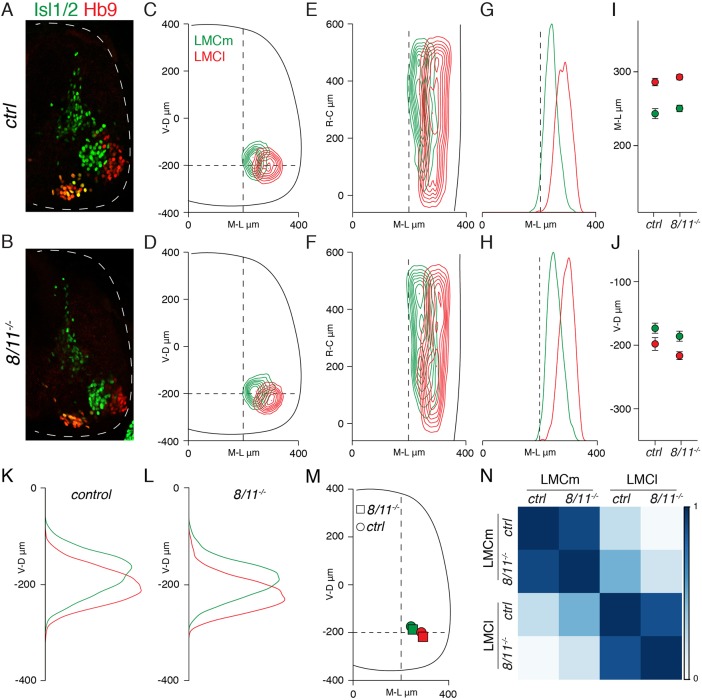
8/11−/− mice are born at expected frequency and do not present any obvious phenotype. Analysis of motor neuron generation and subtype identities did not reveal any difference between 8/11−/− and control mice (Fig. S1G). Next, we studied divisional organization; transverse and longitudinal contour plots did not show any defect in positioning of medial (LMCm) and lateral (LMCl ) LMC neurons (Fig. 1A-F). In addition, we did not observe changes either in motor neuron distribution or average position, both on the mediolateral and dorsoventral axis (Fig. 1G–M). Accordingly, we found that the positions of motor neurons sharing the same identity were highly correlated between control and 8/11−/− embryos (Fig. 1N). Thus, joint elimination of cad-8 and cad-11 does not affect divisional organization.
Fig. 1.
Elimination of cad-8, cad-11 does not perturb divisional organization. (A,B) Organization of Isl1/2+ LMCm (green) and Hb9+ LMCl (red) neurons at lumbar levels in E13.5 control (A) and 8/11−/− (B) embryos. Dashed line delimits spinal cord area. (C,D) Transverse contour plots of LMCm (green) and LMCl (red) neurons in control (C) and 8/11−/− (D) embryos. (E,F) Coronal contour plots of LMCm (green) and LMCl (red) neurons in control (E) and 8/11−/− (F) embryos. (G,H) Mediolateral density plots of LMCm (green) and LMCl (red) neurons in control (G) and 8/11−/− (H) embryos. (I) Mean±s.d. mediolateral position of LMCm (green) and LMCl (red) neurons in control and 8/11−/− embryos. Differences not significant; control vs 8/11−/− LMCm P=0.055; control vs 8/11−/− LMCl P=0.132; unpaired t-test. (J) Mean±s.d. dorsoventral position of LMCm (green) and LMCl (red) neurons in control and 8/11−/− embryos. Differences not significant; control vs 8/11−/− LMCm P=0.055; control vs 8/11−/− LMCl P=0.052; unpaired t-test. (K,L) Dorsoventral density plots of LMCm (green) and LMCl (red) neurons in control (K) and 8/11−/− (L) embryos. (M) Mean mediolateral and dorsoventral positions of LMCm (green) and LMCl (red) neurons in control and 8/11−/− embryos. (N) Correlation analysis of LMC neuron coordinates in control and 8/11−/− embryos. Control LMCm vs 8/11−/− LMCm r>0.8; control LMCl vs 8/11−/− LMCl r>0.8. Scale bar indicates correlation values (r). V-D, ventral to dorsal neuron distribution (μm); R-C, rostral to caudal neuron distribution (μm); M-L, medial to lateral neuron distribution (μm).
8/11 and 6/9/10 specificity groups are not required for pools organization
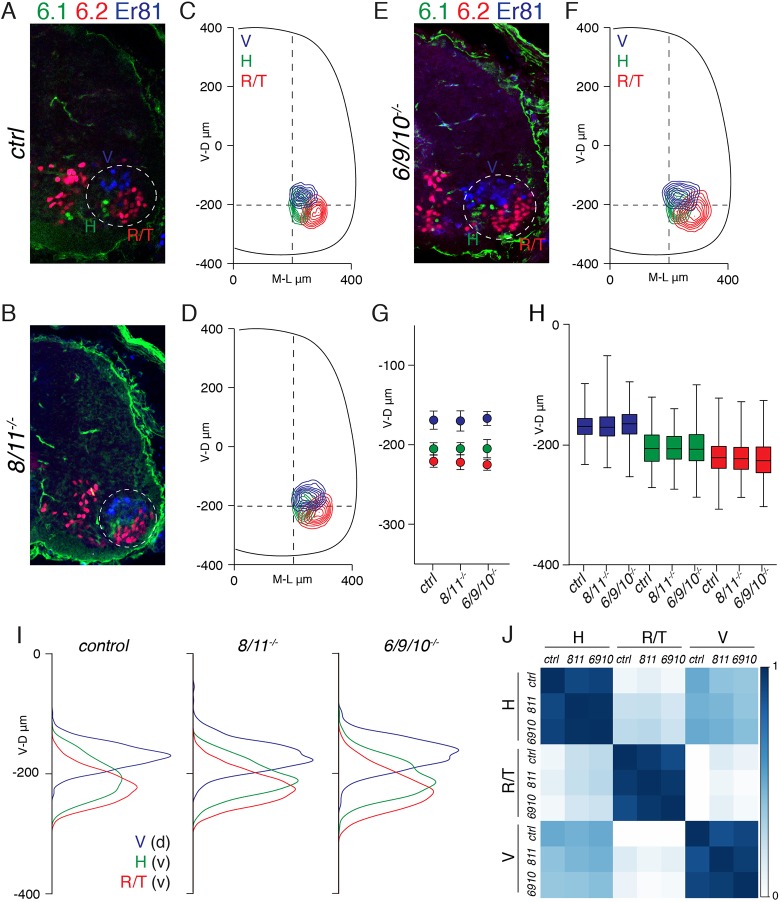
It has been previously shown that motor neuron positioning is achieved following a two-step process. First, N-cadherin–catenin adhesive function, with contributions from reelin and afadin signaling, controls mediolateral segregation of motor neuron divisions (Palmesino et al., 2010; Dewitz et al., 2018). In a second independent phase, whose effectors are unknown, motor neurons are organized into pools on the dorsoventral axis (Dewitz et al., 2018). We asked whether joint elimination of cad-8 and cad-11 could have a selective role in dorsoventral pool segregation and studied the positioning of dorsal (vasti, V, Er81+) and ventral (hamstring, H, Nkx6.1+; rectus femoris/tensor fasciae latae, R/T, Nkx6.2+) motor neurons. Transverse contour analysis indicated that dorsoventral organization was not affected in 8/11−/− embryos (Fig. 2A–D). Density, distribution and average position analyses confirmed that segregation of pools was preserved (Fig. 2G–I). In addition, consistent with the divisional data, we did not observe changes in pool positioning on the mediolateral axis (Fig. S1B–E).
Fig. 2.
Specificity groups cad-8, cad-11 and cad-6, cad-9, cad-10 are dispensable for pool organization. (A,B) Organization of hamstring (H, Nkx6.1+), rectus femoris/tensor fasciae latae (R/T, Nkx6.2+) and vasti (V, Er81+) motor neurons in E13.5 control (A) and 8/11−/− (B) embryos. Dashed line delimits motor neuron area. (C,D) Contour density plots of H (green), R/T (red), and V (blue) neurons in control (C) and 8/11−/− (D) embryos. (E) Organization of H (Nkx6.1+), R/T (Nkx6.2+), and V (Er81+) neurons in E13.5 6/9/10−/− embryos. Dashed line delimits motor neuron area. (F) Contour density plot of H (green), R/T (red), and V (blue) neurons in 6/9/10−/− embryos. (G) Mean±s.d. dorsoventral position of H (green), R/T (red), and V (blue) neurons in control, 8/11−/− and 6/9/10−/− embryos. Differences not significant for all comparisons between genotypes. V: control vs 8/11−/− P=0.762; control vs 6/9/10−/− P=0.966; 8/11−/− vs 6/9/10−/− P=0.637. H: control vs 8/11−/− P=0.947; control vs 6/9/10−/− P=0.999; 8/11−/− vs 6/9/10−/− P=0.942. R/T: control vs 8/11−/− P=0.733; control vs 6/9/10−/− P=0.769; 8/11−/− vs 6/9/10−/− P=0.990; one-way ANOVA and post-hoc Tukey's HSD test. (H) Boxplots of dorsoventral distributions of H (green), R/T (red), and V (blue) neurons in control, 8/11−/− and 6/9/10−/− embryos. The box represents the 25-75th percentiles, and the median is indicated. The whiskers show 0-100th percentiles. (I) Dorsoventral density plots of H (green, ventral), R/T (red, ventral), and V (blue, dorsal) neurons in control, 8/11−/− and 6/9/10−/− embryos. (J) Correlation analysis of H, R/T and V neuron coordinates in control, 8/11−/− and 6/9/10−/− embryos (for all three pool comparisons r>0.8). Scale bar indicates correlation values (r). V-D, ventral to dorsal neuron distribution (μm); M-L, medial to lateral neuron distribution (μm).
Functional redundancy in cad-6, cad-9 and cad-10 has important functions in controlling neuronal interactions in the hippocampus and the retina (Basu et al., 2017; Duan et al., 2018). In order to expand our observation, we investigated the role of this group on motor neuron organization. Triple cad-6, cad-9, cad-10 mutant mice (6/9/10−/−) are viable and fertile (Duan et al., 2018). We found that motor neurons were present at expected numbers with appropriate identities (Fig. S1G). Contour, average position, distribution and density analyses showed that organization of motor pools was similar in control, 8/11−/− and 6/9/10−/− embryos (Fig. 2E–I; Fig. S1D–F). As a consequence, coordinates of H, R/T and V neurons were highly correlated (Fig. 2J). These experiments show that elimination of two different specificity groups does not affect motor neuron organization.
Joint elimination of type II cadherins with N-cadherin reveals type II contributions
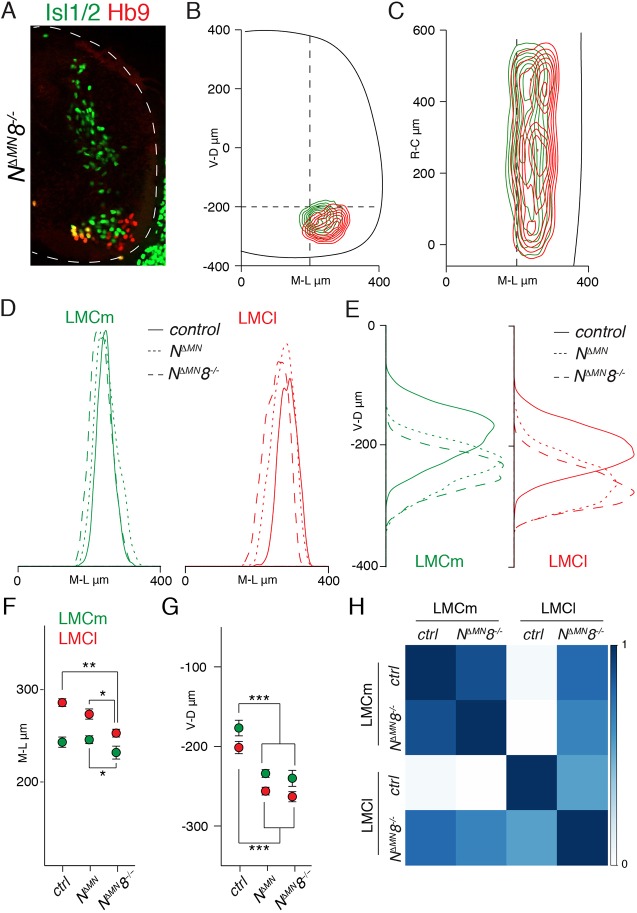
Since any attempt to disrupt motor neuron positioning by eliminating type II cadherin function in mice has failed, we reasoned that another factor might be involved in controlling their activities. According to previous results, such a factor should be ubiquitously expressed in motor neurons and capable of interacting with catenin signaling. N-cadherin, a type I cadherin, fulfills these requirements and its conditional elimination from motor neurons has been shown to cause defects in divisional segregation without impairing dorsoventral organization of pools (Dewitz et al., 2018). Thus, we combined elimination of cadherin-8 with N-cadherin conditional deletion in motor neurons (NΔMN8−/−). NΔMN8−/− mutants are lethal at late embryonic stages. Generation of motor neurons was not affected in NΔMN8−/− embryos (Fig. S1G). We observed a migration defect resulting in post-mitotic motor neurons found in the progenitor area (Fig. S2B). The same phenotype, at lower penetrance, was observed in NΔMN embryos (Fig. S2A-D). Next, we examined the radial glia scaffold at embryonic day (E)13.5 in NΔMN8−/− embryos and did not detect any obvious defect aside from local disruption at the progenitor zone (Fig. S2E,G). However, we cannot exclude the possibility that perturbations at earlier time points may affect motor neuron migration. Since in NΔMN8−/− embryos most LMC neurons migrate out to the ventral horn, we next assessed divisional organization and observed extensive overlap in the positioning of LMCm and LMCl neurons (Fig. 3A-C). Distribution, average position and correlation analyses indicated that the defect is due to impaired mediolateral migration of LMC neurons, with LMCl neurons settling in LMCm-like position (Fig. 3D,F,H). In addition, a ventralization in columnar location was observed (Fig. 3E,G). These phenotypes are reminiscent of the ones described for N-cadherin mutants; however, distribution and average position analyses revealed that the LMC neuron mediolateral migration phenotype in NΔMN8−/− embryos was significantly more severe than in NΔMN embryos, while the dorsoventral positioning phenotype was indistinguishable (Fig. 3D-G) (Dewitz et al., 2018). Thus, these data show that cadherin-8 contributes to mediolateral divisional organization and that elimination of N-cadherin is required to reveal this involvement.
Fig. 3.
Joint elimination of N-cadherin and cadherin-8 perturbs divisional organization. (A) Organization of Isl1/2+ LMCm and Hb9+ LMCl neurons at lumbar levels in E13.5 NΔMN8−/− embryos. Dashed line delimits spinal cord area. (B) Transverse contour plots of LMCm (green) and LMCl (red) neurons in NΔMN8−/− embryos. (C) Coronal contour plots of LMCm (green) and LMCl (red) neurons in NΔMN8−/− embryos. (D) Mediolateral density plots of LMCm (green) and LMCl (red) neurons in control, NΔMN and NΔMN8−/− embryos. (E) Dorsoventral density plots of LMCm (green) and LMCl (red) neurons in control, NΔMN and NΔMN8−/− embryos. (F) Mean±s.d. mediolateral position of LMCm (green) and LMCl (red) neurons in control, NΔMN and NΔMN8−/− embryos. LMCm neurons: control vs NΔMN P=0.258; control vs NΔMN8−/− P=0.091; NΔMN vs NΔMN8−/− *P<0.05. LMCl neurons: control vs NΔMN P=0.156; control vs NΔMN8−/− **P<0.01; NΔMN vs NΔMN8−/− *P<0.05; one-way ANOVA and post hoc Tukey's HSD test. (G) Mean±s.d. dorsoventral position of LMCm (green) and LMCl (red) neurons in control, NΔMN and NΔMN8−/− embryos. LMCm neurons: control vs NΔMN and NΔMN8−/− ***P<0.001; NΔMN vs NΔMN8−/− P=0.254. LMCl neurons: control vs NΔMN and NΔMN8−/− ***P<0.001; NΔMN vs NΔMN8−/− P=0.332; one-way ANOVA and post hoc Tukey's HSD test. (H) Correlation analysis of mediolateral LMC neurons coordinates in control and NΔMN8−/− embryos. Scale bar indicates correlation values (r). V-D, ventral to dorsal neuron distribution (μm); R-C, rostral to caudal neuron distribution (μm); M-L, medial to lateral neuron distribution (μm).
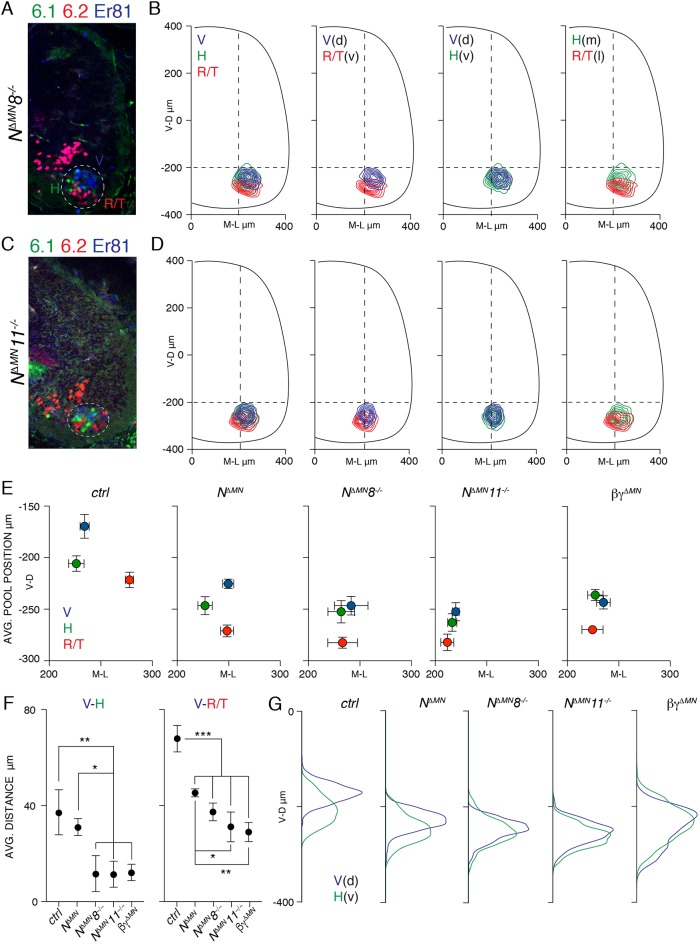
Next, we studied motor pool organization after joint elimination of N-cadherin and type II cadherins. In addition to NΔMN8−/− embryos, we analyzed a double N-cadherin/cad-11 mutant (NΔMN11−/−). Motor neuron generation was not affected in NΔMN11−/− embryos and, as in NΔMN8−/− embryos, a fraction of post-mitotic motor neurons failed to leave the progenitor zone (Fig. S1G, Fig. S2C-D). In both NΔMN8−/− and NΔMN11−/− embryos we observed extensive overlap in motor neuron distribution (Fig. 4A-D). Positional analysis confirmed the positioning defect observed at divisional level, with H and R/T neurons found in similar mediolateral space (Fig. S3C-E). Next, we assessed relative pool positioning and observed that joint inactivation of N-cadherin and a type II cadherin resulted in a spatial reorganization of pools resembling the one found after elimination of β- and γ-catenin (βγΔMN; Fig. 4E). The distance between dorsal and ventral pools was dramatically reduced compared to control and almost undistinguishable from βγΔMN embryos (Fig. 4F). Accordingly, the distribution of motor neurons on the dorsoventral axis in NΔMN8−/− and NΔMN11−/− embryos was mostly overlapping as in βγΔMN (Fig. 4G; Fig. S3A-B). In contrast, dorsal and ventral motor pools were mostly separated in NΔMN embryos, as observed in control embryos (Fig. 4E-G; Fig. S3A-B,F). Taken together, these data indicate that type II cadherins coordinate motor neuron position, initially by contributing to inside-out migration, leading to divisional organization, and later as central effectors of dorsoventral segregation of motor pools within divisions.
Fig. 4.
Joint elimination of N-cadherin and type II cadherins disrupts motor pool dorsoventral organization. (A) Organization of H (Nkx6.1+), R/T (Nkx6.2+) and V (Er81+) neurons in E13.5 NΔMN8−/− embryos. Dashed line delimits motor neuron area. (B) Transverse contour plots of H (green, ventral/medial), R/T (red, ventral/lateral), and V (blue, dorsal) neurons in NΔMN8−/− embryos. (C) Organization of H (Nkx6.1+), R/T (Nkx6.2+) and V (Er81+) neurons in E13.5 NΔMN11−/− embryos. Dashed line delimits motor neuron area. (D) Transverse contour plots of H (green, ventral/medial), R/T (red, ventral/lateral), and V (blue, dorsal) neurons in NΔMN11−/− embryos. (E) Mean±s.d. mediolateral and dorsoventral positions of H (green), R/T (red), and V (blue) neurons in ventral horn of control, NΔMN, NΔMN8−/−, NΔMN11−/− and βγΔMN embryos. (F) Mean±s.d. distance between V and H (V–H), and between V and R/T (V–R/T) neurons in control, NΔMN, NΔMN8−/−, NΔMN11−/− and βγΔMN embryos. V–H: control vs NΔMN P=0.747; control vs NΔMN8−/−, NΔMN11−/− and βγΔMN **P<0.01; NΔMN vs NΔMN8−/−, NΔMN11−/− and βγΔMN *P<0.05; NΔMN8−/− vs NΔMN11−/− P>0.999; NΔMN8−/− vs βγΔMN P>0.999; NΔMN11−/− vs βγΔMN P=0.999. For V–R/T: control vs NΔMN, NΔMN8−/−, NΔMN11−/−, βγΔMN ***P<0.001; NΔMN vs NΔMN8−/− P=0.294; NΔMN vs NΔMN11−/− *P<0.05; NΔMN vs βγΔMN **P<0.01; NΔMN8−/− vs NΔMN11−/− P=0.449; NΔMN8−/− vs βγΔMN P=0.246; NΔMN11−/− vs βγΔMN P=0.969; one-way ANOVA and post hoc Tukey's HSD test. (G) Dorsoventral density plots of H (green, ventral) and V (blue, dorsal) neurons in control, NΔMN, NΔMN8−/−, NΔMN11−/− and βγΔMN embryos. V-D, ventral to dorsal neuron distribution (μm); M-L, medial to lateral neuron distribution (μm).
The importance of classical cadherins for the organization of motor pools has been evident since the first studies in chick and mouse embryos (Price et al., 2002; Demireva et al., 2011). Nevertheless, attempts to define the roles of type II cadherins have failed. There is evidence indicating that classical cadherins may work synergistically in defining adhesive recognition. Binding affinity analysis shows that type II cadherins can be divided into three specificity groups according to their binding preferences, where molecules belonging to the same group bind to each other in a heterophilic manner, but discriminate molecules belonging to different groups (Brasch et al., 2018). Genetic experiments confirmed that redundant function of cad-6, cad-9, cad-10 is at the basis of specific neuronal interactions in the retina and hippocampus (Basu et al., 2017; Duan et al., 2018). These data indicate that elimination of multiple members of a group is necessary to reveal their contributions and support a model based on an adhesive code, where redundancy and combinatorial expression is used to generate developmental programs that are both specific and robust (Jontes, 2018). Surprisingly, our findings show that elimination of all the members of two out of three specificity groups has no effect on motor neuron spatial organization. A role for the remaining group cannot be excluded; however, motor pool organization does not seem to follow a molecular logic based on a redundant adhesive code generated by combinatorial expression of type II cadherins.
Instead, our data show that the contributions of type II cadherins become evident only in the absence of N-cadherin. Almost identical phenotypes are observed by inactivation of N-cadherin with either cadherin-8 or cadherin-11, which did not show any defect when eliminated individually or in conjunction. Thus, cross-talk between type II cadherins and N-cadherin appears to be a central feature for the control of motor neuron pool organization. The molecular and cellular bases for the emerging functions observed after concomitant elimination of N-cadherin and type II cadherins remain unclear. Structural and biophysical studies indicate that binding between type I and type II cadherins is prohibited and there is no evidence of such functional interaction controlling cell adhesive recognition (Patel et al., 2006; Brasch et al., 2012). It has been suggested that differences in adhesive strength may be sufficient to implement developmental programs generating cellular patterns (Steinberg, 2007; Hassan and Hiesinger, 2015). In principle, varying cadherin expression profiles in motor neurons could promote differential adhesive properties to segregate pools. Indeed, it has been shown that changing levels of cadherin expression between otherwise identical cell populations is sufficient to promote segregation (Foty and Steinberg, 2013). Thus, N-cadherin may serve to maintain a basal adhesive level among motor neurons necessary for type II cadherins to modulate relative adhesive strength in different pools. In the future, defining cell surface levels, as well as temporal dynamics, of classical cadherin expression during development will be crucial to understanding the principles controlling the spatial organization of motor neurons.
MATERIALS AND METHODS
Mouse genetics
All experimental procedures were performed according to the policies of the Max Delbrück Center for Molecular Medicine and approved by the Landesamt für Gesundheit und Soziales. β-catfl (Brault et al., 2001), γ-catfl (Demireva et al., 2011), N-cadherinfl (Kostetskii et al., 2005), γ-cat−/− (Ruiz et al., 1996), cad 6−/−; cad 9−/−; cad 10−/− (Duan et al., 2018), cad-8−/− (Suzuki et al., 2007), cad-11−/− (Horikawa et al., 1999) and olig2::Cre (Dessaud et al., 2007) mouse lines have been previously described.
Immunohistochemistry
Embryonic spinal cords were fixed with 4% paraformaldehyde on ice for 90 min, cryo-protected by equilibration with 30% sucrose overnight at 4°C, frozen in OCT (Tissue-Tek) and sectioned at 16 μm using a Leica cryostat. Immunohistochemistry was performed as previously described (Demireva et al., 2011) and images acquired on a Zeiss LSM 800 confocal microscope. Antibodies used were previously described in Dasen et al. (2008); De Marco Garcia and Jessell (2008); Agalliu et al. (2009).
Motor neuron subtype identification
Motor neuron divisional subtypes were identified by the expression of homeobox transcription factors Isl1/2 (LMCm) and Hb9 (LMCl) (Sockanathan and Jessell, 1998). Motor pools occupying different mediolateral and dorsoventral positions at lumbar spinal levels were identified by expression of homeobox and ETS transcription factors (De Marco Garcia and Jessell, 2008). The rectus femoris/tensor fasciae latae (R/T) complex was identified by expression of Nkx6.2, the hamstrings (H) complex was identified by expression of Nkx6.1 and the vasti (V) motor pool was identified by expression of Er81.
Three-dimensional analysis of motor neuron subtype positioning
Motor neuron positions were acquired using three-dimensional analysis as previously described (Dewitz et al., 2018). Briefly, motor neuron cell bodies were assigned Cartesian coordinates (x and y) using the imaging software Imaris (Bitplane) and expressed relative to the midpoint of the spinal cord midline (x,y=0,0). Coordinates were normalized to the size of a standardized spinal cord calculated by measuring mean spinal cord size of mouse embryos at E13.5 (distance from the midline to the lateral edge=365 μm; distance from the midpoint of the midline to the ventral edge=340 μm). The z-coordinates were obtained by tracking the order of histological sections. Datasets were aligned on the z-axis using the appearance of Isl1/2+ motor neurons (for divisional analysis) or Nkx6.1+ motor neurons (for pool analysis) as a starting point and analysis was performed for 512 μm covering approximately the rostral half of the lumbar spinal cord. We analyzed at least n=3 embryos for each genotype. See Table S1 for genotypes, number of embryos and number of sections analyzed per embryo.
Statistical analysis
Positional datasets were analyzed using custom scripts in R project (https://www.r-project.org) as previously described (Dewitz et al., 2018).
Supplementary Material
Acknowledgements
We thank Liana Kosizki and Isabelle Werner for technical help and the Advanced Light Microscope facility at the Max Delbrück Center for assistance with image acquisition and analysis. We are grateful to Peter Robin Hiesinger for invaluable insights and discussions. Stephan Dietrich, Sofia Pimpinella and Sophie Skarlatou provided comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.D., N.Z.; Methodology: C.D., N.Z.; Formal analysis: C.D., N.Z.; Investigation: C.D.; Resources: X.D.; Writing - original draft: C.D., N.Z.; Writing - review & editing: C.D., N.Z.; Supervision: N.Z.; Funding acquisition: N.Z.
Funding
X.D. was supported by the National Institutes of Health (R01 EY030138), a Research to Prevent Blindness CDA Award, a Klingenstein-Simons Neuroscience Fellowship and a Whitehall Foundation grant. C.D. and N.Z. were generously supported by the Deutsche Forschungsgemeinschaft (ZA 885/1-1 and EXC 257 NeuroCure). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.180422.supplemental
References
- Agalliu D., Takada S., Agalliu I., McMahon A. P. and Jessell T. M. (2009). Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron 61, 708-720. 10.1016/j.neuron.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astick M., Tubby K., Mubarak W. M., Guthrie S. and Price S. R. (2014). Central topography of cranial motor nuclei controlled by differential cadherin expression. Curr. Biol. 24, 2541-2547. 10.1016/j.cub.2014.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R., Duan X., Taylor M. R., Martin E. A., Muralidhar S., Wang Y., Gangi-Wellman L., Das S. C., Yamagata M., West P. J. et al. (2017). Heterophilic type II cadherins are required for high-magnitude synaptic potentiation in the hippocampus. Neuron 96, 160-176.e8. 10.1016/j.neuron.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello S. M., Millo H., Rajebhosale M. and Price S. R. (2012). Catenin-dependent cadherin function drives divisional segregation of spinal motor neurons. J. Neurosci. 32, 490-505. 10.1523/JNEUROSCI.4382-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch J., Harrison O. J., Honig B. and Shapiro L. (2012). Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 22, 299-310. 10.1016/j.tcb.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch J., Katsamba P. S., Harrison O. J., Ahlsén G., Troyanovsky R. B., Indra I., Kaczynska A., Kaeser B., Troyanovsky S., Honig B. et al. (2018). Homophilic and heterophilic interactions of type II cadherins identify specificity groups underlying cell-adhesive behavior. Cell Rep. 23, 1840-1852. 10.1016/j.celrep.2018.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O. and Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. [DOI] [PubMed] [Google Scholar]
- Dasen J. S., De Camilli A., Wang B., Tucker P. W. and Jessell T. M. (2008). Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell 134, 304-316. 10.1016/j.cell.2008.06.019 [DOI] [PubMed] [Google Scholar]
- De Marco Garcia N. V. and Jessell T. M. (2008). Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron 57, 217-231. 10.1016/j.neuron.2007.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demireva E. Y., Shapiro L. S., Jessell T. M. and Zampieri N. (2011). Motor neuron position and topographic order imposed by β- and γ-catenin activities. Cell 147, 641-652. 10.1016/j.cell.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E., Yang L. L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B. G. and Briscoe J. (2007). Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720. 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- Dewitz C., Pimpinella S., Hackel P., Akalin A., Jessell T. M. and Zampieri N. (2018). Nuclear organization in the spinal cord depends on motor neuron lamination orchestrated by catenin and afadin function. Cell Rep. 22, 1681-1694. 10.1016/j.celrep.2018.01.059 [DOI] [PubMed] [Google Scholar]
- Duan X., Krishnaswamy A., De la Huerta I. and Sanes J. R. (2014). Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158, 793-807. 10.1016/j.cell.2014.06.047 [DOI] [PubMed] [Google Scholar]
- Duan X., Krishnaswamy A., Laboulaye M. A., Liu J., Peng Y.-R., Yamagata M., Toma K. and Sanes J. R. (2018). Cadherin combinations recruit dendrites of distinct retinal neurons to a shared interneuronal scaffold. Neuron 99, 1145-1154.e9. 10.1016/j.neuron.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty R. A. and Steinberg M. S. (2013). Differential adhesion in model systems. Rev. Dev. Biol. 2, 631-645. 10.1002/wdev.104 [DOI] [PubMed] [Google Scholar]
- Hassan B. A. and Hiesinger P. R. (2015). Beyond molecular codes: simple rules to wire complex brains. Cell 163, 285-291. 10.1016/j.cell.2015.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley C. A., Alaynick W. A., Gallarda B. W., Hayashi M., Hilde K. L., Driscoll S. P., Dekker J. D., Tucker H. O., Sharpee T. O. and Pfaff S. L. (2015). Spinal locomotor circuits develop using hierarchical rules based on motorneuron position and identity. Neuron 87, 1008-1021. 10.1016/j.neuron.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S. and Takeichi M. (2012). Cadherins in brain morphogenesis and wiring. Physiol. Rev. 92, 597-634. 10.1152/physrev.00014.2011 [DOI] [PubMed] [Google Scholar]
- Horikawa K., Radice G., Takeichi M. and Chisaka O. (1999). Adhesive subdivisions intrinsic to the epithelial somites. Dev. Biol. 215, 182-189. 10.1006/dbio.1999.9463 [DOI] [PubMed] [Google Scholar]
- Jontes J. D. (2018). The cadherin superfamily in neural circuit assembly. Cold Spring Harb. Perspect. Biol. 10, a029306 10.1101/cshperspect.a029306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostetskii I., Li J., Xiong Y., Zhou R., Ferrari V. A., Patel V. V., Molkentin J. D. and Radice G. L. (2005). Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 96, 346-354. 10.1161/01.RES.0000156274.72390.2c [DOI] [PubMed] [Google Scholar]
- Montague K., Lowe A. S., Uzquiano A., Knüfer A., Astick M., Price S. R. and Guthrie S. (2017). The assembly of developing motor neurons depends on an interplay between spontaneous activity, type II cadherins and gap junctions. Development 144, 830-836. 10.1242/dev.144063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. (2008). Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem. Soc. Trans. 36, 149-155. 10.1042/BST0360149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmesino E., Rousso D. L., Kao T.-J. J., Klar A., Laufer E., Uemura O., Okamoto H., Novitch B. G. and Kania A. (2010). Foxp1 and Lhx1 coordinate motor neuron migration with axon trajectory choice by gating Reelin signalling. PLoS Biol. 8, e1000446 10.1371/journal.pbio.1000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. D., Ciatto C., Chen C. P., Bahna F., Rajebhosale M., Arkus N., Schieren I., Jessell T. M., Honig B., Price S. R. et al. (2006). Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124, 1255-1268. 10.1016/j.cell.2005.12.046 [DOI] [PubMed] [Google Scholar]
- Price S. R., De Marco Garcia N. V., Ranscht B. and Jessell T. M. (2002). Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell 109, 205-216. 10.1016/S0092-8674(02)00695-5 [DOI] [PubMed] [Google Scholar]
- Romanes G. J. (1964). The motor pools of the spinal cord. Prog. Brain Res. 11, 93-119. 10.1016/S0079-6123(08)64045-5 [DOI] [PubMed] [Google Scholar]
- Ruiz P., Brinkmann V., Ledermann B., Behrend M., Grund C., Thalhammer C., Vogel F., Birchmeier C., Günthert U., Franke W. W. et al. (1996). Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J. Cell Biol. 135, 215-225. 10.1083/jcb.135.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockanathan S. and Jessell T. M. (1998). Motor neuron–derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 94, 503-514. 10.1016/S0092-8674(00)81591-3 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. (2007). Differential adhesion in morphogenesis: a modern view. Curr. Opin. Genet. Dev. 17, 281-286. 10.1016/j.gde.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Sürmeli G., Akay T., Ippolito G. C., Tucker P. W. and Jessell T. M. (2011). Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell 147, 653-665. 10.1016/j.cell.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. C., Furue H., Koga K., Jiang N., Nohmi M., Shimazaki Y., Katoh-Fukui Y., Yokoyama M., Yoshimura M. and Takeichi M. (2007). Cadherin-8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J. Neurosci. 27, 3466-3476. 10.1523/JNEUROSCI.0243-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. (2007). The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 8, 11-20. 10.1038/nrn2043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.