ABSTRACT
The niche controls stem cell self-renewal and differentiation in animal tissues. Although the exocyst is known to be important for protein membrane trafficking and secretion, its role in stem cells and niches has never been reported. Here, this study shows that the exocyst functions in the niche to promote germline stem cell (GSC) progeny differentiation in the Drosophila ovary by directly regulating EGFR membrane trafficking and signaling. Inactivation of exocyst components in inner germarial sheath cells, which form the differentiation niche, causes a severe GSC differentiation defect. The exocyst is required for maintaining niche cells and preventing BMP signaling in GSC progeny by promoting EGFR membrane targeting and signaling through direct association with EGFR. Finally, it is also required for EGFR membrane targeting, recycling and signaling in human cells. Therefore, this study reveals a novel function of the exocyst in niche cells to promote stem cell progeny differentiation by directly controlling EGFR membrane trafficking and signaling in vivo, and also provides important insight into how the niche controls stem cell progeny differentiation at the molecular level.
KEY WORDS: EGFR, GSC, Differentiation, Exocyst, Niche, Stem cell, Drosophila, Human
Summary: The exocyst directly controls EGFR membrane trafficking, recycling and activation in Drosophila and human cells, and is required in the niche to promote germline stem cell differentiation by regulating EGFR signaling.
INTRODUCTION
In adult tissues, stem cells continuously self-renew and differentiate to produce functional differentiated cells for replenishing lost cells caused by injury, disease or aging. Studies in Drosophila and mammals have shown that stem cell self-renewal is tightly controlled by the concerted actions of the niche and intrinsic factors (Fuller and Spradling, 2007; Li and Xie, 2005; Morrison and Spradling, 2008; Xie, 2013). Based on our recent finding in the Drosophila ovary, we propose that stem cell progeny differentiation is also controlled by a distinct ‘differentiation niche’ (Kirilly et al., 2011). Recent studies from our lab and others have further confirmed the existence of the differentiation niche (Fu et al., 2015; Li et al., 2015; Liu et al., 2010, 2015; Lu et al., 2015; Luo et al., 2015; Ma et al., 2014; Wang et al., 2015, 2011). However, it remains largely unknown how this niche controls germline stem cell (GSC) progeny differentiation at the molecular level.
The Drosophila ovary is an attractive system for studying stem cell regulation in relationship to niches because of its well-defined GSC lineage and surrounding somatic cells (Spradling et al., 2011; Xie, 2013). At the apical tip of the ovary lie 12-16 germaria, each carrying two or three GSCs (Lin and Spradling, 1993; Spradling, 1993). In the germarium, five to seven cap cells and GSC-contacting anterior inner germarial sheath cells (ISCs, previously known as escort cells) form the niche for promoting GSC self-renewal (Kirilly et al., 2011; Wang et al., 2011; Xie and Spradling, 2001, 2000). Niche-derived BMP-like Dpp directly controls GSC self-renewal by repressing differentiation (Chen and McKearin, 2003; Song et al., 2004; Xie and Spradling, 1998), and E-cadherin-mediated cell adhesion helps anchor GSCs in the niche for long-term self-renewal (Song et al., 2002). Therefore, the niche controls GSC self-renewal by providing anchorage and repressing differentiation.
Each GSC division generates a differentiating cystoblast (CB), which then undergoes four synchronous divisions to produce an interconnected 16-cell cyst with mitotic 2-cell, 4-cell and 8-cell intermediates. The CBs, mitotic intermediates and 16-cell cysts are encased by ISC cellular processes in the anterior germarium (Decotto and Spradling, 2005; Kirilly et al., 2011; Morris and Spradling, 2011). bam is repressed by BMP signaling in GSCs, and is upregulated in CBs and mitotic cysts (Chen and McKearin, 2003; Song et al., 2004). Bam promotes GSC progeny differentiation by working with other differentiation factors (Xie, 2013). In addition to the Bam-dependent intrinsic mechanisms, the ISC-based differentiation niche promotes GSC progeny differentiation extrinsically (Kirilly et al., 2011). Studies conducted by us and others have demonstrated that ISC cellular process-mediated direct interactions are crucial for GSC progeny differentiation (Banisch et al., 2017; Kirilly et al., 2011; Lu et al., 2015; Maimon et al., 2014; Su et al., 2018; Wang et al., 2015, 2011). In addition, the elimination of ISCs results in the most severe germ cell differentiation defect, further supporting the importance of ISCs in promoting GSC progeny differentiation (Kirilly et al., 2011; Wang et al., 2015, 2011; Wang and Page-McCaw, 2018). Mechanistically, ISCs promote GSC progeny differentiation by preventing BMP signaling through multiple mechanisms. EGFR signaling operates in ISCs to prevent BMP signaling by repressing dally, which encodes a proteoglycan for facilitating Dpp diffusion (Liu et al., 2010), and Wnt signaling functions in ISCs to prevent BMP signaling by maintaining BMP receptor Tkv-mediated Dpp trapping and ISC survival (Luo et al., 2015; Mottier-Pavie et al., 2016; Wang et al., 2015). In addition, Rho prevents BMP signaling by repressing dally and dpp in ISCs, whereas Eggless, Piwi, Lsd1, Hh signaling and the COP9 complex repress dpp in ISCs (Eliazer et al., 2014, 2011; Huang et al., 2017; Jin et al., 2013; Kirilly et al., 2011; Liu et al., 2015; Lu et al., 2015; Ma et al., 2014; Wang et al., 2015, 2011). Tkv acts in ISCs to prevent Dpp diffusion and promote Hh signaling, thereby preventing BMP signaling (Luo et al., 2015; Tseng et al., 2018). Thus, ISCs promote GSC progeny differentiation primarily by preventing BMP signaling.
Long ISC cellular processes should behave like invadosomes because they have to retract from a departing cyst and extend to a new ‘passing-by’ cyst (Kirilly et al., 2011; Morris and Spradling, 2011). Exocytosis can provide the membrane for protrusion (Bretscher, 2008). In Drosophila, exocytosis is controlled by the highly conserved exocyst complex genes, including Sec5, Sec6, Sec10 and Sec15 (Langevin et al., 2005; Murthy et al., 2003, 2005). In this study, we show that the exocyst is required in ISCs themselves to maintain ISCs and their long cellular processes as well as promote GSC progeny differentiation by directly regulating EGFR membrane trafficking and signaling. Moreover, polarized exocytosis toward the apical side of ISCs observed in this study might also provide important insights into the generation and maintenance of ISC cellular processes.
RESULTS
Exocyst components are required in ISCs to promote GSC progeny differentiation
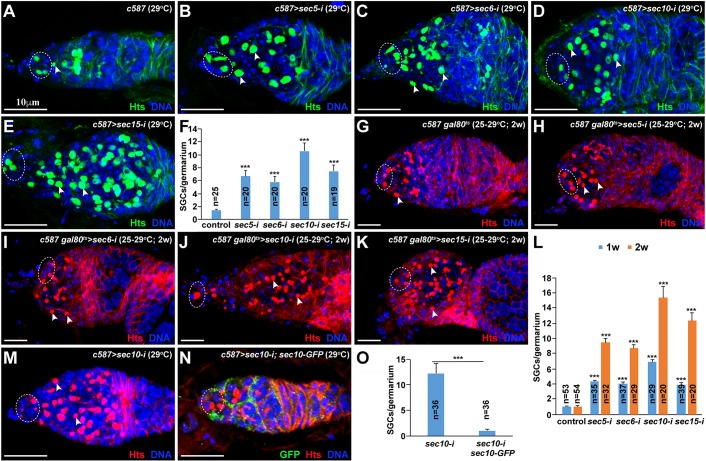
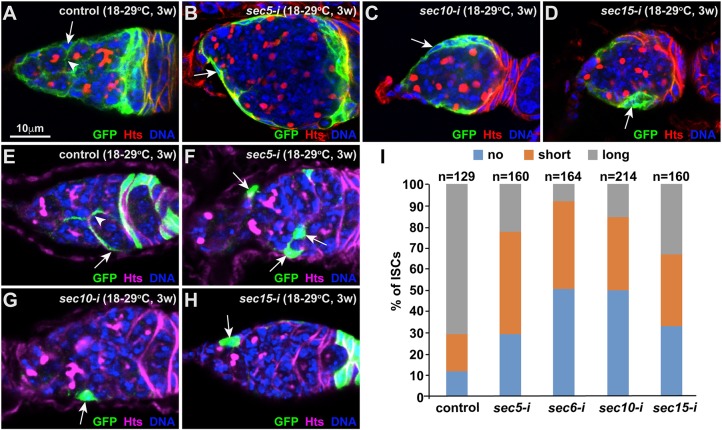
To determine the function of the exocyst in the differentiation niche of the Drosophila ovary, we used the ISC-expressing c587-gal4 driver and UAS-RNAi transgenic strains to knock down Sec5, Sec6, Sec10 and Sec15 specifically in ISCs. The ovaries from the control and Sec knockdown females were labeled for Hu li tai shao (Hts), and GSC and CB numbers were quantified. Hts labels the spherical spectrosome in GSCs/CBs and the branched fusome in cysts (Lin et al., 1994); GSCs can be distinguished from CBs by their direct contact with cap cells (Xie and Spradling, 2000). The control germaria usually contain two or three GSCs and one CB (Fig. 1A,F). Although the Sec5, Sec6, Sec10 and Sec15 knockdown (sec5-i, sec6-i, sec10-i and sec15-i, respectively) germaria contain two or three GSCs as in the control germaria, they carry significantly more spectrosome-containing single germ cells (hereafter referred to as SGCs), which lie posterior to GSCs, than do control germaria, indicating that exocyst components are required in ISCs to promote CB differentiation (Fig. 1B-F). To determine the requirements in adult ISCs for promotion of CB differentiation, we used c587-gal4, tubulin-gal80ts (adult females, which were obtained at 25°C to allow Gal80ts to repress Gal4-driven RNAi knockdown, and were then shifted to 29°C for inactivation of Gal80ts, thereby permitting Gal4-driven RNAi knockdown) to knock down the exocyst complex specifically in adult ISCs because c587 is also expressed in developing ISCs. The 2-week Sec5, Sec6, Sec10 and Sec15 knockdown ovaries accumulate much more SGCs than 1-week knockdown ovaries, and they also carry as many SGCs as knockdown ovaries from females cultured at 29°C throughout development (Fig. 1G-L). These results demonstrate that exocyst components are also required in adult ISCs to promote GSC progeny differentiation.
Fig. 1.
Exocytosis is required in ISCs to promote GSC progeny differentiation. Dashed ovals highlight cap cells and GSCs; arrowheads indicate CBs (spectrosome). (A) Control germarium containing three GSCs and one CB. (B-F) ISC-specific sec5/6/10/15-i germaria carry excess SGCs in addition to the normal two or three GSCs. (F) SGC quantification results. (G-L) Adult ISC-specific sec5/6/10/15-i germaria also carry significantly more SGCs than the control germarium. (L) SGC quantification results. (M-O) Sec10-GFP expression in ISCs (M) fully suppresses the accumulation of SGCs in the sec10-i germarium (N). (O) SGC quantification results. Mean±s.e.m. is shown. n, number of examined germaria. ***P<0.001 (Student's t-test). Scale bars: 10 μm.
Two independent approaches were used to confirm that the germ cell differentiation defects are indeed caused by Sec gene knockdown. First, our quantitative RT-PCR results show that the mRNA expression of Sec5, Sec6, Sec10 and Sec15 in the isolated germaria is efficiently and significantly knocked down by corresponding RNAi lines (Fig. S1). Second, ISC-specific expression of RNAi-resistant sec10-GFP (carrying the nucleotide changes for preventing knockdown, but still encoding a wild-type Sec10 protein) can sufficiently and fully rescue the germ cell differentiation defect caused by Sec10 knockdown (Fig. 1M-O). This result also indicates that the C-terminal GFP-tagged Sec10 is also functional. As knocking down four exocyst components produces similar germ cell differentiation defects, these results demonstrate that exocyst components are required in ISCs to promote GSC progeny differentiation.
To rule out the possibility that Sec knockdown ISCs are functionally converted into cap cells known to promote self-renewal and repress differentiation (Song et al., 2007; Ward et al., 2006; Xie and Spradling, 2000), we used Lamin C as a molecular marker to identify cap cells in the control and Sec knockdown ovaries (Xie and Spradling, 2000) (Fig. S2A). Interestingly, the sec5-i, sec6-i, sec10-i and sec15-i ISCs did not express Lamin C, and knocking down these Sec genes in ISCs does not change the endogenous cap cell number or produce ectopic cap cells (Fig. S2B-D). These results indicate that the germ cell differentiation defects caused by Sec knockdown in ISCs are unlikely to be due to the formation of more cap cells or of ectopic cap cells.
The exocyst is required for the polarized transport of cellular vesicles in ISCs
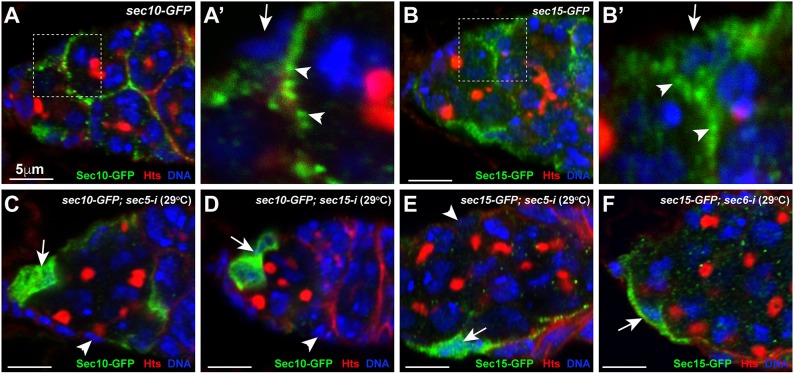
The exocyst is known to be associated with secreted vesicles (SVs) to regulate their membrane tethering in Drosophila and other systems (Langevin et al., 2005; Wu and Guo, 2015). To determine if exocyst components are associated with cellular vesicles, we used Sec10-GFP and Sec15-GFP to visualize the subcellular localization of the exocyst in ISCs. Sec10-GFP shows strong punctate staining underneath the cytoplasmic membrane, which most likely represent individual cellular vesicles (Fig. 2A,A′). Interestingly, these punctate speckles in ISCs are mostly restricted to the apical areas facing germ cells and lying underneath long cellular protrusions. Sec15-GFP, the protein trap line in which GFP is fused in-frame with Sec15 protein, should recapitulate endogenous Sec15 protein expression pattern (Kelso et al., 2004). Interestingly, Sec15-GFP is also primarily expressed in ISCs (Fig. 2B). Consistent with Sec10-GFP subcellular localization in ISCs, Sec15-GFP also exhibits punctate speckles under the surface areas facing germ cells and long cellular protrusions (Fig. 2B,B′). These observations suggest that the exocyst is associated with cellular vesicles in ISCs.
Fig. 2.
The exocyst complex accumulates on cellular vesicles at the apical side and in long cellular processes of ISCs. (A-B′) Sec10-GFP (A,A′) and Sec15-GFP (B,B′) accumulate on cellular vesicles on the apical side (upper arrowhead) and in long cellular processes (lower arrowhead) of ISCs (arrows indicate the basal side). A′ and B′ show high-magnification views of the boxed regions in A and B. (C,D) Sec5 and Sec15 knockdowns cause uniform Sec10-GFP localization in the cytoplasm of ISCs (arrows) and the loss of Sec10-GFP in some of the knockdown ISCs (arrowheads). (E,F) Sec5 and Sec6 knockdowns cause uniform Sec15-GFP localization in the cytoplasm of ISCs (arrows) and the loss of Sec15-GFP in some of the knockdown ISCs (arrowhead). Scale bars: 5 μm.
To determine whether the exocyst is required for membrane vesicle trafficking in ISCs, we examined the expression of Sec10-GFP and Sec15-GFP in Sec knockdown ISCs. Interestingly, sec5-i and sec15-i ISCs lose the Sec10-GFP punctate staining patterns under the cytoplasmic membrane, and instead exhibit uniform Sec10-GFP protein distribution in the cytoplasm (Fig. 2C,D). Consistent with this, sec5-i and sec6-i ISCs show uniform Sec15-GFP protein distribution in the cytoplasm (Fig. 2E,F). Sec10-GFP- and Sec15-GFP-associated cellular vesicles are also localized to their basal side of the Sec knockdown ISCs, which is in contrast with the control ISCs, which show very few Sec10/15-positive vesicles on the basal side (Fig. 2C-F). In addition, some knockdown ISCs also completely lose the Sec10-GFP or Sec15-GFP expression, which might be caused by protein degradation due to the loss of other exocyst components (Fig. 2C-F). Taken together, exocyst components are important for polarized vesicle trafficking and also possibly important for one another's stability in ISCs.
The exocyst maintains ISCs by promoting cell proliferation and survival
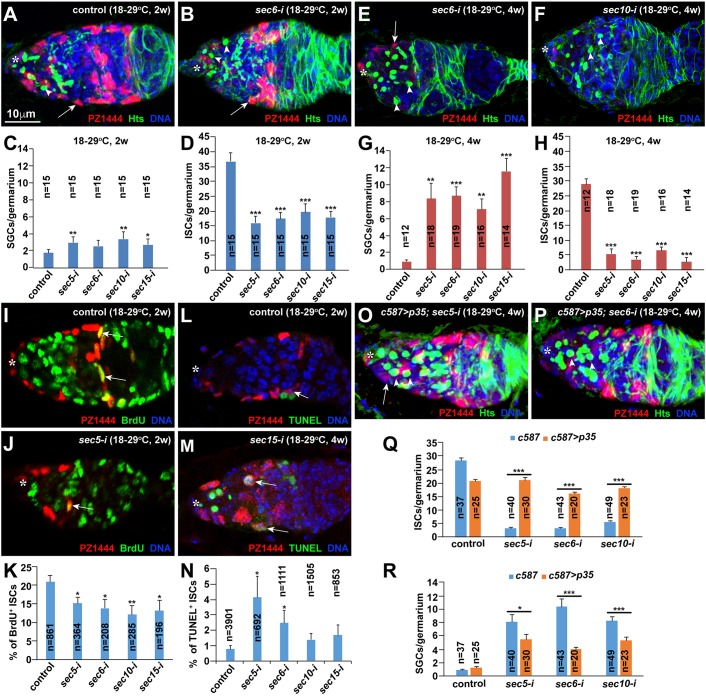
As shown previously (Lu et al., 2015; Wang et al., 2015), c587-gal4-driven UAS-RNAi knockdown is temperature sensitive with little or no knockdown at 18°C and efficient knockdown at 29°C. Consistent with this, sec5-i, sec6-i, sec10-i and sec15-i germaria of females raised at 18°C carry normal numbers of ISCs and CBs, as in control germaria, indicating that these ISCs develop normally and have normal function for supporting GSC progeny differentiation (Fig. S3A-F′). After adult females are cultured at 29°C for 2 or 4 weeks, the sec5-i, sec6-i, sec10-i and sec15-i germaria accumulate significantly more SGCs than the control germaria, further supporting that the exocyst is required in adult ISCs to promote GSC progeny differentiation (Fig. 3A-C,E-G). Intriguingly, the 25-to-29°C shift-based Gal80ts-based conditional Sec gene knockdown produces stronger knockdown phenotypes than the 18-to-29°C shift-based knockdown (2 weeks at 29°C) because the former strategy has leaked expression causing moderate germ cell differentiation defects even at 25°C (Fig. S3G-L). Thus, we used the 18-to-29°C conditional knockdown strategy for all subsequent experiments. Additionally, we also used a PZ1444 lacZ reporter for quantifying ISCs because it labels both ISCs and cap cells, which can be reliably distinguished according to their physical localization and cell size (Margolis and Spradling, 1995; Xie and Spradling, 2000). The sec5-i, sec6-i, sec10-i and sec15-i germaria have significantly fewer ISCs than the control germaria (Fig. 3D,H). These results indicate that the exocyst is required for maintaining adult ISCs and promoting GSC progeny differentiation.
Fig. 3.
The exocyst complex is required in adult ISCs for their maintenance by promoting cell proliferation and preventing apoptosis. Asterisks highlight the cap cell area; arrows indicate ISCs (A,B,E,F,O,P), proliferating ISCs (I,J) or apoptotic ISCs (L,M); arrowheads in O,P indicate spectrosomes. (A-D) sec5/6/10/15-i germaria contain significantly more SGCs and significantly fewer ISCs than the control germaria 2 weeks after temperature shift in the adult stage. (C,D) SGC and ISC quantification results. (E-H) sec5/6/10/15-i germaria show more SGCs and fewer ISCs 4 weeks after the shift than those observed 2 weeks after the shift. (G,H) SGC and ISC quantification results. (I-K) sec5/6/10/15-i germaria contain significantly fewer BrdU-labeled ISCs than the control germaria 2 weeks after the shift. (K) BrdU-positive ISC quantification results. (L-N) sec5/6/10/15-i germaria carry more apoptotic ISCs than the control germaria 2 weeks after the shift. (N) Apoptotic ISC quantification results. (O-R) p35 expression in ISCs can significantly restore ISCs and rescue the germ cell differentiation defects in sec5/6/10-i germaria. (Q,R) SGC and ISC quantification results. Mean±s.e.m. is shown. n, number of examined germaria in C,H,R and number of examined ISCs in K,N. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Scale bars: 10 μm.
ISCs undergo slow turnover, and lost ISCs can be replenished by the proliferation of their neighboring ISCs (Kirilly et al., 2011). To determine whether ISC loss is caused by defective cell proliferation, apoptosis or both, we used bromodeoxyuridine (BrdU) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) labeling to examine the proliferation and apoptosis of control and Sec knockdown ISCs, respectively. Following 3 days of BrdU feeding, about 20% of the control ISCs are BrdU positive (Fig. 3I,K). By contrast, sec5-i, sec6-i, sec10-i and sec15-i ISCs show significantly lower BrdU-positive rates than the control ISCs, and thus the Sec knockdown germaria contain fewer BrdU-positive ISCs (Fig. 3J,K). About 0.7% of the control ISCs are positive for TUNEL labeling (Fig. 3L,N). By contrast, sec5-i, sec6-i, sec10-i and sec15-i germaria tend to have more TUNEL-positive ISCs than control germaria (Fig. 3M,N). The cell death inhibitor p35 is known to prevent apoptosis in Drosophila when overexpressed (Hay et al., 1994). Consistent with this, p35 overexpression can significantly prevent the loss of sec5-i, sec6-i and sec10-i ISCs, and can also significantly rescue the germ cell differentiation defects caused by Sec knockdown (Fig. 3O-R). These results indicate that exocyst components promote ISC maintenance via regulation of cell survival and proliferation.
The exocyst is required in ISCs to promote GSC progeny differentiation partly by preventing BMP signaling
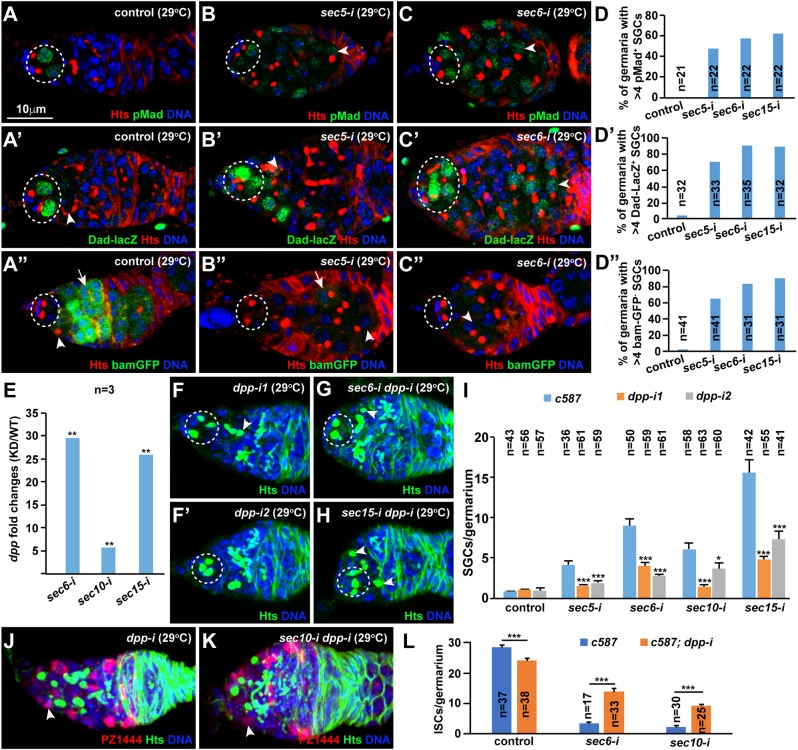
ISCs have been shown to shield GSC progeny from BMP signaling by repressing dpp expression or preventing its diffusion (Kirilly et al., 2011; Liu et al., 2010; Lu et al., 2015; Ma et al., 2014; Wang et al., 2011). BMP signaling activities can be monitored by the expression of phosphorylated Mad (pMad), Dad-lacZ and bam-GFP (Chen and McKearin, 2003; Song et al., 2004; Xie and Spradling, 1998). In the control germaria, GSCs express pMad and Dad-lacZ but not bam-GFP, and CBs and mitotic cysts express bam-GFP, but low or no pMad and Dad-lacZ (Fig. 4A-A″). By contrast, in the sec5-i, sec6-i and sec15-i germaria, those accumulated SGCs lying posteriorly to GSCs often upregulate pMad and Dad-lacZ expression and frequently downregulate bam-GFP expression (Fig. 4B-D″). Based on the expression of pMad, Dad-lacZ and bam-GFP, some of the accumulated SGCs in the Sec knockdown germaria resemble GSCs, and most of them are CB-like, suggesting that those accumulated SGCs are a mixture of GSC-like and CB-like cells. These results further support that the exocyst is required in ISCs to promote GSC progeny differentiation by preventing BMP signaling.
Fig. 4.
The exocyst is required in ISCs to promote GSC differentiation partly by repressing dpp expression. Dashed ovals highlight GSCs; arrowheads and arrows indicate CBs/SGCs, and cysts, respectively. (A-D′) sec5/6/10/15-i germaria have increased pMad (B-D) and Dad-lacZ (B′-D′) expression in some of the accumulated SGCs in comparison with the controls (A,A′). (A″-D″) sec5/6/10/15-i germaria show excess SGCs expressing low bam-GFP compared with the CB and cysts in the control. (D-D″) Quantification results. (E) sec6-i, sec10-i and sec15-i ISCs significantly upregulate dpp mRNA levels based on quantitative RT-PCR results [knockdown (KD) compared with wild type(WT)]. (F-I) dpp knockdown can significantly rescue the germ cell differentiation defect caused by sec5/6/10/15-i ISCs. ISC-specific dpp knockdown germaria contain one (F) or no (F′) CBs. ISC-specific sec dpp double knockdown germaria carry one (G) or two (H) CBs. (I) SGC quantification results. (J-L) dpp knockdown can significantly rescue the sec5/6-i ISC loss (arrowheads indicate ISCs). (L) ISC quantification results. Mean±s.e.m. is shown. n, number of examined germaria. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Scale bar: 10 μm.
To investigate whether the exocyst is required in ISCs to prevent BMP signaling by repressing dpp expression, we used RT-PCR to examine dpp mRNA expression in purified control and knockdown ISCs. Interestingly, dpp is significantly upregulated in the sec6-i, sec10-i and sec15-i ISCs compared with the control (Fig. 4E). As we reported previously (Lu et al., 2015; Ma et al., 2014; Wang et al., 2011), dpp knockdown in adult ISCs has no visible effect on GSC maintenance and GSC progeny differentiation using two independent RNAi lines (Fig. 4F,F′). The sec5-i dpp-i, sec6-i dpp-i, sec10-i dpp-i and sec15-i dpp-i double knockdown germaria contain significantly fewer SGCs than the sec5-i, sec6-i, sec10-i and sec15-i germaria, respectively, indicating that ISC-specific dpp knockdown significantly rescues the germ cell differentiation defects caused by Sec gene knockdown (Fig. 4G-I). Interestingly, dpp knockdown can also partially and significantly prevent the ISC loss caused by Sec6/10 knockdown, suggesting that upregulated BMP signaling also contributes to the ISC loss (Fig. 4J-L). These results indicate that the exocyst is required in ISCs to repress dpp expression and thereby promote GSC progeny differentiation.
The exocyst is required for maintaining long ISC cellular processes
Long ISC cellular protrusions are required to promote GSC progeny differentiation in the Drosophila ovary (Kirilly et al., 2011; Lu et al., 2015). To determine whether the exocyst is required to maintain long ISC cellular processes, we used c587-gal4-driven expression of membrane-tethered CD8-GFP in control and Sec knockdown ISCs. In the control germaria, long GFP-positive ISC cellular processes wrap around the differentiated GSC progeny (Fig. 5A). By contrast, the sec5-i, sec10-i or sec15-i ISCs lack long cellular processes that encase differentiated GSC progeny (Fig. 5B-D). These results indicate that the exocyst is required for the maintenance of long ISC cellular processes.
Fig. 5.
The exocyst is required in ISCs to maintain their cellular processes. Arrows and arrowheads indicate ISCs and their cellular processes, respectively. (A-D) Sec5/10/15-i knockdown ISCs (B-D) lose the long cellular processes wrapping around germ cell cysts in comparison with control (A). (E-I) Individually GFP-marked sec5/6/10/15-i ISCs (F-H) frequently lose their cellular processes compared with the marked control ISCs (E). (I) Quantification of ISC cellular processes based on their length. n, number of examined GFP-labeled ISCs. Scale bars: 10 μm.
As the loss of long ISC cellular processes could be a consequence of the germ cell differentiation defects caused by Sec knockdown (Kirilly et al., 2011), we used the flipase-mediated FLP-out system to label individual control and Sec knockdown ISCs with GFP in adulthood to determine whether exocytosis is required to maintain long ISC cellular processes. In the FLP-out system, one heat shock treatment results in the removal of the transcription stop sequence between the Actin 5C promoter and the yeast gal4 gene, thereby turning on the expression of gal4, which then drives UAS-GFP expression for labeling individual somatic cells, including ISCs (Ito et al., 1997). In the control, GFP-marked individual control ISCs often have long cellular processes; however, some of them have short or no cellular processes, indicating that ISCs likely retract and extend their cellular processes while encasing the bypassing differentiated GSC progeny (Fig. 5E,I). Short ISC cellular processes are defined as those wrapping less than half of the underlying cysts, as long cellular processes of individual ISCs can fully cover the underlying cysts (Kirilly et al., 2011). Interestingly, the GFP-labeled sec5-i, sec6-i, sec10-i and sec15-i ISCs more frequently have no or only short cellular processes compared with control ISCs (Fig. 5F-I). These results demonstrate that the exocyst is required intrinsically to maintain long ISC cellular processes.
Exocyst components are also required to maintain EGFR-MAPK signaling in ISCs
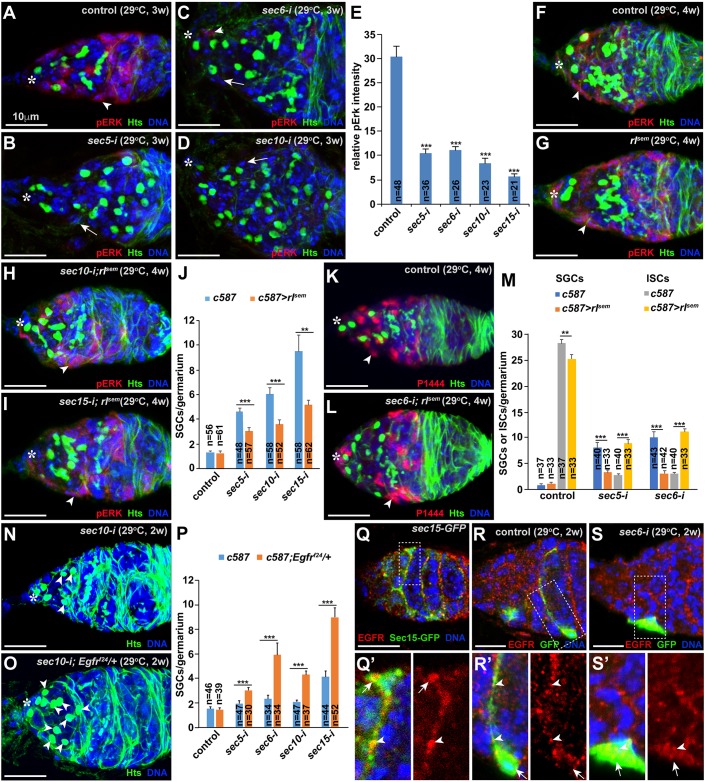
EGFR-MAPK signaling operates in ISCs to promote GSC progeny differentiation (Liu et al., 2010; Schultz et al., 2002). MAPK signaling activity is normally monitored by the expression of phosphorylated MAPK or ERK (pERK). As reported previously (Kirilly et al., 2011; Liu et al., 2010; Schultz et al., 2002), pERK is highly expressed in control ISCs (Fig. 6A). By contrast, the sec5-i, sec6-i, sec10-i and sec15-i ISCs severely decrease or diminish pERK expression (Fig. 6B-E). These results demonstrate that exocyst components are required in ISCs to maintain high MAPK signaling.
Fig. 6.
The exocyst is required in ISCs to maintain pERK expression. Asterisks highlight cap cells. (A-E) sec5/6/10/15-i ISCs (arrowheads) drastically decrease pERK expression in comparison with the control ISC (arrowhead). (E) Quantification of relative fluorescence intensity. (F-J) Expression of constitutively active MAPK (rlsem) can restore pERK expression in ISCs (arrowheads) in comparison with wild-type control ISCs (arrowhead), and can also partially and yet significantly rescue the germ cell differentiation defect caused by sec5/6/10/15-i in ISCs. (J) SGC quantification results. (K-M) ISC-specific rlSEM expression can significantly rescue the germ cell differentiation and ISC loss defect and the ISC loss caused by sec5/6-i (arrowheads indicate ISCs). (M) SGC and ISC quantification results. (N-P) A heterozygous mutation in Egfr can significantly enhance the germ cell differentiation defect caused by sec5/6/10/15-i based on the accumulation of SGCs (arrowheads). (P) SGC quantification results. (Q,Q′) EGFR protein is localized to the Sec15-GFP-positive vesicles on the apical side of the cell body (arrows) and cellular processes (arrowheads). (R,R′) A GFP-marked control ISC (arrow) exhibits EGFR-positive speckles (arrowheads) moving along its cellular process. (S,S′) A GFP-marked sec6-i ISC (arrow) loses its cellular processes, and retains EGFR-positive speckles (arrows) on the basal side (indicated by an arrow). Mean±s.e.m. is shown. n, number of examined germaria. **P<0.01, ***P<0.001 (Student's t-test). Scale bars: 10 μm.
Then, we investigated whether restoration of MAPK signaling in ISCs could rescue the germ cell differentiation defects caused by Sec gene knockdown. rolled (rl) encodes the only MAPK in Drosophila, and rlSEM is a constitutively active MAPK mutant that acts independently of EGFR receptor activation (Oellers and Hafen, 1996). The expression of rlSEM in ISCs does not cause any obvious adverse effect on germ cell development compared with the control (Fig. 6F,G). Interestingly, the expression of rlSEM in Sec knockdown ISCs can restore MAPK activity, and can also significantly rescue the germ cell differentiation and ISC loss defects caused by Sec knockdown (Fig. 6H-M). These results demonstrate that the exocyst is required in ISCs to maintain active MAPK signaling, thereby promoting ISC survival and GSC progeny differentiation.
The exocyst regulates EGFR membrane targeting and signaling
MAPK signaling activity is maintained by EGFR activation in ISCs (Liu et al., 2010; Schultz et al., 2002). As reported previously (Liu et al., 2010), ISC-specific Egfr knockdown causes germ cell differentiation defects by two independent RNAi lines (Fig. S4A-C). Interestingly, Egfr knockdown in adults also results in the ISC loss; the severity of the ISC loss also correlates well with that of the germ cell differentiation defects (Fig. S4C′). In addition, we used individual GFP-labeled Egfr knockdown ISCs to demonstrate that EGFR signaling is also required to directly maintain ISC cellular processes as the exocyst does (Fig. S5). Although control and Egfrf24 heterozygous germaria have similar numbers of CBs, the heterozygous Egfrf24 mutation can significantly enhance the germ cell differentiation defects caused by Sec5, Sec6, Sec10 and Sec15 knockdown in ISCs, demonstrating that EGFR signaling and the exocyst function synergistically in ISCs to promote GSC progeny differentiation (Fig. 6N-P). Interestingly, EGFR proteins are expressed in speckles in ISCs and follicle progenitor cells, which are greatly decreased in the c587-driven Egfr knockdown germaria, suggesting that EGFR protein is present in speckles of ISCs and follicle progenitor cells (Fig. S4D-E′). Interestingly, some EGFR-positive speckles are also positive for Sec15-GFP in control ISCs, suggesting that EGFR is localized to Sec15-positive cellular vesicles (Fig. 6Q,Q′). These results suggest that the exocyst might regulate EGFR trafficking in ISCs to maintain ISCs and promote GSC progeny differentiation.
In individual GFP-labeled control ISCs, EGFR protein speckles are localized to the cell body and cellular processes, but are rarely detected on the basal side facing away from germ cells (Fig. 6R,R′; Fig. S6A-A″). Similarly, we also detected very few Sec10/15-positive vesicles on the basal side of ISCs (Fig. 2A-B′). In individual GFP-labeled sec5-i, sec6-i or sec10-i ISCs, EGFR protein speckles are only present in the cell body owing to the loss of cellular processes (Fig. 6S,S′; Fig. S6B-D″). In addition, the GFP-labeled Sec knockdown ISCs also accumulate more EGFR-positive speckles on the basal side. As EGF ligands responsible for EGFR activation in ISCs are known to come from underneath differentiated germ cells (Liu et al., 2010), our results suggest that the exocyst is required for polarized EGFR-containing vesicle trafficking to cellular processes to maximize EGFR activation by EGF ligands in germ cells, thereby facilitating MAPK signaling.
The exocyst is directly associated with EGFR-carrying vesicles to regulate their membrane trafficking
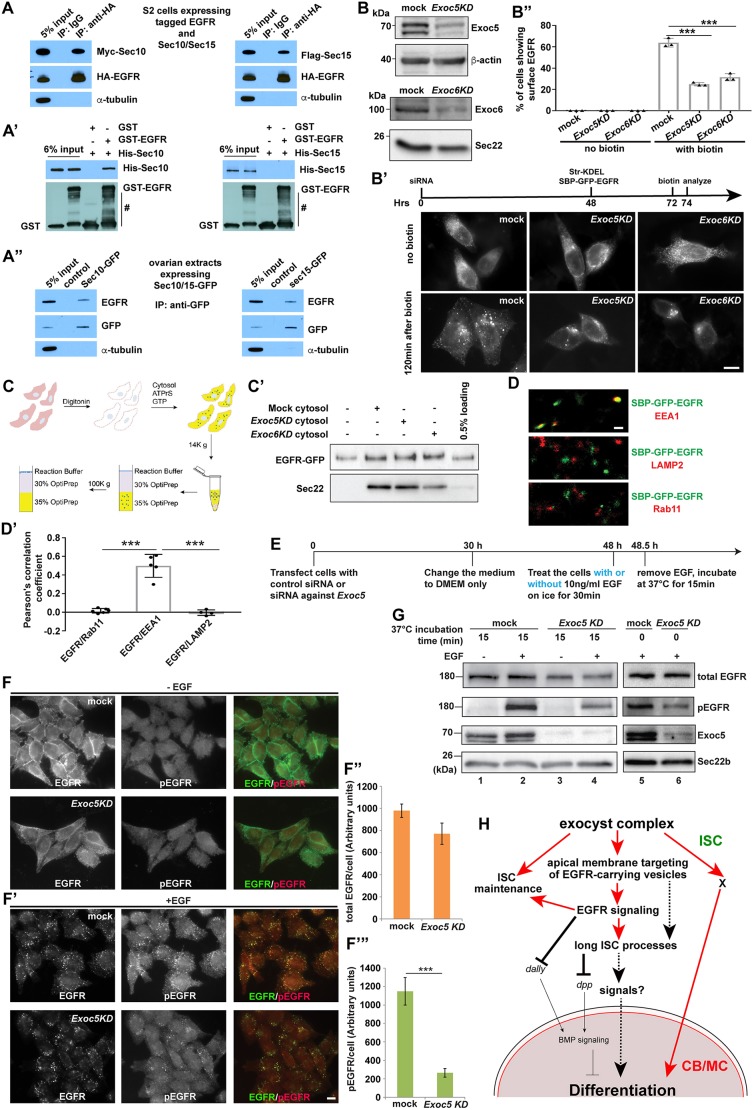
Previous studies suggest that the human exocyst complex can directly interact with human EGFR in cultured cells (Fogelgren et al., 2014; Tan et al., 2015). We investigated whether the Drosophila exocyst also interacts with EGFR in vivo and in vitro. Indeed, HA-tagged membrane-associated EGFR intracellular domain can pull down Myc-tagged Sec10 and Flag-tagged Sec15 in S2 cells, indicating that the exocyst is associated with EGFR in S2 cells (Fig. 7A). In addition, bacterially expressed Sec10, but not Sec15, directly interacts with purified EGFR intracellular domain in vitro, indicating that the exocyst directly binds to EGFR via Sec10-mediated interaction (Fig. 7A′). Moreover, ISC-expressing Sec10-GFP and Sec15-GFP can pull down EGFR protein in ovarian extracts, supporting the in vivo association of the exocyst and EGFR (Fig. 7A″). These results indicate that the exocyst complex recognizes EGFR-containing vesicles for membrane targeting by directly interacting with EGFR.
Fig. 7.
The exocyst is directly associated with EGFR to regulate its membrane targeting in Drosophila ISCs and human cells. (A-A″) HA-EGFR can pull down Myc-Sec10 and Flag-Sec15 in S2 cells (A), but bacterially expressed GST-EGFR can only pull down His-Sec10, not His-Sec15, in vitro (A′; # indicates the degraded GST-EGFR). In ovarian extracts, both Sec10-GFP and Sec15-GFP can pull down EGFR protein (A″). α-tubulin is used in A and A″ as a negative control. (B-B″) EXOC5/6 knockdown (Exoc5KD and Exoc6KD) significantly decreases the efficiency of delivery of SBP-GFP-EGFR to the plasma membrane. In Exoc5/6KD HeLa cells (B′), in which knockdown efficiencies are confirmed by western blots (B), SBP-GFP-EGFR remains localized in the perinuclear puncta in contrast to the mock-transfected cells showing detectable surface-localized SBP-GFP-EGFR. (B″) Quantification of the percentage of cells showing detectable cell surface-localized SBP-GFP-EGFR (mean±s.d.; based on three independent experiments; >50 cells counted for each experiment). (C,C′) The levels of EGFR-GFP in the vesicle fraction are determined by western blots (C′; Sec22 is a cargo protein enriched in vesicles serving as an internal control) after performing the vesicle budding assay described in C. (D,D′) STORM images show that EGFR-GFP punctae are largely overlapped with EEA1 punctae, but not RAB11 and LAMP2 punctae in HeLa cells. (D′) Quantification results. (E-G) EXOC5 is required for EGF-induced EGFR phosphorylation (pEGFR; immunostaining in F,F′ and western blot in G) using the experimental strategy shown in E in HeLa cells. (F″,F‴) Quantification results. Mean±s.d. is shown. n=6 randomly selected fields; >15 cells in each field. ***P<0.001 (Student's t-test). Scale bars: 10 µm (B′,F′); 0.5 µm (D). (H) A model showing that the exocyst regulates ISC maintenance, their cellular processes and thus BMP signaling and GSC progeny differentiation by controlling EGFR membrane targeting and other undefined pathways (X) (red arrows represent the relationships revealed by this study).
Previous studies have shown that the juxtamembrane (JM) domain is important for EGFR to be localized to the basolateral side of epithelial cells (He et al., 2002; Hobert et al., 1997; Kil et al., 1999). In S2 cells, both Myc-Sec10 and Flag-Sec15 fail to be brought down by EGFR lacking the JM domain, but can specifically be pulled down by JM-fused GFP protein (Fig. S7). These results indicate that the exocyst specifically interacts with the JM domain of EGFR, and further suggest that it might be involved in the polarized EGFR trafficking in ISCs.
The exocyst regulates EGFR membrane targeting and recycling to facilitate EGF-induced EGFR phosphorylation in human cells
Because the functions of exocyst components are highly conserved from yeast to human (Schekman, 2010; Wu and Guo, 2015), we investigated whether the exocyst is also required for targeting human EGFR to the plasma membrane in HeLa and HEK293T cells by applying the retention using selective hooks (RUSH) assay (Boncompain et al., 2012). In the RUSH assay, SBP-GFP-EGFR (human EGFR tagged with EGFP and the streptavidin binding peptide) is normally retained in the endoplasmic reticulum (ER) by Str-KDEL (streptavidin fused to the ER retention signal KDEL); upon biotin addition, SBP-GFP-EGFR is rapidly released by Str-KDEL from the ER and is then trafficked to the plasma membrane via cellular vesicles (Fig. S8; Movie 1). In the mock-transfected control HEK293T and HeLa cells, SBP-GFP-EGFR is efficiently trafficked to the cell surface upon biotin addition, but it is still primarily retained inside the Exoc5KD and Exoc6KD cells (EXOC5 and EXOC6 represent Sec10 and Sec15 in humans, respectively) (Fig. 7B-B″; Fig. S9A-C). Consequently, Exoc5KD and Exoc6KD cells had significantly less surface GFP-EGFR protein than the control cells (Fig. 7B″; Fig. S9C). In addition, EXOC5 knockdown causes a significant reduction of surface-localized EGFR in HeLa cells (Fig. S9D,E). Using an in vitro assay that reconstitutes vesicular release of EGFR (Ma et al., 2018) (Fig. 7C), we ruled out the possibility that the exocyst regulates the packaging of EGFR into transport vesicles as EXOC5/6 knockdown does not affect the vesicle-associated EGFR levels in HEK293T cells (Fig. 7C′). Then, we utilized two-color stochastic optical reconstruction microscopy (STORM), which can achieve a spatial resolution of 20 nm (Zhao et al., 2015), to show that EGFR punctae are spatially overlapped with EEA1 punctae, but not RAB11 and LAMP2 punctae, in Exoc5KD HeLa cells (Fig. 7D,D′). EEA1, RAB11 and LAMP2 label early endosomes, recycling endosomes and lysosomes, respectively. These results indicate that the exocyst is important for the delivery of EGFR protein to the plasma membrane, possibly through EEA1-positive early endosomes.
To further determine whether the exocyst is required for EGF-induced EGFR endocytosis and recycling, we examined the surface EGFR levels in control and Exoc5KD HeLa cells after EGF stimulation (Fig. S10A,D). In both control and Exoc5KD cells, EGFR is colocalized with the early endosomal marker EEA1 15 min after EGF stimulation, indicating that EXOC5 is dispensable for EGF-induced EGFR endocytosis (Fig. S10B,C). Interestingly, 60 min after EGF stimulation, EGF-bound EGFR is retrieved back to the plasma membrane or transported to lysosomes for degradation (Fig. S10E). In contrast, Exoc5KD cells have much less EGFR on the plasma membrane than the control cells 60 min after EGF stimulation, indicating that EXOC5 is important for the recycling, but not the endocytosis, of EGF-activated EGFR in human cells (Fig. S10F). Then, we utilized antibodies that specifically recognize phosphorylated EGFR (pEGFR) to monitor EGFR phosphorylation in the control and Exoc5KD HeLa cells based on immunostaining and western blotting (Fig. 7E-G). Remarkably, EGF-induced EGFR phosphorylation is severely and significantly decreased in the Exoc5KD cells compared with the control cells (Fig. 7F-F‴). These results demonstrate that EXOC5 is important for EGF-induced EGFR phosphorylation and EGFR recycling, but not endocytosis.
DISCUSSION
Accumulated experimental evidence demonstrates that ISCs function as a niche to prevent BMP signaling and promote GSC progeny differentiation via multiple signaling pathways (Eliazer et al., 2014, 2011; Kirilly et al., 2011; Li et al., 2015; Liu et al., 2010, 2015; Lu et al., 2015; Luo et al., 2015; Ma et al., 2014; Maimon et al., 2014; Su et al., 2018; Tseng et al., 2018; Wang et al., 2015, 2011; Wang and Page-McCaw, 2018). In addition, long ISC cellular process-mediated interactions are crucial for proper GSC progeny differentiation partly by repressing BMP signaling and also potentially through direct signaling (Banisch et al., 2017; Kirilly et al., 2011; Lu et al., 2015; Maimon et al., 2014; Su et al., 2018; Tseng et al., 2018). However, it remains largely unclear how ISC long cellular processes and ISC-operating signaling pathways are regulated at the cellular level. This study demonstrates that the exocyst is required in adult ISCs to promote EGFR signaling, prevent BMP signaling and maintain ISCs and cellular processes, thereby promoting GSC progeny differentiation (Fig. 7H). This represents the first in vivo study for uncovering the function of the exocyst in stem cell regulation as well as in direct regulation of EGFR membrane trafficking.
This study has provided several significant novel insights into the functions of ISCs as the niche for promoting GSC progeny differentiation. First, this study shows that the exocyst can directly regulate EGFR membrane targeting and signaling in Drosophila by Sec10-mediated physical interaction. The exocyst is required in ISCs to sustain ERK signaling activity, contributing to GSC progeny differentiation. EGFR signaling is the only pathway known to maintain active ERK signaling in ISCs (Liu et al., 2010; Schultz et al., 2002). EGFR protein and Sec10/15-GFP are colocalized in cellular vesicles of ISCs. Mechanistically, Drosophila EGFR is associated with the exocyst in ISCs and S2 cells via interaction with Sec10. Such direct interaction between the exocyst and EGFR is also conserved in vertebrates (Fogelgren et al., 2014). The exocyst knockdown ISCs randomly accumulate EGFR-positive vesicles throughout the cytoplasm, which is in contrast with the preferential localization of EGFR-carrying vesicles on the apical side of ISCs, suggesting that the exocyst might be important for polarized vesicle trafficking in ISCs. Consistent with this idea, Sec10 and Sec15 physically interact with the JM domain of EGFR, which is known to be important for polarized EGFR trafficking and localization in mammalian epithelial cells (Hobert et al., 1997). EGFR-containing cellular vesicles in long cellular processes might help present EGFR for maximizing EGFR signaling activated by germ cell-secreted EGF ligands. This could potentially explain the ERK signaling defect in the exocyst knockdown ISCs mechanistically. Similarly, we have shown that human EXOC5 and EXOC6 are also required for EGFR membrane targeting in human HEK293T and HeLa cells, demonstrating the conserved role of the exocyst in EGFR membrane targeting. Interestingly, in EXOC5 knockdown human cells, EGFR protein accumulates in EEA1-positive early endosomes, suggesting that early endosomes might also participate in EGFR membrane trafficking. Our findings in Drosophila and human cells further support the proposal of one recent study that the exocyst complex can first assemble on the surface vesicle and then promote vesicle fusion to the plasma membrane, and also further suggest that some cargos on the surface of vesicles, such as EGFR, might serve as the anchor for facilitating assembly of the exocyst complex (Ahmed et al., 2018). Thus, this study has revealed an important role of the exocyst in the regulation of EGFR signaling in ISCs by directly controlling EGFR cell-surface trafficking (Fig. 7H).
Second, the exocyst is required in ISCs to promote GSC progeny differentiation partly by preventing BMP signaling. One of the well-defined functions of ISCs in promoting GSC progeny differentiation is to prevent BMP signaling (Eliazer et al., 2014, 2011; Kirilly et al., 2011; Liu et al., 2010, 2015; Lu et al., 2015; Ma et al., 2014; Wang et al., 2011). BMP signaling is necessary and sufficient for GSC self-renewal by preventing differentiation via bam expression (Chen and McKearin, 2003; Song et al., 2004; Xie and Spradling, 1998). Interestingly, exocyst knockdown in ISCs causes elevation of BMP signaling activity in the accumulated SGCs at least partly by upregulating dpp expression. Consistently, ISC-specific dpp knockdown can partially and significantly rescue the germ cell differentiation defect and the ISC loss caused by exocyst knockdown. These results demonstrate that the exocyst is required in ISCs to promote GSC progeny differentiation partly by preventing BMP signaling. EGFR signaling is required in ISCs to prevent BMP signaling by repressing dally expression (Liu et al., 2010). As discussed earlier, the exocyst is required in ISCs to maintain active EGFR signaling. Therefore, the exocyst functions in ISCs to prevent BMP signaling in GSC progeny by repressing dpp expression and maintaining EGFR signaling (Fig. 7H). This is in contrast with the previously demonstrated intrinsic requirement of the exocyst for promoting BMP signaling inside GSCs in the Drosophila testis (Michel et al., 2011).
Third, the exocyst is required in ISCs to promote GSC progeny differentiation at least partly by maintaining ISCs and their cellular processes. Our previous studies have demonstrated that ISCs themselves and their cellular processes are essential for GSC progeny differentiation (Kirilly et al., 2011; Lu et al., 2015; Ma et al., 2014; Wang et al., 2015, 2011). In this study, we show that the exocyst is also required for maintaining ISCs by promoting cell proliferation and survival and for maintaining long ISC cellular processes (Fig. 7H). The exocyst has been shown to regulate polarized exocytosis and be important for membrane trafficking within the cell (Bryant et al., 2010; Langevin et al., 2005; Murthy et al., 2003). In this study, we have shown that Sec10-GFP and Sec15-GFP proteins exhibit punctate patterns underneath the apical membrane of ISCs and in ISC cellular processes, but rarely accumulate on the basal side. These apically targeted cellular vesicles can bring lipid bilayer membranes to the apical side and particularly cellular processes, which could potentially explain the growth and maintenance of ISC cellular processes mechanistically. Consistent with this, the exocyst knockdown GFP-labeled individual ISCs frequently lose their cellular processes. Taken together, our findings reveal several novel in vivo roles of the exocyst in regulating the function and maintenance of the adult stem cell niche, which represents important progress toward a better understanding of stem cell lineage differentiation control (Fig. 7H). In the future, it will be of great interest to investigate whether the exocyst regulates the function of the adult stem cell niche in mammalian systems.
MATERIALS AND METHODS
Drosophila stocks and maintenance
Drosophila stocks were maintained at room temperature on standard cornmeal media unless specified. The information of the following stocks are available from FlyBase (http://flybase.org/): c587, PZ1444, bam-GFP, Dad-lacZ, UAS-CD8GFP, ptc-lacZ, act5C-gal4 and UAS-rlSem. sec15-GFP is the GFP protein trap line (Kelso et al., 2004). The UAS-RNAi knockdown strains used in this study include: Sec5 (BL27526, TH00421.N), Sec6 (BL27314, TH00648.N), Sec10 (BL27483, TH00390.N), Sec15 (BL27499, TH00651.N), ptc (BL28795, TH00660.N), Egfr (BL25781, BL31526 and BL31525), dpp [Tr0047A (Ni et al., 2008), sh2 (Haley et al., 2010)]. To maximize the RNAi-mediated knockdown effect, newly eclosed flies were cultured at 29°C for up to 4 weeks before the analysis of ovarian phenotypes. To obtain individual GFP-labeled RNAi knockdown ISCs, yw hsflp;act>>Stop y+>>gal4-UAS-GFP flies were crossed with RNAi strains at 18°C, and F1 female flies were heat shocked at 37°C for 40 min, and cultured at 29°C for 3 weeks before harvesting their ovaries for the phenotypic analysis as we did previously (Lu et al., 2015).
Immunohistochemistry
For immunohistochemistry, ovaries were dissected, fixed and stained according to the procedures described previously (Xie and Spradling, 1998). The following antibodies were used in this study: monoclonal anti-Hts (1B1, 1:200, Developmental Studies Hybridoma Bank), chicken anti-GFP (1:200, Life Technologies, A10262), rabbit anti-β-galactosidase (1:8000, MP Biomedicals, 08559761), rabbit anti-phosphorylated ERK1/2 (1:200, Cell Signaling Technology, 9101), mouse anti-EGFR (1:50, Sigma-Aldrich, E2906), rabbit monoclonal anti-Smad3 antibody (pS423/pS425) (1:200, Epitomics, ab52903), rabbit anti-Sec5 (1:50, Santa Cruz Biotechnology, sc-292891) and rabbit anti-Sec10 (1:50, Santa Cruz Biotechnology, sc-366727). All images were taken with a Leica SP5 confocal microscope and processed with Leica SP5 software. To measure the fluorescence intensity of pERK in the randomly chosen control and knockdown ISCs, all images were taken under the same parameters at the same time, and were quantified using Leica SP5 software. The fluorescence intensity values were normalized to the background.
Immunofluorescence was performed on HeLa and HEK293T cells as previously described (Ma et al., 2018). Commercial antibodies used were: mouse anti-EGFR (1:500, Santa Cruz Biotechnology, sc-101), rabbit anti-EGFR (1:500 for immunofluorescence, 1:2000 for western blotting, Proteintech, 18986-1-AP-s; RRID: AB_10596476), goat anti-EEA1 (1:500, Santa Cruz Biotechnology, sc-6415; RRID:AB_2096822), mouse anti-LAMP2 (1:500, Developmental Studies Hybridoma Bank, H4B4; RRID:AB_528129), rabbit anti-phosphorylated EGFR (1:500 for immunofluorescence, 1:2000 for western blotting, Cell Signaling Technology, 3777s; RRID:AB_2096270). To quantify EGFR levels, at least six representative fields, each containing over 15 cells, were taken in each control and Exoc5KD experimental group under identical exposure times and scaling conditions. Fluorescence intensities were quantified using ImageJ as follows: (1) a single fixed threshold was manually chosen and applied to all images; (2) total fluorescence in each field was determined using ImageJ measure functions and normalized to the total number of cells in that field.
BrdU and TUNEL labeling
For BrdU labeling, flies were fed with yeast paste mixed with 10 µM BrdU (Sigma-Aldrich) for 3 days before the dissection. The dissected ovaries were fixed with 4% paraformaldehyde for 15 min and washed, and then incubated in DNase I buffer for 5 min, and in 20 units DNase I at 37°C for 1 h. Then the ovaries were washed, and incubated with rat anti-BrdU monoclonal antibody (rat mAb-BrdU, 1:400, Abcam, ab6326). TUNEL labeling was performed using the TUNEL Apoptosis Detection Kit (Yeasen) according to the published procedure (Zhu and Xie, 2003).
qRT-PCR on sorted GFP-positive ISCs
For fluorescence-based quantitative real-time PCR (qPCR), GFP-labeled control and knockdown ISCs were sorted from 400-600 ovaries for each genotype by fluorescence-activated cell sorting, and total RNA was extracted using Trizol (Thermo Fisher Scientific). RNA was amplified using CellAmp Whole Transcriptome Amplification Kit (Takara), and qPCR was performed to quantify the expression.
Co-immunoprecipitation using Drosophila ovarian extracts and S2 cells
S2 cells were grown at 25°C in HyClone SFX-Insect Cell Culture Media (Thermo Fisher Scientific). Transfections were performed using X-treme GENE HP transfection reagent (Roche, 6366546001) according to the manufacturer's instructions. pAWH-EGFRTM-ICD (aa 808-1377; EGFR-PB transmembrane domain and intracellular domain; FlyBase ID: FBpp0071571), pAMW-Sec10 and pAFW-Sec15 plasmids were constructed according to the Gateway Cloning methods (K240020; 11791019; Thermo Fisher Scientific). To construct the HA-tagged JM domain deletion EGFR construct (EGFRJMΔ-HA), amino acid sequence LRPSNIGANLCKLRIVKDAELRKGGVLG was removed from pAWH-EGFRTM-ICD. To generate the GFP-tagged JM domain (GFP-EGFRJM), LRPSNIGANLCKLRIVKDAELRKGGVLG was fused to the 3′ end of GFP CDS.
For S2 co-immunoprecipitation experiments, 12 ml S2 cells were transfected with the indicated plasmids. For in vivo co-immunoprecipitation experiments, 200 pairs of ovaries of each genotype (C587 overexpressed UAS-Sec10-GFP, UAS-Sec15-GFP) were digested with type II collagenase (Worthington, 50D11833), and eggs were filtered and removed. Cells were then lysed with 800 μl ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, and a mixture of protease inhibitors). The supernatant of the lysates was incubated with 2 μg mouse anti-HA (H3663; Sigma-Aldrich) plus 40 μl Protein A/G PLUS-Agarose (sc-2003; Santa Cruz Biotechnology) or 25 μl GFP-Trap agarose (gta-10; ChromoTek). The agarose beads were washed and incubated with 5% bovine serum albumin at 4°C for 1 h, and then added to the lysates. The agarose/antibody/lysate mix was incubated overnight at 4°C. After six washes with lysis buffer, the bound complexes were eluted with 2×SDS sample buffer and subjected to SDS-PAGE and immunoblotting. Mouse anti-Flag (1:2000, Sigma-Aldrich, F1804), mouse anti-HA (1:2000, Sigma-Aldrich, H3663), mouse anti-Myc (1:2000, Sigma-Aldrich, M5546) or chicken anti-GFP (1:2000, Invitrogen, A10262) antibodies were used for western blotting.
In vitro protein binding
The intracellular domain of EGFR (aa 865-1377; EGFR-PB intracellular domain; FlyBase ID: FBpp0071571) was cloned into the XhoI of pGEX4T1, full-length Sec10 or Sec15 were cloned into the BamHI/NotI of pET32a(+). After induced expression, bacterial was lysed with B-PER Bacterial Protein Extraction Reagent (Thermo Fisher Scientific, 90078). The inclusion body was dissolved in 15 ml 8 M urea (in 1×TBS, pH 7.4). The proteins were then dialyzed with Slide-A-Lyzer G2 Dialysis Cassettes (Thermo Fisher Scientific, 88252) in 5L 1×TBS, pH 7.4 for 24-48 h at 4°C. After the dialysis, proteins were concentrated with Amicon Ultra-2 Centrifugal Filter Unit (Millipore, UFC201024). 10 μg GST or GST-EGFRICD was mixed with 10 μg His-Sec10 or His-Sec15 in 500 μl buffer A (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.001% Triton X-100), respectively, then 40 μl glutathione agarose (Thermo Fisher Scientific, 16100) was added. The protein-agarose mix was incubated at room temperature for 2 h with shaking. After six washes with buffer A, the bound complexes were eluted with 40 μl 2×SDS sample buffer and subjected to SDS-PAGE and immunoblotting. Rabbit anti-GST (1:2000, Sigma-Aldrich, G7781) or mouse anti-His (1:2000 Sigma-Aldrich, H1029) antibodies were used for western blotting.
RUSH assay in human HeLa and HEK293 cells
EGFR expression constructs were generated by a standard molecular cloning procedure. The DNA fragment encoding E-cadherin within the plasmid Str-KDEL_SBP-EGFP-Ecadherin (Addgene, Plasmid #65286) was replaced with a DNA fragment encoding human EGFR (amino acids 31-1210). HeLa and HEK293T cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum and 1% penicillin streptomycin mix (Invitrogen). Transfection of siRNA or DNA constructs into HeLa cells or HEK293T cells was performed as described (Guo et al., 2013; Ma et al., 2018). siRNAs against human EXOC5 and EXOC6 were purchased from Ribobio. The commercial antibodies rabbit anti-Exoc5 (1:2000, Proteintech, 17593-1-AP) and rabbit anti-Exoc6 (1:2000, Proteintech, 12723-1-AP) were used to verify knockdown efficiencies using western blotting. Images were acquired with a Zeiss Axioobserver Z1 microscope system or Leica STED TCS SP5 II confocal laser scanning microscope.
For the RUSH transport assay, HeLa cells or HEK293T cells were transfected with plasmids encoding Str-KDEL and SBP-EGFP-EGFR for 24 h. To release the SBP-EGFP-EGFR from the ER, cells were treated with 40 μM D-biotin (Sigma-Aldrich) and 100 ng/μl cycloheximide (Sigma-Aldrich) for the indicated time.
STORM imaging
The imaging buffer for two-color STORM was designed for the two-dye combination of Alexa 647 and Alexa 750 (Zhao et al., 2015). The buffer contained 10% (w/v) glucose, 25 mM Tris(2-carboxyethyl) phosphine hydrochloride solution (TCEP; Sigma-Aldrich, 646547), 2 mM cyclooctatetraene (Sigma-Aldrich, 138924), 560 µg/ml glucose oxidase, 40 µg/ml catalase, 50 mM Tris-HCl pH 8.0, 1 mM ascorbic acid and 1 mM methyl viologen. The composition of the imaging buffer provided matched and balanced switching characteristics for both dyes (Zhao et al., 2015). The sample was mounted on a customized glass-bottom chamber filled with imaging buffer. After the region of interest was identified, the laser power was increased to 4 kW/cm2 in both channels enabling rapid ‘blinking’ of dye molecules for single molecule detection and localization. The blinking was recorded by an EMCCD at 30 Hz for 15,000-20,000 frames based on the abundance of proteins. When each frame was captured, the peak-finding algorithm recognized the sites of blinking, followed by the fitting algorithm, which determined the centroid of each blinking with nanometer accuracy. These centroids were registered to the final super-resolution image. In addition, active sample locking was applied to stabilize the sample with nanometer accuracy in the xy plane and z-axis during acquisition. Home-build software was used to generate the localization histogram plotting the cross-section of each protein. Colocalizations of the STORM images were performed using the FIJI colocalization Test function. Each super-resolution image used for quantification is the image showing the localization patterns of the indicated protein in the whole juxtanuclear area of each individual cell.
Supplementary Material
Acknowledgements
We would like to thank Developmental Studies Hybridoma Bank and Bloomington Drosophila Stock Center for reagents, and the Ni and Xie lab members for comments and discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.M., R.T., Y.H., J.N., Y.G., T.X.; Validation: R.T.; Formal analysis: Y.M., R.T., Y.H., J.N., Y.G., T.X.; Investigation: Y.M., R.T., Y.H., J.W.; Resources: D.M., Y.Z.; Data curation: Y.M., R.T., Y.H., P.K.L.; Writing - original draft: Y.M., R.T., Y.H., Y.G., T.X.; Writing - review & editing: J.N., Y.G., T.X.; Supervision: J.N., Y.G., T.X.; Project administration: J.N., Y.G., T.X.; Funding acquisition: J.N., Y.G., T.X.
Funding
This work was supported by Tsinghua-Peking Joint Center for Life Sciences (Y.M.), the Ministry of Science and Technology of China (2013CB35102 to J.N.), the National Natural Science Foundation of China (31371496 to J.N.; 31871421 to Y.G.), Hong Kong Research Grants Council (26100315, 16101116, 16102218, AoE/M-05/12 and C4002-17G to Y.G.), the National Institutes of Health (R01HD097664 to T.X.), and the Stowers Institute for Medical Research (T.X.). Deposited in PMC for release after 12 months.
Data availability
Original data underlying this manuscript can be accessed from the Stowers Original Data Repository at http://www.stowers.org/research/publications/libpb-1431
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.174615.supplemental
References
- Ahmed S. M., Nishida-Fukuda H., Li Y., McDonald W. H., Gradinaru C. C. and Macara I. G. (2018). Exocyst dynamics during vesicle tethering and fusion. Nat. Commun. 9, 5140 10.1038/s41467-018-07467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisch T. U., Maimon I., Dadosh T. and Gilboa L. (2017). Escort cells generate a dynamic compartment for germline stem cell differentiation via combined Stat and Erk signalling. Development 144, 1937-1947. 10.1242/dev.143727 [DOI] [PubMed] [Google Scholar]
- Boncompain G., Divoux S., Gareil N., de Forges H., Lescure A., Latreche L., Mercanti V., Jollivet F., Raposo G. and Perez F. (2012). Synchronization of secretory protein traffic in populations of cells. Nat. Methods 9, 493-498. 10.1038/nmeth.1928 [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. (2008). Exocytosis provides the membrane for protrusion, at least in migrating fibroblasts. Nat. Rev. Mol. Cell Biol. 9, 916 10.1038/nrm2419-c3 [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodríguez-Fraticelli A. E., Peränen J., Martín-Belmonte F. and Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035-1045. 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. and McKearin D. (2003). Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13, 1786-1791. 10.1016/j.cub.2003.09.033 [DOI] [PubMed] [Google Scholar]
- Decotto E. and Spradling A. C. (2005). The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501-510. 10.1016/j.devcel.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Eliazer S., Palacios V., Wang Z., Kollipara R. K., Kittler R. and Buszczak M. (2014). Lsd1 restricts the number of germline stem cells by regulating multiple targets in escort cells. PLoS Genet. 10, e1004200 10.1371/journal.pgen.1004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S., Shalaby N. A. and Buszczak M. (2011). Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 108, 7064-7069. 10.1073/pnas.1015874108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B., Zuo X., Buonato J. M., Vasilyev A., Baek J.-I., Choi S. Y., Chacon-Heszele M. F., Palmyre A., Polgar N., Drummond I. et al. (2014). Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am. J. Physiol. Renal Physiol. 307, F1334-F1341. 10.1152/ajprenal.00032.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Geng C., Wang H., Yang Z., Weng C., Li H., Deng L., Liu L., Liu N., Ni J. et al. (2015). Twin promotes the maintenance and differentiation of germline stem cell lineage through modulation of multiple pathways. Cell Rep. 13, 1366-1379. 10.1016/j.celrep.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Fuller M. T. and Spradling A. C. (2007). Male and female Drosophila germline stem cells: two versions of immortality. Science 316, 402-404. 10.1126/science.1140861 [DOI] [PubMed] [Google Scholar]
- Guo Y., Zanetti G. and Schekman R. (2013). A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. eLife 2, e00160 10.7554/eLife.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B., Foys B. and Levine M. (2010). Vectors and parameters that enhance the efficacy of RNAi-mediated gene disruption in transgenic Drosophila. Proc. Natl. Acad. Sci. USA 107, 11435-11440. 10.1073/pnas.1006689107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B. A., Wolff T. and Rubin G. M. (1994). Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121-2129. [DOI] [PubMed] [Google Scholar]
- He C., Hobert M., Friend L. and Carlin C. (2002). The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, including a dominant signal with a polyproline core. J. Biol. Chem. 277, 38284-38293. 10.1074/jbc.M104646200 [DOI] [PubMed] [Google Scholar]
- Hobert M. E., Kil S. J., Medof M. E. and Carlin C. R. (1997). The cytoplasmic juxtamembrane domain of the epidermal growth factor receptor contains a novel autonomous basolateral sorting determinant. J. Biol. Chem. 272, 32901-32909. 10.1074/jbc.272.52.32901 [DOI] [PubMed] [Google Scholar]
- Huang J., Reilein A. and Kalderon D. (2017). Yorkie and Hedgehog independently restrict BMP production in escort cells to permit germline differentiation in the Drosophila ovary. Development 144, 2584-2594. 10.1242/dev.147702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y. and Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761-771. [DOI] [PubMed] [Google Scholar]
- Jin Z., Flynt A. S. and Lai E. C. (2013). Drosophila piwi mutants exhibit germline stem cell tumors that are sustained by elevated Dpp signaling. Curr. Biol. 23, 1442-1448. 10.1016/j.cub.2013.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso R. J., Buszczak M., Quinones A. T., Castiblanco C., Mazzalupo S. and Cooley L. (2004). Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 32, D418-D420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil S. J., Hobert M. and Carlin C. (1999). A leucine-based determinant in the epidermal growth factor receptor juxtamembrane domain is required for the efficient transport of ligand-receptor complexes to lysosomes. J. Biol. Chem. 274, 3141-3150. 10.1074/jbc.274.5.3141 [DOI] [PubMed] [Google Scholar]
- Kirilly D., Wang S. and Xie T. (2011). Self-maintained escort cells form a germline stem cell differentiation niche. Development 138, 5087-5097. 10.1242/dev.067850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Rossé C., Racine V., Sibarita J.-B., Aresta S., Murthy M., Schwarz T., Camonis J. and Bellaiche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365-376. 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Li L. and Xie T. (2005). Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605-631. 10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Li C., Kan L., Chen Y., Zheng X., Li W., Zhang W., Cao L., Lin X., Ji S., Huang S. et al. (2015). Ci antagonizes Hippo signaling in the somatic cells of the ovary to drive germline stem cell differentiation. Cell Res. 25, 1152-1170. 10.1038/cr.2015.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. and Spradling A. C. (1993). Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159, 140-152. 10.1006/dbio.1993.1228 [DOI] [PubMed] [Google Scholar]
- Lin H., Yue L. and Spradling A. C. (1994). The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120, 947-956. [DOI] [PubMed] [Google Scholar]
- Liu M., Lim T. M. and Cai Y. (2010). The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci. Signal. 3, ra57 10.1126/scisignal.2000740 [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhong G., Chai P. C., Luo L., Liu S., Yang Y., Baeg G.-H. and Cai Y. (2015). Coordinated niche-associated signals promote germline homeostasis in the Drosophila ovary. J. Cell Biol. 211, 469-484. 10.1083/jcb.201503033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Wang S., Gao Y., Mao Y., Yang Z., Liu L., Song X., Ni J. and Xie T. (2015). COP9-Hedgehog axis regulates the function of the germline stem cell progeny differentiation niche in the Drosophila ovary. Development 142, 4242-4252. 10.1242/dev.124768 [DOI] [PubMed] [Google Scholar]
- Luo L., Wang H., Fan C., Liu S. and Cai Y. (2015). Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J. Cell Biol. 209, 595-608. 10.1083/jcb.201409142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang S., Do T., Song X., Inaba M., Nishimoto Y., Liu L.-P., Gao Y., Mao Y., Li H. et al. (2014). ZPiwi is required in multiple cell types to control germline stem cell lineage development in the Drosophila ovary. PLoS ONE 9, e90267 10.1371/journal.pone.0090267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Li B., Wang R., Lau P. K., Huang Y., Jiang L., Schekman R. and Guo Y. (2018). A mechanism for differential sorting of the planar cell polarity proteins Frizzled6 and Vangl2 at the trans-Golgi network. J. Biol. Chem. 293, 8410-8427. 10.1074/jbc.RA118.001906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon I., Popliker M. and Gilboa L. (2014). Without children is required for Stat-mediated zfh1 transcription and for germline stem cell differentiation. Development 141, 2602-2610. 10.1242/dev.109611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J. and Spradling A. (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797-3807. [DOI] [PubMed] [Google Scholar]
- Michel M., Raabe I., Kupinski A. P., Pérez-Palencia R. and Bökel C. (2011). Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche. Nat. Commun. 2, 415 10.1038/ncomms1426 [DOI] [PubMed] [Google Scholar]
- Morris L. X. and Spradling A. C. (2011). Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138, 2207-2215. 10.1242/dev.065508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J. and Spradling A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598-611. 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier-Pavie V. I., Palacios V., Eliazer S., Scoggin S. and Buszczak M. (2016). The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev. Biol. 417, 50-62. 10.1016/j.ydbio.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M., Garza D., Scheller R. H. and Schwarz T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433-447. 10.1016/S0896-6273(03)00031-X [DOI] [PubMed] [Google Scholar]
- Murthy M., Ranjan R., Denef N., Higashi M. E., Schupbach T. and Schwarz T. L. (2005). Sec6 mutations and the Drosophila exocyst complex. J. Cell Sci. 118, 1139-1150. 10.1242/jcs.01644 [DOI] [PubMed] [Google Scholar]
- Ni J.-Q., Markstein M., Binari R., Pfeiffer B., Liu L.-P., Villalta C., Booker M., Perkins L. and Perrimon N. (2008). Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5, 49-51. 10.1038/nmeth1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellers N. and Hafen E. (1996). Biochemical characterization of rolledSem, an activated form of Drosophila mitogen-activated protein kinase. J. Biol. Chem. 271, 24939-24944. 10.1074/jbc.271.40.24939 [DOI] [PubMed] [Google Scholar]
- Schekman R. (2010). Charting the secretory pathway in a simple eukaryote. Mol. Biol. Cell 21, 3781-3784. 10.1091/mbc.e10-05-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C., Wood C. G., Jones D. L., Tazuke S. I. and Fuller M. T. (2002). Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523-4534. [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C. H., Doan C. and Xie T. (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855-1857. 10.1126/science.1069871 [DOI] [PubMed] [Google Scholar]
- Song X., Wong M. D., Kawase E., Xi R., Ding B. C., McCarthy J. J. and Xie T. (2004). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353-1364. 10.1242/dev.01026 [DOI] [PubMed] [Google Scholar]
- Song X., Call G. B., Kirilly D. and Xie T. (2007). Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134, 1071-1080. 10.1242/dev.003392 [DOI] [PubMed] [Google Scholar]
- Spradling A. C. (1993). Developmental genetics of oogenesis. In The Development of Drosophila Melanogaster (ed. Bate M. and Martinez Arias A.), pp. 1-71. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Spradling A., Fuller M. T., Braun R. E. and Yoshida S. (2011). Germline stem cells. Cold Spring Harbor Perspect. Biol. 3, a002642.. 10.1101/cshperspect.a002642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. H., Rastegri E., Kao S. H., Lai C. M., Lin K. Y., Liao H. Y., Wang M. H. and Hsu H. J. (2018). Diet regulates membrane extension and survival of niche escort cells for germline homeostasis via insulin signaling. Development 145, dev159186 10.1242/dev.159186 [DOI] [PubMed] [Google Scholar]
- Tan X., Thapa N., Sun Y. and Anderson R. A. (2015). A kinase-independent role for EGF receptor in autophagy initiation. Cell 160, 145-160. 10.1016/j.cell.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-Y., Su Y.-H., Yang S.-M., Lin K.-Y., Lai C.-M., Rastegari E., Amartuvshin O., Cho Y., Cai Y. and Hsu H.-J. (2018). Smad-independent BMP signaling in somatic cells limits the size of the germline stem cell pool. Stem Cell Rep. 11, 811-827. 10.1016/j.stemcr.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pan L., Wang S., Zhou J., McDowell W., Park J., Haug J., Staehling K., Tang H. and Xie T. (2011). Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation. PLoS Genet. 7, e1002426 10.1371/journal.pgen.1002426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Gao Y., Song X., Ma X., Zhu X., Mao Y., Yang Z., Ni J., Li H., Malanowski K. E. et al. (2015). Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. Elife 4, e08174 10.7554/eLife.08174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. and Page-McCaw A. (2018). Wnt6 maintains anterior escort cells as an integral component of the germline stem cell niche. Development 145, dev158527 10.1242/dev.158527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. J., Shcherbata H. R., Reynolds S. H., Fischer K. A., Hatfield S. D. and Ruohola-Baker H. (2006). Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr. Biol. 16, 2352-2358. 10.1016/j.cub.2006.10.022 [DOI] [PubMed] [Google Scholar]
- Wu B. and Guo W. (2015). The exocyst at a glance. J. Cell Sci. 128, 2957-2964. 10.1242/jcs.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. (2013). Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: concerted actions of niche signals and intrinsic factors. WIREs Dev. Biol. 2, 261-273. 10.1002/wdev.60 [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. C. (1998). decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251-260. 10.1016/S0092-8674(00)81424-5 [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. C. (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328-330. 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. (2001). The drosophila ovary: an in vivo stem cell system. In Stem Cell Biology (ed. Marshak D. R., Gardner R. L. and Gottlieb D.), pp. 129-148. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Zhao T., Wang Y., Zhai Y., Qu X., Cheng A., Du S. and Loy M. M. T. (2015). A user-friendly two-color super-resolution localization microscope. Opt. Express 23, 1879-1887. 10.1364/OE.23.001879 [DOI] [PubMed] [Google Scholar]
- Zhu C. H. and Xie T. (2003). Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development 130, 2579-2588. 10.1242/dev.00499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.